Abstract
Objective
Based on surgical outcomes of patients with infratentorial meningiomas surgically treated at our institution, we analyzed the predictors for surgical resection, recurrence, complication, and survival.
Methods
Of surgically treated 782 patients with intracranial meningioma, 158 (20.2%) consecutive cases of infratentorial location operated on between April 1993 and May 2013 at out institute were reviewed retrospectively. The patients had a median age of 57.1 years (range, 16--77 years), a female predominance of 79.7%, and a mean follow-up duration of 48.4 months (range, 0.8--242.2 months).
Results
Gross total resection (Simpson's grade I & II) was achieved in 81.6% (129/158) of patients. Non-skull base location was an independent factor for complete resection. The recurrence rate was 13.3% (21/158) and the 5-, 10-, and 15-year recurrence rates were 8.2%, 12.0%, and 13.3%, respectively. Benign pathology, postoperative KPS over than 90, low peritumoral edema, and complete resection were significantly associated with longer recurrence-free survival rate. The 5-, 10-, and 15-year survival rates were 96.2%, 94.9%, and 94.9%, respectively. Benign pathology, postoperative KPS over than 90 and complete resection were significantly associated with a longer survival rate. The permanent complication rate was 13% (21/158). Skull base location and postoperative KPS less than 90 were independent factors for the occurrence of permanent complication.
Conclusion
Our experience shows that infratentorial meningiomas represent a continuing challenge for contemporary neurosurgeons. Various factors are related with resection degree, complications, recurrence and survival.
Keywords: Complication, Intracranial meningioma, Infratentorial, Recurrence, Surgical outcome, Survival
INTRODUCTION
As reported in Henschen, a petrous ridge meningioma was first described by Rokitansky in 18556). In 1938, Harvey Cushing described seven cases of meningiomas. Only one did well and, accordingly, he emphasized a high surgical risk in dealing with infratentorial meningiomas25). Because of its deep location, narrow visual field of operation and proximity to cranial nerves, brain stem and important blood vessels, surgical intervention for this pathology have a high lethal and crippled rate16). Clinical presentation and microsurgical anatomy, particularly in regard to cranial nerve dislocation, critically depend on the tumor's dural origin22). Infratentorial meningiomas include cerebellar convexity, tentorial, cerebellopontine angle, jugular foramen, petroclival, peritorcula, and foramen magnum.
The ideal primary treatment of these tumors is total surgical resection5). However, infratentorial meningiomas have been a challenge for operation, because of its close proximity to critical vascular and neurologic structures and slow growth25). In the early series, the surgical try to remove these lesions was associated with high rates of morbidity and mortality9). As developing radiologic tools, microsurgical techniques and surgical approaches of these tumors, surgical management has been evolving and shown good outcomes along with a decrease in surgical morbidity and pamortality2,11,20,28). Despite these improvements, surgery for infratentorial meningiomas is still associated with high morbidity and complications. In the present study, we analyzed surgical outcomes of patients with infratentorial meningiomas surgically treated at our institution to postulate the predictors for surgical resection, recurrence, complication, and survival.
MATERIALS AND METHODS
Patients
The study is in compliance with the Declaration of Helsinki (Sixth Revision, 2008). This study fulfils all the requirements for patient anonymity was approved by the institutional review board. Between April 1993 and May 2013, 782 case of intracranial meningioma were surgically resected at our institution. Among these cases, 158 patients (20.2%) were diagnosed with infratentorial meningioma by means of radiological studies [non-enhanced and enhanced computed tomographic (CT) scanning and magnetic resonance imaging (MRI)] and their surgical records. Recurred cases were excluded from this study.
Analysis variables
To define the clinical and radiological characteristics of the patients, several variables such as age, sex, presenting symptoms, pathology, tumor features (size, location, peritumoral edema), preoperative and postoperative KPS, postoperative transient/permanent complications, recurrence and survival rate were evaluated. These clinicoradiological variables are summarized in Table 1.
Table 1.
Clinical characteristics of 158 patients with infratentorial meningiomas
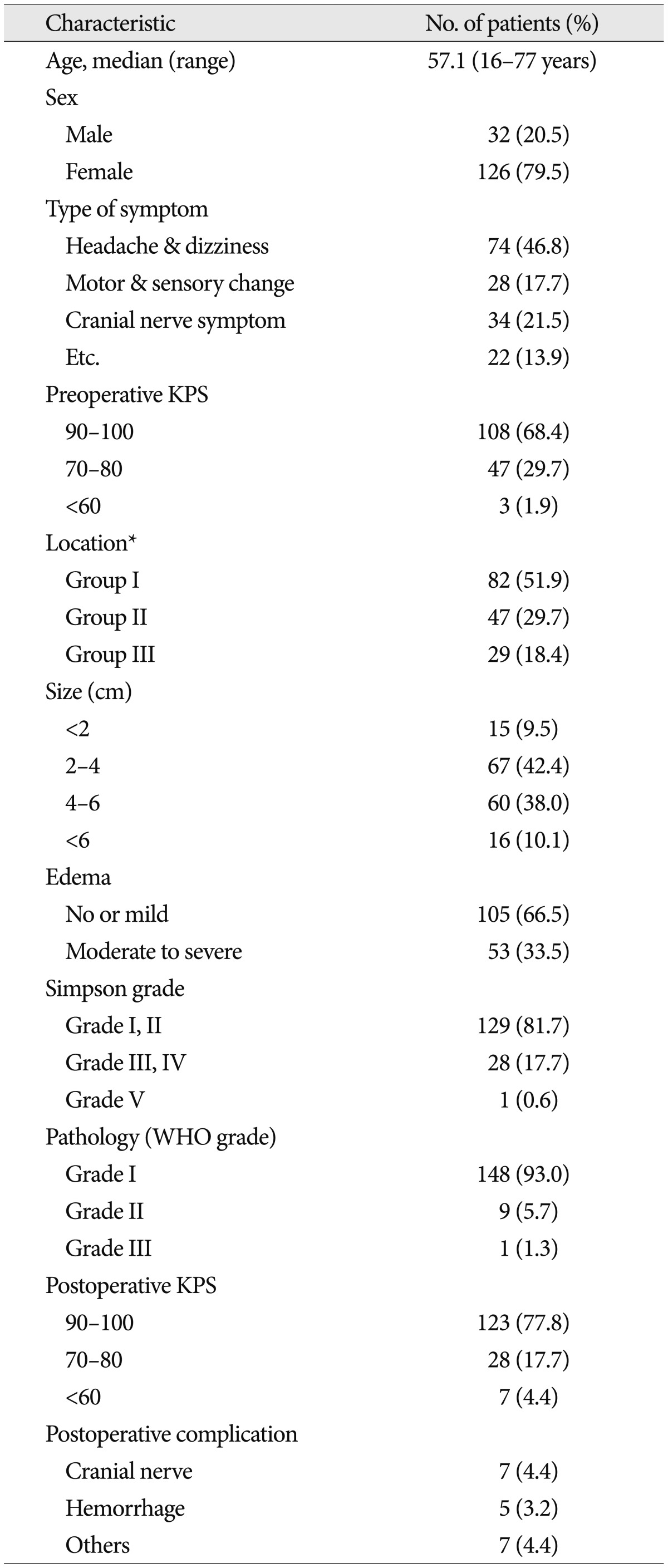
*Group I : cerebellopontine angle and cerebellar convexity, Group II : tentorial & peritorcular, Group III : skull base locations including petroclival, foramen magnum, and jugular foramen. WHO : World Health Organization, KPS : Karnofsky performance scale
The patients consisted of 32 males and 126 females (female predominance, 79.7%), with a median age of 57.1 years (range, 16-77 years). The most common presenting symptoms were headache, followed by dizziness and disturbance of gait. Some patients presented with mental change, another with cranial nerve palsy. The exact location of the patients was decided by radiological study and operation records, with regard to the origin of the mass, including tentorial meningioma in 39 patients, cerebellopontine angle (CPA) in 75 patietns, petroclival in 21 patients, peritorcular in 8 patients, cerebellar convexity in 7 patients, foramen magnum in 6 patients, and jugular foramen in 2 patients.
