Abstract
Objective
Statins are commonly prescribed cholesterol-lowering drugs. Preclinical studies suggest that statins may possess cancer preventive properties. The primary objective of this meta-analysis was to determine the association between statin use and risk of liver cancer.
Design
Meta-analysis.
Setting
International.
Participants
A comprehensive literature search of PubMed, BIOSIS Previews, Web of Science, EMBASE, EBSCO and Cochrane Library was conducted through March 2014. The effect estimate was reported as pooled relative risk (RR) with 95% CIs, using the random-effects model.
Results
A total of 12 studies (1 individual patient data analysis of 22 randomised controlled trials, 5 cohorts and 6 case–controls) were qualified for this meta-analysis, involving 5 640 313 participants including 35 756 liver cancer cases. Our results indicated a significant risk reduction of liver cancer among all statin users (RR=0.58, 95% CIs 0.51 to 0.67). The difference of the study designs can partly explain the significant heterogeneity found in the overall analysis (I2=65%, p=0.0006). No evidence of publication bias was observed in this meta-analysis. Similar risk reductions were found in the subgroups analysis of Western and Asian countries, lipophilic and hydrophilia statins. There was a trend towards more risk reductions in subgroups with higher baseline risk, inadequate adjustment and higher cumulative dosage of statin use.
Conclusions
This meta-analysis suggests that statin is associated with a significant risk reduction of liver cancer when taken daily for cardiovascular event prevention. However, this preventive effect might be overestimated due to the exposure period, the indication and contraindication of statins and other confounders. Statins might be considered as an adjuvant in the treatment of liver cancer.
Strengths and limitations of this study.
Statins are commonly prescribed as cholesterol-lowering drugs. In this comprehensive meta-analysis, we demonstrate that statin use is associated with a significant risk reduction of liver cancer.
The difference of the study designs is part of the reason explaining the significant heterogeneity found in the overall analysis.
However, this preventive effect might be overestimated due to the exposure period, the indication and contraindication of statins and other confounders.
Statins might be considered as an adjuvant in the treatment of liver cancer.
Introduction
Statins are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase, widely used to reduce plasma cholesterol levels and the risk of cardiovascular events.1 Although there is a concern over their possible carcinogenicity raised in rodent studies,2 preclinical studies indicate that statins have anticancer properties in vitro and in vivo, through inhibiting angiogenesis, inducing apoptosis, and suppressing tumour growth and metastasis.3–5
However, higher concentrations of statins are typically required to induce these effects, raising questions concerning the therapeutic relevance of statins on cancer.6 To date, clinical studies regarding the cancer incidence associated with statin administration have highlighted conflicting results. Moreover, a large number of meta-analyses have concluded that there was no association between statin use and risk of overall cancer,7–10 or cancer of breast,11 stomach12 or pancreas.13 There is only a modest protective effect of statins in prostate cancer14 and colorectal cancer.15
On the contrary, recent studies reported encouraging results for risk reduction of liver cancer among all statin users. Previous meta-analysis, conducted by Singh et al16 through including 10 studies, found that statin users were less likely to develop hepatocellular carcinoma (HCC) than statin non-users. However, Singh et al included the ALERT, LIPS and MEGA trials twice by including three individual patient data (IPD) analysis of randomised controlled trials (RCTs).17–19 Meanwhile, some factors of stratification were not considered in their analyses, such as dose and timing of exposure to statins, and the selection of controls and confounders, which might limit the evaluation of cancer risk.20 Furthermore, lipophilic statins are accompanied by an extensive first-pass effect at the hepatic level.21 It is plausible that lipophilic statins may have better liver cancer preventive qualities than hydrophilic statins.22
Therefore, we performed this updated meta-analysis to assess the association between statin use and risk of liver cancer, involving the recently published studies and conducting more subgroup analyses based on the factors aforementioned. Our results demonstrated that statin use was associated with an over 40% risk reduction in liver cancer, which may have a significant translational potential in clinical practice. However, there were some confounders that might overestimate this preventive effect of statins.
Materials and methods
Literature search strategy
This meta-analysis was conducted following the PRISMA guidelines.23
The systematic computerised search for eligible studies were performed on the database of PubMed, BIOSIS Previews, Web of Science, EMBASE, EBSCO and Cochrane Library, covering all studies published from their inception to 5 March 2014. The following terms were searched with the subjects (MeSH terms) as well as text-word search strategies: “(Statin OR HMG-CoA reductase inhibitors OR Atorvastatin OR Cerivastatin OR Fluvastatin OR Lovastatin OR Pravastatin OR Rosuvastatin OR Simvastatin) AND (Hepatocellular OR Hepatic OR Intrahepatic OR Interlobular OR Liver) AND (Carcinoma OR Sarcomas OR Angiosarcoma OR Cancer OR Neoplasm). Additionally, the relevant reviews and retrieved articles were searched manually for more eligible studies.
In study searching, only the original researches, published in the form of peer review articles or meeting abstracts, were included. No language restrictions were imposed. However, the studies we included were all published in English.
Study selection
The inclusion criteria were: (1) RCTs, cohort studies or case–control studies; (2) original studies that assessed the effect of statin use on the risk of liver cancer, compared with placebo or no treatment; (3) liver cancer cases identified according to the International Classification of Diseases codes (ICD) and (4) studies with estimate of relative risk (risk ratio, RR) of liver cancer, or with data sufficient to calculate it.
