Abstract
Background
No conventional creatinine- or cystatin C-based glomerular filtration rate (GFR) estimation equation performed consistently outstandingly in elderly Chinese in our previous studies. This research aimed to further evaluate the performance of some recently proposed estimation equations based on creatinine and cystatin C, alone or combined, in this specific population.
Materials and methods
The equations were validated in a population totaling 419 participants (median age 68 [range 60–94] years). The estimated GFR (eGFR) calculated separately by ten equations was compared with the reference GFR (rGFR) measured by the 99mTc-DTPA renal dynamic imaging method.
Results
Median serum creatinine, cystatin C, and rGFR levels were 0.93 mg/L, 1.13 mg/L, and 74.20 mL/min/1.73 m2, respectively. The Chinese population-developed creatinine- and cystatin C-based (Cscr-cys) equation yielded the least median absolute difference (8.81 vs range 9.53–16.32, P<0.05, vs the Chronic Kidney Disease Epidemiology Collaboration serum creatinine equation), the highest proportion of eGFR within 15% and 30% of rGFR (P15 and P30, 55.13 and 85.44, P<0.05 and P<0.01, vs the Chronic Kidney Disease Epidemiology Collaboration serum creatinine equation), and the lowest root mean square error (14.87 vs range 15.30–22.45) in the whole cohort. A substantial agreement of diagnostic consistency between eGFR and rGFR (with a kappa 0.61–0.80) was also observed with the Cscr-cys equation. Moreover, measures of performance in the Cscr-cys equation were consistent across normal to mildly injured GFR strata and individuals aged ≤80 years. Among all the Cscr-cys equations, the elderly Chinese-developed creatinine-based (CEscr) equation performed best in this specific population. Nevertheless, none of the equations achieved ideal manifestation in the moderately to severely GFR-injured group or in individuals aged ≥80 years.
Conclusion
The Cscr-cys equation appeared to be optimal in elderly Chinese among the investigated equations. If cystatin C is not available, the CEscr equation is an acceptable alternative. A multicenter study with abundant subjects to develop an apposite formula for elderly Chinese is assumed to be essential.
Keywords: elderly Chinese, creatinine, cystatin C, glomerular filtration rate, equation
Introduction
Chronic kidney disease (CKD) has increasingly been considered a research and public health priority.1 CKD prevalence markedly increases with age.2,3 Surveys have shown that aged CKD patients have increasingly accounted for >50% of all the CKD patients.4,5 According to the sixth national census in China in 2010, individuals aged ≥60 years numbered close to 179 million, accounting for 13.26% of the total Chinese population, which increased by 3.36% from the previous census conducted 10 years ago.6 An accurate, convenient, and reproducible method to improve early recognition and diagnosis of CKD in the elderly is necessary but challenging.7 It is necessary because with lifestyle modification, an aging population, complicated disease distribution, and increasingly complex polypharmacy, accurately estimating glomerular filtration rate (GFR) and detecting and managing decreased kidney function in the elderly will have profound socioeconomic and public health consequences in developing countries like People’s Republic of China.8 Meanwhile, estimating renal function remains challenging mainly due to the fact that some endogenous markers are likely to be suboptimal in those patients prone to alteration and variation of their physical and pathological conditions.9
With increasing emphasis on the earlier detection and management of CKD, GFR has generally been considered the vital indicator to evaluate kidney function. The clearance rates of some exogenous materials have been considered the standard methods of GFR measurement. However, the standard methods are costly, time consuming, not routinely available, and have limited application for periodic GFR monitoring. Since the severity-based international classification of renal diseases directly relies on GFR values,10 the accuracy of GFR-estimating equations and those of serum creatinine (scr) and cystatin C (cys C) assays has become a hot topic, common to clinicians and laboratories.11 Traditionally, concentration of creatinine is influenced by factors including age, muscle mass, diet, sex, and ethnicity.12 Although cys C is altered by factors like inflammation, smoking status, corticosteroid treatment, and C-reactive protein level,13–15 it is often presented as a promising alternative marker of kidney function,16 due to its production rate being consistent and not dependent on sex, age, body weight, or diet.17
Our previous study indicated that no conventional creatinine- or cys C-based equation performed consistently satisfyingly in this specific population.18,19 The creatinine- and/or cys C-based equations employed in our present study were elderly based or newly developed in recent years.20–26 How accurately these equations are to estimate GFR in elderly Chinese is an essential question that needs to be answered in order to further establish a suitable equation for renal function assessment in this population.
Materials and methods
Participants
A total of 419 elderly Chinese participants aged ≥60 years, with or without CKD, at the First Affiliated Hospital of Nanjing Medical University, Jiangsu, People’s Republic of China, between December 2009 and March 2014, were consecutively enrolled in the study. All participants were of Chinese origin. Exclusion criteria included: 1) severe heart failure, acute renal failure, pleural or abdominal effusion, serious edema or malnutrition, skeletal muscle atrophy, amputation, or ketoacidosis; and 2) those on cimetidine or trimethoprim or hemodialysis therapy. All participants provided their written informed consent to take part in this study, and study approval was obtained from the Nanjing Medical University Ethics Committee.
Scr/Cys C assay and calibration
All participants had plasma creatinine and cys C measured at the Department of Clinical Laboratory of The First Affiliated Hospital of Nanjing Medical University. Scr concentration was assayed by isotope dilution mass spectrometry traceable standardized enzymatic method (Kehua Dongling Diagnostic Products Co., Ltd., Shanghai, People’s Republic of China), with a reported coefficient of variation of 6%, reference range 44–136 mmol/L, traceable to the National Institute of Standards and Technology Standard Reference Material for creatinine (SRM 967). Cys C concentration was examined by the particle-enhanced immunoturbidimetry method (Leadman Biomedical Co., Ltd., Beijing, People’s Republic of China), with a reported coefficient of variation of 8%, reference range 0.60–1.55 mg/L, which was calibrated against the international certified reference material ERM-DA471. Both fasting serum samples were assayed on an Olympus AU5400 autoanalyzer (Olympus Co., Tokyo, Japan) in strict accordance with the manufacturer’s instructions.