Tumor locations were classified into 3 groups according to their origin site (Group I, cerebellopontine angle & cerebellar convexity; Group II, tentorial & peritorcular; Group III, petroclival & other skull base location). Based on T1-weighted enhanced & T2-weighted non-enhanced MRI, the maximal size of the tumor and peri-tumoral edema was evaluated. Four centimeter was cut-off value as the size parameter (<4 cm vs. ≥4 cm). According to the peri-tumoral edema, the patients were divided into two groups [none or minimal (<5 mm in edema thickness) vs. moderate or severe (≥5 mm in edema thickness)].
The extent of tumor resection was classified according to the Simpson's classification22). Gross total resection was defined as Simpson's Grades I and II resection without any visible tumor remnant on surgical finding and follow-up MRI. All tumors were graded pathologically according to the World Health Organization (WHO) classification system. According to the pamortality had been classified by WHO grade that grade I in 148 patients, grade II & III in 10 (9 patients in Grade II & 1 in III). On follow-up MRI, new enhancing mass in completely resected case or re-growing symptomatic mass in non-completely resected case was regarded as a recurred lesion. Surgery-related complication was defined as a new developed neurological deficit or aggravated pre-existing deficit. The postoperative complications were separated of transient and permanent. The transient complication was defined as the new or aggravated postoperative problem resolved generally within 3-6 months after the operation. If the problem persisted or needed another operation, it was regarded as the permanent complication. The pre- and post-operative clinical status was quantified retrospectively using the KPS. The time to measure of post-operative KPS was 6 months after the operation. Good performance status was defined as above KPS 80. All surviving patients were monitored; the mean duration of follow-up was 48.4 months with a range of 0.8 to 242.2 months.
Statistical analysis
Recurrence-free survival (RFS) was calculated as the time from surgery to the date of recurrence. The probability of RFS was analyzed according to the Kaplan-Meier method and compared with the log-rank test. Overall survival was also calculated as the time from surgery to the date of death or last follow-up visit. For the multivariate analysis, independent prognostic factors were determined using the Cox's proportional hazards model. The relation between resection degree/permanent complication occurrence and categorical variables was compared by using a chi-square test or Fisher-exact probability test. Furthermore, binary logistic regression test was applied for multivariate analysis. All statistical analyses were performed using SPSS version 20.0 software program for Windows (Chicago, IL, USA). The level of significance was set at p<0.05.
RESULTS
Resection