The exclusion criteria were: (1) study design not meeting the inclusion criteria; (2) studies without estimate of RR, or without sufficient data to calculate it or (3) studies with duplicated or overlap reports.
Data extraction
Two independent investigators (MS and XC) extracted data from the eligible studies using a predefined data collection form. The differences of data extraction were resolved by consensus referring back to the original article. The extracted information included: (1) studies: first author, year of publication, study design, location, patient populations, period and follow-up; (2) statins: type, dosage or duration of statin use and (3) liver cancer: case identification, number of liver cancers, crude RR with 95% CIs, adjusted RR reflecting the greatest degree of control for confounders and confounders for adjustment (including variables for matching). When the RR data were not available, the RR with 95% CIs were calculated from the raw data in original studies.
We extracted different measurements of effect estimates from original studies, such as RR, OR, HR and Observed/Expected ratio. Owing to the fact that the incidence of liver cancer was low in all studies, these different measurements can be used to provide similar estimates of RR.
Methodological quality assessment
Of note, the included RCT was pooled analysis of other RCTs, therefore it is inappropriate to assess the methodological quality. The methodological quality of cohort and case–control studies were assessed on the Newcastle-Ottawa Scale,24 including eight items that were categorised into three categories: selection (four items, one star each), comparability (one item, up to two stars) and exposure/outcome (three items, one star each). A ‘star’ presents a ‘high’-quality choice of each item.
Statistical analysis
The overall meta-analysis was first performed, followed by the subgroup analyses, based on study design, baseline risk of liver cancer, confounding adjustment, study location and pharmacokinetics. Meanwhile, we conducted subgroup analyses based on studies that reported RR estimates for higher cumulative dosage of statin use, when appropriate data were available.
To take into account the heterogeneity and provide a more conservative estimate, the inverse variance method was used to estimate the pooled RRs and corresponding 95% CIs, and data were pooled using a random effects model. Heterogeneity was assessed using the χ2 statistic (P) together with the Higgins I2 statistic (I2), a p value <0.10 was considered statistically significant for heterogeneity; and an I2 value >50% was considered a measure of severe heterogeneity.25
Publication bias was assessed using the Begg's test and the Egger's test.26 Influence analysis was performed to investigate the influence of a single study on the overall meta-analysis estimate, by omitting one study in each turn. Test for interaction was applied to identify the difference between pooled RRs from subgroup analysis using the method described by Altman and Bland.27 All statistical tests were two-sided and p<0.05 was considered statistically significant, unless otherwise specified. Software Review Manager (RevMan5.2, Copenhagen) and STATA (Stata V.11.2, Texas, USA) were used for the statistical analysis.
Results
Study selection
Figure 1 illustrates the process of study selection for the meta-analysis. Of the 1424 potentially relevant references identified by electric and manual search, 142 were selected for full-text review after screening titles and abstracts. Finally, a total of 12 studies were included, with one IPD analysis,19 five cohort studies,28–32 and six case–control studies.33–38 One case–control study was presented solely in abstract form.33
Figure 1.
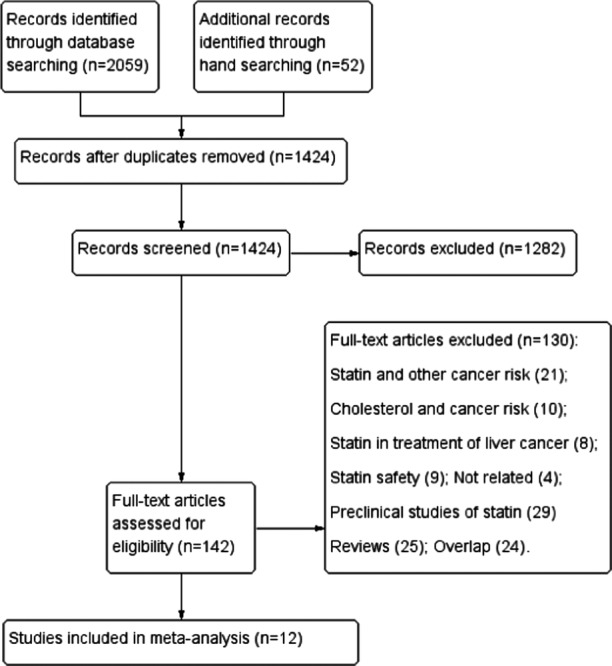
Flow chart of study selection in the present meta-analysis.
Of note, the cohort study conducted by Friedman et al29 reported RR estimates separately for different genders (male and female), we considered these two reports as separate studies. Therefore, a total of 13 reports were included for the present meta-analysis.
Study characteristics
Table 1 summarises the characteristics of qualified studies in this meta-analysis. The 12 studies, involving 5 640 313 participants with 35 756 liver cancer cases, were published between 2005 and 2013. The ‘RCT’ in the present study was pooled analysis of 22 clinical trials,19 which investigated statin therapy in cardiovascular event prevention and reported the occurrence of liver cancer as an adverse event. The observational studies were conducted with the local or national health databases, statin exposures were identified by linkage to prescription databases, and the controls were matched mainly by age, sex and index date. Except for one cohort, which adopted ICD-10 C22,28 all other studies identified liver cancer cases according to the ICD-9 155. Of note, two cohorts were restricted to patients with hepatitis B virus (HBV)31 and hepatitis C virus (HCV) infections32; one case–control only included patients with diabetes mellitus34; and two observational studies included patients aged at least 45 years.30 35
Table 1.
