Abstract
Post-mortem bacterial culture and specific biochemical tests are currently performed to characterize the etiologic agent of bovine tuberculosis. Cultures take up to 90 days to develop. A diagnosis by molecular tests such as PCR can provide fast and reliable results while significantly decreasing the time of confirmation. In the present study, a nested-PCR system, targeting rv2807, with conventional PCR followed by real-time PCR, was developed to detect Mycobacterium tuberculosis complex (MTC) organisms directly from bovine and bubaline tissue homogenates. The sensitivity and specificity of the reactions were assessed with DNA samples extracted from tuberculous and non-tuberculous mycobacteria, as well as other Actinomycetales species and DNA samples extracted directly from bovine and bubaline tissue homogenates. Regarding the analytical sensitivity, DNA of the M. bovis AN5 strain was detected up to 1.5 pg by nested-PCR, whereas DNA of M. tuberculosis H37Rv strain was detected up to 6.1 pg. The nested-PCR system showed 100% analytical specificity for MTC when tested with DNA of reference strains of non-tuberculous mycobacteria and closely-related Actinomycetales. A clinical sensitivity level of 76.7% was detected with tissues samples positive for MTC by means of the culture and conventional PCR. A clinical specificity of 100% was detected with DNA from tissue samples of cattle with negative results in the comparative intradermal tuberculin test. These cattle exhibited no visible lesions and were negative in the culture for MTC. The use of the nested-PCR assay to detect M. tuberculosis complex in tissue homogenates provided a rapid diagnosis of bovine and bubaline tuberculosis.
Keywords: bovine and bubaline tuberculosis, nested-PCR, real-time PCR, tissue, sanitary inspection
Introduction
Tuberculosis is an infectious-chronic disease caused by members of the Mycobacterium tuberculosis complex (MTC), including M. tuberculosis, M. africanum, M. bovis, M. microti, M. caprae, M. mungi, M. orygis, M. pinnipedii and M. cannetii (Brosch et al., 2002). Although M. bovis is the most common agent of tuberculosis in cattle, other species of the MTC have been confirmed, including M. tuberculosis (Ameni et al., 2011), M. caprae (Sahraoui et al., 2009) and M. africanum (Weber et al., 1998).
In Brazil, the control of bovine and bubaline tuberculosis involves culling infected cattle based on the intradermal reaction to tuberculin purified protein derivative (PPD). Epidemiologic surveillance is also carried out in abattoirs, as well as sanitary inspections of all animals slaughtered for consumption purposes (Brasil, 2004).
With the advent of economic globalization, tariff barriers have been substituted by sanitary barriers. There is growing pressure from importing markets for a definitive diagnosis of tuberculosis in cattle that exhibit lesions compatible with tuberculosis (LCT) in the exporting country.
Although bacteriological cultures are a reliable, definitive method to detect MTC, they require a significant amount of time (up to 90 days) because members of this complex grow slowly when compared with general bacteriological standards (Costello et al., 1998; Miller et al., 2002; Schmitt et al., 2002; Drobniewski et al., 2003; Hines et al., 2006; Lisle et al., 2008).
In this sense, it is worth pointing out that molecular diagnostic systems based on real-time PCR technology are faster and provide more automation possibilities (Soini and Musser, 2001). Although many real-time PCR systems have been developed to detect MTC directly from biological samples in humans, the development of procedures to detect MTC in cattle tissue homogenates has been very limited (Taylor et al., 2001; Parra et al., 2008; Thacker et al., 2011; Costa et al., 2013; Araújo et al., 2014). The main constraints are the difficulty in extracting mycobacterial DNA from cattle samples, since they have few bacteria and the structure of the biological sample itself exhibits strong fibrosis and calcification, which hinder the DNA detection process (Liébana et al., 1995). Another difficulty involves the concentration of viable mycobacterial DNA, which is usually low, when compared to the concentration of host DNA (Liébana et al., 1995). A third problem is that cattle with PPD positive reactions do not always exhibit visible lesions during slaughter in the abattoir (Costello et al., 1997), due to recent infections with a low mycobacterial charge.
To overcome sensitivity problems in detecting pathogenic mycobacteria directly from bovine/bubaline tissues, the aim of the present study was to describe nested-PCR, with a combination of conventional PCR and real-time PCR, to detect MCT in tissue homogenates.
Materials and Methods
Biological samples
Table 1 displays the reference bacterial strains used for analytical sensitivity and specificity testing and optimization of the nested-PCR. These include members of the Mycobacterium tuberculosis complex (Mycobacterium bovis and Mycobacterium tuberculosis), the Mycobacterium avium complex (Mycobacterium avium), atypical non-tuberculosis mycobacteria (Mycobacterium abscessus, Mycobacterium fortuitum, Mycobacterium gordonae, Mycobacterium kansasii and Mycobacterium smegmatis), and non-Mycobacterium Actinomycetales (Corynebacterium pseudotuberculosis, Rhodococcus equi).