Commonly used approaches were lateral or midline suboccipital. For some cases, far lateral, subtemporal, or orbitozygomatic route was selected to resect the lesion located on skull base region. In our series, 129 patients (81.6%, 129/158) were classified into Simpson's grade I and II, 28 patients (17.7%) into grade III and IV and 1 patient (0.6%) into grade V. On univariate analysis, female (p=0.019), non-skull base location (p<0.001) and postoperative KPS over than 90 (p=0.012) were significantly related with the possibility of complete resection (Table 2). However, only non-skull base location was associated with complete resection, on multivariate analysis [hazard ratio (HR) : 0.162, 95% confidence index (CI) : 0.065-0.405, p<0.001].
Table 2.
Univariate and multivariate analysis for resection predictors in patients with infratentorial meningiomas
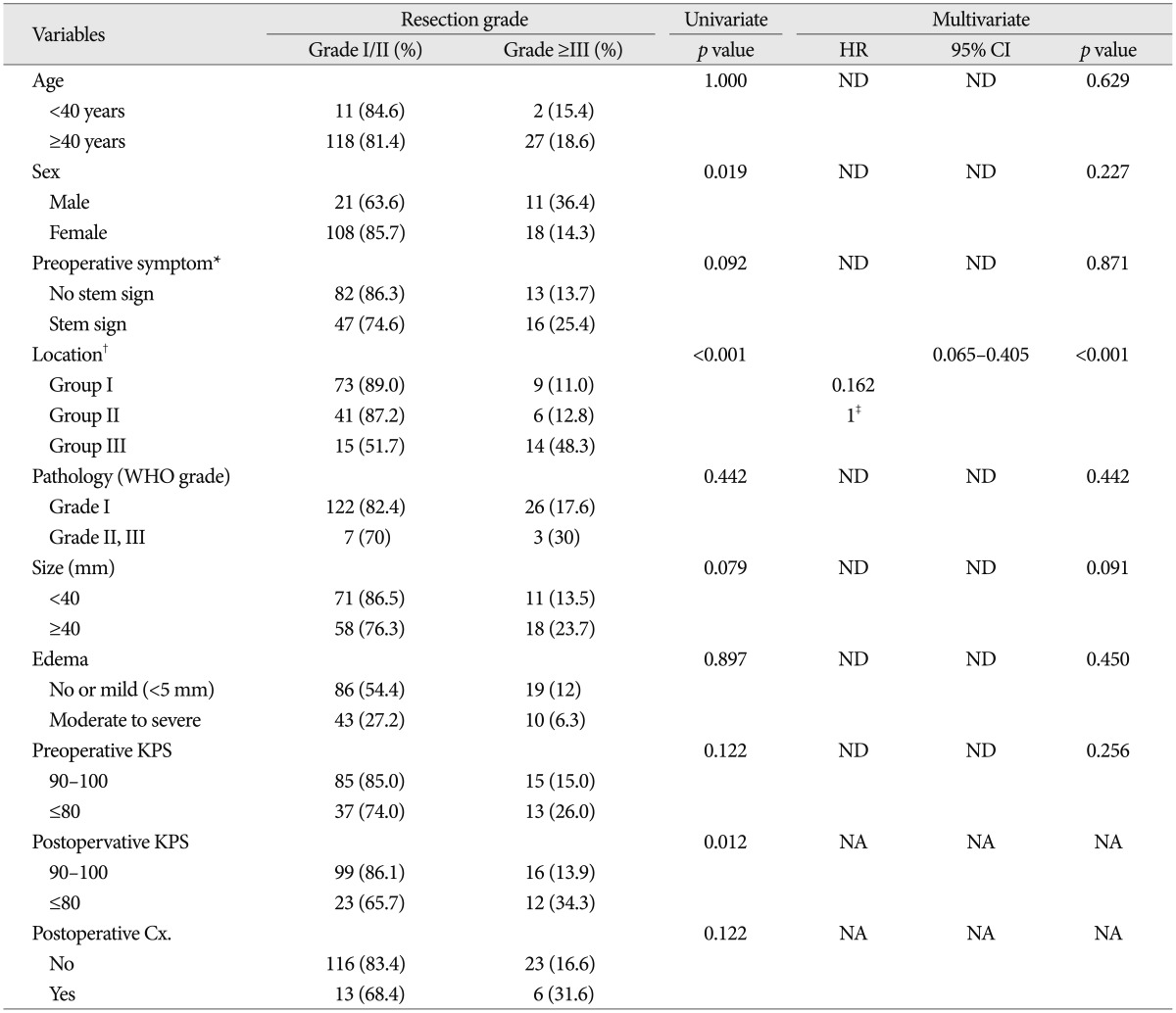
*No stem sign : headache, dizziness, seizure, Stem sign : mental changes, motor weakness, cranial nerve deficit, †Group I : cerebellopontine angle & cerebellar convexity, Group II : tentorial & peritorcular, Group III : skull base locations including petroclival, foramen magnum, and jugular foramen, ‡Reference variable : non-skullbase location groups (Group I+II). Cx : complication, KPS : Karnofsky performance scale, NA : non-available, ND : non-detected, WHO : World Health Organization
Recurrence
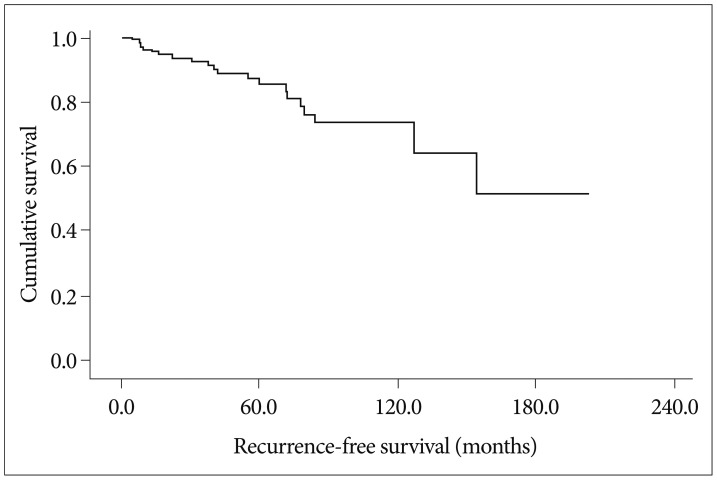
In this study, recurrence rate was 13.3% (21/158) and the 5-, 10-, and 15-year recurrence rates were 8.2%, 12.0%, and 13.3%, respectively (Fig. 1). The results of analyses of the variables that could be correlated with recurrence are shown in Fig. 2 and Table 3.
Fig. 1.
Overall recurrence-free survival. The incidence of recurrence rate is 13.3% (21/158) with the mean recurrence time of 149.7 months 95% confidence index : 128.3--171.2 months, the median recurrence time has not reached.
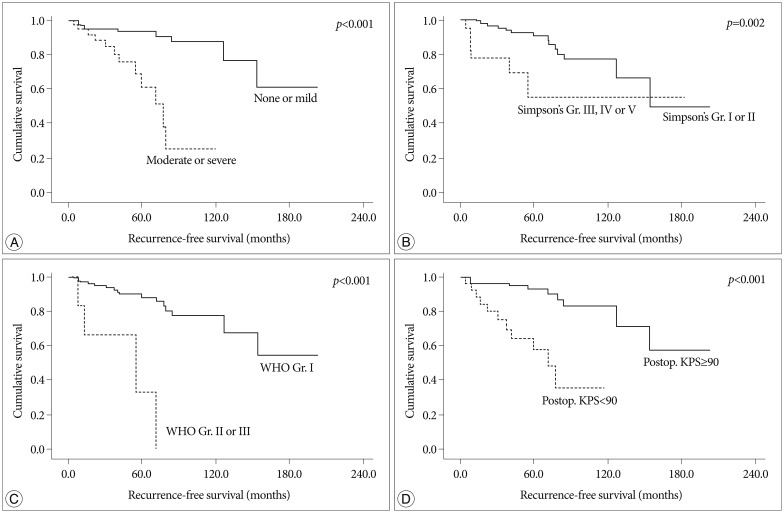
Fig. 2.
Kaplan-Meier analysis of recurrence-free survival for enrolled patients according to independent predictors on multivariate analysis (overall comparison was estimated using a log-rank test). A : Peritumoral edema. B : Simpson's grade. C : Pathology. D : Postoperative Karnofsky performance scale.
Table 3.
Univariate and multivariate analysis for recurrance predictors in patients with infratentorial meningiomas
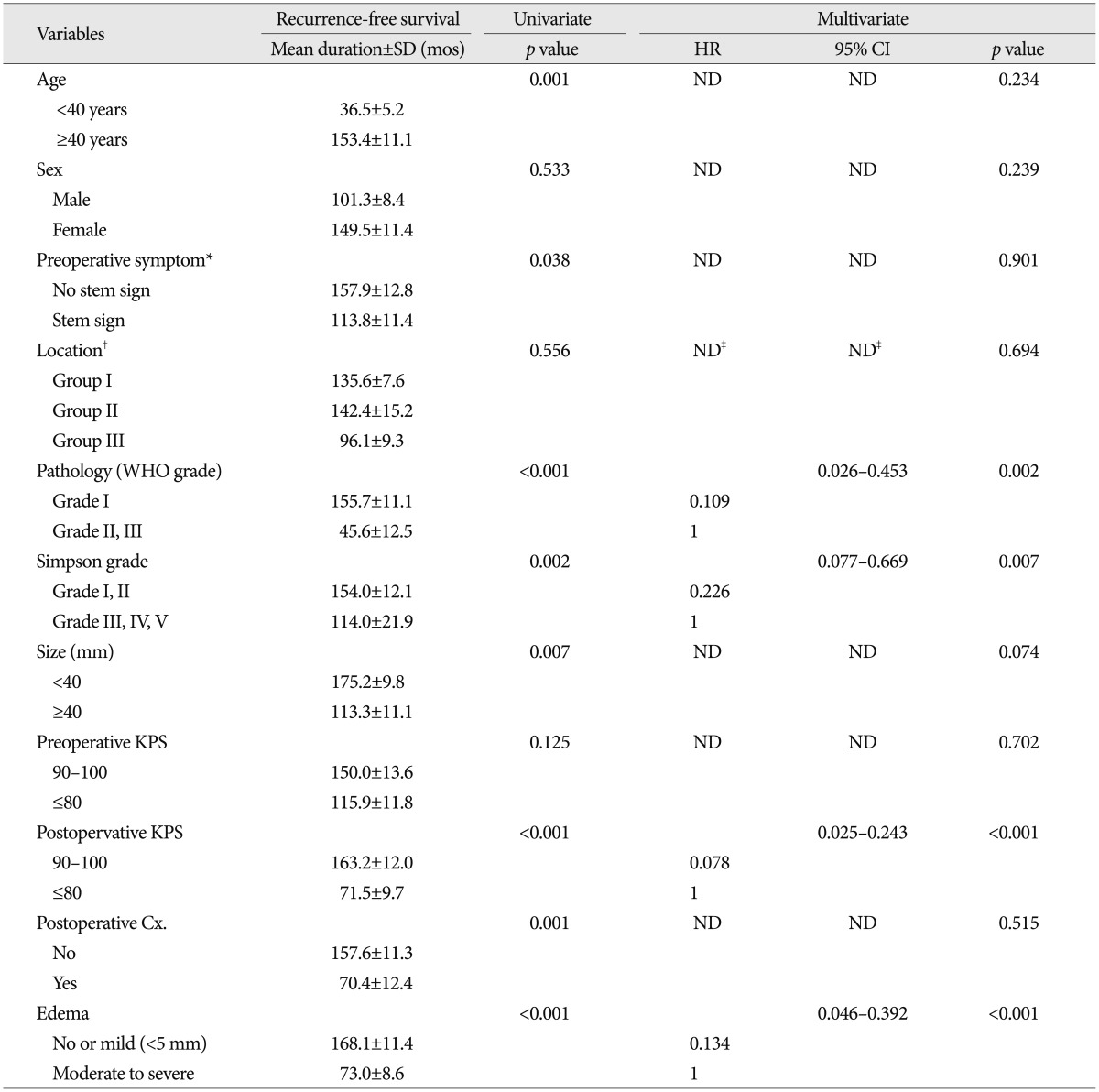
*No stem sign : headache, dizziness, seizure, Stem sign : mental changes, motor weakness, cranial nerve deficit, †Group I : cerebellopontine angle & cerebellar convexity, Group II : tentorial & peritorcular, Group III : skull base location including petroclival, foramen magnum, and jugular foramen, ‡Reference variable : non-skullbase location groups (Group I+II). Cx : complication, KPS : Karnofsky performance scale, ND : non-detected, WHO : World Health Organization
