Abstract
Mycobacterium bovis is the main causative agent of animal tuberculosis (TB) and it may cause TB in humans. Molecular typing of M. bovis isolates provides precise epidemiological data on issues of inter- or intra-herd transmission and wildlife reservoirs. Techniques used for typing M. bovis have evolved over the last 2 decades, and PCR-based methods such as spoligotyping and mycobacterial interspersed repetitive unit-variable number tandem repeat (MIRU-VNTR) have been extensively used. These techniques can provide epidemiological information about isolates of M. Bovis that may help control bovine TB by indicating possible links between diseased animals, detecting and sampling outbreaks, and even demonstrating cases of laboratory cross-contamination between samples. This review will focus on techniques used for the molecular typing of M. bovis and discuss their general aspects and applications.
Keywords: tuberculosis, bovine, diagnosis, genotyping, Mycobacterium bovis
Introduction
Bovine tuberculosis (BTB) has been detected in cattle throughout the world. According to disease timelines available in the Worldwide Animal Health Information Database (OIE, 2009), 109 countries reported the presence of Mycobacterium bovis infections and/or clinical diseases in their cattle herds at some time in 2005–2010. In developed countries that have a tradition of cattle farming, the prevalence of BTB has reached very low levels because of strict control policies. In several of these countries, the disease has been eradicated. Conversely, in developing countries, despite recently implanted control mea-sures, considerable economic losses consistently occur in regions such as Brazil and Argentina, where there is intense cattle breeding (Ruggiero et al., 2007).
The first attempt to differentiate M. bovis isolates based on DNA sequence polymorphism was done by Collins and de Lisle in 1985 (Collins and De Leslie, 1985). They performed restriction digestion and agarose gel electrophoresis of genomic DNA. Identification of insertion sequences in M. tuberculosis and M. bovis genomes led to the development of the restriction fragment length polymorphism (RFLP) typing method (Thierry et al., 1990), a technique still in use nowadays. Knowledge of the genomic sequence of M. bovis and M. tuberculosis has facilitated the development of high-throughput molecular typing techniques that allow greater insight into the epidemiology, evolution, and population structure of M. bovis. Polymorphic GC Repeat Sequence (Roring et al., 1998; Ross et al., 1992), direct repeats (DR) regions (Hermans et al., 1991), and variable number of tandem repeats (VNTR) (Supply et al., 2000) have all been exploited as typing methods.
These tools can help to determine the source of infection and outbreaks, understand the relationship between different outbreaks, and identify wild animal reservoir of M. bovis. In addition, they can provide insight into the risk factors for BTB transmission by allowing identification of the dynamics of this disease (Brosch et al., 2002; Garnier et al., 2003; Zumarraga et al., 2005). In this review, we describe the main techniques used for genotyping and their application in characterizing M. bovis isolates.
Restriction Fragment Length Polymorphism (RFLP)
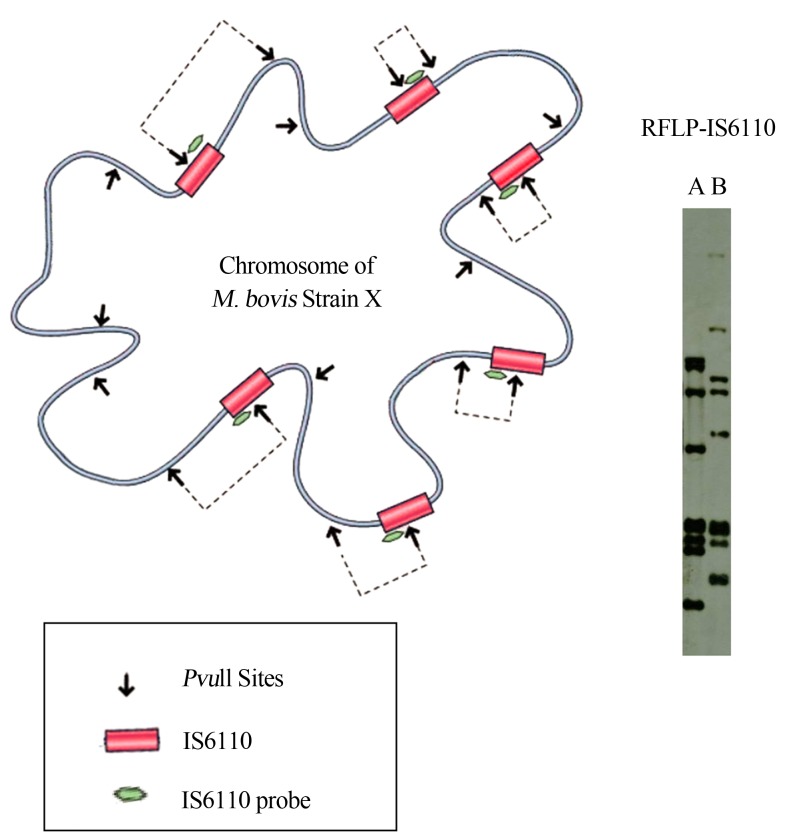
Strain differentiation by using RFLP analysis has proven to be a very useful tool for epidemiologic studies of tuberculosis. RFLP based on the presence of the insertion sequence IS6110 has been widely used as a genetic marker (Otal et al., 1991). IS6110 fingerprinting via RFLP has been standardized by using PvuII as the restriction enzyme of choice to digest mycobacterial genomic DNA (Figure 1) (Brosch et al., 2002; Gutierrez et al., 1995; Thierry et al., 1990). After electrophoresis of digested DNA on agarose gel, Southern blotting is carried out. Polymorphic banding patterns is revealed after hybridization by using a fragment of IS6110 as a probe (Durr et al., 2000). This insertion sequence is present in up to 20 copies in M. tuberculosis, thus enabling the application of IS6110-RFLP as the gold standard genotyping technique for this organism. In contrast, only 1–5 copies of IS6110 are found in M. bovis, which limits the ability of this element to discriminate between different M. bovis strains (Aranaz et al., 1996; Aranaz et al., 1999; van et al., 1994; van, 2001).