Study characteristics
| Studies | Study design | Patient population | Study period | Cases defined | Follow-up (years) | Statin types | Dosage/duration of statin use |
|---|---|---|---|---|---|---|---|
| Emberson et al,19 UK | RCT | IPD analysis of 22 RCTs | – | ICD-9 155 | 5.1 (Me) | A, F, L, P, R and S | 5.1 years (Me) |
| Friis et al,28 North Jutland | Cohort | General population (CPR) | 1989–2002 | ICD-10 C22 | 3.3 (M) | Unspecified | ≥2 Rx |
| Friedman et al,29 USA | Cohort | General population (KPMCP) | 1994–2003 | ICD-9-CM 155 | >2 | A, L and S (97.6%) | ≥1 Rx |
| Marelli et al,30 USA | Cohort | General older population (men ≥45 and women ≥55 years; GE Centricity) | 1990–2009 | ICD-9 155 | 4.6 (M) | Unspecified | ≥1 cDDD |
| Tsan et al,31 Taiwan | Cohort | Patients with HBV infection (NHIRD) | 1997–2008 | ICD-9 155 | 9.9 (M) | A, F, L, P, R and S | ≥28 cDDDs |
| Tsan et al,32 Taiwan | Cohort | Patients with HCV infection (NHIRD) | 1999–2010 | ICD-9 155 | 10.7 (M) | A, F, L, P, R and S | ≥28 cDDDs |
| Khurana et al,33 USA | Case–control | General population (VISN) | 1997–2002 | ICD-9 155 | NR | Unspecified | ≥1 Rx |
| El-Serag et al,34 USA | Case–control | Diabetes patients (VA) | 1997–2002 | ICD-9-CM 155 | 2.4 (M) | A, C, F, L, P and S | 1.6 years (M) |
| Chiu et al,35 Taiwan | Case–control | Older patients (≥50 years; NHIRD) | 2005–2008 | ICD-9-CM 155 | NR | A, F, L, P, R and S | ≥1 cDDD |
| Lai et al,36 Taiwan | Case–control | General population (NHIRD) | 2000–2009 | ICD-9-CM 155 | 1.4 (M) | A, F, L, P, R and S | ≥1 Rx |
| Leung et al,37 Taiwan | Case–control | General population (NHIRD) | 2000–2008 | ICD-9-CM 155 | 4.1 (M) | Unspecified | >0.5 years |
| Chaiteerakij et al,38 USA | Case–control | Hyperlipidaemia patients (Mayo Clinic) | 2000–2010 | ICD-9-CM 155 | >1 | Unspecified | ≥1 Rx |
Duration of follow-up: When the follow-up periods of statin user and non-user were different, only the shorter period was shown, and all periods were transformed to years.
≥1 cDDD=more than 1 cumulative defined daily dose before the diagnosis of liver cancer; A, atorvastatin; C, cerivastatin; CM, clinical modification; CRP, the Central Population Register of Danish citizens; F, fluvastatin; GE Centricity, the General Electric Centricity database; HBV, hepatitis B virus; HCV, hepatitis C virus; ICD-9 or ICD-10, International Classification of Diseases, Ninth Revision or Tenth Revision; IPD, individual patient data; KPMCP, the Kaiser Permanente Medical Care Program in northern California; L, lovastatin; M, mean; Mayo Clinic, Mayo Clinic (Rochester, MN); Me, median; NHIRD, the Taiwanese National Health Insurance research database; non-statin, non-statin cholesterol-lowering drug(s) only; NR, not reported; P, pravastatin; R, rosuvastatin; RCT, randomised controlled trials; Rx, prescriptions; S, simvastatin; VA, Veterans Affairs national databases; VISN, Veterans Integrated Service Networks 16 Veteran Affairs database.
Table 2 summarises the data of the included studies. In the RCT19 and one cohort study,30 the RR with 95% CIs were calculated from the 2×2 tables defined by the incidence of liver cancer and statin use status. The observational studies reported different measurements of RR estimates with adjustment by confounders. Several observational studies adopted the important risk factors of liver cancer for adjustments,31 32 34–36 such as HBV infection, HCV infection, cirrhosis and alcoholic liver disease or non-alcoholic fatty liver disease (NAFLD).39 Of note, only two studies adjusted for cholesterol level,30 38 and no study adjusted for metabolic syndrome, which might also influence the risk of liver cancer.39
Table 2.