On univariate analysis, age, pathology, postoperative KPS, postoperative complication, edema, size, preoperative symptom and Simpson's grade showed statistical significance. The patient's age at the operation was important factor for RFS [mean value, 36.5±5.2 months (group <40 years) vs. 155.5±12.9 months (40≤ group <60 years), 107.6±6.5 months (group ≥60 years), p=0.004]. The younger-aged group showed shorter RFS than the older-aged group (mean value, 36.5±5.2 months vs. 153.4±11.1 months, p=0.001). The more aggressive pathology group (WHO grade II and III) showed the recurrence in earlier postoperative period than the benign group (mean value, 45.6±12.5 months vs. 155.7±11.1 months, p<0.001). The size of tumor was also an important factor [mean value, 175.2±9.8 months (group <4 cm) vs. 107.7±12.9 months (4 cm≤ group <6 cm), 106.3±22.8 months (group ≥6 cm), p=0.026]. In the group with large-size tumor (≥4 cm), the RFS was shorter than small-size group (mean value, 175.2±9.8 months vs. 113.3±11.1 months, p=0.007). The patient with brain stem or cranial nerve sign showed a shorter RFS time, compared to the patient with other minor symptoms (value, 157.9±12.8 months vs. 113.8±11.4 months, p=0.038). In regard to the peritumoral edema, higher edema group (moderate or severe) was significantly associated with shorter RFS (mean value, 168.1±11.4 months vs. 73.0±8.6 months, p<0.001). Not surprisingly, non-complete resection group (Simpson's grade III-V) demonstrated a shorter RFS compared to complete resection group (mean value, 154.0±12.1 months vs. 114.0±21.9 months, p=0.002). The absence of postoperative complication (mean value, 157.6±11.3 months vs. 70.4±12.4 months, p=0.001) and postoperative KPS over 90 (mean value, 163.2±12.0 months vs. 71.5±9.7 months, p<0.001) were significantly related with recurrence in a longer follow-up period.
On multivariate analysis, pathologic grade, postoperative KPS, peritumoral edema and resection degree were independent predictable factors for tumor recurrence. Benign pathology (HR 0.109, 95% CI 0.026-0.453, p=0.002), postoperative KPS over 90 (HR 0.078, 95% CI 0.025-0.243, p<0.001), low peritumoral edema (HR 0.134, 95% CI 0.046-0.392, p<0.001), and complete resection (HR 0.226, 95% CI 0.077-0.669, p=0.007) were significantly associated with longer RFS time.
Overall survival
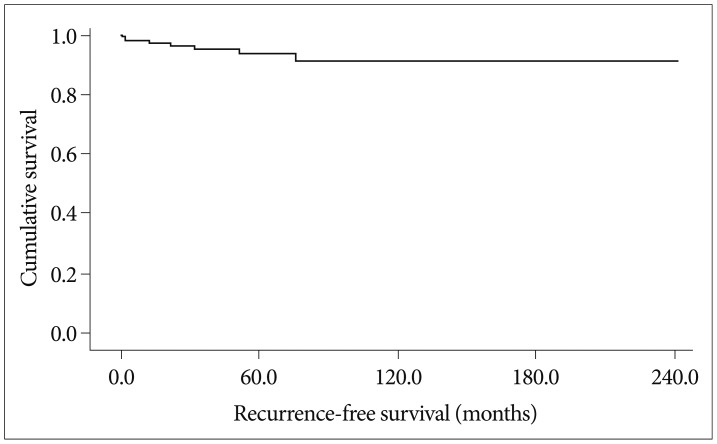
The incidence of death rate was 5.0% over follow-up period (8/158). The surgery-related mortality which the death occurred within one month after resection was 3.16% in this study (5/158). The 5-, 10-, and 15-year survival rates were 96.2%, 94.9%, and 94.9%, respectively (Fig. 3). The results of analyses of the variables that could be correlated with survival time are shown in Fig. 4 and Table 4.
Fig. 3.
Overall survival. The incidence of death rate was 5.0% (8/158) with the mean survival time of 224.1 months 95% confidence index : 211.3-237.0 months, the median survival time was not reached.
