Abstract
Oncogenic HPV genotypes are strongly associated with premalignant and malignant cervical lesion. The purpose was to determine human papillomavirus (HPV) prevalence and genotypes, and to estimate cervical cancer risk factor associations. Cervical samples were obtained from 251 women seeking gynecological care at the Pelotas School of Medicine Clinic. This is a cross-sectional study. HPV-DNA was amplified by nested-PCR using MY09/11 and GP5/6 primers, and the sequencing was used for genotyping. Sociodemographic and behavioral risk factors were obtained by closed questionnaire, and its relationship to HPV infection prevalence were analyzed. Statistical analyses were performed using SPSS 16.0 software, and differences were considered significant at p < 0.05. As results, the prevalence of HPV infection was 29.9%. The most frequent genotype was HPV-16 (41.3%), followed by HPV-18 (17.3%), and HPV-33 (9.3%). Others nine HPV genotypes were also found. On this population, prevalence of oncogenic HPV genotypes was high, but does not seem to confer relationship with the risk factors investigated. Future investigations in larger populations are necessary, for the proposition of more appropriated monitoring strategies and treatment according to the Brazilian health service reality, as well as patients.
Keywords: HPV, SDT, molecular epidemiology, cervical cancer
Introduction
The human papillomavirus (HPV) is one of the most common etiologic agent of sexually transmitted diseases, with high prevalence rates. Actually, more than 100 HPV types have been described, of which 40 can infect the mucosa of the anogenital tract in both, men and women. According to their oncogenic potential, those viruses are classified as high- or low-risk genotypes (Bernard et al., 2010; De Villiers et al., 2011). The high-risk oncogenic genotypes are those that are strongly associated with premalignant and malignant cervical lesions, mainly HPVs 16 and 18, being also responsible for 70% of invasive cervical cancer (CC) cases in Brazil (Muñoz et al., 2006).
In Brazil, cervical cancer is the second most common type of cancer in the female population, and the fourth major cause of death in women. For 2012, 17.540 new cases were expected, 17 cases per 100,000 women (MS, 2011). Highly accurate techniques and epidemiologic studies demonstrate the relationship between the development of cervical cancer and HPV. HPV-DNA has been detected in 99.7% of cervical cancer cases worldwide (Fernandes et al., 2004).
Although HPV infection is considered an integral part of cervical cancer development, an association between HPV infection, oncogenic genotypes persistence, and other risk factors, determine the development of cervical intraepithelial neoplasia (CIN), and cervical cancer (Wang et al., 2010). The risk factors include youth, a high number of sexual partners throughout life, early sexual activity, tobacco smoking, low socioeconomic status, and genetic factors (Marlow et al., 2007; Kapeus et al., 2009; Louie et al., 2009), as well the 16 and/or 18 HPVs genotypes infection, responsible for 70% of invasive cervical cancer (CC) cases in Brazil (Muñoz et al., 2006). In addition, infections caused by human immunodeficiency virus (HIV), bacterial vaginosis (BV), Trichomonas vaginalis (TV), and by Chlamydia trachomatis (CT) were involved as co-factors for cervical cancer development, adjuvants of the neoplastic process (King et al., 2011, Nam et al., 2009).
Based on these data, our study aimed to detect the prevalence of HPV infection, to identify HPV genotypes, and to analyze cervical cancer risk factors in a female population representative of Pelotas, Rio Grande do Sul, Brazil.
Materials and Methods
Study population
This is a cross-sectional study. From May 2010 to May 2011, 251 women seeking gynecologic care at the gynecological ambulatory clinic at the Pelotas Federal University School of Medicine (UFPel) fulfilling eligibility criteria (not pregnant, sexually active, and not menstruating), and agreeing to participate in the study, were sequentially selected. The study used a closed questionnaire adapted from a previous study (Silveira and Santos, 2006), applied by a female trained interviewer. Routine gynecological exams (cervicitis indicators, visual inspection with acetic acid and Lugol’s iodine), were realized, and included in the questionnaire, as well the patient’s recorded information (last Pap test result).
The study was approved by the Ethics Committee of the Faculty of Medicine - Federal University of Pelotas in June 2009, and informed consent was obtained from all participants. All procedures were carried out in accordance with the guidelines of the Helsinki Declaration.