Study data
| Studies | Intervention/cases |
Control |
Measurements of effect estimates | Crude RR with 95% CIs | Adjusted RR with 95% CIs | Confounders for adjustment | ||
|---|---|---|---|---|---|---|---|---|
| Number of event/number of exposure | Number of total | Number of event/ number of exposure | Number of total | |||||
| Emberson et al,19 UK | 35 | 67 258 | 33 | 67 279 | RR | 1.06 (0.66 to 1.71)* | 1.06 (0.66 to 1.71)* | Randomisation |
| Friis et al,28 North Jutland | 1 | 12 251 | 166 | 334 754 | OR | NA | 1.16 (0.46 to 2.90) | 1,2, 16, 21, 23 |
| Friedman et al (male),29 USA | 32 | 192 598 | NA | 1 904 876 | HR | NA | 0.49 (0.34 to 0.70) | 16 |
| Friedman et al (female),29 USA | 10 | 169 261 | NA | 1 976 332 | HR | NA | 0.40 (0.21 to 0.75) | |
| Marelli et al,30 USA | 13 | 45 857 | 24 | 45 857 | RR | 0.31 (0.14 to 0.68)* | 0.31 (0.14 to 0.68)* | 1–5, 14, 16–18, 26, 27 |
| Tsan et al,31 Taiwan | 58 | 2785 | 963 | 30 628 | HR | 0.66 (0.51 to 0.86) | 0.47 (0.36 to 0.61) | 1, 2, 7, 8, 11, 12 |
| Tsan et al,32 Taiwan | 1378 | 35 023 | 26 505 | 225 841 | HR | 0.42 (0.39 to 0.46) | 0.53 (0.49 to 0.58) | 1, 2, 7, 8, 11, 13 |
| Khurana et al,33 USA | NA | NA | NA | NA | OR | NA | 0.52 (0.41 to 0.67) | 1, 11, 13 |
| El-Serag et al,34 USA | 447 | 1303 | 2766 | 5212 | OR | 0.46 (0.40 to 0.52) | 0.74 (0.64 to 0.87) | 1–3, 6, 8, 9, 11–13, 21, 24, 28 |
| Chiu et al,35 Taiwan | 117 | 1166 | 195 | 1166 | OR | 0.53 (0.41 to 0.69) | 0.62 (0.45 to 0.83) | 1, 2, 8, 9, 11, 12, 20, 29 |
| Lai et al,36 Taiwan | 255 | 3480 | 1635 | 13 920 | OR | 0.61 (0.52 to 0.72) | 0.71 (0.56 to 0.89) | 1, 2, 8–13, 22, 24, 25 |
| Leung et al,37 Taiwan | 26 | 424 | 6851 | 33 781 | HR | 0.45 (0.30 to 0.67) | 0.44 (0.28, 0.72) | 1, 2, 11, 15, 20, 21, 23 |
| Chaiteerakij et al,38 USA | 72 | 165 | 165 | 256 | OR | NA | 0.6 (0.4 to 0.9) | 1–3, 8, 11, 17, 22, 28, 30 |
The RR with an asterisk mark (*) was calculated based on the raw data. The others, crude or adjusted, were extracted from the original paper; confounders for adjustment: 1=age, 2=sex, 3=race, 4=BMI, 5=smoking status, 6=ethanol intake, 7=socioeconomic status, 8=cirrhosis, 9=alcoholic liver disease, 10=non-alcoholic fatty liver disease, 11=diabetes mellitus, 12=HBV infection, 13=HCV infection, 14=concomitant diagnoses (unspecified), 15=Charlson score, 16=calendar year, 17=cholesterol (total cholesterol, VLDL, LDL or triglycerides), 18=prostate-specific antigen, 19=resection extent, 20=other lipid-lowering agents, 21=cardiovascular medications (aspirin, non-steroidal anti-inflammatory medications or ACE inhibitors), 22=metformin or thiazolidinedione, 23=hormone-replacement therapy, 24=HCV treatment, 25=HBV treatment, 26=medications taken (unspecified), 27=number of office visits, 28=propensity to use statins, 29=hospital stay, 30=biliary tract diseases.
BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; LDL, low-density lipoprotein; NA, not applicable; RR, relative risk; VLDL, very low-density lipoprotein.
Methodological quality
For the cohort and case–control studies, the median score was 7 on the Newcastle-Ottawa Scale, with a range 5–8 (see online supplementary table S1). These results indicated that the observational studies were of a reasonably good quality.
Overall meta-analysis
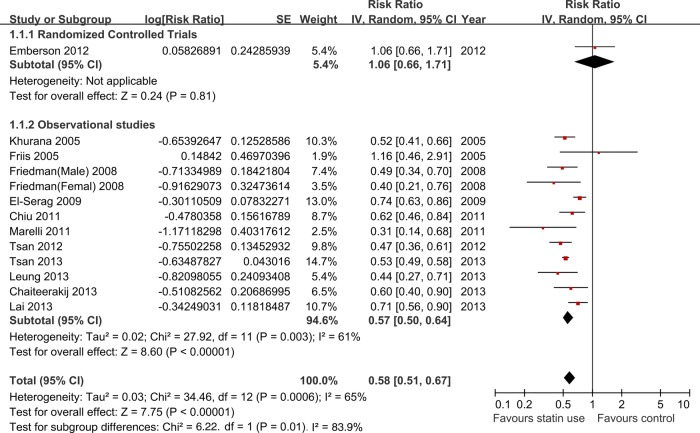
Figure 2 depicts the forest plot of RR estimate with 95% CIs from individual studies and overall meta-analysis. In the overall meta-analysis, pooled results showed a statistically significant decrease in liver cancer risk among all statin users (RR=0.58, 95% CIs 0.51 to 0.67). Of note, a statistically significant heterogeneity was observed (I2=65%, p=0.0006). The p values of Begg's test and Egger's test were 0.669 and 0.749, respectively, both suggesting there was no evidence of publication bias. In the influence analysis, the omission of any individual studies did not alter the direction and magnitude of the observed effect (see online supplementary figure S1).