Figure 1.
Representation of the M. bovis chromosome with the IS6110 region (red), PvuII (arrow) restriction sites and the 245 bp (green) IS6110 probe used for Southern blotting. Different banding patterns result from the number and position of IS6110 copies, as well as polymorphism in the adjacent region where the PvuII site is located. Different strains produce distinct banding patterns as shown in this example with strains A and B.
One additional limitation of the RFLP-based typing systems is that they require a well-grown culture for DNA extraction. The time lag between isolation of M. bovis from a clinical sample and the growth of a mycobacterial culture is often too long. This problem can be circumvented with the use of several complementary biomarkers such as those based in the polymorphic IS6110 region, and by using direct repeats (DR) and polymorphic GC-rich repeat sequences (PGRS) as probes (Cousins et al., 1998; van Embden et al., 1996).
Polymorphic GC-Rich Repeat Sequence (PGRS)
The PGRS method is similar to standardized IS6110 fingerprinting in that it requires purified DNA for Southern blot hybridization and banding pattern analysis. PGRS fingerprinting has proven to be useful for differentiating strains with fewer than 6 copies of IS6110 that could not readily be differentiated by IS6110 fingerprinting (van et al., 1993; Yang et al., 2000).
The PGRS-based RFLP probe is the single most discriminatory of the probes currently available for M. bovis strain typing and can be present in up to 30 copies in members of the M. tuberculosis complex. PGRS is present in multiple copies interspersed throughout the genome and it exhibits a high level of polymorphism between unrelated isolates. However, the result of a PGRS DNA fingerprint is relatively complex because it contains many bands, making it potentially difficult to interpret. For the same reason, computer-assisted band analysis, particularly when the final image is less than ideal (O’Brien et al., 2000), can also be difficult.
Polymerase Chain Reaction (PCR) Based Techniques
Techniques based on DNA amplification via PCR, such as spoligotyping and MIRU-VNTR (Burgos et al., 2004), have become tools for epidemiological studies of bovine tuberculosis transmission, and have given prominence to a modern field of research known as molecular epidemiology. The agility and speed in detecting M. bovis can be decisive in the choice of these methods, which differentiate the species and different isolates of the same species at the DNA level.
Spoligotyping
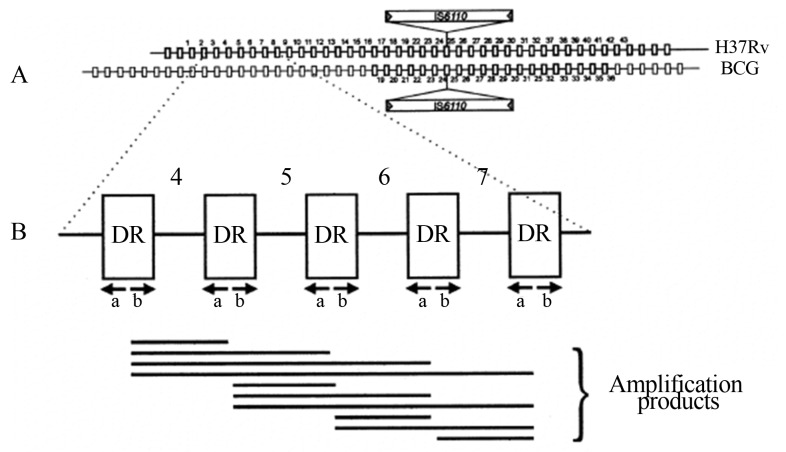
Spoligotyping (from “spacer oligotyping”) is based on the direct repeat region (DR), a DNA polymorphism present in a particular chromosomal locus that was first described by Hermans et al... (1991). This chromosomal region contains a large number of DRs of 36 bp each, interspersed with a spacer DNA of 35–41 bp in length. When DR regions of several isolates are compared, it is observed that the order of the spacers is about the same in all isolates; however, deletions and insertions of DRs occur (Figure 2). Polymorphisms in various isolates comprise the presence or absence of spacers of known sequence. This characteristic is used to determine genetic similarity among strains (Kontsevaya et al., 2011). Spoligotyping can easily distinguish between M. tuberculosis and M. bovis, can be used with DNA extracted from a bacterial culture as well as directly from a specimen, and has been used to identify the clonal nature of the isolates (O’Brien et al., 2000; Zanini et al., 2001; Zumarraga et al., 1999).