Measurement and estimation of GFR
All participants had a 99mTc-DTPA renal dynamic imaging measurement as the reference glomerular filtration rate (rGFR). We used 99mTc-DTPA as a gold standard method, since it has an excellent agreement with inulin clearance and is widely applied in clinical practice. After measuring height and weight, drinking 300 mL water, and emptying the bladder, participants received a bolus injection in the elbow vein of 185 MBq 99mTc-DTPA (purity 95%–99%, Senke Co., Ltd., Nanjing, People’s Republic of China). The 99mTc-DTPA renal dynamic imaging measurement was performed, and after image acquisition rGFR was automatically calculated by a computer with the Gates method.
Estimated glomerular filtration rate (eGFR) was calculated separately from GFR-estimating equations, including the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation based on scr (CKD-EPIscr),20 the CKD-EPI equation based on cys C (CKD-EPIcys),21 the CKD-EPI equation based on scr and cys C (CKD-EPIscr-cys),21 the Japanese equation based on scr (Jscr),22 the Japanese equation based on cys C (Jcys),23 the Berlin Initiative Study (BIS) equation based on scr (BISscr),24 the BIS equation based on scr and cys C (BISscr-cys),24 the Chinese elderly equation based on scr (CEscr),25 the Chinese equation based on cys C (Ccys), and the Chinese equation based on scr and cys C (Cscr-cys),26 which are presented in detail in Table 1.
Table 1.
Equations to estimate glomerular filtration ratea
| Name | Year | Sex | scr | cys C | Equation |
|---|---|---|---|---|---|
| CKD-EPIscr | 2009 | Female | ≤0.7 | 144×(scr/0.7)−0.329×0.993age(×1.159, if black) | |
| >0.7 | 144×(scr/0.7)−1.209×0.993age(×1.159, if black) | ||||
| Male | ≤0.9 | 141×(scr/0.9)−0.411×0.993age(×1.159, if black) | |||
| >0.9 | 141×(scr/0.9)−1.209×0.993age(×1.159, if black) | ||||
| CKD-EPIcys | 2012 | Female | ≤0.8 | 133×(cys C/0.8)−0.499×0.996age×0.932 | |
| >0.8 | 133×(cys C/0.8)−1.328×0.996age×0.932 | ||||
| Male | ≤0.8 | 133×(cys C/0.8)−0.499×0.996age | |||
| >0.8 | 133×(cys C/0.8)−1.328×0.996age | ||||
| CKD-EPIscr-cys | 2012 | Female | ≤0.7 | ≤0.8 | 130×(scr/0.7)−0.248×(cys C/0.8)−0.375×0.995age(×1.08, if black) |
| >0.8 | 130×(scr/0.7)−0.248×(cys C/0.8)−0.711×0.995age(×1.08, if black) | ||||
| >0.7 | ≤0.8 | 130×(scr/0.7)−0.601×(cys C/0.8)−0.375×0.995age(×1.08, if black) | |||
| >0.8 | 130×(scr/0.7)−0.601×(cys C/0.8)−0.711×0.995age(×1.08, if black) | ||||
| Male | ≤0.9 | ≤0.8 | 135×(scr/0.9)−0.207×(cys C/0.8)−0.375×0.995age(×1.08, if black) | ||
| >0.8 | 135×(scr/0.9)−0.207×(cys C/0.8)−0.711×0.995age(×1.08, if black) | ||||
| >0.9 | ≤0.8 | 135×(scr/0.9)−0.601×(cys C/0.8)−0.375×0.995age(×1.08, if black) | |||
| >0.8 | 135×(scr/0.9)−0.601×(cys C/0.8)−0.711×0.995age(×1.08, if black) | ||||
| Jscr | 2009 | Female | 194×scr−1.094×age−0.287×0.739 | ||
| Male | 194×scr−1.094×age−0.287 | ||||
| Jcys | 2012 | Female | 104×cys C−1.019×0.996age×0.929–8 | ||
| Male | 104×cys C−1.019×0.996age–8 | ||||
| BISscr | 2012 | Female | 3,736×scr−0.87×age−0.95×0.82 | ||
| Male | 3,736×scr−0.87×age−0.95 | ||||
| BISscr-cys | 2012 | Female | 767×scr−0.40×cys C−0.61×age−0.57×0.87 | ||
| Male | 767×scr−0.40×cys C−0.61×age−0.57 | ||||
| CEscr | 2013 | Female | ≤0.7 | 140×(scr/0.7)−0.176×0.993age | |
| >0.7 | 127×(scr/0.7)−0.616×0.993age | ||||
| Male | ≤0.9 | 128×(scr/0.9)−0.015×0.993age | |||
| >0.9 | 119×(scr/0.9)−0.688×0.993age | ||||
| Ccys | 2013 | 78.64×cys C−0.964 | |||
| Cscr-cys | 2013 | Female | 173.9×scr−0.184×cys C−0.725×age−0.193×0.89 | ||
| Male | 173.9×scr−0.184×cys C−0.725×age−0.193 |
Note:
scr was shown as mg/dL, cys C was shown as mg/L, age was shown as years.