Table 1.
Bacterial strains used to assess the analytical specificity or sensitivity of the nested-PCR.
| Bacterial strains | Strain/origin |
|---|---|
| Corynebacterium pseudotuberculosis | LGCM/FIOCRUZ* |
| Mycobacterium abscessus | ATCC 19977/FIOCRUZ |
| Mycobacterium avium | ATCC 25291/FIOCRUZ |
| Mycobacterium bovis | AN5 strain, Ministry of Agriculture – LANAGRO-MG |
| Mycobacterium fortuitum | ATCC 6841/FIOCRUZ |
| Mycobacterium gordonae | ATCC 14470/FIOCRUZ |
| Mycobacterium kansasii | ATCC 12478/FIOCRUZ |
| Mycobacterium tuberculosis | H37Rv/FIOCRUZ |
| Mycobacterium smegmatis | ATCC 700044/FIOCRUZ |
| Rhodococcus equi | ATCC 6939/FIOCRUZ |
Oswaldo Cruz Foundation, National Institute for Quality Control in Health.
DNA was also obtained from the following sources: culture samples isolated from lesions of cattle naturally-infected with M. bovis from the Ministry of Agriculture – LANAGRO, MG, Brazil (n = 50); the Biological Institute in São Paulo, Brazil (n = 42); DNA isolated from 170 culture samples of M. tuberculosis isolated from the sputum of humans with tuberculosis (LABMAM, Fiocruz, RJ, Brazil); DNA from culture samples (n = 3) of M. avium from LANAGRO-MG. The M. bovis and M. avium strains were identified using standard biochemical methods (samples from LANAGRO-MG) or by PCR with primers JB21 and JB22 for MCT (Rodriguez et al., 1995). The M. tuberculosis strains were identified using spoligotyping (Kamerbeek et al., 1997) and PCR for polymorphism of the pncA gene (Barouni et al., 2004).
The AN5 reference strain of M. bovis (Canevari-Castelão et al., 2014) was cultured in a Stonebrink medium, while the other Mycobacterium sp. reference strains were cultured in Lowenstein Jensen media. Non-mycobacterium strains were not cultured and the DNA was purified directly from lyophilized bacterial suspension.
Bacterial strains DNA isolation
DNA of reference bacteria was purified with the DNEasy Blood & Tissue kit (Qiagen), following the manufacturer’s instructions. The quality and concentration of the DNA samples were assessed using spectrophotometry (NanoDrop ND-1000, Thermo Scientific) and electrophoresis in 0.8% agarose gel, stained with SYBR Gold (Invitrogen).
Primers and probes
Based on the DNA sequences of MTC members available through the GenBank-NCBI (http://www.ncbi.nlm.nih.gov) and Basic Local Alignment Search Tool (BLAST, http://blast.ncbi.nlm.nih.gov) programs, specific targets were selected for DNA amplification. The probe and primers for nested-PCR were designed using Primer Express v2.0 software (Applied Biosystems).
The target selected for amplification was rv2807 (ID: 888907), a 443 bp gene that encodes for a hypothetical protein of the M. tuberculosis complex.
Two sets of primers were designed: outer primers, for conventional PCR amplification; and the internal primers and probe, for TaqMan MGB real-time PCR amplification. The first reaction was included to enrich the MTC DNA, since the higher relative concentration of host DNA isolated from cattle tissues may interfere with the amplification of the target gene. Table 2 displays the probe and primer sequences.
Table 2.
Primers and probes used with first conventional PCR and second real-time PCR for Mycobacterium tuberculosis complex (MTC).
| Target gene | DNA sequences (5′-3′) |
|---|---|
| rv2807 of Mycobacterium tuberculosis complex | Forward outer MTC: GGCGGTGGCGGAGTTGAAGGCGATGAG Reverse outer MTC: GCCGCGAGCGAGTCTGGGCGATGTC Forward internal MTC: CATTGCTGCGTAATTCGATCA Probe MTC: 6FAM CATCCACACCTGTTCG MGBNFQ Reverse internal MTC: GACCTTGGGCGCCTCAT |
Nested-PCR standardization
First step conventional PCR was carried out in a volume of 25 uL, containing the following: 10 mM Tris-HCl (pH 8.3); 50 uM KCl; 1.5 mM MgCl2; 0.2 mM of each dNTP; 7.5 pmol of each primer; 1.25 U of Taq DNA polymerase (Sigma) and 400 ng of DNA.
Second step real-time PCR reactions for MTC were performed in a volume of 12.5 uL, containing the following: 6.25 uL of TaqMan Master Mix (ref 4352042, Applied Biosystems); 600 nM of each primer; 100 nM of the probe and 3 uL of the first step PCR reaction.
