Abstract
Brucella is an intracellular pathogen capable of infecting animals and humans. The aim of this study was to identify Brucella spp in sera of high risk individuals by a polymerase chain reaction (PCR)-based method. A total of 180 patients suspected to have Brucellosis were examined by serological tests. To establish a PCR protocol for diagnosis of active brucellosis, DNA was extracted from the serum samples by using a commercial kit. PCR amplification was done for detection of Brocella DNA using BCSP31 target gene and IS711 locus. The PCR assay showed that an amplicon of 223 bp was obtained in 73.8% (133/180) of the tested sera using primers (B4/B5) derived from a gene encoding the 31-kDa Brucella abortus antigen. In another PCR, an amplicon of 498 bp was obtained in 63.8% (115/180) of the samples using Brucella abortus-specific primers derived from a locus adjacent to the 3′-end of IS711, and also an amplicon of 731 bp was produced in 4.4% (8/180) of the tested samples using Brucella melitensis-specific primers. When the Wright method was used as a gold standard, the sensitivity and specificity of the PCR technique for genus identification were found to be 96 and 80.7%, respectively. However, the sensitivity value obtained with the species-specific PCR method was 82%, and specificity was similar to that previous reported. This is the first report of a high frequency of Brucella abortus in patients suspicious of Brucellosis from the Zanjan province.
Keywords: PCR, serologic tests, Brucella abortus, human Brucellosis
Introduction
Brucellosis remains a main source of disease in humans and animal husbandry worldwide (Corbel, 1997).- About half a million human brucellosis cases are reported annually. However according to the WHO (1997) estimates, the true frequency of the disease is 10–25-times higher than the reported number. The highest annual incidence rates are reported from the Middle Eastern countries, such as Syria, Iraq, Iran, and Saudi Arabia (Pappas et al., 2006). In Iran, where Brucellosis is endemic, the incidence of the disease is up to 34 per 100,000 per year in certain areas (Najafi et al., 2011).
Brucellosis is caused by several species of Gram-negative facultative intracellular bacteria of the genus Brucella that can be transmitted to humans by direct animal contact or their products; it is an occupational hazard to those involved in certain professions including farmers, veterinarians, and laboratory and slaughterhouse workers (Sofian et al., 2008). As a consequence of, there is severe human suffering from the disease, which may cause enormous economic losses in endemic regions.
The clinical picture of brucellosis is so strange and protean that it can be easily bewildered with other infectious and noninfectious diseases, leading to diagnostic delays and late onset of therapy (Al Dahouk and Nockler, 2011). Therefore, laboratory confirmation is needed for detection of Brucella. However, there is no completely reliable and satisfactory diagnostic procedure for brucellosis (Bower and Chudnoff, 1948).
There are currently three major approaches for the diagnosis of brucellosis including microbiological, serological, and molecular techniques (Alves et al., 2010). The diagnostic standard remains the isolation of Brucella from blood cultures or host tissues (Queipo-Ortuno et al., 2005). The accuracy of blood cultures is reaching 70–80% of cases in acute forms produced by Brucella melitensis. However, in cases of chronic forms and in patients with focal complications and also infections caused by Brucella abortus and Brucella suis, culture positive results rarely exceed 30–50% (Young, 1995). Furthermore, blood culture is a time-consuming and represents a potential hazard of infection for lab personnel as Brucella species are class III pathogens (Bricker, 2002). In the absence of adequate culture facilitates the diagnosis of brucellosis depends on serological tests (Alves et al., 2010), such as the Rose Bengal test, serum agglutination test, Coombs test, and immune capture test, but the specificity of these techniques are low in endemic areas, in persons exposed professionally to Brucella or in patients with relapse or a recent history of brucellosis (Christopher et al., 2010). Cross-reactivity may occur with other Gram negative bacteria that can affect the specificity. There are also some restrictions in the detection of causative agent in early phases of the disease (Young, 1991; Ariza et al., 1992). Alternatively, molecular techniques could be used for diagnosis of human brucellosis, especially that these kinds of procedures are useful for diagnosis of several infectious diseases caused by fastidious or slowly growing bacteria and also have detected the small amounts of DNA in different samples (Romero et al., 1995; Al Dahouk and Nockler, 2011).
The main aim of this project was to investigate the utility of PCR for the diagnosis of brucellosis and determination of brucella species from the serum samples of high risk individuals, and to compare the PCR protocol’s sensitivity with the conventional standard tube agglutination diagnostic method.