Abbreviations: BISscr, Berlin equation based on scr; BISscr-cys, Berlin equation based on scr and cys C; Ccys, Chinese equation based on cys C; CEscr, Chinese elderly equation based on scr; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CKD-EPIcys, CKD-EPI equation based on cys C; CKD-EPIscr, CKD-EPI equation based on scr; CKD-EPIscr-cys, CKD-EPI equation based on scr and cys C; Cscr-cys, Chinese equation based on scr and cys C; Jscr, Japanese equation based on scr; Jcys, Japanese equation based on cys C; scr, serum creatinine; cys C, cystatin C.
Statistical analysis
Owing to non-normal distribution of datasets (P<0.001, Kolmogorov–Smirnov test), nonparametric statistics were used in our study. Bias, precision, and accuracy were measured to evaluate the performance of each equation as proposed by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative. Bias was defined as the median difference and the median absolute difference as well. The median difference was calculated between eGFR and rGFR (eGFR–rGFR); the median absolute difference between eGFR and rGFR was defined as the median value of absolute difference (|eGFR–rGFR|). The precision was demonstrated as the interquartile range of the median differences. Accuracy was assessed as the proportion of eGFR within 15%, 30%, and 50% of rGFR (P15, P30, P50) and also as root mean square error (RMSE). Wilcoxon matched-pairs signed rank test was used to compare the bias, and the McNemar test was used to compare P15, P30, and P50 values. The CKD-EPIscr equation has been recommended by the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guidelines for the Evaluation and Management of Chronic Kidney Disease.10 Therefore, all comparisons between equations were made against the CKD-EPIscr equation. A kappa test was used to compare the diagnosis consistency of GFR stratification between the eGFR and rGFR: kappa value 0.21–0.40 is considered mild agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement, and 0.81–1.00 near perfect agreement. Bland–Altman analysis was measured and plotted to intuitively compare eGFR with rGFR. P<0.05 was considered statistically significant. All statistical analyses were carried out using SPSS software (version 17.0; SPSS, Chicago, IL, USA) and MedCalc for Windows (version 11.6.1.0; Medcalc Software, Mariekerke, Belgium).
Results
Participant characteristics
Overall, participants were a median of 68 (25th to 75th percentile, 63–74) years old and 62.80% were male. Median scr, cys C, and rGFR levels were 0.93 mg/L, 1.13 mg/L, and 74.20 mL/min/1.73 m2, respectively. The aforementioned equations were used to calculate the eGFRs in the entire cohort. The median values for the eGFRs varied from 56.21 mL/min/1.73 m2 to 75.29 mL/min/1.73 m2. A prevalence of rGFR <60 mL/min/1.73 m2 was 30.80%. Only 9.80% of those aged ≥80 years are included in the present study. Detailed demographic characteristics are shown in Table 2.
Table 2.
Baseline characteristics of enrolled elderly subjectsa
| All subjects | rGFR <60 mL/min/1.73 m2 | rGFR ≥60 mL/min/1.73 m2 | |
|---|---|---|---|
| Age (years) | 68 (63–74) | 69 (64–75) | 68 (63–74) |
| Age category | |||
| 60–69 | 234 (55.9) | 67 (52.0) | 167 (57.6) |
| 70–79 | 144 (34.4) | 47 (36.4) | 97 (33.5) |
| >80 | 41 (9.8) | 15 (11.6) | 26 (9.0) |
| BUN (mmol/L) | 5.86 (4.73–7.67) | 8.20 (6.15–12.24) | 5.37 (4.27–6.62) |
| scr (mg/L) | 0.93 (0.77–1.20) | 1.46 (1.10–1.90) | 0.84 (0.68–0.98) |
| cys C (mg/L) | 1.13 (0.96–1.43) | 1.72 (1.33–2.48) | 1.02 (0.90–1.17) |
| rGFR (mL/min/1.73 m2) | 74.2 (54.40–88.20) | 44.6 (33.25–52.75) | 81.3 (73.45–95.73) |
| rGFR category | |||
| ≥60 | 290 (69.2) | / | / |
| <60 | 129 (30.8) | / | / |
| eGFR (mL/min/1.73 m2) | |||
| CKD-EPIscr | 75.3 (55.1–90.52) | 47.0 (32.96–53.4) | 85.1 (72.4–92.3) |
| CKD-EPIcys | 63.1 (45.1–79.6) | 35.7 (22.6–50.3) | 72.2 (60.2–84.5) |
| CKD-EPIscr-cys | 70.0 (50.3–83.9) | 39.2 (27.3–53.8) | 77.3 (67.9–89.5) |
| Jscr | 56.2 (42.8–68.3) | 36.5 (25.1–47.3) | 64.2 (53.4–75.1) |
| Jcys | 61.0 (45.0–73.8) | 35.9 (23.1–50.0) | 68.2 (58.0–77.8) |
| BISscr | 67.1 (51.9–80.1) | 46.4 (35.6–57.5) | 74.4 (64.3–83.7) |
| BISscr-cys | 64.4 (49.0–74.9) | 40.5 (30.3–52.3) | 69.5 (61.6–80.7) |
| CEscr | 69.6 (57.7–81.7) | 52.8 (43.1–62.1) | 74.8 (67.0–83.5) |
| Ccys | 69.9 (55.7–81.8) | 46.6 (32.8–59.7) | 77.5 (67.6–87.3) |
| Cscr-cys | 70.0 (55.2–80.3) | 45.9 (34.7–59.3) | 75.4 (67.8–85.3) |
| Primary disease or comorbid conditions | |||
| Nephritis | 45 (10.7) | 19 (14.7) | 26 (9.0) |
| Kidney neoplasm | 130 (31.0) | 51 (39.5) | 79 (27.2) |
| Hematological disease | 16 (3.8) | 7 (5.4) | 9 (3.1) |
| Hypertension | 139 (33.2) | 59 (45.7) | 80 (27.6) |
| Coronary heart disease | 38 (9.1) | 16 (12.4) | 22 (7.6) |
| Diabetes mellitus | 85 (20.3) | 31 (24.0) | 54 (18.6) |
Notes:
Values for continuous variables expressed as median (25th to 75th percentile); values for categorical values expressed as number (percentage). Conversion factors for units: scr in mg/L to μmol/L ×88.4.