Figure 2.
(A) Structure of the DR locus in the mycobacterial genome. Multiple DRs in the chromosomes of M. tuberculosis and M. bovis (depicted as rectangles) are interspersed with unique spacers varying in length from 35 to 41 bp. The (numbered) spacers used correspond to 37 spacers from M. tuberculosis H37Rv and 6 from M. bovis BCG. The site of integration of insertion element IS6110 is depicted. (B) Principle of in vitro amplification of the DR region by using PCR. Any DR in the DR region may serve as a target for these primers; therefore, the amplified DNA is composed of a mixture of a large number of different-size fragments (Kamerbeek et al., 1997).
Spoligotyping is reportedly useful for identifying sources of infection, transmission of tuberculosis (TB) between species, and the stability of tuberculosis strains for long periods of time in closed populations, indicating that Mycobacterium is clonal (Cousins et al., 1998). In an ecological setting, spoligotyping is a rapid and inexpensive option that can be used to search for a relationship between strains (Zumarraga et al., 2012). Because strains of M. bovis from cattle usually contain few copies of IS6110, IS6110-RFLP is not the best method for distinguishing strains of M. bovis (Allix et al., 2006). Spoligotyping has proven to be a practical and discriminatory method for large-scale studies of the epidemiology of M. bovis as well as for the differentiation of M. bovis from M. tuberculosis, because the former lacks spacers 39–43 (Kamerbeek et al., 1997). Furthermore, there is an international database holding over 1900 spoligotype patterns from around the world (Lazzarini et al., 2012).
The main disadvantage of spoligotyping is that all genetic polymorphisms are restricted to a single genomic locus, the DR cluster, which limits the resolution. While having the advantages of being considerably faster and less labor-intensive than RFLP analysis, spoligotyping alone does not usually provide sufficient discrimination among strains of M. bovis to be used as a sole typing method, and is thus often combined with supplementary techniques (Costello et al., 1999; Cousins et al., 1998; McLernon et al., 2010; Roring et al., 1998).
Variable Number Tandem Repeat (VNTR)
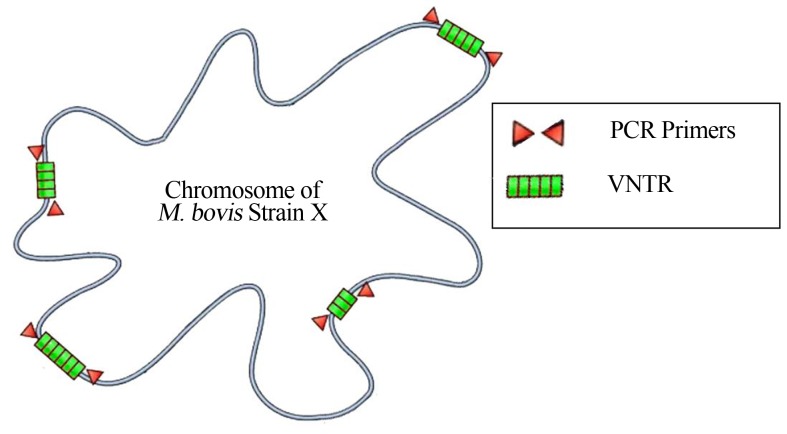
Tandemly repeated sequences are dispersed by thousands of copies in virtually all higher eukaryote genomes. Loci with short sequence repeats of 1 ± 13 bp are generally referred to as microsatellites, and those with 10 ± 100 bp sequence repeats as minisatellites (Figure 3). Many of these loci show hypervariability in their repeat numbers in humans and in animals, and are therefore called VNTR loci (Supply et al., 2000). VNTR sequences are also found in bacteria, and have been used to genotype many species. Polymorphism at a tandem repeat (TR) locus can occur either as a result of nucleotide sequence changes between individual repeat units or as a result of variations in the number of repeat units, thereby creating allelic variants. VNTR typing is based upon repeat number polymorphisms within these tandemly arranged repetitive DNA sequences.
Figure 3.
Scheme of the M. bovis chromosome with VNTR (green) loci, a variation in the number of short, repeated segments contained in a specific locus. Amplification of a specific PCR primer (red) flanking the locus produces DNA fragments whose lengths vary within strains. The number of repeats per locus varies among strains.
There are several VNTR loci in the genome of M. bovis, and hence VNTR typing provides a greater resolution than spoligotyping alone (Roring et al., 2002). Many of these TR loci display hypervariability, enabling their exploitation for strain typing in numerous bacterial species. Originally, 6 VNTR loci, described as exact TR A through F (ETR-A, -B, -C, -D, -E, and -F), were reported and applied to M. tuberculosis isolates (Frothingham and Meeker-O’Connell, 1998).