Figure 2.
Overall meta-analysis of statin use and liver cancer risk.
Subgroup analyses and test for interaction
We first performed preplanned subgroup analyses based on study design, baseline risk of liver cancer, confounding adjustment and study location (table 3).
Table 3.
Subgroup analyses of included studies
| Study design | |||||
|---|---|---|---|---|---|
| Subgroup | Number of studies (reports) | Summary RR (95% CIs) | Heterogeneity, I2 (%) | Heterogeneity, p value | Pinteraction |
| RCT | 1 | 1.06 (0.66 to 1.71) | – | – | 0.01 |
| Observational studies | 11 (12) | 0.57(0.50 to 0.64) | 61 | 0.003 | |
| Observational studies | |||||
| Cohort studies | 5 (6) | 0.51 (0.44 to 0.58) | 18 | 0.30 | 0.04 |
| Case–control studies | 6 | 0.63 (0.54 to 0.73) | 46 | 0.10 | |
| Baseline risk of liver cancer | |||||
| Higher baseline risk | 4 | 0.52 (0.47 to 0.59) | 16 | 0.31 | 0.08 |
| General population | 8 (9) | 0.63 (0.52 to 0.75) | 59 | 0.01 | |
| Confounding adjustment | |||||
| Adequate adjustment | 6 | 0.64 (0.53 to 0.77) | 81 | 0.0001 | 0.08 |
| Inadequate adjustment | 6 (7) | 0.51 (0.43 to 0.60) | 3 | 0.40 | |
| Study location | |||||
| Western studies | 7 (8) | 0.61 (0.48 to 0.76) | 64 | 0.007 | 0.54 |
| Asian studies | 5 | 0.56 (0.48 to 0.64) | 51 | 0.09 | |
| Pharmacokinetic | |||||
| Lipophilic statins | 5 (6) | 0.57 (0.50 to 0.65) | 50 | 0.08 | 0.86 |
| Hydrophilia statins | 3 | 0.59 (0.41 to 0.84) | 50 | 0.13 | |
| Higher cumulative dosage of statin | 6 | 0.53 (0.36 to 0.79) | 90 | <0.0001 | – |
RR higher baseline risk of liver cancer: patients with older age, HBV or HCV infection. Adequate adjustment: RCT or studies, which adjusted for at least four of seven important confounders, such as HBV infection, HCV infection, cirrhosis, alcoholic liver disease, NAFLD, HBV treatment or HCV treatment; lipophilic statins: atorvastatin, fluvastatin, lovastatin or simvastatin; hydrophilia statins: pravastatin or rosuvastatin; higher cumulative dosage of statin use: >180 cumulative defined daily dose or duration of statin use >0.5 years before the diagnosis of liver cancer.
HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, non-alcoholic fatty liver disease; RCT, randomised controlled trials; RR, relative risk.
The RCT showed there is no significant association between statin use and risk of liver cancer (RR=1.06, 0.66 to 1.71). But the observational studies indicated a significant decrease of liver cancer risk among all statin users (RR=0.57, 0.50 to 0.64; I2=61%, p=0.003; figure 2). Furthermore, we found a greater risk reduction in the subgroup analysis of cohort studies (RR=0.51, 0.44 to 0.58; I2=18%, p=0.30) than in the case–control studies (RR=0.63, 0.54 to 0.73; I2=46%, p=0.10; see online supplementary figure S2).
Test for interaction showed significant results between subgroups of the RCT and observational studies (Pinteraction=0.01, Z=2.47), and between subgroups of the cohort and case–control studies (Pinteraction=0.04, Z=−2.03). These results indicated that the difference of the study designs was partly the reason why there was severe heterogeneity in the overall analysis (table 3).
In the subgroup analysis of the four studies with higher baseline risk of liver cancer,30–32 35 defined as patients with older age, HBV or HCV infection, there was a trend towards more decrease of liver cancer risk (RR=0.52, 0.47 to 0.59; I2=16%, p=0.31) than in the other eight studies with general population19 28 29 33 34 36–38 (RR=0.63, 0.52 to 0.75; I2=59%, p=0.01; see online supplementary figure S3).
We defined the RCT or studies adjusted for at least four of seven important confounders, such as HBV infection, HCV infection, cirrhosis, alcoholic liver disease, NAFLD, HBV treatment or HCV treatment,39 were adjusted adequately. Subgroup analysis of these six studies19 31 32 34–36 found a trend towards less decrease of liver cancer risk (RR=0.64, 0.53 to 0.77; I2=81% p=0.0001) than the other six studies28–30 33 37 38 (RR=0.51, 0.43 to 0.60; I2=3%, p=0.40; see online supplementary figure S4).
Subgroup analyses based on study location found a similar risk reduction of liver cancer in Western countries (RR=0.61, 0.48 to 0.76; I2=64%, p=0.007) and in Asian countries (RR=0.56, 0.48 to 0.64; I2=51%, p=0.09; see online supplementary figure S5).
Besides the overall RR estimates, some studies reported different RR estimates for different pharmacokinetics and dosage of statin use (see online supplementary table S2). We conducted further subgroup analyses based on these available data.