First step amplifications were carried out in an MJ Mini Bio-Rad thermocycler. Initial denaturation was carried out at 95 °C for 4 min, followed by 35 cycles of denaturation at 95 °C for 90 s, annealing at 65 °C for 30 s, and extension at 72 °C for 45 s. A single 72 °C final extension step was carried out for 3 min. Real-time PCR amplifications were carried out in a StepOne Plus thermocycler (Applied Biosystems, USA). Initial denaturation was carried out at 95 °C for 10 min, followed by 35 cycles of denaturation at 95 °C for 15 s and annealing/extension at 62 °C for 30 s.
For all the nested-PCR reactions, the DNA of M. bovis AN5 and M. tuberculosis H37Rv was used for positive controls. No DNA was used for the negative control.
Primers for MTC were tested for analytical sensitivity with serial dilutions of DNA from reference strains of M. bovis AN5 and M. tuberculosis H37Rv, in triplicate, with one reaction mix for each replicate. DNA samples were only tested by real-time PCR, conventional PCR or nested-PCR (both conventional and real-time reactions).
The primers/probe for MTC were tested for sensitivity with DNA from 92 cultures of M. bovis from naturally-infected cattle, provided by LANAGRO and the Biological Institute, as well as 170 DNA samples from cultures of M. tuberculosis from naturally-infected humans.
The primers/probe for MTC were tested for specificity with 50 ng of DNA of Corynebacterium pseudotuberculosis, Mycobacterium abscessus, Mycobacterium avium, Mycobacterium fortuitum, Mycobacterium gordonae, Mycobacterium kansasii, Mycobacterium smegmatis, and Rhodococcus equi. To test for PCR inhibitors, aliquots of DNA from the above species, used to assess specificity, were spiked with DNA of M. bovis AN5 and tested by nested-PCR. The amplification conditions were the same as those for the specific target.
Direct detection of MTC in bovine and water buffalo tissues
Direct detection of MTC in tissue homogenates was carried out with 113 bovines and 46 water buffaloes (Bubalus bubalis) from the following groups:
Seventy-six comparative intradermal tuberculin test (CITT) positive cattle, including 45 cattle with LCT and 31 with no visible lesions (NVL). These animals, of different ages and zootechnical categories (dairy and beef cattle), were culled following the guidelines of the Brazilian National Program for the Control and Eradication of Bovine Brucellosis and Tuberculosis (Brasil, 2004).
Twenty-three CITT negative cattle with no visible lesions (NVL). These animals came from a mixed exploitation farm (dairy and beef cattle), with a previous history of bovine tuberculosis.
Sixty cattle with no history of CITT, including 59 animals with LCT and 1 animal with NVL.
Comparative intradermal tuberculin tests were conducted following the guidelines of the Brazilian National Program for the Control and Eradication of Bovine Brucellosis and Tuberculosis (Brasil, 2004). A positive CITT reaction was defined as a relative increase in skin thickness at the injection site for bovine PPD that was at least 4 mm greater than the increase in skin thickness at the injection site for avian PPD (Brasil, 2004).
In the present study, lesions compatible with tuberculosis (LCT) were obtained from hepatic, iliac, mandibular, mediastinal, mesenteric, pre-scapular, retropharyngeal and tracheobronchial lymph nodes, as well as from the lungs, tonsils, liver or diaphragm. When cattle exhibited no visible lesions (NVL), hepatic, mediastinal, mesenteric, retropharyngeal and tracheobronchial lymph nodes were collected.
The organs were kept on ice until they reached the laboratory, where they were stored at 30 °C until processing. The organs were thawed and divided into two samples: one for culturing and the other for DNA isolation.
For DNA isolation, the samples were cut into pieces of 100 mg, corresponding to the transition between gross lesions and apparently healthy areas. These pieces were completely homogenized with 1 mL of phosphate buffered saline (PBS). From these tissue suspensions, 200 uL was used for DNA isolation with the DNEasy Blood & Tissue kit (Qiagen), following the manufacturer’s instructions. Nested-PCR reactions were carried out as described above.
For culturing, the samples were thawed and homogenized with an equivalent amount of sterile sand and water. The tissue suspensions were filtered through sterile gauze and centrifuged at 1200 g for 15 min. The sediments were suspended with 2 mL of sterile water, decontaminated using Petroff’s method, and cultured in a Stonebrink medium. The cultures were incubated at 37 °C and searched for bacterial colonies for at least 90 days, with weekly checks. The smears from the isolated colonies were stained using the Ziehl-Neelsen method (ZN) for acid-fast bacilli (AFB). All the AFB + cultures were analyzed by PCR using the primers JB21 and JB22 for MTC (Rodriguez et al., 1995).