However, the level of discrimination found in M. tuberculosis or M. bovis isolates using the 5 ETRs (A through E) was not as good as that achieved with either spoligotyping or IS6110-RFLP typing (Collins et al., 1994; Good and Duignan, 2011). Therefore, another set of polymorphic repeats, termed as mycobacterial interspersed repetitive units (MIRU-VNTR) (Roring et al., 2002; Supply et al., 2000; Supply et al., 2006), has been proposed. Initially, these involved a 12-loci set, which was considered efficient for epidemiological purposes (Supply et al., 2001a), but some limitations were found with regard to the discriminatory power (Garcia, V et al., 2006; Scott et al., 2005). Furthermore, a 15- or 24-loci subset has been shown to ensure better discrimination (Supply et al., 2006).
The challenge is to compile standardized molecular fingerprinting patterns originating from highly networked, multi-centric, genotypic analysis in databases for inter-laboratory use and for further references. Rapid genotyping methods are needed to overcome low reproducibility, not proven application-less discriminatory methods such as MIRU-VNTR (Viedma et al., 2011). By analyzing the intended purpose and possibilities for each method (Table 1), it is possible to use a combination of typing methods and thus accurately identify differences among strains.
Table 1.
Molecular typing methods for Mycobacterium bovis.
| Method | Advantages | Disadvantages |
|---|---|---|
| IS6110-RFLP | The number of copies and their positions in the genome may vary from isolate to isolate (van Embden et al., 1993), providing identical profiles of isolates from animals involved in recent transmission chains, and different genetic patterns in isolates from animals not associated with infection (Aranaz et al., 1999). | Requires large amounts of DNA (1–2 μg) and technical skills; it is slow and has little discriminating power in isolates with less than 6 IS6110 copies (Gutacker et al., 2002); there is difficulty in reproducing results and comparing them among different laboratories (Supply et al., 2001b); and the majority of M. bovis isolates have a low number of IS6110 copies-in general, only 1 or 2 (Haddad et al., 2004). |
| PGRS | Higher discriminating power in isolates with 6 or less IS6110 copies (Rozo and Ribón, 2010); useful tool for confirming the identity of strains matched by IS6110 or to type low-copy number stains (Kanduma et al., 2003); for the majority of M. bovis strains, except those originating from certain animal species like goats, PGRS sequences, in terms of copy number, much more polymorphic than the DR region, which is more polymorphic than the IS6110; it is considered more stable than the IS6110-RFLP patterns and shows 100% reproducibility. | Requires large amounts of high quality DNA (Asgharzadeh and Kafil, 2007; Doroudchi et al., 2000) and technical skills; it has lower discriminating power in isolates with multiple copies (Bauer et al., 1999); the large number of bands produced by this technique makes interpretation of the gels difficult, limiting its application as a primary typing technique (Kanduma et al., 2003). |
| Spoligotyping | This technique is fast, robust, low cost and can differentiate strains of M. bovis and M. tuberculosis; the results can be fully expressed in a simple, digital format, which facilitates inter- and intra-laboratory results comparisons, as has been demonstrated for VNTR; this method may differentiate better when the location of more spacers is investigated; it can potentially be applied directly to pathological samples (Kamerbeek et al., 1997). | The discriminatory power of this method is lower than IS6110-RFLP typing when high copy number strains are being analyzed. |
| VNTR | Powerful approach to high-resolution genotyping of isolates (Supply et al., 2001b); it is reproducible, fast and specific for M. tuberculosis complex isolates (Supply et al., 2001b); potential approach to detect and genotype bacteria of the M. tuberculosis complex directly in a range of clinical samples (Roring et al., 2002); the results can be fully expressed in a simple, digital format, which facilitates inter- and intra-laboratory results comparisons; it has shown 100% reproducibility. | The discriminatory power of this method is lower than IS6110-RFLP (for high copy number strains) and spoligotyping (Kremer et al., 1999); detection via electrophoresis is inexpensive, but automated detection involving fluorescence is not. |
Implications of Molecular Typing in Epidemiology
Within the last 10 years, many techniques have been developed or adapted for typing M. tuberculosis complex isolates. In this review, we have presented an overview of the main techniques used for the differentiation of M. bovis isolates. For the majority of them, a specific and polymorphic genetic region is involved. In comparison to the majority of other bacterial groups, the genome of mycobacteria has a high GC content (GC% is 65%), and its polymorphism is very limited compared to its genome size (4.4 Mb). However, some regions are highly polymorphic, either due to variations in number and/or position, or because of variations in primary structure. These areas of higher polymorphism appear to correspond essentially to segments of genes encoding proteins where variability provides a selective advantage to the bacteria, such as antibiotic-resistance proteins, antigens involved in escaping the immune response, or non-coding sequences (insertion sequences or repeated sequences) that are probably involved in inducing variability in neighboring genetic areas.
Implementation of molecular methods for M. bovis typing requires an analysis of their advantages and disadvantages with regard to tuberculosis surveillance and control programs. Their use enables the detection of epidemiological links among samples (Pheiffer et al., 2005), and contributes to better understanding of BTB transmission dynamics. Some studies have shown that laboratories should determine the discriminatory power for each molecular method to enable better selection, implementation, and combination according to specific conditions found in each laboratory and the particular features of a geographic region (Rozo and Ribón, 2010).