Materials and Methods
Subjects and serum samples
A total of 160 serum samples were collected from high risk individuals to brucellosis referred to “Danesh” private diagnostic laboratory in Khodabandeh district and also two governmental diagnostic laboratories in Zanjan, Iran, over a period of 8 month (January–September 2011).
All patients had clinical signs similar to brucellosis. The diagnosis was performed using serologic techniques: serum samples were analyzed before starting an antibiotic treatment by the standard tube agglutination test (STA) and also complementary tests including 2-ME (2- Mercaptoethanol) and Coombs test were investigated for further confirmation.
In the current study, a positive STA titer was defined as either equal or greater than 1:160, and the Coombs’ Wright was considered as either equal or greater than 1:80, according to the standard methods (Colmenero et al., 2007). Samples based on Wright data in the laboratory record forms were further divided into three subgroups (Wright > 1:160, Wright > 1:160, and Wright Negative).
Isolation of DNA from clinical serum samples
DNA was isolated from serum samples (100 μL) with the High yield DNA Purification kit (Cinnagen, Tehran, Iran), according to the supplier’s manual. Concentration and purity of the DNA samples were determined spectrophotometrically (Eppendorf, Germany) by reading A260 and A280.
DNA amplification by two different targets
The B4 and B5 primers (Table 1) described previously (Baily et al., 1992), were used for detection of Brucella genus which encodes a protein of Brucella abortus 31 kDa, BCSP31. BCSP31-PCR reaction consisted of 12.5 μL 2× PCR master mixes (Fermentas, USA), 1 μg DNA template, 100 nM of each primers, and nuclease free water up to 25 μL. PCR profile was performed on a thermocycler (iCycler, Bio-Rad, USA) using the following parameters: Denaturation primal at 95 °C for 5 min, 35 cycles of template denaturation at 94 °C for 1 min, 30 s of primer annealing at 64 °C, and 60 s of primer extension at 72 °C with a final extension cycle at 72 °C for 7 min.
Table 1.
Olignucleotides for detection of Brucella used in this study.
| No. | Target | Olignucleotide sequence | Amplified product |
|---|---|---|---|
| 1 | BCSP31 | B4: 5′-TGGCTCGGTTGCCAATATCAA-3′ B5: 5 ′-CGCGCTTGCCTTTCAGGTCTG-3 |
223 bp |
| 2 | IS711, B. abortus | F: 5′-TGCCGATCACTTAAGGGCCTTCAT-3′ R: 5′-GAC GAACGGAATTTTTCCAATCCC-3′ |
498 bp |
| 3 | IS711, B. melitensis | F: 5′-TGCCGATCACTTAAGGGCCTTCAT-3′ R: 5′-AAA TCGCGTCCTTGCTGGTCTGA-3′ |
731 bp |
The other primers described previously (Bricker, 2002) specific for Brucella spp including, forward primers were derived from insertion sequence 711 (IS711) is unique to identification of Brucella species but the reverse primers are different and were derived from B. abortus and B. melitensis specific locus on chromosomal DNA (Table 1). IS711-PCR assay were done in a total volume of 25 μL containing the same mixture were used for BCSP31-PCR.
The amplification programs for B. abortus and B. melitensis consisted of initial DNA denaturation at 95 °C for 3 min and then cycled 35 times at 95 °C for 90 s, 65 °C for 1 min, and 72 °C for 1 min. We performed a final extension step of 5 min at 72 °C. The reaction products (5 μL) were detected by electrophoresis on 1.5% agarose gel and visualized with UV transilluminator after staining with 2 μg/mL ethidium bromide to determine the size of amplified products.
DNA isolated from B. melitensis 16 M provided from Department of Bacterial Vaccines and Antigens Production, Pasteur Institute of Iran and B. abortus from the department of biotechnology, faculty of pharmacy, ZUMS, Iran were used as the positive control. The negative control strain was Escherichia coli ATCC 8739 for determination of specificity assay and to detect the contamination during the extraction stage of the DNA and/or primer. Water was used as negative control. All samples were carried out in duplicate.
Results
Study Patients
180 suspected patients to brucellosis included in this study. From the total of 128 Wright-positive patients (mean age of 40 years; ranging 15–77 yr), 41 (32%) were female and 87 (67%) were male. Ninety three (72.6%) patients lived in rural areas, 107 (83.6%) had direct contacts with animals or animal products, and 21 (16.4%) acquired their infections by consuming un-pasteurized dairy products.