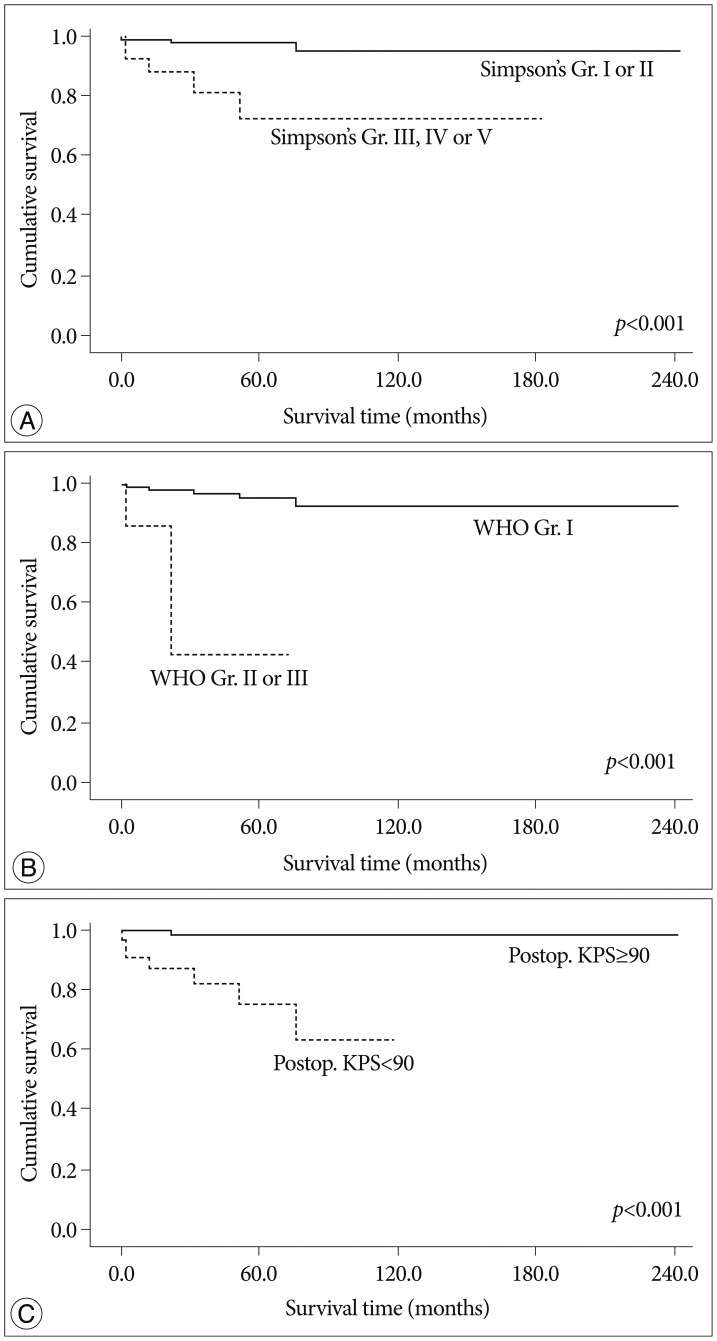
Fig. 4.
Kaplan-Meier analysis of overall survival for enrolled patients according to independent predictors on multivariate analysis (overall comparison was estimated using a log-rank test). A : Simpson's grade. B : Pathology. C : Postoperative Karnofsky performance scale.
Table 4.
Univariate and multivariate analysis for death predictors in patients with infratentorial meningiomas
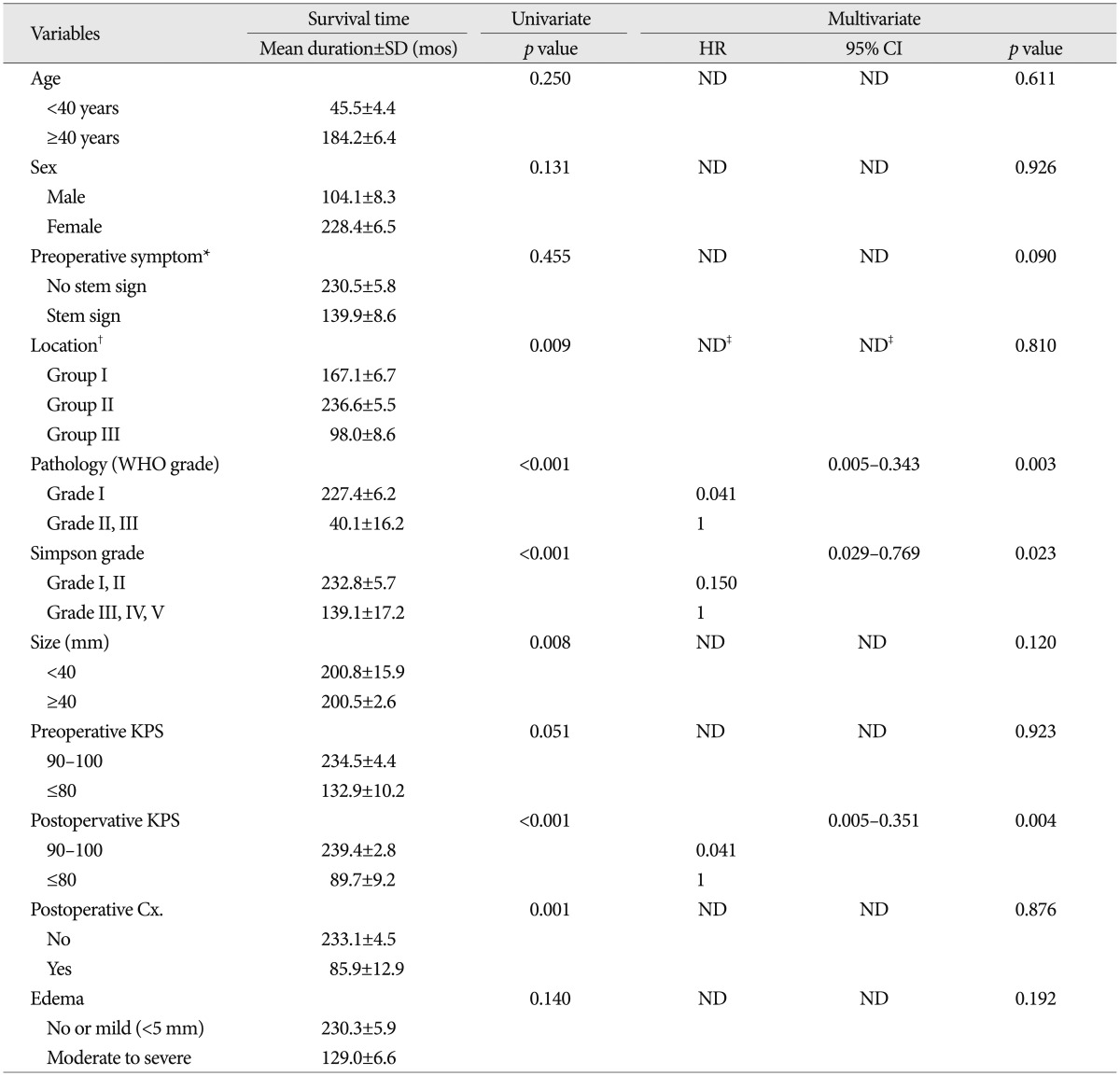
*No stem sign : headache, dizziness, seizure, Stem sign : mental changes, motor weakness, cranial nerve deficit, †Group I : cerebellopontine angle & cerebellar convexity, Group II : tentorial & peritorcular, Group III : skull base location including petroclival, foramen magnum, and jugular foramen, ‡Reference variable : non-skullbase location groups (Group I+II). Cx : complication, KPS : Karnofsky performance scale, ND : non-detected, WHO : World Health Organization
On univariate analysis, pathology, location, size, postoperative complication and KPS, and Simpson's grade showed statistical significance. The more aggressive pathology group (WHO grade II and III) showed a shorter survival time than the benign group (mean value, 40.1±16.2 months vs. 227.4±6.2 months, p<0.001). The tumor location was important factor for survival time [mean value, 167.1±6.7 months (group I) vs. 236.6±5.5 months (group II), 98.0±8.6 months (group III), p=0.009]. The group located at skull base showed shorter survival time than the non-skull base group (mean value, 98.0±8.6 months vs. 232.3±6.3 months, p=0.002). The size of tumor is also important variable in survival time [mean value, 200.5±2.6 months (group <4 cm) vs. 192.1±20.8 months (4 cm≤ group <6 cm), 152.7±11.2 months (group ≥6 cm), p=0.023]. In larger-sized group (≥4 cm), the survival time was shorter compared that of small-size group (mean value, 200.5±2.6 months vs. 200.8±15.9 months, p=0.008). Interestingly, non-complete resection group (Simpson's grade III-V) demonstrated a shorter survival time than the complete resection group (mean value, 232.8±5.7 months vs. 139.1±17.2 months, p<0.001). With regard of permanent complication after surgical resection, the presence of complication was significantly related with shorter survival time (mean value, 233.1±4.5 months vs. 85.9±12.9 months, p=0.001). The patient with low level of postoperative KPS (<90) also showed shorter survival time, compared to the patient with higher score (mean value, 239.4±2.8 months vs. 89.7±9.2 months, p<0.001). Our study showed that the patient lesser than 90 in preoperative KPS survived shorter than the patients of high level KPS, with a marginal statistical significance (mean value, 234.5±4.4 months vs. 132.9±10.2 months, p=0.051).