Genotyping of bacterial isolates or PCR products is increasingly becoming a standard tool for epidemiological disease control and eradication. Distinguishing M. bovis strains at the molecular level provides important insights into the sources of infection and identification of practices or environments, thereby aiding the spread and maintenance of tuberculosis (Medeiros et al., 2010). More importantly, transmission routes between livestock and wildlife may be identified by strain typing. In addition, transmission routes of BTB within livestock via animal movements become evident; this is a prerequisite for targeted disease control aiming at testing all potentially exposed animals (Schiller et al., 2010).
PCR-based techniques used for strain fingerprinting have proven to be of useful in relating outbreaks of TB to sources of infection. Epidemiologically related isolates have similar fingerprints that differ from those that are epidemiologically unrelated. Therefore, a desirable characteristic for typing is related to its stability within a strain and diversity within a species.
DNA fingerprinting of M. bovis for molecular epidemiology has been used to study transmission of bovine tuberculosis in Latin America and other parts of the world (PARREIRAS et al., 2012). However, there are a few reports in Brazil with regard to characterization of M. bovis (Figueiredo et al., 2011; Rodriguez et al., 2004; Zanini et al., 2005) that revealed the occurrence of a high genetic diversity; however, these studies were conducted using a limited number of isolates (Zumarraga et al., 1999).
Conclusion
Tuberculosis caused by M. bovis is important for public health, animal health, and animal production. Whatever the epidemiological context, the need for techniques permitting differentiation of isolates at the molecular level is evident. These tools would help determine the origin of outbreaks, increase the understanding with regard to the link between different outbreaks, show the relationship between domestic TB and wild TB, and identify the source of infection. No typing technique developed so far can be used on its own. Each technique has its advantages and disadvantages that must be considered when choosing the one to be implemented in the laboratory. Using spoligotyping in combination with MIRU-VNTR seems to be the best choice as both have the advantages of being PCR-based, with improved discriminatory power when combined.
Hopefully, in the future we will have new and improved techniques for typing M. bovis. It is conceivable that a lab-on-a-chip approach will be capable of not only detecting M. bovis from a clinical sample but also typing the pathogen at the same time. Regarding the target locus, it is likely that single nucleotide polymorphisms in specific genes will be used for molecular epidemiology.
References
- Allix C, Walravens K, Saegerman C, Godfroid J, Supply P, Fauville-Dufaux M. Evaluation of the epidemiological relevance of variable-number tandem-repeat genotyping of Mycobacterium bovis and comparison of the method with IS6110 restriction fragment length polymorphism analysis and spoligotyping. J Clin Microbiol. 2006;44:1951–1962. doi: 10.1128/JCM.01775-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranaz A, Liebana E, Gomez-Mampaso E, Galan JC, Cousins D, Ortega A, Blazquez J, Baquero F, Mateos A, Suarez G, Dominguez L. Mycobacterium tuberculosis subsp. caprae subsp. nov.: a taxonomic study of a new member of the Mycobacterium tuberculosis complex isolated from goats in Spain. Int J Syst Bacteriol. 1999;49:1263–1273. doi: 10.1099/00207713-49-3-1263. [DOI] [PubMed] [Google Scholar]
- Aranaz A, Liebana E, Mateos A, Dominguez L, Vidal D, Domingo M, Gonzolez O, Rodriguez-Ferri EF, Bunschoten AE, Van Embden JD, Cousins D. Spacer oligonucleotide typing of Mycobacterium bovis strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. J Clin Microbiol. 1996;34:2734–2740. doi: 10.1128/jcm.34.11.2734-2740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgharzadeh M, Kafil HS. Current trends in Molecular Epidemiology Studies of Mycobacterium tuberculosis. 2007:108–115. [Google Scholar]
- Bauer J, Andersen AB, Kremer K, Miorner H. Usefulness of spoligotyping To discriminate IS6110 low-copy-number Mycobacterium tuberculosis complex strains cultured in Denmark. J Clin Microbiol. 1999;37:2602–2606. doi: 10.1128/jcm.37.8.2602-2606.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, Garnier T, Gutierrez C, Hewinson G, Kremer K, Parsons LM, Pym AS, Samper S, van SD, Cole ST. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci USA. 2002;99:3684–3689. doi: 10.1073/pnas.052548299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos MV, Mendez JC, Ribon W. Molecular epidemiology of tuberculosis: methodology and applications. Biomedica. 2004;24(Supp 1):188–201. [PubMed] [Google Scholar]