Cattle were considered positive for tuberculosis when at least one tissue sample exhibited positive amplification in the nested-PCR or when AFB positive cultures were confirmed in the PCR with primers JB21 and JB22.
Statistical analysis
Fischer’s exact test or the chi-squared test was carried out to assess associations between the categorical variables. Results were considered statistically significant when p ≤ 0.05.
Results
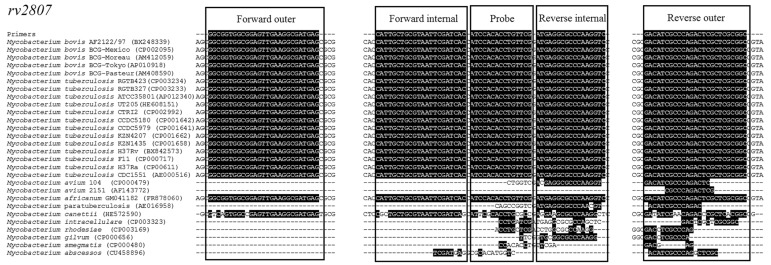
In silico analysis of the primer and probe sequences is shown in Figure 1. Complete identity was detected with MTC species, such as M. bovis (including BCG), M. tuberculosis, M. africanum, except M. canettii, which revealed partial identity, as well as M. abscessus, M. avium, M. gilvum, M. intracellulare, M. paratuberculosis, M. rhodesiae, M. smegmatis and M. ulcerans (Figure 1).
Figure 1.
BLASTn analysis of the probe and primer sequences for rv2807 of Mycobacterium tuberculosis complex.
The DNA sequences of the primers and probe for rv2807 were also conserved in 7 M. bovis isolates from Argentina and 10 M. bovis isolates from Brazil, the genomes of which were sequenced (data not shown).
With regards to analytical sensitivity, the primers and probe for MTC were tested by nested-PCR, real-time PCR and conventional PCR approaches. DNA of M. bovis AN5 was detected up to the following values: 390.6 pg with conventional PCR; 24.4 pg with real-time PCR and 1.5 pg with nested-PCR. DNA of M. tuberculosis H37Rv was detected up to the following values: 6.1 pg with conventional PCR; 24.4 pg with real-time PCR and 6.1 pg with nested-PCR.
Of the 50 DNA samples isolated from cultures of cattle naturally-infected with M. bovis (Ministry of Agriculture – LANAGRO), 49 (98.8%) were positive in the nested-PCR for MTC. All of the 42 (100%) DNA samples isolated from cultures of M. bovis, from the Biological Institute in São Paulo, were positive in the nested-PCR for MTC. With regards to the DNA of 170 culture samples of M. tuberculosis, isolated from sputum of humans with tuberculosis (LABMAM, Fiocruz, RJ, Brazil), 100% were positive in the nested-PCR for MTC.
The analytical specificity of the nested-PCR for MTC was tested using the DNA of C. pseudotuberculosis, M. abscessus, M. avium, M. fortuitum, M. gordonae, M. kansasii, M. smegmatis and R. equi. No amplification was detected when 50 ng of DNA of these microorganisms was used. Positive amplifications were recorded with the positive control DNA of M. bovis AN5 and M. tuberculosis H37Rv.
DNA aliquots of non-target microorganisms were spiked with the DNA of M. bovis AN5 to test for PCR inhibitors. Amplifications were detected, showing that there was no interference by PCR inhibitors.
Tissue samples from 159 bovines/bubalines were tested directly by nested-PCR for MTC. The nested-PCR and culture results are shown in Table 3.
Table 3.
Nested-PCR for Mycobacterium tuberculosis complex and culture results of 159 bovine and bubaline tissue homogenates.
| Status | Total number | Test | Number of positives (%) | p-value |
|---|---|---|---|---|
| CITT + and LCT | 45 | Nested-PCR + | 32 (71.1) | 0.318 |
| AFB + cultures* | 37 (82.2) | |||
| CITT + and NVL | 31 | Nested-PCR + | 17 (54.8) | 0.124 |
| AFB + cultures* | 10 (32.2) | |||
| CITT- and NVL | 23 | Nested-PCR + | 0 (0.0) | 0.999 |
| AFB + cultures* | 1 (4.3) | |||
| No CITT and LCT | 59 | Nested-PCR + | 33 (55.9) | 0.015 |
| AFB + cultures* | 19 (32.2) | |||
| No CITT and NVL | 1 | Nested-PCR + | 0 (0.0) | |
| AFB + cultures* | 1 (100.0) |
CITT = Comparative intradermal tuberculin test. LCT = Lesions compatible with tuberculosis. NVL = no visible lesions. AFB= Acid-fast bacilli.
Confirmed by PCR with primers JB21 and JB22 (Rodriguez et al., 1995).