Abbreviations: BISscr, Berlin equation based on scr; BISscr-cys, Berlin equation based on scr and cys C; BUN, blood urea nitrogen; Ccys, Chinese equation based on cys C; CEscr, Chinese elderly equation based on scr; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CKD-EPIscr, CKD-EPI equation based on scr; CKD-EPIscr-cys, CKD-EPI equation based on scr and cys C; CKD-EPIcys, CKD-EPI equation based on cys C; Cscr-cys, Chinese equation based on scr and cys C; eGFR, estimated glomerular filtration rate; Jcys, Japanese equation based on cys C; Jscr, Japanese equation based on scr; rGFR, reference glomerular filtration rate (using the 99mTc-DTPA renal dynamic imaging method); scr, serum creatinine; cys C, cystatin C.
Overall performance of the equations
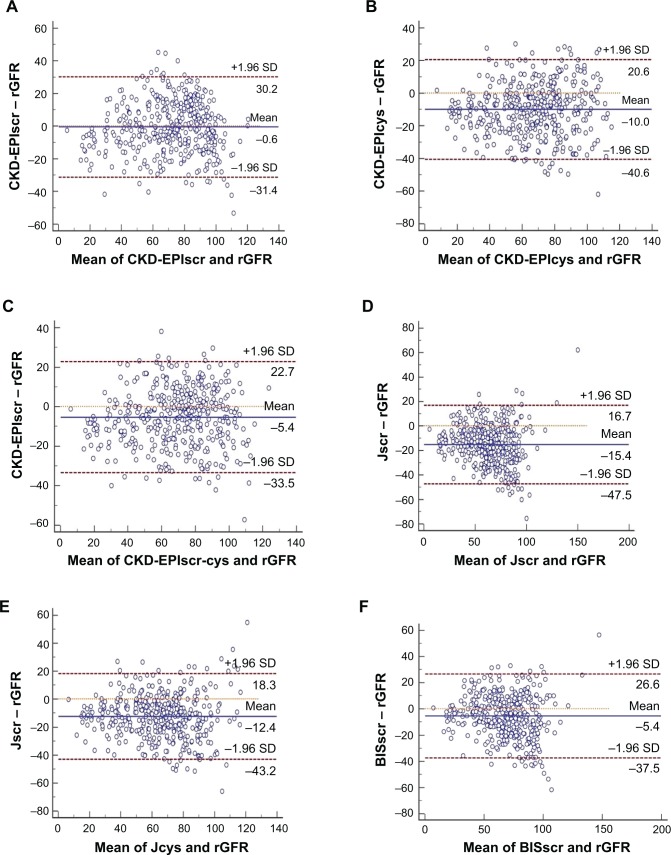
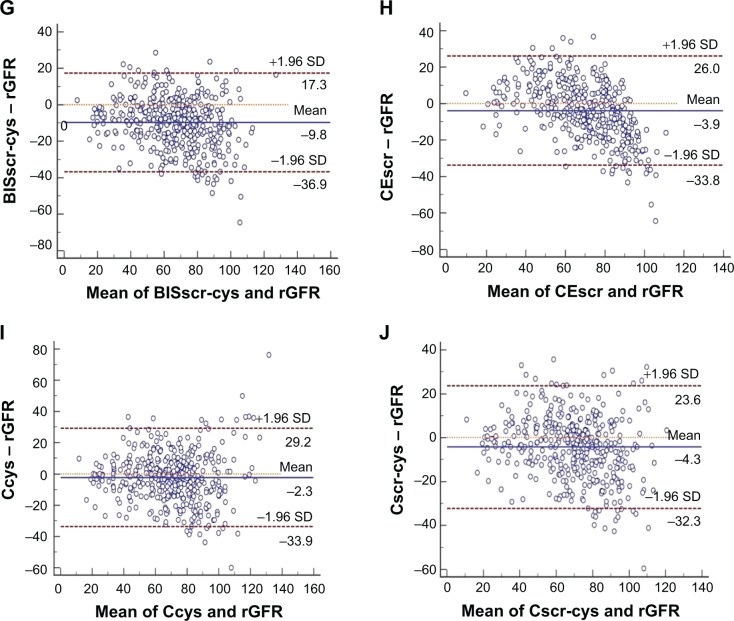
All equations tended to underestimate GFR in the elderly. The median absolute difference of the Cscr-cys equation was least among all equations (8.81 vs a range from 9.53 to 16.32, P<0.01, in comparison with the CKD-EPIscr equation). The Cscr-cys equation also yielded the highest P15 and P30 (55.13 and 85.44, P<0.05 and P<0.01, vs CKD-EPIscr equation, respectively) and least RMSE (14.87 vs range 15.30–22.45) in the whole cohort. The CEscr possessed performance only inferior to the Cscr-cys equation, with a secondly ranked P30 (83.05, P<0.05, in comparison with the CKD-EPIscr equation) and P15 (52.51). Three CKD-EPI equations performed not as well as previously expected, while two Japanese equations achieved the worst performance of all employed equations in our study (Table 3). Bland–Altman analysis demonstrated a consistent result (Figure 1).
Table 3.