According to the different pharmacokinetics, statins can be classified as lipophilic statins (atorvastatin, fluvastatin, lovastatin and simvastatin) and hydrophilia statins (pravastatin and rosuvastatin).21 Subgroup analysis of lipophilic statins29 31 34–36 found a significant decrease of liver cancer risk (RR=0.57, 0.50 to 0.65; I2=50%, p=0.08). There was a similar result among users of hydrophilia statins31 35 36 (RR=0.59, 0.41 to 0.84; I2=50%, p=0.13; see online supplementary figure S6).
Test for interaction showed non-significant results for subgroups with different baseline risk, confounding adjustment, study location or pharmacokinetics (Pinteraction=0.08, 0.08, 0.54 and 0.86, respectively; table 3). Therefore, there is no strong evidence to support a different preventive effect of statins on liver cancer in these subgroups.
Subgroup analysis of six studies with higher cumulative dose of statin use, defined as statin use over 180 cumulative defined daily doses (cDDDs) or 0.5 years (cumulative duration), showed a trend towards more risk reduction of liver cancer (RR=0.53, 0.36 to 0.79), but with a high degree of heterogeneity (I2=90%, p<0.00001; see online supplementary figure S7).
Discussion
This present meta-analysis represents the most comprehensive review to date on the association between statin use and liver cancer risk, by including 12 studies (1 IPD analysis of 22 RCTs, 5 cohort studies and 6 case–control studies) and involving 5 640 313 participants with 35 756 liver cancer cases. Overall, we found that statin use was associated with an over 40% risk reduction in liver cancer compared with non-users (RR=0.58, 95% CIs 0.51 to 0.67). This result was in line with the previous three meta-analyses: Singh et al16 included 10 studies and suggested statin users were less likely to develop HCC (OR 0.63, 95% CIs 0.52 to 0.76), Pradelli et al40 and Zhang et al41 included 5 and 7 observational studies and found a summary RR of 0.58 (95% CIs 0.46 to 0.74) and 0.61 (95% CIs 0.49 to 0.76), respectively.
The IPD analysis of 22 RCTs showed there is no significant association between statin use and risk of liver cancer. The significant risk reduction of liver cancer among all statin users was seen primarily in the observational studies, and this preventive effect was relatively more convincing in the cohorts than in the case–controls. There were some reasons to explain the different findings between RCTs and observational studies.
First, the exposure period to statins might be shorter than the period to carcinogenesis and the latency to diagnosis in the cohorts and the case–controls. The observational studies defined statin use varying in dosage and duration, from patients who received ≥1 cDDD or >1 Rx of statins to more than 0.5 years (table 1). On the other hand, the median period of statin use was 5.1 years in the RCTs. Although there was a trend towards more risk reduction of liver cancer with higher cumulative dose of statin use, this defect might still result in overestimating the cancer-preventive effect of statins in the observational studies.
Second, clinical studies demonstrated that higher serum total cholesterol concentration was associated with decreased risk of liver cancer (see online supplementary table S3).42–44 Meanwhile, there were inverse associations between use of non-statin lipid-lowering drugs and risk of liver cancer.35 38 Meanwhile, because of the contraindication, statins might not be prescribed to patients with chronic liver disease, which is a known risk factor of liver cancer. Unfortunately, the observational studies included in this analysis seldom adopted these factors for adjustment. Actually, subgroup analysis of studies with adequate adjustment showed a trend towards less risk reduction, indicating the potential of overestimation of this preventive effect by confounders.
Third, the RCTs including a lower risk population (patients with cardiovascular disease rather than HBV/HCV infection), might not be powerful enough to investigate the liver cancer outcomes, which were much rarer than cardiovascular events. In addition, subgroup analysis of studies with higher baseline risk showed a trend towards a greater decrease of liver cancer risk.
These reasons suggested that the observed modulation of cancer incidence cannot be ascribable to a direct statin-mediated effect20; the exposure period, the indication (eg, hyperlipidaemia) and contraindication (eg, chronic liver disease) of statins might overestimate its cancer-preventive effect.
We found similar results in Western and Asian countries, which were different from the meta-analysis conducted by Singh et al, which concluded that the inverse association of statins with HCC was stronger in the Asian population. Considering four more studies we included, this difference might be caused by the insufficient data in their meta-analysis. Based on the pharmacokinetics, it is plausible that lipophilic and hydrophilic statins will differ in their liver cancer prevention qualities.21 22 However, subgroup analysis of lipophilic and hydrophilic statins showed similar results.
Besides the limitations described previously, there were some other limitations that should be noted. First, a significant heterogeneity was observed in the present meta-analysis, which might result from the difference in study design. Results of subgroup analyses would also be limited by this heterogeneity. Second, the adherence to statin therapy is known to be associated with a healthy lifestyle, which might affect the cancer outcome.45 Such information is hard to capture in databases or medical records in the observational studies.46 Third, five observational studies were conducted using the Taiwanese National Health Insurance Research Database (NHIRD).31 32 35–37 Although they were not all in the same period, these studies might contain overlapping groups of patients. These limitations aforementioned could lead to confounding of overall results from the present study, and should be considered in future studies aiming at confirming the protective effects of statins on human cancer risk.
The strengths of our meta-analysis were as follows: First, we performed a much more comprehensive search and more subgroup analyses, compared with the previous meta-analyses; second, the methodological quality of the included studies was reasonably good; third, publication bias, due to the tendency of not publishing small studies with null results, was not found in our meta-analysis.