In Brazil, CITT is considered a reference in vivo confirmatory test to identify cattle infected with M. bovis. Of the 76 CITT+ cattle recorded in the present study, 47 animals were positive in the culture (61.8%) and 49 were positive in the MTC nested-PCR (64.4%) (p = 0.86).
Of the 45 CITT + bovines and bubalines with LCT, 32 (71.1%) were positive for MTC in the nested-PCR and 37 (82.2) were positive in the culture, as confirmed by JB21/JB22 PCR (p = 0.318). Of the 31 CITT + bovines and bubalines with NVL, 17 (54.8%) were positive for MTC in the nested-PCR and 10 (32.2%) were positive in the culture, as confirmed by JB21/JB22 PCR (p = 0.124).
Detection of positive cattle for tuberculosis was statistically higher in the CITT+/LCT+ group than in the CITT+/NVL group for both the MCT nested-PCR (p = 0.0001) and culture (p = 0.0001) results.
Of the 76 CITT+ animals, 31 (40.7%) exhibited NVL during meat inspection. Of these animals, 7 (22.5%) were positive both in the culture and in the MTC nested-PCR.
In the group of 21 CITT+/NVL animals with negative cultures, there were 10 positive animals in the nested-PCR for MCT.
Of the 59 bovines and bubalines with LCT but not previously tested by the CITT, 33 (55.9%) were positive in the MTC nested-PCR and 19 (32.2%) were positive in the culture, as confirmed by JB21/JB22 PCR (p = 0.015).
In the analysis of 23 CITT– cattle from a farm with a history of bovine tuberculosis, there were no positive animals in the nested-PCR for MTC. However, one animal (4.3%) was positive in the culture, as confirmed by JB21/JB22 PCR.
In the analyses of the 104 animals that exhibited LCT, there were 65 animals positive in the MTC nested-PCR (62.5%) and 56 animals positive in the culture (53.8%) (p = 0.261).
Considering the presence of lesions compatible with tuberculosis, confirmed by the culture and PCR in a reference post-mortem test, there were 56 positive bovines and bubalines, of which 43 animals were positive in the MTC nested-PCR, determining a clinical sensitivity of 76.7%. There were 22 animals exhibiting NVL, with negative cultures and negative CITT, of which 22 were negative in the MTC nested-PCR, determining a clinical specificity of 100%.
Discussion
The aim of the present study was to develop a rapid post-mortem diagnostic system of bovine and bubaline tuberculosis, applicable directly to bovine clinical samples. The objective was to develop an accurate method that could substantially reduce the time between the detection of LCT and the etiological diagnosis (2 days), when compared to the traditional method of culturing, which takes up to 90 days.
One of the problems with detecting M. tuberculosis complex directly from lesions that are compatible with tuberculosis is that tissues generally exhibit strong fibrosis and calcification, which decrease the access to the mycobacterial DNA (Liébana et al., 1995). Three different commercial DNA purification kits were tested using tissues of cattle showing lesions caused by M. bovis. The best results were recorded by the DNEasy Blood & Tissue kit (Qiagen), in terms of the quality of the DNA and detection through MCT nested-PCR (data not shown).
Analysis of serial dilutions of DNA from reference strains of M. bovis AN5 revealed a higher analytical sensitivity in nested-PCR than in real-time PCR alone or conventional PCR, although this difference was more notable when comparing nested-PCR or real-time PCR with conventional PCR. With DNA of M. tuberculosis H37Rv, nested-PCR showed a similar sensitivity as conventional PCR, and both were more sensitive than real-time PCR alone. Although conventional PCR was not expected to exhibit the same analytical sensitivity as nested-PCR in the detection of M. tuberculosis DNA, the former requires post-amplification product processing (e.g., gel electrophoresis), which is time-consuming. The impact of the use of the nested-PCR approach will probably be greater in relation to clinical sensitivity, as in this case, since PCR inhibitors and atypical mycobacteria may interfere with the PCR performance. The choice for a nested-PCR strategy was also considered by Thacker et al. (2011), Costa et al. (2013) and Araújo et al. (2014), resulting in the increase of the clinical sensitivity in detecting M. bovis directly from tissue samples.
In silico analysis of the DNA primer and probe sequences for MTC showed complete identity with members of MTC, with the exception of M. canettii, which showed partial identity. However, M. canettii is a human pathogen (Brosch et al., 2002) and is believed to be rare and confined to eastern African countries (Reddington et al., 2011).