From 52 Wright-negative serum samples (mean age of 38 years; ranging 10–70 yr), 37 cases (71%) were female and 15 (28%) were male. Thirty six (69.2%) cases had contact with livestock or their products, and 16 (30.8%) had a history of consuming non-pasteurized dairy products.
Testing of serum samples with BCSP31-PCR
Detection of Brucella genus with B4 and B5 primers are shown in Figure 1. As expected, the bcsp31 gene amplicon size was 223 bp. Among the 128 Wright positive patients, 123 (96.1%) samples were positive by B4 and B5 primers, and there were only 5 (3.9%) false negative results for Wright-positive samples. A total of 10 out of 52 (19.23%) Wright-negative cases were positive by PCR (Table 2).
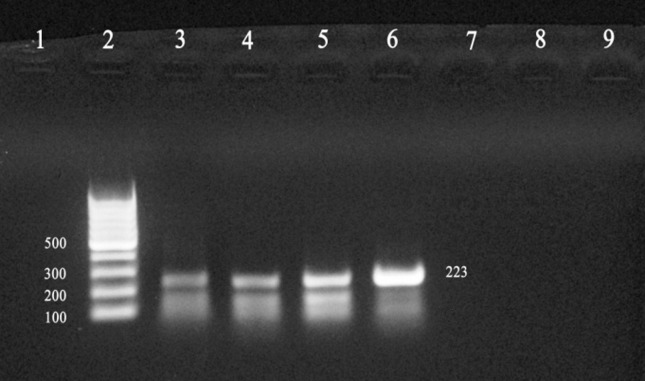
Figure 1.
Identification of DNA amplified fragments by agarose gel electrophoresis and ethidium bromide staining. An amplicon size of 223 bp was obtained by PCR using Baily’s primers (B4, B5) and template DNA from reference Brucella spp and patient sera. Lanes: 1, Negative control; 2, 1 kb Ladder; 3, 4, Wright positive sera; 5, 6, B. abortus and B. melitensis; 7, 8, Wright negative sera; 9, E. coli.
Table 2.
Table 2. The results of the PCR amplification of the BCSP31 target gene by using B4 and B5 primers.
| Standard tube agglutination (Wright) | Positive | Negative | Total |
|---|---|---|---|
| Wright > 1:160 | 102 (96.22%) | 4 (3.78) | 106 (100%) |
| Wright < 1:160 | 21 (95.45%) | 1 (4.55%) | 22 (100%) |
| Wright Negative | 10 (19.23%) | 42 (80.77%) | 52 (100%) |
| Total | 133 | 47 | 180 |
Testing of serum samples with IS711-PCR
Detection of Brucella spp. in serum samples with B. abortus and B. melitensis specific primers (IS711) produced amplicons of 498 and 731 bp for B. abortus and B. melitensis, respectively (Figures 2 and 3). Amplicons with a molecular size of 498 bp were obtained from 115/180 (63.9%) of serum samples. Of the 115 PCR positive serum samples, 105/115 (91.3%) was obtained from Wright-positive and 10/115 (8.7%) from Wright-negative. However, in the PCR targeting IS711 for detection of B. melitensis showed that an amplicon of the 731 bp was obtained, only from 8/128 (6.25%) cases of Wright-positive samples (Table 3). Comparison of PCR and serology techniques in the detection of brucellosis is indicated on Table 4.
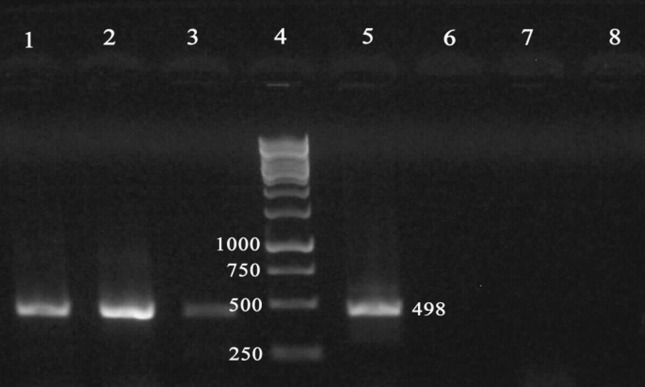
Figure 2.
Identification of DNA amplified fragments by agarose gel electrophoresis and ethidium bromide staining. An amplicon of 498 bp was obtained by PCR using B. abortus - specific primers and Brucella DNA as template. Lanes: 1, 2, 3, serum DNAs from patients diagnosed with brucellosis; 4, 1 kb Ladder; 5, Positive control for B. abortus; 6, E.coli DNA; 7, B. melitensis 16 M strain; 8, Negative control.