- Collins DM, De Leslie GW. DNA restriction endonuclease analysis of Mycobacterium bovis and other members of the tuberculosis complex. J Clin Microbiol. 1985;21:562–564. doi: 10.1128/jcm.21.4.562-564.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DM, Radford AJ, de Leisle GW, Billman-Jacobe H. Diagnosis and epidemiology of bovine tuberculosis using molecular biological approaches. Vet Microbiol. 1994;40:83–94. doi: 10.1016/0378-1135(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Costello E, O’Grady D, Flynn O, O’Brien R, Rogers M, Quigley F, Egan J, Griffin J. Study of restriction fragment length polymorphism analysis and spoligotyping for epidemiological investigation of Mycobacterium bovis infection. J Clin Microbiol. 1999;37:3217–3222. doi: 10.1128/jcm.37.10.3217-3222.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins DV, Skuce RA, Kazwala RR, Van Embden JD. Towards a standardized approach to DNA fingerprinting of Mycobacterium bovis. International Union Against Tuberculosis and Lung Disease, Tuberculosis in Animals Subsection. Int J Tuberc Lung Dis. 1998;2:471–478. [PubMed] [Google Scholar]
- Doroudchi M, Kremer K, Basiri EA, Kadivar MR, van SD, Ghaderi AA. IS6110-RFLP and spoligotyping of Mycobacterium tuberculosis isolates in Iran. Scand J Infect Dis. 2000;32:663–668. doi: 10.1080/003655400459595. [DOI] [PubMed] [Google Scholar]
- Durr PA, Hewinson RG, Clifton-Hadley RS. Molecular epidemiology of bovine tuberculosis. I. Mycobacterium bovis genotyping. Rev Sci Tech. 2000;19:675–688. doi: 10.20506/rst.19.3.1241. [DOI] [PubMed] [Google Scholar]
- Figueiredo EE, Ramos DF, Medeiros L, Silvestre FG, Lilenbaum W, Silva JT, Paschoalin VM, Dellagostin OA. Multiple strains of Mycobacterium bovis revealed by molecular typing in a herd of cattle. Vet J. 2011;193:296–298. doi: 10.1016/j.tvjl.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Frothingham R, Meeker-O’Connell WA. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology. 1998;144:1189–1196. doi: 10.1099/00221287-144-5-1189. [DOI] [PubMed] [Google Scholar]
- Garcia dV, Alonso RN, Andres S, Martinez LM, Ruiz Serrano MJ, Bouza E. Evaluation of alternatives to RFLP for the analysis of clustered cases of tuberculosis. Int. J. Tuberc Lung Dis. 2006;10:454–459. [PubMed] [Google Scholar]
- Garnier T, Eiglmeier K, Camus JC, Medina N, Mansoor H, Pryor M, Duthoy S, Grondin S, Lacroix C, Monsempe C, Simon S, Harris B, Atkin R, Doggett J, Mayes R, Keating L, Wheeler PR, Parkhill J, Barrell BG, Cole ST, Gordon SV, Hewinson RG. The complete genome sequence of Mycobacterium bovis. Proc Natl Acad Sci USA. 2003;100:7877–7882. doi: 10.1073/pnas.1130426100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M, Duignan A. Perspectives on the History of Bovine TB and the Role of Tuberculin in Bovine TB Eradication. Vet Med Int. 2011;2011:410–470. doi: 10.4061/2011/410470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutacker MM, Smoot JC, Migliaccio CA, Ricklefs SM, Hua S, Cousins DV, Graviss EA, Shashkina E, Kreiswirth BN, Musser JM. Genome-wide analysis of synonymous single nucleotide polymorphisms in Mycobacterium tuberculosis complex organisms: resolution of genetic relationships among closely related microbial strains. Genetics. 2002;162:1533–1543. doi: 10.1093/genetics/162.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M, Samper S, Gavigan JA, Garcia Marin JF, Martin C. Differentiation by molecular typing of Mycobacterium bovis strains causing tuberculosis in cattle and goats. J Clin Microbiol. 1995;33:2953–2956. doi: 10.1128/jcm.33.11.2953-2956.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad N, Masselot M, Durand B. Molecular differentiation of Mycobacterium bovis isolates. Review of main techniques and applications. Res Vet Sci. 2004;76:1–18. doi: 10.1016/s0034-5288(03)00078-x. [DOI] [PubMed] [Google Scholar]
- Hermans PW, van SD, Bik EM, de Haas PE, Dale JW, Van Embden JD. Insertion element IS987 from Mycobacterium bovis BCG is located in a hot-spot integration region for insertion elements in Mycobacterium tuberculosis complex strains. Infect Immun. 1991;59:2695–2705. doi: 10.1128/iai.59.8.2695-2705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerbeek J, Schouls L, Kolk A, van AM, van SD, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, Van EJ. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanduma E, McHugh TD, Gillespie SH. Molecular methods for Mycobacterium tuberculosis strain typing: a users guide. J Appl Microbiol. 2003;94:781–791. doi: 10.1046/j.1365-2672.2003.01918.x. [DOI] [PubMed] [Google Scholar]
- Kontsevaya IS, Nikolayevsky VV, Balabanova YM. Molecular Epidemiology of Tuberculosis: Objectives, Methods, and Prospects. 2011:1–9. [Google Scholar]