The analytical specificity of the nested-PCR was analyzed in vitro. No amplification was detected when the primers/probe for MTC were used with DNA of the atypical mycobacteria M. abcessus, M. avium, M. fortuitum, M. gordonae, M. kansasii and M. smegmatis, nor with the DNA of R. equi and C. pseudotuberculosis. This is particularly significant since environmental mycobacteria present in lymph nodes submitted for diagnostic testing can confound assays that lack sufficient specificity (Thacker et al., 2011). Furthermore, closely-related Actinomycetales, such as R. equi and C. pseudotuberculosis, may cause lesions to be mistaken for tuberculosis (Flynn et al., 2001; Sahraoui et al., 2009).
No statistically significant differences were found between the nested-PCR for MTC and the culture in terms of the detection of CITT+/LCT+ or CITT+/NVL animals. However, the nested-PCR and culture exhibited a higher sensitivity in detecting CITT+/LCT+ animals than those with CITT+/NVL. This is a clear indicator of the mycobacterial charge in each group of lesions. The same tendency of higher PCR sensitivity in the group of cattle with LCT was detected by Parra et al. (2008). However, in that study, the PCR failed to detect positive animals in the NVL group when they were associated with negative cultures. In the group of CITT+/NVL animals in the present study, nested-PCR for MTC was able to detect positive animals for tuberculosis, even when they exhibited negative cultures.
Lower rates of positivity were found by nested-PCR and culture in the group of animals with no CITT that exhibited LCT during meat inspection. One of the possible reasons for this result is the presence of granulomatous lesions caused by other microorganisms. Isolation of atypical mycobacteria in cattle with disseminated tuberculosis lesions is uncommon, owing to the non-progressive, chronic character of the infections. However, some cases of disseminated disease have been reported (Oloya et al., 2007). Lesions caused by other non-mycobacterial microorganisms, such as Rhodococcus, Actinobacillus, Arcanobacterium, and Nocardia, among others (Lisle et al., 2002), can also be mistaken for tuberculosis.
In Brazil, cattle with CITT+ results or lesions compatible with tuberculosis during routine abattoir inspections are considered positive for tuberculosis. With regards to the culture confirmed by PCR in a reference post-mortem test, a clinical sensitivity of 76.7% was detected. These results were obtained in a short period of time by nested-PCR (2 days), in contrast with the culture, which takes up to 90 days, and exhibited 100% clinical specificity.
Several methodologies have previously been employed to increase sensitivity of real-time PCR when detecting Mycobacterium sp. directly from tissue homogenates. Parra et al. (2008) used a capture probe to isolate mycobacterial DNA from tissue homogenates, achieving a lower sensitivity (65.6%) than that reported herein. Taylor et al. (2007) reported a sensitivity of 70% when performing PCR directly on tissue homogenates, although the sensitivity increased to 91% when PCR was only performed on DNA isolated from lesions excised from the tissues rather than whole tissue homogenates. The limitation of this method is that the assay can only be performed on tissues that have visible lesions, thus excluding samples without readily apparent lesions. Thacker et al. (2011) used a similar strategy to the present study, with a first conventional PCR and a second real-time reaction, although the authors targeted IS6110, detecting 66.7% of the culture positive samples. Costa et al. (2013) evaluated a nested real-time PCR for IS6110, detecting a diagnostic sensitivity and specificity of 98.2% and 88.7%, respectively. Araújo et al. (2014) tested a nested-PCR targeting the TbD1 region of M. bovis, detecting a clinical sensitivity of 76.0% with tissue samples from animals that exhibited positive results in the CITT, as well as from those with LCT that rendered positive cultures; and detected a clinical specificity of 100% with tissue samples from animals with CITT- results, with NVL and negative cultures.
One of the concerns about nested-PCR, particularly in relation to real-time reactions, is the possibility of cross-contamination. Throughout the DNA extraction procedure, gloves were changed frequently. DNA purification was carried out on a biosafety level 3 cabinet with the PCR set-up on a laminar flow PCR cabinet with UV light. Separate sets of micropipettes were used for DNA purification and PCR set-up. Filter tips were used routinely. Surfaces and equipment in contact with sample tubes were cleaned before each assay.
During meat inspection at abattoir level, the main concern is animals showing visible lesions, which are considered inappropriate for consumption. However, in the bovine/bubaline samples of the present study, 40.7% of the CITT+ animals exhibited NVL, from which 22.5% were positive for MTC both in the culture and the nested-PCR. This raises concerns that zoonotic transmission, such as that of M. bovis, the main MTC species found in cattle, can survive the cooking process (Van der Merwe et al., 2009). For this reason, sanitary policies involving PCR testing of CITT+/NVL animals should be considered.
All of the 23 CITT- cattle were also negative in the nested-PCR, although one animal was positive in the culture, which was confirmed with JB21/JB22 PCR. The farm on which these animals were raised had a history of bovine tuberculosis. Cattle with advanced stages of tuberculosis may not react in the CITT, but surprisingly, this animal exhibited NVL.