On multivariate analysis, pathology, resection degree and postoperative KPS were independent predictable factors for survival rate. Benign pathology (HR 0.041, 95% CI 0.005-0.343, p=0.003), complete resection (HR 0.150, 95% CI 0.029-0.769, p=0.023) and postoperative KPS over 90 (HR 0.041, 95% CI 0.005-0.351, p=0.004) were significantly associated with a longer survival rate.
Complication
Permanent complication rate was 13% (21/158) in our study. Most common permanent complication is cranial nerve injury (42.9%) followed by operation-related hemorrhage or infarction, motor weakness, hydrocephalus, and infection. The results of analyses of the variables that could be correlated with permanent complications are shown in Table 5.
Table 5.
Univariate and multivariate analysis for permanent complication predictors in patients with infratentorial meningiomas
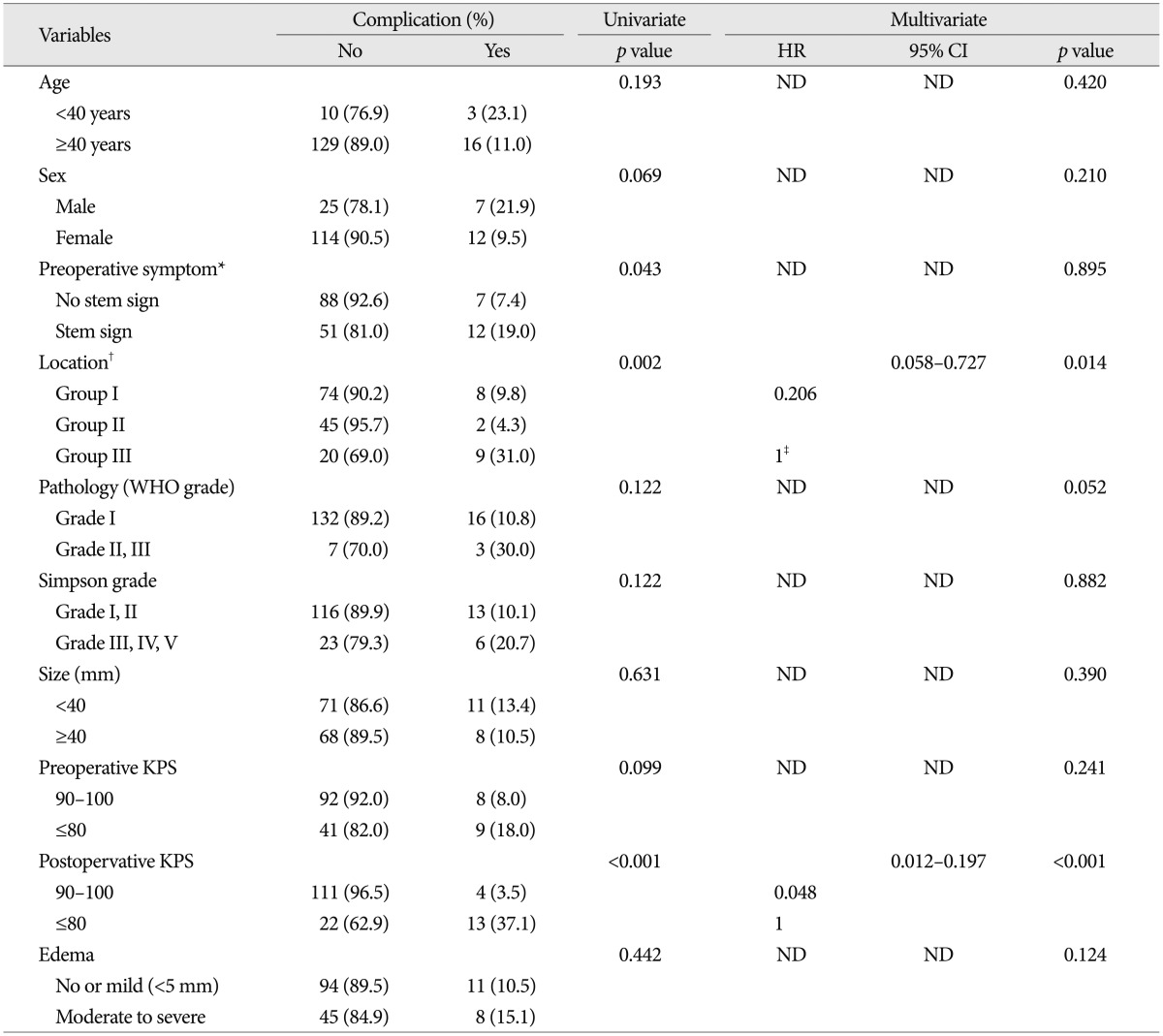
*No stem sign : headache, dizziness, seizure, Stem sign : mental changes, motor weakness, cranial nerve deficit, †Group I : cerebellopontine angle & cerebellar convexity, Group II : tentorial & peritorcular, Group III : skull base location including petroclival, foramen magnum, and jugular foramen, ‡Reference variable : non-skullbase location groups (Group I+II). Cx : complication, KPS : Karnofsky performance scale, ND : non-detected, WHO : World Health Organization
On univariate analysis, location, preoperative symptom, and preoperative KPS showed statistical significance. The tumor location was important factor for development of permanent complication (8.1% in group I vs. 2.3% in group II, 28.0% in group III, p=0.002). The group located at skull base showed higher possibility of permanent complication than the non-skull base group (28.0% vs. 6.0%, p=0.001). The patient with brain stem or cranial nerve sign showed higher possibility for postoperative complication, compared to the patient with other minor symptoms (19.0% vs. 7.4%, p=0.043). The patient with low level of postoperative KPS (<90) was also significantly associated with permanent complication (37.1% vs. 3.5%, p<0.001).
On multivariate analysis, location and postoperative KPS were independent predictable factors for development of permanent complication. Non-skull base location (HR 0.206, 95% CI 0.058-0.727, p=0.014) and postoperative KPS over 90 (HR 0.048, 95% CI 0.012-0.197, p<0.001) were significantly related with a higher complication rate.
DISCUSSION
A rising number of patients with intracranial meningiomas have been operated on in recent years and a few surgical series were reported in the literature22). And, surgical results with infratentorial meningiomas have much improved during the last two decades, but radical removal continues producing a high morbidity rate and still occasional mortality19).