- Kremer K, van SD, Frothingham R, Haas WH, Hermans PW, Martin C, Palittapongarnpim P, Plikaytis BB, Riley LW, Yakrus MA, Musser JM, van Embden JD. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol. 1999;37:2607–2618. doi: 10.1128/jcm.37.8.2607-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini LC, Rosenfeld J, Huard RC, Hill V, Lapa e Silva JR, DeSalle R, Rastogi N, Ho JL. Mycobacterium tuberculosis spoligotypes that may derive from mixed strain infections are revealed by a novel computational approach. Infect Genet Evol. 2012;12:798–806. doi: 10.1016/j.meegid.2011.08.028. [DOI] [PubMed] [Google Scholar]
- McLernon J, Costello E, Flynn O, Madigan G, Ryan F. Evaluation of mycobacterial interspersed repetitive-unit-variable-number tandem-repeat analysis and spoligotyping for genotyping of Mycobacterium bovis isolates and a comparison with restriction fragment length polymorphism typing. J Clin Microbiol. 2010;48:4541–4545. doi: 10.1128/JCM.01175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros LS, Marassi CD, Figueiredo EES, Lilenbaum W. Potential application of new diagnostic methods for controlling bovine Tuberculosis in Brazil. Braz J Microbiol. 2010;41:531–541. doi: 10.1590/S1517-83822010005000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien R, Flynn O, Costello E, O’Grady D, Rogers M. Identification of a novel DNA probe for strain typing Mycobacterium bovis by restriction fragment length polymorphism analysis. J Clin Microbiol. 2000;38:1723–1730. doi: 10.1128/jcm.38.5.1723-1730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE. Annual Animal Disease Status. Bovine Tuberculosis 2009 [Google Scholar]
- Otal I, Martin C, Vincent-Levy-Frebault V, Thierry D, Gicquel B. Restriction fragment length polymorphism analysis using IS6110 as an epidemiological marker in tuberculosis. J Clin Microbiol. 1991;29:1252–1254. doi: 10.1128/jcm.29.6.1252-1254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreiras PM, Andrade GI, Nascimento TF, Oelemann MC, Gomes HM, Alencan AP, Assis RA, Mota PMPC, Pereiras MAS, Lobato FCF, Lage AP, Suffys PN. Spoligotyping and variable number tandem repeat analysis of Mycobacterium bovis isolates from cattle in Brazil. Mem Inst Oswaldo Cruz. 2012;107:64–73. doi: 10.1590/s0074-02762012000100009. [DOI] [PubMed] [Google Scholar]
- Pheiffer C, Betts JC, Flynn HR, Lukey PT, van HP. Protein expression by a Beijing strain differs from that of another clinical isolate and Mycobacterium tuberculosis H37Rv. Microbiology. 2005;151:1139–1150. doi: 10.1099/mic.0.27518-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez CAR, Zumarraga M, Oliveira EMD, Cataldi A, Romano MI, Otto HH, Bonafé VL, Ferreira Neto JS. Caracterização molecular de isolados de Mycobacterium bovis do Estado de São Paulo Brasil, utilizando a técnica de spoligotyping. Arq Inst Biol. 2004;71:277–282. [Google Scholar]
- Roring S, Brittain D, Bunschoten AE, Hughes MS, Skuce RA, Van Embden JD, Neill SD. Spacer oligotyping of Mycobacterium bovis isolates compared to typing by restriction fragment length polymorphism using PGRS, DR and IS6110 probes. Vet Microbiol. 1998;61:111–120. doi: 10.1016/s0378-1135(98)00178-3. [DOI] [PubMed] [Google Scholar]
- Roring S, Scott A, Brittain D, Walker I, Hewinson G, Neill S, Skuce R. Development of variable-number tandem repeat typing of Mycobacterium bovis: comparison of results with those obtained by using existing exact tandem repeats and spoligotyping. J Clin Microbiol. 2002;40:2126–2133. doi: 10.1128/JCM.40.6.2126-2133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross BC, Raios K, Jackson K, Dwyer B. Molecular cloning of a highly repeated DNA element from Mycobacterium tuberculosis and its use as an epidemiological tool. J Clin Microbiol. 1992;30:942–946. doi: 10.1128/jcm.30.4.942-946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozo AJC, Ribón W. Molecular tools for Mycobacterium tuberculosis genotyping. Rev salud pública. 2010;12:510–521. [PubMed] [Google Scholar]
- Ruggiero AP, Ikuno AA, Ferreira VCA, Roxo E. Tuberculose bovina: Alternativas para o diagnóstico. Arq Inst Biol. 2007;74:55–65. [Google Scholar]
- Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, Orloski KA, Buddle BM, Thacker TC, Lyashchenko KP, Waters WR. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound Emerg Dis. 2010;57:205–220. doi: 10.1111/j.1865-1682.2010.01148.x. [DOI] [PubMed] [Google Scholar]