Conclusions
The use of the nested-PCR assay to detect M. tuberculosis complex in tissue homogenates provided a rapid diagnosis of bovine and bubaline tuberculosis. A large-scale study and an inter-laboratory validation of the method are required to determine whether the method is adequate for the Brazilian National Program for the Control and Eradication of Bovine Brucellosis and Tuberculosis.
Acknowledgments
The authors would like to thank CNPq (processes 578278/2008, 479394/2011-3 and 310165/2010-5), FUNDECT (process 23/200.152/2009 and 23/200.582/2012), and CAPES (PhD fellowship) for financial support and the Fundação Oswaldo Cruz - Instituto Nacional de Controle de Qualidade em Saúde for providing bacterial reference samples.
References
- Ameni G, Vordermeier M, Firdessa R, Aseffa A, Hewinson G, Gordon SV, Berg S. Mycobacterium tuberculosis infection in grazing cattle in central Ethiopia. Vet J. 2011;188:359–361. doi: 10.1016/j.tvjl.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo CP, Osório AL, Jorge KS, Ramos CA, Filho AF, Vidal CE, Roxo E, Nishibe C, Almeida NF, Júnior AA, Silva MR, Neto JD, Cerqueira VD, Zumárraga MJ, Araújo FR. Detection of Mycobacterium bovis in bovine and bubaline tissues using nested-PCR for TbD1. PLoS One. 2014;9:e91023. doi: 10.1371/journal.pone.0091023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouni AS, Augusto CJ, Lopes MT, Zanini MS, Salas CE. A pncA polymorphism to differentiate between Mycobacterium bovis and Mycobacterium tuberculosis. Mol Cell Probes. 2004;18:167–170. doi: 10.1016/j.mcp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Brasil. Manual Técnico. Ministério da Agricultura, Pecuária e Abastecimento; Brasília: 2004. Programa Nacional de Controle e Erradicação da Brucelose e Tuberculose – PNCEBT. [Google Scholar]
- Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, Garnier T, Gutierrez C, Hewinson G, Kremer K, Parsons LM, Pym AS, Samper S, van Soolingen D, Cole ST. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci USA. 2002;99:3684–3689. doi: 10.1073/pnas.052548299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canevari-Castelão AB, Nishibe C, Moura A, de Alencar AP, de Azevedo Issa M, Hodon MA, Mota PM, Sales EB, Fonseca AA, Júnior, Almeida NF, Araújo FR. Draft genome sequence of Mycobacterium bovis strain AN5, used for production of Purified Protein Derivative. Genome Announc. 2014;2:e00277. doi: 10.1128/genomeA.00277-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P, Ferreira AS, Amaro A, Albuquerque T, Botelho A, Couto I, Cunha MV, Viveiros M, Inácio J. Enhanced detection of tuberculous mycobacteria in animal tissues using a semi-nested probe-based real-time PCR. PLoS One. 2013;8:e81337. doi: 10.1371/journal.pone.0081337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E, Doherty ML, Monaghan ML, Quigley FC, O’Reilly PF. A study of cattle-to-cattle transmission of Mycobacterium bovis infection. Vet J. 1998;155:245–250. doi: 10.1016/s1090-0233(05)80019-x. [DOI] [PubMed] [Google Scholar]
- Costello E, Egan JW, Quigley FC, O’Reilly PF. Performance of the single intradermal comparative tuberculin test in identifying cattle with tuberculous lesions in Irish herds. Vet Rec. 1997;141:222–224. doi: 10.1136/vr.141.9.222. [DOI] [PubMed] [Google Scholar]
- Lisle GW, Kawakami RP, Yates GF, Collins DM. Isolation of Mycobacterium bovis and other mycobacterial species from ferrets and stoats. Vet Microbiol. 2008;132:402–407. doi: 10.1016/j.vetmic.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Drobniewski FA, Gibson A, Ruddy M, Yates MD. Evaluation and utilization as a public health tool of a national molecular epidemiological tuberculosis outbreak database within the United Kingdom from 1997 to 2001. J Clin Microbiol. 2003;41:1861–1868. doi: 10.1128/JCM.41.5.1861-1868.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn O, Quigley F, Costello E, O’Grady D, Gogarty A, Mc Guirk J, Takai S. Virulence-associated protein characterisation of Rhodococcus equi isolated from bovine lymph nodes. Vet Microbiol. 2001;78:221–228. doi: 10.1016/s0378-1135(00)00297-2. [DOI] [PubMed] [Google Scholar]
- Hines N, Payeur JB, Hoffman LJ. Comparison of the recovery of Mycobacterium bovis isolates using the BACTEC MGIT 960 system, BACTEC 460 system, and Middlebrook 7H10 and 7H11 solid media. J Vet Diagn Invest. 2006;18:243–250. doi: 10.1177/104063870601800302. [DOI] [PubMed] [Google Scholar]
- Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan FA, Chaudhry ZI, Ali MI, Khan S, Mumtaz N, Ahmad I. Detection of Mycobacterium avium subsp. paratuberculosis in tissue samples of cattle and buffaloes. Trop Anim Health Prod. 2010;42:633–638. doi: 10.1007/s11250-009-9467-8. [DOI] [PubMed] [Google Scholar]
- Liébana E, Aranaz A, Mateos A, Vilafranca M, Gomez-Mampaso E, Tercero JC, Alemany J, Suarez G, Domingo M, Dominguez L. Simple and rapid detection of Mycobacterium tuberculosis complex organisms in bovine tissue samples by PCR. J Clin Microbiol. 1995;33:33–36. doi: 10.1128/jcm.33.1.33-36.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisle GW, Bengis RG, Schmitt SM, O’Brien DJ. Tuberculosis in free-ranging wildlife: detection, diagnosis and management. Rev Sci Tech. 2002;21:317–334. doi: 10.20506/rst.21.2.1339. [DOI] [PubMed] [Google Scholar]
- Miller JM, Jenny AL, Payeur JB. Polymerase chain reaction detection of Mycobacterium tuberculosis complex and Mycobacterium avium organisms in formalin-fixed tissues from culture-negative ruminants. Vet Microbiol. 2002;87:15–23. doi: 10.1016/s0378-1135(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Oloya J, Kazwala R, Lund A, Opuda-Asibo J, Demelash B, Skjerve E, Johansen TB, Djønne B. Characterisation of mycobacteria isolated from slaughter cattle in pastoral regions of Uganda. BMC Microbiol. 2007;7:95. doi: 10.1186/1471-2180-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra A, García N, García A, Lacombe A, Moreno F, Freire F, Moran J, Hermoso de Mendoza J. Development of a molecular diagnostic test applied to experimental abattoir surveillance on bovine tuberculosis. Vet Microbiol. 2008;127:315–324. doi: 10.1016/j.vetmic.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Reddington K, O’Grady J, Dorai-Raj S, Niemann S, van Soolingen D, Barry T. A novel multiplex real-time PCR for the identification of mycobacteria associated with zoonotic tuberculosis. PLoS One. 2011;6:e23481. doi: 10.1371/journal.pone.0023481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JG, Mejia GA, Del Portillo P, Patarroyo ME, Murillo LA. Species-specific identification of Mycobacterium bovis by PCR. Microbiology. 1995;141:2131–2138. doi: 10.1099/13500872-141-9-2131. [DOI] [PubMed] [Google Scholar]
- Sahraoui N, Müller B, Guetarni D, Boulahbal F, Yala D, Ouzrout R, Berg S, Smith NH, Zinsstag J. Molecular characterization of Mycobacterium bovis strains isolated from cattle slaughtered at two abattoirs in Algeria. BMC Vet Res. 2009;5:4. doi: 10.1186/1746-6148-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt SM, O’Brien DJ, Bruning-Fann CS, Fitzgerald SD. Bovine tuberculosis in Michigan wildlife and livestock. Ann NY Acad Sci. 2002;969:262–268. doi: 10.1111/j.1749-6632.2002.tb04390.x. [DOI] [PubMed] [Google Scholar]
- Soini H, Musser JM. Molecular diagnosis of mycobacteria. Clin Chem. 2001;47:809–814. [PubMed] [Google Scholar]
- Taylor GM, Worth DR, Palmer S, Jahans K, Hewinson RG. Rapid detection of Mycobacterium bovis DNA in cattle lymph nodes with visible lesions using PCR. BMC Vet Res. 2007;3:12. doi: 10.1186/1746-6148-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Hughes MS, Skuce RA, Neill SD. Detection of Mycobacterium bovis in bovine clinical specimens using real-time fluorescence and fluorescence resonance energy transfer probe rapid-cycle PCR. J Clin Microbiol. 2001;39:1272–1278. doi: 10.1128/JCM.39.4.1272-1278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker TC, Harris B, Palmer MV, Waters WR. Improved specificity for detection of Mycobacterium bovis in fresh tissues using IS6110 real-time PCR. BMC Vet Res. 2011;7:50. doi: 10.1186/1746-6148-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe M, Bekker JL, van der Merwe P, Michel AL. Cooking and drying as effective mechanisms in limiting the zoonotic effect of Mycobacterium bovis in beef. J S Afr Vet Assoc. 2009;80:142–145. doi: 10.4102/jsava.v80i3.189. [DOI] [PubMed] [Google Scholar]
- Weber A, Reischl U, Naumann L. Demonstration of Mycobacterium africanum in a bull from North Bavaria. Berl Munch Tierarztl Wochenschr. 1998;111:6–8. [PubMed] [Google Scholar]