Overall performance of the equationsa
| Bias
|
Precision
|
Accuracy
|
|||||
|---|---|---|---|---|---|---|---|
| Median difference | Median absolute difference | IQR of the median difference | P15 | P30 | P50 | RMSE | |
| CKD-EPIscr | −1.47 | 10.77 | 21.49 | 48.45 | 79.00 | 92.84 | 15.70 |
| CKD-EPIcys | −9.05* | 12.13* | 19.61 | 41.05** | 71.84* | 93.32 | 18.50 |
| CKD-EPIscr-cys | −5.09** | 10.21 | 18.74 | 51.55 | 78.76 | 95.23** | 15.30 |
| Jscr | −15.67* | 16.32* | 20.21 | 29.36* | 62.29* | 93.56 | 22.45 |
| Jcys | −11.26* | 13.27* | 18.36 | 39.62** | 68.97* | 92.84 | 20.00 |
| BISscr | −5.20** | 10.70 | 21.58 | 47.26 | 79.71 | 95.70* | 17.20 |
| BISscr-cys | −8.65** | 10.70 | 16.31 | 48.93 | 79.71 | 96.90* | 16.94 |
| CEscr | −3.66 | 9.92 | 19.75 | 52.51 | 83.05** | 94.03 | 15.74 |
| Ccys | −1.74 | 9.53 | 19.14 | 51.31 | 82.10 | 95.47 | 16.24 |
| Cscr-cys | −3.20 | 8.81* | 17.62 | 55.13** | 85.44* | 95.94** | 14.87 |
Notes:
Median absolute difference = median value of absolute difference (|eGFR–rGFR|)
P<0.01
P<0.05.
Abbreviations: BISscr, Berlin equation based on scr; BISscr-cys, Berlin equation based on scr and cys C; Ccys, Chinese equation based on cys C; CEscr, Chinese elderly equation based on scr; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CKD-EPIcys, CKD-EPI equation based on cys C; CKD-EPIscr, CKD-EPI equation based on scr; CKD-EPIscr-cys, CKD-EPI equation based on scr and cys C; Cscr-cys, Chinese equation based on scr and cys C; eGFR, estimated glomerular filtration rate; IQR, the interquartile range of difference; Jcys, Japanese equation based on cys C; Jscr, Japanese equation based on scr; P15, the proportion of eGFR within 15% of rGFR; P30, the proportion of eGFR within 30% of rGFR; P50, the proportion of eGFR within 50% of rGFR; rGFR, reference glomerular filtration rate; RMSE, root mean square error; scr, serum creatinine; cys C, cystatin C.
Figure 1.
Bland–Altman analysis measured and plotted to intuitively compare eGFR with rGFR. Horizontal solid lines represent the mean difference between methods. Horizontal dashed lines represent 95% limits of agreement. GFR was measured in mL/min/1.73 m2. (A) CKD-EPIscr, (B) CKD-EPIcys, (C) CKD-EPIscr-cys, (D) Jscr, (E) Jcys, (F) BISscr, (G) BISscr-cys, (H) CEscr, (I) Ccys, and (J) Cscr-cys.
Abbreviations: BISscr, Berlin equation based on scr; BISscr-cys, Berlin equation based on scr and cys C; Ccys, Chinese equation based on cys C; CEscr, Chinese elderly equation based on scr; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CKD-EPIcys, CKD-EPI equation based on cys C; CKD-EPIscr, CKD-EPI equation based on scr; CKD-EPIscr-cys, CKD-EPI equation based on scr and cys C; Cscr-cys, Chinese equation based on scr and cys C; eGFR, estimated glomerular filtration rate; Jcys, Japanese equation based on cys C; Jscr, Japanese equation based on scr; rGFR, reference glomerular filtration rate; scr, serum creatinine; cys C, cystatin C; SD, standard deviation.
As displayed in Table 4, substantial diagnosis consistencies were achieved with a kappa value of 0.643 and 0.716 by the Cscr-cys equation (divided into two stages with a threshold GFR of 60 mL/min/1.73 m2 as well as 45 mL/min/1.73 m2). The CEscr equation yielded the lowest misclassification rate (14.32%) with a threshold of 60 mL/min/1.73 m2 while the Cscr-cys equation possessed the lowest misclassification rate (7.40%) with a threshold of 45 mL/min/1.73 m2.
Table 4.
Diagnosis consistency and misclassification rate of the equationsa
| Threshold 60 mL/min/1.73 m2
|
Threshold 45 mL/min/1.73 m2
|
|||
|---|---|---|---|---|
| Kappa | Misclassification rate | Kappa | Misclassification rate | |
| CKD-EPIscr | 0.632 | 15.5% | 0.638 | 9.8% |
| CKD-EPIcys | 0.599 | 19.3% | 0.607 | 12.9% |
| CKD-EPIscr-cys | 0.662 | 15.0% | 0.705 | 8.6% |
| Jscr | 0.406 | 31.5% | 0.574 | 14.8% |
| Jcys | 0.569 | 18.1% | 0.601 | 13.1% |
| BISscr | 0.594 | 18.1% | 0.662 | 9.1% |
| BISscr-cys | 0.614 | 18.1% | 0.680 | 9.6% |
| CEscr | 0.654 | 14.3% | 0.529 | 10.5% |
| Ccys | 0.651 | 14.8% | 0.687 | 8.1% |
| Cscr-cys | 0.643 | 15.3% | 0.716 | 7.4% |
Note:
eGFR and rGFR were given in mL/min/1.73 m2.
Abbreviations: BISscr, Berlin equation based on scr; BISscr-cys, Berlin equation based on scr and cys C; Ccys, Chinese equation based on cys C; CEscr, Chinese elderly equation based on scr; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CKD-EPIcys, CKD-EPI equation based on cys C; CKD-EPIscr, CKD-EPI equation based on scr; CKD-EPIscr-cys, CKD-EPI equation based on scr and cys C; Cscr-cys, Chinese equation based on scr and cys C; eGFR, estimated glomerular filtration rate; Jcys, Japanese equation based on cys C; Jscr, Japanese equation based on scr; rGFR, reference glomerular filtration rate; scr, serum creatinine; cys C, cystatin C.