Sample collection and processing
Two cervical samples were collected from each patient. The first, with an Ayre’s spatula was placed on slides for Bacterial vaginosis (BV) and Trichomonas vaginalis (TV) analysis by the Gram method. The second sample was collected with a cytobrush, and placed into 1.5 mL eppendorf tubes containing 300 mL of Cell Lysis Solution (Puregene™ DNA Extraction Kit, Gentra Systems Minneapolis, MN). The tubes were submitted to digestion using 1.5 μL of Proteinase K (10 mg/mL, New England Biolabs, MA), and incubated overnight at room temperature. The genomic material (DNA) was extracted, according to manufacturer specifications. As control for extracted DNA quality, the human TP53 gene PCR was performed, using the primers previously described (Lin et al., 2008). PCR were performed in a final reaction volume of 12 μL, and was carried out with one cycle 94 °C for 3 min, followed by 40 cycles at 94 °C for 30 s, 57 °C for 30, 72 °C for 30 s and a final extension for 3 min at 72 °C (Thurow et al., 2011).
HPV detection was carried out using nested-PCR (nPCR) technique, which is performed in two rounds: the first using MY09/11 primers (Manos et al., 1989) and amplifying a 450 pb fragment, the second used GP5/6 internal primers (Snijders et al., 1990), which amplify the 140 pb fragment. The MY90/11 PCR reaction was performed in a final volume of 25 μL, and the conditions were as follows: 40 cycles of denaturation (1 min at 95 °C), annealing (1 min at 55 °C), and extension (1 min at 72 °C) (Gravitt et al., 2000). In the second round, the conditions were 40 cycles of denaturation (30 s at 94 °C), annealing (30 s at 45 °C), and extension (30 s at 72 °C) (Husnjak et al., 2000). Both PCR reactions were preheated for 9 min at 95 °C, and a final extension for 5 min at 72 °C. All PCR products were visualized on a 2.0% agarose gel with GelRed™ (Biotium Inc., CA).
HPV sequencing
HPV positive PCR products were purified previously using the Gel Band purification kit (GE Healthcare, USA) according the manufacturer instructions. The sequencing was performed in a MegaBACE 1000 DNA sequencer (GE Healthcare, USA), using Dynamic ET-terminator technology. Chromatograms were assembled and analyzed using the ContigExpress® module of the Vector NTI 10.0 suite (Invitrogen, USA). The assembled sequences were submitted to BLAST alignment (www.nci.nlm.gov/BLAST), against sequences available in GenBank.
Statistical analyses
Chi-square (χ2) test was used to evaluate the association between HPV presence (and their HR/LR genotypes) with the variables of questionnaire. A multivariate analysis was made using logistic regression applied to a hierarchical model. The analysis was performed using SPSS 16.0 software (SPSS, Chicago, IL). Differences were considered significant at p < 0.05.
Results
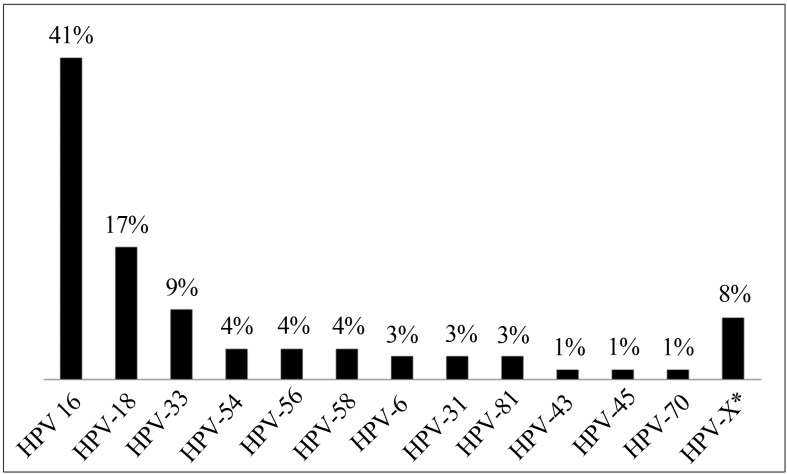
Four hundred women were approached to participate, but only 251 were considered eligible for the study. The mean age was 33.3 years old (median 34 years old), ranging from 18 to 45 years old. Most of the samples did not present endocervical mucupurulent discharge (77.2%), friability (70.8%), or cervical motion tenderness (84.6%). The visual inspection with acetic acid (VIA) and visual inspection with Lugol’s iodine (VILI) were negative for sub-clinical lesions in 85.9% and 71.1% of the participants, respectively. The Gram test detected BV in 9.2% of the women, and TV in 2.8%. Of the Pap smear results, 54.2% were normal, 38.6% inflammatory, 0.8% with ASCUS, 0.4% showed presence HPV, 2.1% indicated NIC I, and 0.4% indicated NIC II. Colposcopies were abnormal in 5% of the participants (data not show). The behavioral and sociodemographic characteristics are showed on Table 1. HPV was detected in 29.9% (N = 75), HR-HPV in 25.3%, and LR-HPV in 2.9% of the samples (Table 2). The most frequent genotype was HPV-16 (41.3%), followed by HPV-18 (17.3%), and HPV-33 (9.3%). The genotypes HPV-6 (2.7%), −31 (2.7%), −43 (1.3%), −45 (1.3%), −54 (4.0%), −56 (4.0%), −58 (4.0%), −70 (1.3) and −81 (2.7%) were also found. Six samples (8.0%) could not classified by sequencing due to overlap of the sequence-peaks. These were categorized as HPV-X (Figure 1).