Of note, preclinical studies have indicated that statins possess synergism with other therapeutic agents in vitro and in vivo for liver cancer.47 48 Some clinical studies have also demonstrated that statins would prolong survival in patients with advanced liver cancer (see online supplementary table S4),49–52 and are associated with risk reduction of recurrence after curative surgery in patients of HBV-related HCC.53 Therefore, considerable interest exists in adjunctive therapy with statins for liver cancer. In fact, there were some RCTs ongoing to determine the effectiveness of pravastatin, when used in combination with sorafenib, in the treatment of liver cancer (see online supplementary table S5).
Currently, physicians are less likely to prescribe statins for patients with chronic liver disease, based on the concerns about statin-induced liver injury.31 However, there were number of studies that have demonstrated their safe use and even salutary effects.54–56 Meanwhile, the risk of serious statin-related liver injury appears to be no greater than the background incidence of this rare event.57 Therefore, considering their benefits for cardiovascular event prevention and the potential effect in liver cancer prevention and treatment, statins should not be denied to these patients.
In conclusion, our results suggest that statin use is associated with significant risk reduction of liver cancer when taken daily for cardiovascular event prevention. However, this preventive effect might be overestimated due to the exposure period, indication and contraindication of statins, and other confounders. Statins might be considered as an adjuvant in the treatment of liver cancer.
Supplementary Material
Acknowledgments
The authors thank Medjaden Bioscience Limited and Gui Lv for assisting in the preparation and revision of this manuscript.
Footnotes
Contributors: XC had the original idea. MS, XC and WG worked together to develop an appropriate theoretical framework and design. XC developed the search. MS and XC were involved in the selection process. MS and XC extracted relevant data. XC and WG performed the statistical analysis and all authors were involved in the data interpretation. MS and BN wrote the manuscript draft and revised the draft based on input from the other authors. All authors revised it critically for content and approved the final version.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–78 [DOI] [PubMed] [Google Scholar]
- 2.Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA 1996;275:55–60 [PubMed] [Google Scholar]
- 3.Demierre MF, Higgins PD, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer 2005;5:930–42 [DOI] [PubMed] [Google Scholar]
- 4.Zeichner S, Mihos CG, Santana O. The pleiotropic effects and therapeutic potential of the hydroxy-methyl-glutaryl-CoA reductase inhibitors in malignancies: a comprehensive review. J Cancer Res Ther 2012;8:176–83 [DOI] [PubMed] [Google Scholar]
- 5.Lonardo A, Loria P. Potential for statins in the chemoprevention and management of hepatocellular carcinoma. J Gastroenterol Hepatol 2012;27:1654–64 [DOI] [PubMed] [Google Scholar]
- 6.Shimoyama S. Statins are logical candidates for overcoming limitations of targeting therapies on malignancy: their potential application to gastrointestinal cancers. Cancer Chemother Pharmacol 2011;67:729–39 [DOI] [PubMed] [Google Scholar]
- 7.Dale KM, Coleman CI, Henyan NN, et al. Statins and cancer risk: a meta-analysis. JAMA 2006;295:74–80 [DOI] [PubMed] [Google Scholar]
- 8.Browning DRL, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer 2007;120:833–43 [DOI] [PubMed] [Google Scholar]
- 9.Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: a systematic review and meta-analysis. Eur J Cancer 2008;44:2122–32 [DOI] [PubMed] [Google Scholar]
- 10.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Undela K, Srikanth V, Bansal D. Statin use and risk of breast cancer: a meta-analysis of observational studies. Breast Cancer Res Treat 2012;135:261–9 [DOI] [PubMed] [Google Scholar]
- 12.Shimoyama S. Statins and gastric cancer risk. Hepatogastroenterology 2011;58:1057–61 [PubMed] [Google Scholar]
- 13.Cui X, Xie Y, Chen M, et al. Statin use and risk of pancreatic cancer: a meta-analysis. Cancer Causes Control 2012;23:1099–111 [DOI] [PubMed] [Google Scholar]
- 14.Bansal D, Undela K, D'Cruz S, et al. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS ONE 2012;7:e46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardou M, Barkun A, Martel M. Effect of statin therapy on colorectal cancer. Gut 2010;59:1572–85 [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Singh PP, Singh AG, et al. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 2013;144:323–32 [DOI] [PubMed] [Google Scholar]
- 17.Stein EA, Corsini A, Gimpelewicz CR, et al. Fluvastatin treatment is not associated with an increased incidence of cancer. Int J Clin Pract 2006;60:1028–34 [DOI] [PubMed] [Google Scholar]
- 18.Matsushita Y, Sugihara M, Kaburagi J, et al. Pravastatin use and cancer risk: a meta-analysis of individual patient data from long-term prospective controlled trials in Japan. Pharmacoepidemiol Drug Saf 2010;19:196–202 [DOI] [PubMed] [Google Scholar]
- 19.Emberson JR, Kearney PM, Blackwell L, et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS ONE 2012;7:e29849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gazzerro P, Bifulco M. Statins and cancer in gastroenterology: new insight? Gastroenterology 2013;144:1572–3 [DOI] [PubMed] [Google Scholar]