GFR- and age-specific subgroup performance of the equations
In subgroups with rGFR ≥60 mL/min/1.73 m2, the CEscr equation acquired the highest P30 (91.72), followed by the Cscr-cys equation. Relative smaller bias and higher precision were also achieved by the two equations (Table 5). Consistent with the whole cohort, the Cscr-cys equation yielded the best performance in the 60–69 year subgroup and the 70–79 year subgroup, with the smallest median absolute difference (9.06 and 7.91, respectively), highest P30 (86.75 and 85.42, respectively), and least RMSE (14.91 and 14.54, respectively). Performance of the CEscr equation was preceded only by the Cscr-cys equation in the <80 year subgroups. The BISscr-cys equation also performed well in the 60–69 year subgroup. None of the employed equations achieved an ideal P30 value under the conditions of rGFR <60 mL/min/1.73 m2 or age ≥80 years, although the Cscr-cys equation achieved the best performance among the included ten equations (Tables 5 and 6).
Table 5.
GFR-specific performance of the equationsa
| rGFR <60 mL/min/1.73 m2
|
rGFR ≥60 mL/min/1.73 m2
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Bias
|
Precision
|
Accuracy
|
Bias
|
Precision
|
Accuracy
|
|||
| Median absolute difference | IQR of the median difference | P30 | RMSE | Median absolute difference | IQR of the median difference | P30 | RMSE | |
| CKD-EPIscr | 11.71 | 24.15 | 55.04 | 16.67 | 10.31 | 21.91 | 89.66 | 15.27 |
| CKD-EPIcys | 9.91 | 15.02 | 55.81 | 14.94 | 13.18* | 20.93 | 78.97* | 19.88 |
| CKD-EPIscr-cys | 10.86 | 17.29 | 54.26 | 14.31 | 9.95 | 19.79 | 89.66 | 15.73 |
| Jscr | 9.87 | 13.54 | 57.36 | 13.56 | 20.16* | 22.48 | 64.48* | 25.42 |
| Jcys | 9.66 | 13.86 | 55.81 | 14.62 | 15.61* | 19.87 | 74.83* | 21.98 |
| BISscr | 8.86* | 17.79 | 72.09* | 12.59 | 12.08* | 22.52 | 83.10* | 18.90 |
| BISscr-cys | 8.04* | 13.00 | 71.32* | 11.60 | 12.20* | 17.45 | 83.45* | 18.83 |
| CEscr | 9.83* | 14.72 | 63.57 | 13.94 | 9.93 | 18.04 | 91.72 | 17.50 |
| Ccys | 7.71* | 15.39 | 70.54* | 13.00 | 10.50 | 19.30 | 87.24 | 17.49 |
| Cscr-cys | 6.72* | 14.69 | 73.64* | 12.20 | 9.74 | 18.39 | 90.69 | 15.92 |
Notes:
Median absolute difference = median value of absolute difference (|eGFR–rGFR|);
P<0.01.
Abbreviations: BISscr, Berlin equation based on scr; BISscr-cys, Berlin equation based on scr and cys C; Ccys, Chinese equation based on cys C; CEscr, Chinese elderly equation based on scr; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CKD-EPIcys, CKD-EPI equation based on cys C; CKD-EPIscr, CKD-EPI equation based on scr; CKD-EPIscr-cys, CKD-EPI equation based on scr and cys C; Cscr-cys, Chinese equation based on scr and cys C; eGFR, estimated glomerular filtration rate; IQR, the interquartile range of difference; Jcys, Japanese equation based on cys C; Jscr, Japanese equation based on scr; P30, the proportion of eGFR within 30% of rGFR; rGFR, reference glomerular filtration rate; RMSE, root mean square error; scr, serum creatinine; cys C, cystatin C.
Table 6.
Age-specific performance of the equations
| Bias | Precision | Accuracy | Bias | Precision | Accuracy | Bias | Precision | Accuracy | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Median absolute difference | IQR of the median difference | P30 | RMSE | Median absolute difference | IQR of the median difference | P30 | RMSE | Median absolute difference | IQR of the median difference | P30 | RMSE | |
|
|
|
|
||||||||||
| Age 60–69 years | Age 70–79 years | Age 80+ years | ||||||||||
| Cscr-cys | 9.06 | 17.13 | 86.75* | 14.91 | 7.91** | 16.60 | 85.42 | 14.54 | 10.53 | 24.04 | 78.05 | 15.80 |
| Ccys | 9.54 | 17.32 | 83.33 | 15.98 | 9.33 | 18.70 | 81.25 | 15.40 | 9.97 | 23.13 | 78.05 | 20.10 |
| CEscr | 9.71 | 19.99 | 8r5.90** | 15.93 | 10.10 | 20.73 | 82.64 | 15.23 | 10.71 | 23.12 | 68.29 | 16.44 |
| BISscr-cys | 10.94 | 16.54 | 83.76** | 16.46 | 10.53 | 15.91 | 77.78 | 16.93 | 12.42** | 24.44 | 63.41 | 19.47 |
| BISscr | 10.13 | 20.69 | 83.76** | 16.28 | 12.16** | 19.15 | 77.78 | 17.58 | 10.88 | 27.12 | 63.41 | 20.66 |
| Jcys | 14.00* | 18.11 | 70.51** | 20.28 | 12.04** | 10.43 | 69.44** | 18.75 | 14.90* | 25.13 | 58.54 | 22.54 |
| Jscr | 16.14* | 20.26 | 64.96* | 22.49 | 17.57* | 19.28 | 60.42* | 22.34 | 13.44* | 27.27 | 53.66** | 22.57 |
| CKD-EPIscr-cys | 10.21 | 18.38 | 79.91 | 15.08 | 9.55 | 20.21 | 79.17 | 15.03 | 12.13 | 22.53 | 70.73 | 17.34 |
| CKD-EPIcys | 12.13** | 18.85 | 72.22 | 18.90 | 10.90 | 19.92 | 72.92 | 17.41 | 14.32** | 23.41 | 65.85 | 19.91 |
| CKD-EPIscr | 11.21 | 22.48 | 79.06 | 15.57 | 9.58 | 19.16 | 80.56 | 15.64 | 10.23 | 25.84 | 73.17 | 16.69 |
Notes:
Median absolute difference = median value of absolute difference (|eGFR–rGFR|)
P<0.01
P<0.05.