- Scott AN, Menzies D, Tannenbaum TN, Thibert L, Kozak R, Joseph L, Schwartzman K, Behr MA. Sensitivities and specificities of spoligotyping and mycobacterial interspersed repetitive unit-variable-number tandem repeat typing methods for studying molecular epidemiology of tuberculosis. J Clin Microbiol. 2005;43:89–94. doi: 10.1128/JCM.43.1.89-94.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rusch-Gerdes S, Willery E, Savine E, de HP, van DH, Roring S, Bifani P, Kurepina N, Kreiswirth B, Sola C, Rastogi N, Vatin V, Gutierrez MC, Fauville M, Niemann S, Skuce R, Kremer K, Locht C, van SD. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol. 2006;44:4498–4510. doi: 10.1128/JCM.01392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supply P, Lesjean S, Savine E, Kremer K, van SD, Locht C. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J Clin Microbiol. 2001;39:3563–3571. doi: 10.1128/JCM.39.10.3563-3571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supply P, Lesjean S, Savine E, Kremer K, van SD, Locht C. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J Clin Microbiol. 2001;39:3563–3571. doi: 10.1128/JCM.39.10.3563-3571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supply P, Mazars E, Lesjean S, Vincent V, Gicquel B, Locht C. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol Microbiol. 2000;36:762–771. doi: 10.1046/j.1365-2958.2000.01905.x. [DOI] [PubMed] [Google Scholar]
- Thierry D, Brisson-Noel A, Vincent-Levy-Frebault V, Nguyen S, Guesdon JL, Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol. 1990;28:2668–2673. doi: 10.1128/jcm.28.12.2668-2673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick TM. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Embden JD, van SD, Heersma HF, De Neeling AJ, Jones ME, Steiert M, Grek V, Mooi FR, Verhoef J. Establishment of a European network for the surveillance of Mycobacterium tuberculosis, MRSA and penicillin-resistant pneumococci. J Antimicrob Chemother. 1996;38:905–907. doi: 10.1093/jac/38.5.905. [DOI] [PubMed] [Google Scholar]
- van SD. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J Intern Med. 2001;249:1–26. doi: 10.1046/j.1365-2796.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- van SD, de Haas PE, Haagsma J, Eger T, Hermans PW, Ritacco V, Alito A, Van Embden JD. Use of various genetic markers in differentiation of Mycobacterium bovis strains from animals and humans and for studying epidemiology of bovine tuberculosis. J Clin Microbiol. 1994;32:2425–2433. doi: 10.1128/jcm.32.10.2425-2433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van SD, de Haas PE, Hermans PW, Groenen PM, Van Embden JD. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J Clin Microbiol. 1993;31:1987–1995. doi: 10.1128/jcm.31.8.1987-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viedma DG, Mokrousov I, Rastogi N. Innovations in the molecular epidemiology of tuberculosis. Enferm Infecc Microbiol Clin. 2011;29:8–13. doi: 10.1016/S0213-005X(11)70012-X. [DOI] [PubMed] [Google Scholar]
- Yang ZH, Ijaz K, Bates JH, Eisenach KD, Cave MD. Spoligotyping and polymorphic GC-rich repetitive sequence fingerprinting of Mycobacterium tuberculosis strains having few copies of IS6110. J Clin Microbiol. 2000;38:3572–3576. doi: 10.1128/jcm.38.10.3572-3576.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini MS, Moreira EC, Lopes MT, Oliveira RS, Leao SC, Fioravanti RL, Roxo E, Zumarraga M, Romano MI, Cataldi A, Salas CE. Mycobacterium bovis: polymerase chain reaction identification in bovine lymphonode biopsies and genotyping in isolates from Southeast Brazil by spolygotyping and restriction fragment length polymorphism. Mem Inst Oswaldo Cruz. 2001;96:809–813. doi: 10.1590/s0074-02762001000600012. [DOI] [PubMed] [Google Scholar]
- Zanini MS, Moreira EC, Salas CE, Lopes MT, Barouni AS, Roxo E, Telles MA, Zumarraga MJ. Molecular typing of Mycobacterium bovis isolates from south-east Brazil by spoligotyping and RFLP. J Vet Med B Infec Dis Vet Public Health. 2005;52:129–133. doi: 10.1111/j.1439-0450.2005.00835.x. [DOI] [PubMed] [Google Scholar]
- Zumarraga MJ, Arriaga C, Barandiaran S, Cobos-Marin L, de WJ, Estrada-Garcia I, Figueiredo T, Figueroa A, Gimenez F, GOMES HM, Gonzalez YMJ, Macias A, Milian-Suazo F, Rodriguez CA, Santillan MA, SUFFYS PN, Trangoni MD, Zarraga AM, Cataldi A. Understanding the relationship between Mycobacterium bovis spoligotypes from cattle in Latin American Countries. Res Vet Sci. 2012 doi: 10.1016/j.rvsc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Zumarraga MJ, Martin C, Samper S, Alito A, Latini O, Bigi F, Roxo E, Cicuta ME, Errico F, Ramos MC, Cataldi A, van SD, Romano MI. Usefulness of spoligotyping in molecular epidemiology of Mycobacterium bovis-related infections in South America. J Clin Microbiol. 1999;37:296–303. doi: 10.1128/jcm.37.2.296-303.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumarraga MJ, Meikle V, Bernardelli A, Abdala A, Tarabla H, Romano MI, Cataldi A. Use of touch-down polymerase chain reaction to enhance the sensitivity of Mycobacterium bovis detection. J Vet Diagn Invest. 2005;17:232–238. doi: 10.1177/104063870501700303. [DOI] [PubMed] [Google Scholar]