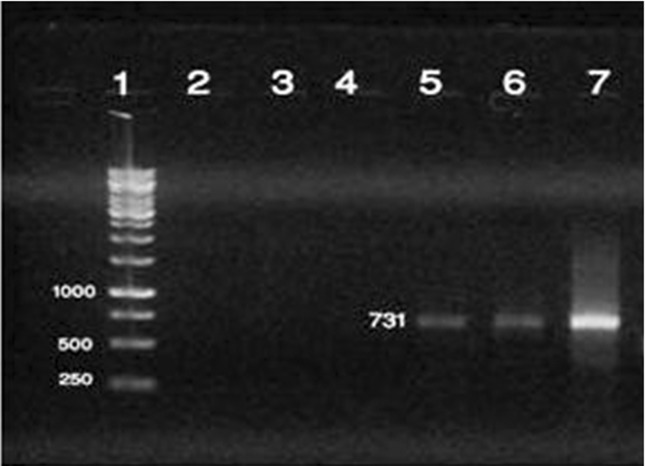
Figure 3.
Identification of DNA amplified fragments by agarose gel electrophoresis and ethidium bromide staining. An amplicon size of 731 bp was obtained by PCR using B. melitensis - specific primers and Brucella DNA as template. Lanes: 1, 1 kb Ladder; 2, Negative control; 3, E.coli DNA; 4, B. abortus; 5, 6, serum DNAs from patients diagnosed with brucellosis; 7, Positive control for B. melitensis 16 M strain.
Table 3.
Table 3. The results of the PCR amplification for B. abortus and B. melitensis.
| Standard tube agglutination (Wright) | PCR Positive B. melitensis | PCR Positive B. abortus | PCR Negative | Total |
|---|---|---|---|---|
| Wright > 1:160 | 6 (5.66%) | 87 (82.08%) | 13 (12.26%) | 106 (100%) |
| Wright < 1:160 | 2 (9.1%) | 18 (81.8%) | 2 (9.1%) | 22 (100%) |
| Wright Negative | 0 | 10 (19.23%) | 42 (80.77%) | 52 (100%) |
| Total | 8 | 115 | 57 | 180 |
Table 4.
Comparison of the PCR results with the Wright Method for detection of brucellosis.
| PCR Brucellosis | Wright | Total | |
|---|---|---|---|
|
|
|||
| Positive | Negative | ||
| Positive | 113 | 10 | 123 |
| Negative | 15 | 42 | 57 |
| Total | 128 | 52 | 180 |
Discussion
The diagnosis of brucellosis is challenging as culturing of Brucellae and sero-conversion are time consuming (Al Dahouk and Nockler, 2011). Therefore, molecular techniques like as PCR are promising alternatives for the diagnosis of infectious diseases caused by fastidious or slowly growth microorganisms such as Brucellae. In this study, we evaluated a PCR assay for detection of Brucella spp in human serum samples as templates, and also epidemiology of brucellosis in the Zanjan province of Iran. In the present study, by using of B4 and B5 primers, 123 out of 128 Wright-positive samples were positive for brucellosis. This observation is in agreement with the suggestions of researchers that SAT titers of less than 1:160 should not be ignored without further follow ups. On the other hand, SAT titers of 1:160 are not indicative of active infection, especially in Brucella endemic areas (Mantur et al., 2006; El Kholy et al., 2009; Gemechu et al., 2011).
Additionally, we compared the sensitivity and specificity of two targets, bcsp31 and IS711 genes, in PCR in comparison with STA. In the literature, sensitivities and specificities of various PCR assays targeting the bcsp31 gene to detect Brucella DNA in human blood or serum samples have varied from 50% to 100% and 60% to 100%, respectively (Queipo-Ortuno et al., 1997; Navarro et al., 1999; Al-Attas et al., 2000; Al-Ajlan et al., 2011). Our results indicated 91.6% efficiency for sensitivity of 96% and specificity of 80.7%, using STA as gold standard.
Our results indicate that the species-specific PCR assay with primers IS711 detects higher numbers of B. abortus DNA in both Wright-positive and -negative serum samples than B. melitensis. These findings are significantly different from PCR results that were reported by Khosravi (2006) in Iran and Elfaki (2005) in Saudi Arabia, respectively. In these studies, a large number of B. melitensis DNA was detected with using the same primers. The sensitivity and specificity of PCR-IS711 compared to STA as gold standard was found to be 88.2% and 80.7%, respectively.