In intracranial meningioma resection, surgical outcome and its predictive factors were not fully identified. Some authors mentioned the presence of peritumoral edema as a predictor of poor outcome in elderly patients3,8,13), whereas others did not find any relationship between edema and unfavorable outcome10,23). Tumors size has been considered as a predictive factor for postoperative outcome by some authors3,8). In other reports, however, tumor size did not have any significant influence on surgical morbidity10,15,24). Some authors have pointed out that outcome is less favorable in elderly patients with meningiomas located at the base of the skull, especially the posterior fossa1,2,4,5,20). Other authors reported that KPS present a relationship of postoperative outcome13,29). In consideration of previous studies, surgery for infratentorial meningioma is still associated with significant postoperative deficits, thus confirming that these lesions represent a great challenge even for contemporary neurosurgeons25).
Since the first classification of infratentorial meningiomas was proposed by Cushing and Eisenhardt in 1938, Castellano and Ruggiero subdivided these tumors into five groups; cerebellar convexity, tentorium, posterior petrous, clivus and foramen magnum meningiomas9). Based on this classification, total 158 patients with infratentorial meningiomas underwent surgery during 20 years in out hospital (20.2%, in total 782 patients with intracranial meningiomas).
Resection
Although a radical resection of these histologically benign tumors is the ideal objective of surgical therapy, this is not always possible25). During surgical planning, it should be kept in mind that meningiomas show a marked tendency to invade dura, nerves, and surrounding bone21). The dura surrounding the tumor should be resected and the bony invasion should be drilling until normal bone is seen27). And vascular encasement, although representing an additional surgical problem, by itself did not generally prevent the pursuance of gross total removal25). In fact, as noted by Sekhar et al.28), blood vessels are surrounded by an arachnoid plane that can be carefully dissected by experienced surgeons. Subtotal resection carries a lower risk of morbidity than radical excision, but residual tumor may lead some patients to the initial clinical scenario sooner or later, and reoperation is usually less successful and more risky than initial surgery, particularly when the patient is given radiotherapy after the initial operation18,28). At the present moment the majority of authors recommend subtotal resection for old patients or when there are factors defying complete removal7,12,17). The critical factors influencing the possibility of radical and safe resection of meningiomas are tumor-vascular relationships and the integrity of the arachnoid plane between the tumor and the brainstem19). In our series, non-skull base location was a significant factor for complete resection.
Recurrence
The recurrence rate of infratentorial meningiomas is particularly difficult to estimate because of differences in the reported series regarding the rates of radical excision, the methods of assessment of complete removal, the follow-up periods, the average age of the patients and the percentage of patients receiving postoperative radiotherapy19). Some studies have shown that meningiomas may recur after an apparently radical excision, but it is clear that completeness of resection is the main factor preventing regrowth in all locations1,4,17). Others indicated that frozen section analysis for intraoperative pathological sample is appropriate since a diagnosis of meningioma will direct that bone adjacent to the tumor site of origin be removed to reduce recurrence rate30). In our series, non-benign pathology, non-complete resection, moderate or severe degree of peritumoral edema, and low postoperative KPS (<90) were independent factors for short RFS in multivariate analysis. It is not difficult to anticipate that meningioma with high pathological grade or non-complete resection frequently recurred after the surgery. The postoperative KPS was higher, the recurrence rate was lower. It means, in our opinion, that the group of postoperative KPS under 80 tend to have subtotal resection because of intraoperative events, such as accidental injuries or poor dissection plane of cranial nerves, surrounding en-passing vessels, or brain stem, even though initial attempt for gross total resection. Therefore, these groups seem to have lower postoperative KPS, have higher recurrence rate. The more have edema, the more have recurrence rate. This is probably because the patients have moderate to severe edema tend to have high-grade pathology, large size, or poor dissection plane, leading to eventual recurrence of tumor.
Survival and complication
Considering that the goal of surgery of meningiomas is gross total resection with minimal morbidity and low mortality14), single-stage total removal should be attempted27). But the attempt for aggressive surgical resection continues producing a high morbidity rate and still occasional mortality19). The perioperative mortality rate in the surgical management of infratentorial meningiomas has varied from 0-15.7%25). In our series, the total death rate during the follow-up period was 5.0% (8/158) of case. The postoperative mortality rate, as defined patients ware expired within 30 days after initial operation, was 3.16% (5/158). Non-benign pathology, non-complete resection, and low postoperative KPS (<90) were independent factors for short survival in multivariate analysis. In our study, the group of gross total resection has more survival rate. It is presumably related with recurrence rate and postoperative condition. Patients with high postoperative KPS represented lower complication rate and good postoperative condition. For this reason, the postoperative KPS was significant predictive factor of survival.
As previously reported by several authors, the most common postoperative complication was found to be cranial nerve paresis or palsy2,11,20,26). In our study, the permanent neurological deficits occurred in 12% (19/158) of case and the most common type is cranial nerve injury (47%, 9/19). In multivariate analysis, skull base location and low postoperative KPS were independent factors for the occurrence of permanent complication. Because of more difficult anatomical factor in petroclival, foramen magnum or jugular foramen, surgical resection for these lesions have high risk of permanent complication, even though experienced neurosurgeon.
CONCLUSION
In this study, gross-total resection was achieved in 81.6% of the cases and related with non-skull base location. The recurrence rate was 13.3% and longer RFS was associated with benign pathology, postoperative KPS over than 90, low peritumoral edema, and complete resection. The 5-, 10-, and 15-year overall survival rates were 96.2%, 94.9%, and 94.9%, respectively. Benign pathology, postoperative KPS over than 90 and complete resection were independent factor for a longer survival. The permanent complication was developed in 13% of the patients and related with skull base location and postoperative KPS less than 90. Our results show relatively low morbidity and mortality rates, and good surgical resection and low recurrence rate, compare to the results of past, due to significant advances contemporary neurosurgical techniques, neuromonitoring and neuroanestehsia. However, infratentorial meningioma still has been a challenge for neurosurgeon because of their close to critical vascular and nerves structure. To achieve the goal that a little more lower morbidity and mortality, the surgeon need to consider of these factors and try to appropriate surgical planning of each case.