Table 1.
Distribution of socioeconomic, demographic and behavioral characteristics.
| Variable | (%) |
|---|---|
| Age (years) | |
| 18 – 24 | 17.5 |
| 25 – 30 | 21.9 |
| 31 – 35 | 17.9 |
| 36 – 39 | 19.9 |
| 40 – 45 | 22.7 |
| Skin color | |
| White | 65.7 |
| Black | 12.4 |
| Others | 21.9 |
| Reading ability | |
| Yes | 98.0 |
| No | 2.0 |
| Schooling (years) | |
| 0 | 2.0 |
| 1–4 | 12.9 |
| 5–8 | 37.5 |
| 9–11 | 14.9 |
| ≥ 12 | 32.7 |
| Marital status | |
| Married/consensual union | 81.3 |
| Single | 11.2 |
| Separated/divorced/widowed | 7.6 |
| Live alone | |
| Yes | 2.8 |
| No | 97.2 |
| Family Income (minimum wages) | |
| 0 – 1 | 25.5 |
| 2 | 41.3 |
| 3 | 23.5 |
| ≥ 4 | 9.7 |
| Cigarette smoking | |
| Current smoker | 19.9 |
| Ex-smoker | 24.7 |
| Never smoker | 55.4 |
| Alcohol consume (last 4 weeks) | |
| At least once a week | 17.1 |
| Less than once a week | 29.5 |
| Never | 53.4 |
| Pregnancy | |
| Yes | 81.7 |
| No | 18.3 |
| Use of condom (in life) | |
| Yes | 90.0 |
| No | 10.0 |
| Sexual activity began (years) | |
| ≤ 12 | 2.8 |
| 13 – 15 | 33.1 |
| 16 – 18 | 49.8 |
| ≥ 19 | 14.3 |
| Sexual partners (in life) | |
| 1 | 30.8 |
| 2 – 4 | 44.4 |
| 5 – 8 | 15.2 |
| ≥ 9 | 9.6 |
| STD detection (last 12 months) | |
| Yes | 6.8 |
| No | 93.2 |
| Last Pap test | |
| Never | 4.4 |
| < 3 years | 74.9 |
| > 3 years | 15.1 |
| Don’t remember | 5.6 |
Table 2.
HPV detection and oncogenic potential.
| Variables | Number | (%) |
|---|---|---|
| HPV | 251 | |
| Positive | 29.9 | |
| Negative | 70.1 | |
| HPV oncogenic potential | 69* | |
| High risk | 89.9 | |
| Low risk | 10.1 |
HPV positives by nPCR.
Figure 1.
HPV genotypes identified by sequencing.
The bivariate analyses didn’t show statistic significance in relation to behavioral characteristics, although it was observed that HPV infection occurred mostly in women who were ex-smokers (35.5%), had consumed alcohol at least once a week (34.9%), had never been pregnant (45.7%), began sexual activities between 13–15 years of age (35.4%), had from 2 to 4 sexual partners in life (26.9%), had STD detected in the last 12 months (35.3%), and whose last Pap test was in less than 3 years (32.3%). Cervicitis indicators showed that 30.1% of the samples did not present endocervical mucupurulent discharge, or friability at (31.6%); cervical motion tenderness was observed in 31.6% of the women (Table 3). The VIA inspection was positive in 34.3% of the participants, and VILI inspection in 29.6%. The presence of BV and TV was observed for 31.8% and 28.6% of the patients respectively. The cytological results for HPV presence, and NIC II was confirmed by nPCR for all (p = 0.362). The colposcopy results were abnormal in 41.7% of the HPV positive cases (p = 0.379). In relation to high or low risk HPV infection, and variables, it was observed that all high risk HPV infections showed a direct association (100%) with NIC I and NIC II Pap test results (p = 0.470). No statistical difference was also found in the multivariate analyses (data not show).