In our study, we used serum samples instead of whole-blood for PCR assay. It has been previously reported that there are fewer inhibitors in the serum than in whole blood, and that the DNA extraction from serum is more efficient compared with whole blood (Zerva et al., 2001). Despite our satisfactory results, some patients with positive STA showed up as negative by PCR. These false-negatives could be result the presence of either a number of organisms below the detection limit or degradation of target DNA in the serum samples (Romero et al., 1995). The fact that the Wright-negative subjects were among the false positive samples could be due to a consistent exposed to the disease agent, examples of who include Veterinarian, Animal husbandry, and housekeeper /farmers. This finding corroborates well with the fact that most infections are found among housekeeper/farmer family and sheep/goats farmers (Kazemi et al., 2008).
In summary, PCR appears to offer several advantages over conventional methods: it is easy to perform; it is rapid; and it is safe for laboratory staffs because the serum based PCR-assay will reduce to risk of handling the microorganism in the laboratory. Therefore, the use of the BCSP, IS711- based PCR assays described here is a promising method for detection of the Brucella genus and also identifying Brucella spp in clinical samples.
Acknowledgments
We are indebted to Dr. Araghi for providing us the serum samples. We would like to thank laboratory staffs of Amir-Al-Momenin and vali-Asr hospital in Zanjan and the Department of Biotechnology, Faculty of Pharmacy, ZUMS (Zanjan, Iran) for providing access to the laboratory facilities. We would also like to thank Dr. Mehrdad Pedram (Department of Biotechnology, Medical Genetics and Molecular Biology, ZUMS) for his careful editing of the manuscript and valuable comments.
References
- Al-Ajlan HH, Ibrahim AS, Al-Salamah AA. Comparison of different PCR methods for detection of Brucella spp. in human blood samples. Pol J Microbiol. 2011;60:27–33. [PubMed] [Google Scholar]
- Al-Attas RA, Al-Khalifa M, Al-Qurashi AR, Badawy M, Al-Gualy N. Evaluation of PCR, culture and serology for the diagnosis of acute human brucellosis. Ann Saudi Med. 2000;20:224–228. doi: 10.5144/0256-4947.2000.224. [DOI] [PubMed] [Google Scholar]
- Al Dahouk S, Nockler K. Implications of laboratory diagnosis on brucellosis therapy. Expert Rev Anti Infect Ther. 2011;9:833–845. doi: 10.1586/eri.11.55. [DOI] [PubMed] [Google Scholar]
- Alves CJ, Figueiredo SM, Azevedo SS, Clementino IJ, Keid LB, Vasconcellos SA, Batista CSA, Rocha VCM, Higino SS. Detection of Brucella ovis in ovine from Paraíba State, in the Northeast region of Brazil. Brazilian Journal of Microbiology. 2010;41:365–367. doi: 10.1590/S1517-838220100002000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza J, Pellicer T, Pallares R, Foz A, Gudiol F. Specific antibody profile in human brucellosis. Clin Infect Dis. 1992;14:131–140. doi: 10.1093/clinids/14.1.131. [DOI] [PubMed] [Google Scholar]
- Baily GG, Krahn JB, Drasar BS, Stoker NG. Detection of Brucella melitensis and Brucella abortus by DNA amplification. J Trop Med Hyg. 1992;95:271–275. [PubMed] [Google Scholar]
- Bower AG, Chudnoff JS. Laboratory Procedures in Diagnosis of Brucellosis. Calif Med. 1948;69:131–132. [PMC free article] [PubMed] [Google Scholar]
- Bricker BJ. PCR as a diagnostic tool for brucellosis. Vet Microbiol. 2002;90:435–446. doi: 10.1016/s0378-1135(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Christopher S, Umapathy BL, Ravikumar KL. Brucellosis: review on the recent trends in pathogenicity and laboratory diagnosis. J Lab Physicians. 2010;2:55–60. doi: 10.4103/0974-2727.72149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenero JD, Munoz-Roca NL, Bermudez P, Plata A, Villalobos A, Reguera JM. Clinical findings, diagnostic approach, and outcome of Brucella melitensis epididymoorchitis. Diagn Microbiol Infect Dis. 2007;57:367–372. doi: 10.1016/j.diagmicrobio.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kholy AA, Gomaa HE, El Anany MG, Abd El Rasheed E. Diagnosis of human brucellosis in Egypt by polymerase chain reaction. East Mediterr Health J. 2009;15:1068–1074. [PubMed] [Google Scholar]