Abbreviations: BISscr, Berlin equation based on scr; BISscr-cys, Berlin equation based on scr and cys C; Ccys, Chinese equation based on cys C; CEscr, Chinese elderly equation based on scr; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CKD-EPIcys, CKD-EPI equation based on cys C; CKD-EPIscr, CKD-EPI equation based on scr; CKD-EPIscr-cys, CKD-EPI equation based on scr and cys C; Cscr-cys, Chinese equation based on scr and cys C; eGFR, estimated glomerular filtration rate; IQR, the interquartile range of difference; Jcys, Japanese equation based on cys C; Jscr, Japanese equation based on scr; P30, the proportion of eGFR within 30% of rGFR; rGFR, reference glomerular filtration rate; RMSE, root mean square error; scr, serum creatinine; cys C, cystatin C.
Discussion
The early period of CKD is always asymptomatic, which means people do not get identified or treated until the disease has progressed to near end-stage kidney failure. It is important to improve early recognition and diagnosis of CKD,7 which requires not only public awareness of timely medical examination but also an accurate, convenient, and reproducible method to assess kidney function. No single equation has been recommended to assess GFR in the elderly so far. Therefore, it is very important to validate the available equations in the elderly Chinese, considering the rapidly aging population and vast amount of CKD patients.
Cys C is often presented as a promising alternative marker of kidney function.18 Two meta-analyses concluded cys C to be superior to creatinine in accurately predicting GFR.27,28 Indeed, cys C is freely filtrated, completely reabsorbed, and then catabolized within the cells of proximal convoluted tubule. Furthermore, being secreted by all the nucleated cells, its production rate is persistent and independent of sex, age, body weight, or diet.19 Serum cys C-based equations are supposed to be advantageous over the creatinine-based equations because of the inherent advantages of cys C. However, a multivariate analysis adjusted for the level of renal function revealed that serum cys C levels may be influenced by multiple factors independent of renal function, especially high C-reactive protein levels.14 Cys C-based equations may be biased for patients suffering from thyroid diseases or diseases typically treated with steroids, eg, chronic obstructive lung disease, or autoimmune diseases such as sarcoidosis, vasculitis, and rheumatoid and skin diseases.13 There is still no explicit evidence for superiority of cys C-based equations in the elderly in clinical practice. After the proposed CKD-EPIscr-cys equation, equations based on a combination of different markers (cys C together with scr) have increasingly attracted attention of clinicians.
Typically and understandably, a single equation is impossible to work equally well in all populations. It always performs best in the development scope and its application to a wide range needs verification. Several studies have been undertaken to validate the contemporary GFR-estimating equations. In the original development and validation dataset, the CKD-EPIscr-cys equation performed better than equations based on either marker alone, but the CKD-EPIcys equation did not show obvious advantages over the CKD-EPIscr equation.21 Our previous study indicated that the CKD-EPIscr-cys equation achieved ideal accuracy in nonelderly individuals with normally or mildly injured GFR.29 Validation in the European elderly population suggested that the CKD-EPI equations performed as well in older people as in the younger population. The combined markers-based equation appeared most ideal, while the benefit of either marker-based equation was marginal.30 Evaluation performed by Liu et al31 indicated that the CKD-EPIscr-cys equation was more suitable than the CKD-EPIscr equation for the elderly population. Horio et al23 suggested that the CKD-EPIcys equation performed well in Japanese individuals without race modification. The Berlin Initiative Study demonstrated that the BISscr-cys equation should be used to estimate GFR in persons aged ≥70 years with normal or mild to moderately reduced kidney function, while the BISscr equation was also an acceptable alternative.24 A study comparing the performances of BISscr, MDRD, and CKD-EPI equations in estimating GFR in older patients demonstrated that BISscr was the most reliable for assessing renal function in older white patients, especially in those with CKD stages 1–3.32 Another study to evaluate the application of the scr-based equations indicated that the BISscr equation may be the optimal one for elderly Chinese CKD patients.33 The newly developed Chinese population-based equations (CEscr and Cscr-cys) performed outstandingly in the original development dataset.
Our present study demonstrated that the calculation of eGFR with a combination of scr and cys C more accurately yielded considerable performance improvement compared with either marker alone, probably owing to the lesser overall effects of non-GFR determinants of either marker when both markers are included. Comparing against creatinine-based equations, the cys C-based equations appeared to have no obvious superiority. The principal finding of the present study was that the Cscr-cys formula had better capability to accurately evaluate GFR in the entire range of participants, particularly in participants aged 60–80 years or with normally or mildly injured GFR. The CEscr equation, a typical creatinine-based equation, was another well-performing formula with impressive accuracy. Several studies have shown that the mortality risk associated with a given eGFR level is attenuated in the elderly, which would be closer to 45 mL/min/1.73 m2 in this population.34–37 Hence, diagnostic consistency was tested with a threshold of 45 mL/min/1.73 m2 besides a constantly defined threshold of 60 mL/min/1.73 m2. Substantial agreement of diagnostic consistency between eGFR and rGFR was observed in the Cscr-cys equation with both thresholds.