Table 3.
Frequency distribution of HPV infection according Cervicitis indicators, visual inspection, bacterial infection and Pap test results.
| Variables | HPV infection | |
|---|---|---|
|
|
||
| Positive (%) | Negative (%) | |
| Endocervical mucupurulent discharge | ||
| Yes | 28.1 | 71.9 |
| No | 30.1 | 69.9 |
| p = 0.773 | ||
| Friablility | ||
| Yes | 26.0 | 74.0 |
| No | 31.6 | 98.4 |
| p = 0.379 | ||
| Cervical motion tenderness | ||
| Yes | 31.6 | 68.4 |
| No | 29.7 | 70.3 |
| p = 0.813 | ||
| Visual inspection with acetic acid (VIA) | ||
| Positive | 34.3 | 65.7 |
| Negative | 28.6 | 71.4 |
| p = 0.497 | ||
| Visual inspection with Lugol’s iodine (VILI) | ||
| Positive | 29.6 | 70.4 |
| Negative | 29.1 | 70.9 |
| p = 0.946 | ||
| Vaginosis | ||
| Positive | 31.8 | 68.2 |
| Negative | 29.0 | 71.0 |
| p = 0.783 | ||
| Trichomonas vaginalis | ||
| Positive | 28.6 | 71.4 |
| Negative | 29.3 | 70.7 |
| p = 0.967 | ||
| Pap test results | ||
| Normal | 28.1 | 71.9 |
| Inflammatory | 34.1 | 65.9 |
| ASCUS | 0 | 100.0 |
| HPV | 100.0 | 0 |
| NIC I | 40.0 | 60.0 |
| NIC II | 100.0 | 0 |
| Inconclusive | 25.5 | 75.0 |
| p = 0.362 | ||
| Colposcopy | ||
| Normal | 29.7 | 70.3 |
| Abnormal | 41.7 | 58.3 |
| p = 0.379 | ||
Discussion
Brazil has a population of 69.14 million women who are at risk for cervical cancer development, and current estimates indicate that 14.1% of the women in the general population have harbored a cervical HPV infection at some time in their lives. A total 70.7% of invasive cervical cancers are attributed to HPV-16 or -18 (WHO, 2010). HPV infection prevalence in Brazil varies between 7% and 43%, according to a systematic review of the literature performed in four Brazilian regions (Ayres and Silva, 2010). In this study, the prevalence was within the expected range (29.9%). Two other studies were realized in the same state in Southern Brazil: on the first was found a higher HPV infection prevalence (60%) in HIV negative women, (with or without cervical lesions) than reported here (Entiauspe et al., 2010). In the second study Rosa et al (2008) found that HPV infection was lower (12.3%). In the Northern regions, Noronha et al. (2011) conducted a screening study in 1021 women in the city of Belém, and observed an HPV prevalence of 12.4%.
In this study, the most prevalent genotype was HPV-16, followed by HPV-18. These findings are consistent with Oliveira-Silva et al. (2011), which in a study performed in Rio de Janeiro (with PCR for DNA-HPV detection in 297 HPV positive women), noted a prevalence of 28% for HPV-16, and 14.4% for HPV-18, differing by only a third from the most prevalent genotype, which was reported for HPV-45 (7.6%), and in our study was observed for HPV-33 (9.3%). However, Mendonça et al. (2010) carried out a study with 248 women in the city of Recife, and also observed that the HPV-33 genotype was the third most prevalent (13.9%), a similar percentage to the present study. The high prevalence of oncogenic genotypes found in this study indicates the need for frequent monitoring, and suggests nPCR technique for DNA-HPV detection as an alternative to early identification of women at high risk for cervical cancer development. Previous studies have already demonstrated that the use of primers pair MY09/11 and GP5/GP6 in a nPCR assay increases the sensitivity of HPV detection compared with PCR assay (Seaman et al., 2010; Coser et al., 2011).