- Elfaki MG, Uz-Zaman T, Al-Hokail AA, Nakeeb SM. Detection of Brucella DNA in sera from patients with brucellosis by polymerase chain reaction. Diagn Microbiol Infect Dis. 2005;53:1–7. doi: 10.1016/j.diagmicrobio.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Gemechu MY, Gill JP, Arora AK, Ghatak S, Singh DK. Polymerase chain reaction (PCR) assay for rapid diagnosis and its role in prevention of human brucellosis in Punjab, India. Int J Prev Med. 2011;2:170–177. [PMC free article] [PubMed] [Google Scholar]
- Kazemi B, Yousefi Namin SA, Dowlatshahi M, Bandepour M, Kafilzadeh F, Gachkar L, Mahmoudinejad F, Samarghandi AM, Mardani M. Detection of Brucella by Peripheral Blood PCR and Comparison with Culture and Serological Methods in Suspected Cases. Iranian J Public Health. 2008;37:96–102. [Google Scholar]
- Khosravi AD, Abassi E, Alavi SM. Isolation of Brucella melitensis and Brucella abortus from brucellosis patients by conventional culture method and polymerase chain reaction technique. Pak J Med Sci. 2006;22:396–400. [Google Scholar]
- Mantur BG, Biradar MS, Bidri RC, Mulimani MS, Veerappa Kariholu P, Patil SB, Mangalgi SS. Protean clinical manifestations and diagnostic challenges of human brucellosis in adults: 16 years’ experience in an endemic area. J Med Microbiol. 2006;55:897–903. doi: 10.1099/jmm.0.46097-0. [DOI] [PubMed] [Google Scholar]
- Najafi N, Ghassemian R, Davoody AR, Tayebi A. An unusual complication of a common endemic disease: clinical and laboratory aspects of patients with brucella epididymoorchitis in the north of Iran. BMC Res Notes. 2011;4:286. doi: 10.1186/1756-0500-4-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro E, Fernandez JA, Escribano J, Solera J. PCR assay for diagnosis of human brucellosis. J Clin Microbiol. 1999;37:1654–1655. doi: 10.1128/jcm.37.5.1654-1655.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- Queipo-Ortuno MI, Colmenero JD, Reguera JM, Garcia-Ordonez MA, Pachon ME, Gonzalez M, Morata P. Rapid diagnosis of human brucellosis by SYBR Green I-based real-time PCR assay and melting curve analysis in serum samples. Clin Microbiol Infect. 2005;11:713–718. doi: 10.1111/j.1469-0691.2005.01202.x. [DOI] [PubMed] [Google Scholar]
- Queipo-Ortuno MI, Morata P, Ocon P, Manchado P, Colmenero JD. Rapid diagnosis of human brucellosis by peripheral-blood PCR assay. J Clin Microbiol. 1997;35:2927–2930. doi: 10.1128/jcm.35.11.2927-2930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero C, Gamazo C, Pardo M, Lopez-Goni I. Specific detection of Brucella DNA by PCR. J Clin Microbiol. 1995;33:615–617. doi: 10.1128/jcm.33.3.615-617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofian M, Aghakhani A, Velayati AA, Banifazl M, Eslamifar A, Ramezani A. Risk factors for human brucellosis in Iran: a case-control study. Int J Infect Dis. 2008;12:157–161. doi: 10.1016/j.ijid.2007.04.019. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Fact sheet No. 173. WHO; Geneva, Switzerland: 1997. Brucellosis. [Google Scholar]
- Young EJ. Serologic diagnosis of human brucellosis: analysis of 214 cases by agglutination tests and review of the literature. Rev Infect Dis. 1991;13:359–372. doi: 10.1093/clinids/13.3.359. [DOI] [PubMed] [Google Scholar]
- Young EJ. An overview of human brucellosis. Clin Infect Dis. 1995;21:283–289. doi: 10.1093/clinids/21.2.283. quiz 290. [DOI] [PubMed] [Google Scholar]
- Zerva L, Bourantas K, Mitka S, Kansouzidou A, Legakis NJ. Serum is the preferred clinical specimen for diagnosis of human brucellosis by PCR. J Clin Microbiol. 2001;39:1661–1664. doi: 10.1128/JCM.39.4.1661-1664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]