References
- 1.Adegbite AB, Khan MI, Paine KW, Tan LK. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58:51–56. doi: 10.3171/jns.1983.58.1.0051. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mefty O, Fox JL, Smith RR. Petrosal approach for petroclival meningiomas. Neurosurgery. 1988;22:510–517. doi: 10.1227/00006123-198803000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Arienta C, Caroli M, Crotti F, Villani R. Treatment of intracranial meningiomas in patients over 70 years old. Acta Neurochir (Wien) 1990;107:47–55. doi: 10.1007/BF01402612. [DOI] [PubMed] [Google Scholar]
- 4.Ayerbe J, Lobato RD, de la Cruz J, Alday R, Rivas JJ, Gómez PA, et al. Risk factors predicting recurrence in patients operated on for intracranial meningioma. A multivariate analysis. Acta Neurochir (Wien) 1999;141:921–932. doi: 10.1007/s007010050398. [DOI] [PubMed] [Google Scholar]
- 5.Baroncini M, Thines L, Reyns N, Schapira S, Vincent C, Lejeune JP. Retrosigmoid approach for meningiomas of the cerebellopontine angle : results of surgery and place of additional treatments. Acta Neurochir (Wien) 2011;153:1931–1940. doi: 10.1007/s00701-011-1090-6. discussion 1940. [DOI] [PubMed] [Google Scholar]
- 6.Bassiouni H, Hunold A, Asgari S, Stolke D. Meningiomas of the posterior petrous bone : functional outcome after microsurgery. J Neurosurg. 2004;100:1014–1024. doi: 10.3171/jns.2004.100.6.1014. [DOI] [PubMed] [Google Scholar]
- 7.Bricolo AP, Turazzi S, Talacchi A, Cristofori L. Microsurgical removal of petroclival meningiomas : a report of 33 patients. Neurosurgery. 1992;31:813–828. doi: 10.1227/00006123-199211000-00001. discussion 828. [DOI] [PubMed] [Google Scholar]
- 8.Buhl R, Hasan A, Behnke A, Mehdorn HM. Results in the operative treatment of elderly patients with intracranial meningioma. Neurosurg Rev. 2000;23:25–29. doi: 10.1007/s101430050027. [DOI] [PubMed] [Google Scholar]
- 9.Castellano F, Ruggiero G. Meningiomas of the posterior fossa. Acta Radiol Suppl. 1953;104:1–177. [PubMed] [Google Scholar]
- 10.Cornu P, Chatellier G, Dagreou F, Clemenceau S, Foncin JF, Rivierez M, et al. Intracranial meningiomas in elderly patients Postoperative morbidity and mortality Factors predictive of outcome. Acta Neurochir (Wien) 1990;102:98–102. doi: 10.1007/BF01405421. [DOI] [PubMed] [Google Scholar]
- 11.Couldwell WT, Fukushima T, Giannotta SL, Weiss MH. Petroclival meningiomas : surgical experience in 109 cases. J Neurosurg. 1996;84:20–28. doi: 10.3171/jns.1996.84.1.0020. [DOI] [PubMed] [Google Scholar]
- 12.Cudlip SA, Wilkins PR, Johnston FG, Moore AJ, Marsh HT, Bell BA. Posterior fossa meningiomas : surgical experience in 52 cases. Acta Neurochir (Wien) 1998;140:1007–1012. doi: 10.1007/s007010050208. [DOI] [PubMed] [Google Scholar]
- 13.Djindjian M, Caron JP, Athayde AA, Février MJ. Intracranial meningiomas in the elderly (over 70 years old) A retrospective study of 30 surgical cases. Acta Neurochir (Wien) 1988;90:121–123. doi: 10.1007/BF01560565. [DOI] [PubMed] [Google Scholar]
- 14.Gerganov V, Bussarsky V, Romansky K, Popov R, Djendov S, Dimitrov I. Cerebellopontine angle meningiomas Clinical features and surgical treatment. J Neurosurg Sci. 2003;47:129–135. discussion 135. [PubMed] [Google Scholar]
- 15.Gijtenbeek JM, Hop WC, Braakman R, Avezaat CJ. Surgery for intracranial meningiomas in elderly patients. Clin Neurol Neurosurg. 1993;95:291–295. doi: 10.1016/0303-8467(93)90104-o. [DOI] [PubMed] [Google Scholar]
- 16.Jiang YG, Xiang J, Wen F, Zhang LY. Microsurgical excision of the large or giant cerebellopontine angle meningioma. Minim Invasive Neurosurg. 2006;49:43–48. doi: 10.1055/s-2005-919151. [DOI] [PubMed] [Google Scholar]
- 17.Kokkino AJ, Abdel Aziz KM, Tew JM., Jr Honored guest presentation : contemporary treatment of skull base meningiomas. Clin Neurosurg. 2000;46:554–574. [PubMed] [Google Scholar]
- 18.Levine ZT, Buchanan RI, Sekhar LN, Rosen CL, Wright DC. Proposed grading system to predict the extent of resection and outcomes for cranial base meningiomas. Neurosurgery. 1999;45:221–230. doi: 10.1097/00006123-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Lobato RD, Gonzaáez P, Alday R, Ramos A, Lagares A, Alen JF, et al. Meningiomas of the basal posterior fossa. Surgical experience in 80 cases. Neurocirugia (Astur) 2004;15:525–542. doi: 10.1016/s1130-1473(04)70439-x. [DOI] [PubMed] [Google Scholar]
- 20.Mayberg MR, Symon L. Meningiomas of the clivus and apical petrous bone. Report of 35 cases. J Neurosurg. 1986;65:160–167. doi: 10.3171/jns.1986.65.2.0160. [DOI] [PubMed] [Google Scholar]
- 21.Molony TB, Brackmann DE, Lo WW. Meningiomas of the jugular foramen. Otolaryngol Head Neck Surg. 1992;106:128–136. doi: 10.1177/019459989210600202. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura M, Roser F, Dormiani M, Vorkapic P, Samii M. Surgical treatment of cerebellopontine angle meningiomas in elderly patients. Acta Neurochir (Wien) 2005;147:603–609. doi: 10.1007/s00701-005-0517-3. discussion 609-610. [DOI] [PubMed] [Google Scholar]
- 23.Papo I. Intracranial meningiomas in the elderly in the CT scan era. Acta Neurochir (Wien) 1983;67:195–204. doi: 10.1007/BF01401421. [DOI] [PubMed] [Google Scholar]
- 24.Proust F, Verdure L, Toussaint P, Bellow F, Callonec F, Menard JF, et al. [Intracranial meningioma in the elderly. Postoperative mortality, morbidity and quality of life in a series of 39 patients over 70 years of age] Neurochirurgie. 1997;43:15–20. [PubMed] [Google Scholar]
- 25.Roberti F, Sekhar LN, Kalavakonda C, Wright DC. Posterior fossa meningiomas : surgical experience in 161 cases. Surg Neurol. 2001;56:8–20. doi: 10.1016/s0090-3019(01)00479-7. discussion 20-21. [DOI] [PubMed] [Google Scholar]
- 26.Samii M, Tatagiba M. Experience with 36 surgical cases of petroclival meningiomas. Acta Neurochir (Wien) 1992;118:27–32. doi: 10.1007/BF01400723. [DOI] [PubMed] [Google Scholar]
- 27.Sanna M, Bacciu A, Falcioni M, Taibah A, Piazza P. Surgical management of jugular foramen meningiomas : a series of 13 cases and review of the literature. Laryngoscope. 2007;117:1710–1719. doi: 10.1097/MLG.0b013e3180cc20a3. [DOI] [PubMed] [Google Scholar]
- 28.Sekhar LN, Swamy NK, Jaiswal V, Rubinstein E, Hirsch WE, Jr, Wright DC. Surgical excision of meningiomas involving the clivus : preoperative and intraoperative features as predictors of postoperative functional deterioration. J Neurosurg. 1994;81:860–868. doi: 10.3171/jns.1994.81.6.0860. [DOI] [PubMed] [Google Scholar]
- 29.Umansky F, Ashkenazi E, Gertel M, Shalit MN. Surgical outcome in an elderly population with intracranial meningioma. J Neurol Neurosurg Psychiatry. 1992;55:481–485. doi: 10.1136/jnnp.55.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voss NF, Vrionis FD, Heilman CB, Robertson JH. Meningiomas of the cerebellopontine angle. Surg Neurol. 2000;53:439–446. doi: 10.1016/s0090-3019(00)00195-6. discussion 446-447. [DOI] [PubMed] [Google Scholar]