Recent data (Vigitel, 2011) show that there has occurred a large decrease in the number of smokers in Brazilian population, going from 16.2% in year of 2006 to less than 15% in 2011. These data show that the state capital of Rio Grande do Sul, Porto Alegre, has the largest number of smokers (23%), higher than the one found in this study. The survey research also identifies that there was an increase in Pap tests realized in the last three years (89.6%) in women who had more than 12 years schooling. This study also observed a higher percentage of Pap tests realized during the same period (< 3 years), but didn’t found a relation with higher schooling. Some studies correlate potential risk factors with HPV infection. In this study, it wasn’t possible to observe this relationship. One of the explanations could be the increasing awareness of condom use and smoking health harms that may be contributed to these findings.
Since the study is clinical based, the results should be considered as preliminary, giving an indication of the high prevalence of HPV infection by oncogenic genotypes found in women from Pelotas, but not being capable of correlating the infection with behavioral and socio-demographic factors. Further population based studies from the same geographical area are needed. The current research helps to identify regional differences of HPV genotypes, given the large area and population differences exist in Brazil, and is a contribution to the medical care of the population of Pelotas and to research centers studying human papillomavirus.
Acknowledgments
The authors are grateful to Brazilian National Research Council (CNPq).
Footnotes
Conflict of Interest
None declared.
References
- Ayres ARG, Silva GA. Cervical HPV infection in Brazil: systematic review. Rev Sau Publ. 2010;44:963–974. doi: 10.1590/s0034-89102010000500023. [DOI] [PubMed] [Google Scholar]
- Bernard HU, Burk RD, Chen Z, van Doorsaler K, zur Hausen H, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70–79. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coser J, Boeira TR, Fonseca ASK, Ikuta N, Lunge VR. Human papillomavirus detection and typing using a nested-PCR-RFLP assay. Braz J Infect Dis. 2011;15:467–472. doi: 10.1016/s1413-8670(11)70229-x. [DOI] [PubMed] [Google Scholar]
- De Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Entiauspe LG, Teixeira LO, Mendonza-Sassi RA, Gonçalves CV, Gonçalves P, Martinez AMB. Human papillomavirus: prevalence and genotypes found among HIV-positive and negative women at a reference center in the far south of Brazil. Rev Soc Bras Med Trop. 2010;43:260–263. doi: 10.1590/s0037-86822010000300009. [DOI] [PubMed] [Google Scholar]
- Fernandes APM, Gonçalves MAG, Simões RT, Quintana SM, Duarte G, Donadi EA. Influência da infecção pelo HIV-1 sobre a presença do HPV em lesões do colo uterino. J Bras Doenças Sex Transm. 2004;16:21–25. [Google Scholar]
- Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlee F, Hildesheim A, Schiffman MH, Scott DR, Apple RJ. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husnjak K, Grce M, Magdic L, Pavelic K. Comparison of five different polymerase chain reaction methods for detection of human papillomavirus in cervical cell specimens. J Virol Methods. 2000;88:125–134. doi: 10.1016/s0166-0934(00)00194-4. [DOI] [PubMed] [Google Scholar]
- Kapeu AS, Luostarinen T, Jellum E, Dillner J, Hakama M, Koskela P, Lenner P, Love A, Mahlamaki E, Thoresen S, Tryggvadottir L, Wadell G, Youngman L, Lehtinen M. Is smoking an independent risk factor for invasive cervical cancer? A nested case-control study within Nordic biobanks. Am J Epidemiol. 2009;169:480–488. doi: 10.1093/aje/kwn354. [DOI] [PubMed] [Google Scholar]
- King CC, Jamieson DJ, Wiener J, Cu-Uvin S, Klein RS, Rompalo AM, Shah KV, Sobel JD. Bacterial vaginosis and the natural history of human papillomavirus. Infect Dis Obstet Gynecol. 2011;2011:319460. doi: 10.1155/2011/319460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Huang HI, Wang LH, Tsai CC, Lung O, Dai CY, Yu ML, Ho CK, Chen CH. Polymorphisms of COX-2-765G > C and p53 codon 72 and risks of oral squamous cell carcinoma in a Taiwan population. Oral Oncol. 2008;44:798–804. doi: 10.1016/j.oraloncology.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Louie KS, de Sanjosé S, Diaz M, Castellsague X, Herrero R, Meijer CJ, Shah K, Franceschi S, Muñoz N, Bosch FX. Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Braz J Cancer. 2009;100:1191–1197. doi: 10.1038/sj.bjc.6604974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manos MM, Ting Y, Wright DK, Lewis AI, Broker TR, Wolinsky SM. The use of polymerase chain reaction amplification for the detection of genital human papillomaviruses. Cancer Cells. 1989;7:209–214. [Google Scholar]