- 21.Gazzerro P, Proto MC, Gangemi G, et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev 2012;64:102–46 [DOI] [PubMed] [Google Scholar]
- 22.Gronich N, Rennert G. Beyond aspirin—cancer prevention with statins, metformin and bisphosphonates. Nat Rev Clin Oncol 2013;10:625–42 [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 25.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.METABIAS: Stata module to test for small-study effects in meta-analysis [program], 2009
- 27.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friis S, Poulsen AH, Johnsen SP, et al. Cancer risk among statin users: a population-based cohort study. Int J Cancer 2005;114:643–7 [DOI] [PubMed] [Google Scholar]
- 29.Friedman GD, Flick ED, Udaltsova N, et al. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf 2008;17:27–36 [DOI] [PubMed] [Google Scholar]
- 30.Marelli C, Gunnarsson C, Ross S, et al. Statins and risk of cancer a retrospective Cohort analysis of 45,857 matched Pairs from an electronic medical records database of 11 million adult Americans. J Am Coll Cardiol 2011;58:530–7 [DOI] [PubMed] [Google Scholar]
- 31.Tsan YT, Lee CH, Wang JD, et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol 2012;30:623–30 [DOI] [PubMed] [Google Scholar]
- 32.Tsan YT, Lee CH, Ho WC, et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol 2013;31:1514–21 [DOI] [PubMed] [Google Scholar]
- 33.Khurana V, Saluja A, Caldito G, et al. Statins are protective against hepatocellular cancer in patients with hepatitis C virus infection: half a million US veterans’ study. Gastroenterology 2005;128(4, Suppl 2):A714 [Google Scholar]
- 34.El-Serag HB, Johnson ML, Hachem C, et al. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology 2009;136:1601–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu HF, Ho SC, Chen CC, et al. Statin use and the risk of liver cancer: a population-based case-control study. Am J Gastroenterol 2011;106:894–8 [DOI] [PubMed] [Google Scholar]
- 36.Lai SW, Liao KF, Lai HC, et al. Statin use and risk of hepatocellular carcinoma. Eur J Epidemiol 2013;28:485–92 [DOI] [PubMed] [Google Scholar]
- 37.Leung HW, Chan AL, Lo D, et al. Common cancer risk and statins: a population-based case-control study in a Chinese population. Expert Opin Drug Saf 2013;12:19–27 [DOI] [PubMed] [Google Scholar]
- 38.Chaiteerakij R, Yang JD, Harmsen WS, et al. Risk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer risk. Hepatology 2013;57:648–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh S, Singh PP, Roberts LR, et al. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2014;11:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pradelli D, Soranna D, Scotti L, et al. Statins and primary liver cancer: a meta-analysis of observational studies. Eur J Cancer Prev 2013;22:229–34 [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Gao C, Fang L, et al. Statin use and risk of liver cancer: a meta-analysis of 7 studies involving more than 4.7 million patients. World J Meta-Anal 2013;1:130–7 [Google Scholar]
- 42.Ahn J, Lim U, Weinstein SJ, et al. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev 2009;18:2814–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iso H, Ikeda A, Inoue M, et al. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer 2009;125:2679–86 [DOI] [PubMed] [Google Scholar]
- 44.Kitahara CM, de Gonzalez AB, Freedman ND, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol 2011;29:1592–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol 2007;166:348–54 [DOI] [PubMed] [Google Scholar]
- 46.Boudreau DM, Yu O, Johnson J. Statin use and cancer risk: a comprehensive review. Expert Opin Drug Saf 2010;9:603–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim W, Yoon JH, Kim JR, et al. Synergistic anti-tumor efficacy of lovastatin and protein kinase C-beta inhibitor in hepatocellular carcinoma. Cancer Chemother Pharmacol 2009;64:497–507 [DOI] [PubMed] [Google Scholar]
- 48.Polo MP, Crespo R, de Bravo MG. Geraniol and simvastatin show a synergistic effect on a human hepatocarcinoma cell line. Cell Biochem Funct 2011;29:452–8 [DOI] [PubMed] [Google Scholar]
- 49.Kawata S, Yamasaki E, Nagase T, et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer 2001;84:886–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lersch C, Schmelz R, Erdmann J, et al. Treatment of HCC with pravastatin, octreotide, or gemcitabine—a critical evaluation. Hepatogastroenterology 2004;51:1099–103 [PubMed] [Google Scholar]
- 51.Graf H, Jungst C, Straub G, et al. Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion 2008;78:34–8 [DOI] [PubMed] [Google Scholar]
- 52.Georgescu EF, Badulescu F, Dumitrescu D, et al. Lovastatin may enhance cytostatic effects of sorafenib in hepatic carcinoma. Primary results of a pilot study. Hepatol Int 2011;5:423 [Google Scholar]
- 53.Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 2012;308:1906–14 [DOI] [PubMed] [Google Scholar]
- 54.Lewis JH, Mortensen ME, Zweig S, et al. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 2007;46:1453–63 [DOI] [PubMed] [Google Scholar]
- 55.Nelson A, Torres DM, Morgan AE, et al. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo-controlled trial. J Clin Gastroenterol 2009;43:990–4 [DOI] [PubMed] [Google Scholar]
- 56.Lewis JH. Clinical perspective: statins and the liver-harmful or helpful? Dig Dis Sci 2012;57:1754–63 [DOI] [PubMed] [Google Scholar]
- 57.Bader T. The myth of statin-induced hepatotoxicity. Am J Gastroenterol 2010;105:978–80 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.