- Marlow LA, Waller J, Wardle J. Public awareness that HPV is a risk factor for cervical cancer. Braz J Cancer. 2007;97:691–694. doi: 10.1038/sj.bjc.6603927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça VG, Guimarães MJB, Filho JLL, Mendonça CG, Martins DBG, Crovella S, Alencar LCA. Infecção cervical por papilomavírus humano: genotipagem viral e fatores de risco para lesão intraepitelial de alto grau e câncer de colo do útero. Rev Bras Ginecol Obstet. 2010;32:476–485. doi: 10.1590/s0100-72032010001000002. [DOI] [PubMed] [Google Scholar]
- Ministério da Saúde do Brasil Instituto Nacional do Câncer. Estimativas 2012: Incidência de Câncer no Brasil. INCA; Rio de Janeiro: 2011. p. 35. [Google Scholar]
- Muñoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24(Suppl 3):S3–1-S310. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- Nam KH, Kim YT, Kim SR, Kim SW, Kim JW, Lee MK, Nam EJ, Jung YW. Association between bacterial vaginosis and cervical intraepithelial neoplasia. J Gynecol Oncol. 2009;20:39–43. doi: 10.3802/jgo.2009.20.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha VL, Cruz EM, Pinho CN, Mello WA, Villa LL, Russomano FB. Human Papillomavirus (HPV) in Women Screened to Cervical Uterine Cancer, Belém - Pará - Brazil. J Bras Doenças Sex Transm. 2011;23:5–11. [Google Scholar]
- Oliveira-Silva M, Lordello CX, Zardo LMG, Bonvicino CR, Moreira MAM. Human Papillomavirus in Brazilian women with and without cervical lesions. Virol J. 2011;8:p4. doi: 10.1186/1743-422X-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MI, Fachel JMG, Rosa DD, Medeiros LRM, Igansi CN, Bozzetti MC. Persistence and clearance of human papillomavirus infection: a prospective cohort study. Am J Obstet Gynecol. 2008;199:e617. doi: 10.1016/j.ajog.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Seaman WT, Andrews E, Couch M, Kojic EM, Cu-Uvin S, Palefsky J, Deal AM, Webster-Cyriaque J. Detection and quantification of HPV in genital and oral tissueas and fluids by real time PCR. Virology J. 2010;7:194. doi: 10.1186/1743-422X-7-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira MF, Santos IS. Impact of an educational intervention to promote condom use among the male partners of HIV positive women. J Eval Clin Pract. 2006;12:102–111. doi: 10.1111/j.1365-2753.2005.00626.x. [DOI] [PubMed] [Google Scholar]
- Snijders PJ, van den Brule AJ, Schrijnemakers HF, Snow G, Meijer CJ, Walboomers JM. The use of general primers in the polymerase chain reaction permits the detection of a broad spectrum of human papillomavirus genotypes. J Gen Virol. 1990;71(Pt1):173–181. doi: 10.1099/0022-1317-71-1-173. [DOI] [PubMed] [Google Scholar]
- Thurow HS, Haack R, Hartwig FP, Oliveira IO, Dellagostin AO, Gigante DP, Horta BL, Collares T, Seixas FK. TP53 gene polymorphism: importance to cancer, ethnicity and birth weight in a Brazilian cohort. J Biosci. 2011;36:823–831. doi: 10.1007/s12038-011-9147-5. [DOI] [PubMed] [Google Scholar]
- Vigitel - Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico, Ministério da Saúde do Brasil. [Acessed 01 Mar 2013];Sistema de Monitoramento de Fatores de Risco e Proteção para Doenças Crônicas Não Transmissíveis por meio de Inquérito Telefônico. 2011 Available on http://portal.saude.gov.br.
- Wang SS, Gonzalez P, Yu K, Porras C, Li Q, Safaeian M, Rodriguez AC, Sherman ME, Bratti C, Schiffman M, Wacholder S, Burk SD, Herrero R, Chanock SJ, Hildesheim A. Common Genetic Variants and Risk for HPV Persistence and Progression to Cervical Cancer. PLOS ONE. 2010;5:e8667. doi: 10.1371/journal.pone.0008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO/ICO Information Centre on HPV and Cervical Cancer. Human Papillomavirus and Related Cancers in Brazil. [Acessed on 01 Mar 2013];Summary Report 2010. 2010 Available on http://www.who.int/hpvcentre.