Under circumstances of rGFR <60 mL/min/1.73 m2 and age ≥80 years, none of the equations achieved ideal accuracy. Demonstrable improvements of the equation based on a combination of scr and cys C were not detected. Due to the fact that the nonrenal clearance of cys C is substantially higher in moderately to severely injured kidney function individuals,38 serum cys C might be an inappropriate GFR marker in advanced kidney failure. If cys C is dependent on body composition, this may help the interpretation of minor or even no improvement performance with cys C-based equations and proposals approved by some investigators that cys C was affected by demographic and anthropometric variables.14,39 Since it is more expensive to measure serum cys C than scr (approximately US $8.5 vs $0.75),40 besides accuracy is unacceptable in moderately–severely damaged kidney function elderly subjects, the Cscr-cys equation is not recommended in elderly with advanced kidney function. β-trace protein and symmetric dimethylarginine may be potential ideal markers to further improve equation performance in the future.30
The discrepancy of equation performance among equations could be induced in part by racial, ethic, and regional variations in muscle mass and diet.41,42 Considering the racial difference, we suggest that the addition of the Chinese racial factor may allow performance improvement. Additionally, differences in original development group, such as the difference of the constituent ratio of the subjects’ age, sex, GFR stages, and complications, may be the reason for the discrepancy in performance. Inconsistent assay methods and various measurements of rGFR could also attribute to the differing applicability. With the complex primary and comorbid disease conditions in the elderly, we suppose that research into factors that affect scr and cys C levels independent of GFR could potentially ameliorate the performance of GFR-estimating equations in clinical practice. Moreover, taking into account clinical practice and public health priorities, other endogenous filtration markers should also be taken as potential ideal indicators to predict GFR.
There are several strengths in our study. First of all, few data were available regarding the performance of elderly-based or recently described creatinine- and/or cys C-based equations in an elderly Chinese population. Furthermore, our study covers a proportion of elderly individuals who might not suffer with CKD, which means we evaluate the equations in a more general elderly population. Additionally, to truly identify the application of estimating equations in the elderly, subgroup analysis in different GFR stages and age status was also conducted.
Several limitations of our study should be pointed out. To begin with, the 99mTc-DTPA renal dynamic imaging method was still utilized to measure rGFR in this study, which was distinct from the plasma clearance of exogenous filtration markers used in other equations’ development datasets. Therefore, the variability in the measurement of rGFR may partially affect the valid values. However, before a unified rGFR is performed globally, this study may provide some exploratory information for clinicians and researchers. Moreover, the unbalanced subgroups (the limited number of participants aged ≥80 years) in the present study might result in selective bias, so the validation of GFR-estimating equations in the elderly (age ≥80 years) should be conducted with more individuals and interpreted with caution.
Conclusion
In conclusion, there is inevitably no ideal “one size fits all” equation, and clinicians need to be careful of potential limitations in their application and to interpret the results in a comprehensive clinical context. More research is warranted in clinical practice for the older age categories. A more accurate GFR-estimating equation can be developed if the equation is specifically derived from elderly subjects. Until a more accurate GFR-estimating equation is developed to estimate kidney function in elderly Chinese, we recommend the use of the CSscr-cys equation instead of conventional equations for early detection and management of CKD and for assessing prognosis. If cys C is not available, the CEscr equation is an acceptable alternative.
Acknowledgments
This work was supported by the Innovation of Science and Technology Achievement Transformation Fund of Jiangsu Province (BL2012066), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions JX10231801 and the National Natural Science Foundation of China (H0511-81070588 and H0511-81370843), with grants from the Major State Basic Research Development Program of China (2013CB530803) and the Issue of Cadres Health Care in Jiangsu Province (BJ14007).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J Intern Med. 2010;268(5):456–467. doi: 10.1111/j.1365-2796.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 2.Carter JL, Stevens PE, Irving JE, Lamb EJ. Estimating glomerular filtration rate: comparison of the CKD-EPI and MDRD equations in a large UK cohort with particular emphasis on the effect of age. QJM. 2011;104(10):839–847. doi: 10.1093/qjmed/hcr077. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 4.Cepoi V, Onofriescu M, Segall L, Covic A. The prevalence of chronic kidney disease in the general population in Romania: a study on 60,000 persons. Int Urol Nephrol. 2012;44(1):213–220. doi: 10.1007/s11255-011-9923-z. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J, Selvin E, Steven LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 6.National Bureau of Statistics of China China Statistical Yearbook 2011. [Accessed August 12, 2014]. Available from: http://www.stats.gov.cn/tjsj/ndsj/2011/indexee.htm.
- 7.Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med. 2002;137(6):479–486. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]
- 8.Nugent RA, Fathima SF, Feigl AB, Chyung D. The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clin Pract. 2011;118(3):c269–c277. doi: 10.1159/000321382. [DOI] [PubMed] [Google Scholar]
- 9.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933–1953. [PubMed] [Google Scholar]
- 10.Levey AS, Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 11.Delanaye P, Cohen EP. Formula-based estimates of the GFR: equations variable and uncertain. Nephron Clin Pract. 2008;110(1):c48–c53. doi: 10.1159/000151436. [DOI] [PubMed] [Google Scholar]
- 12.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 13.Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C serum concentrations underestimate glomerular filtration rate in renal transplant patients. Clin Chem. 1999;45:1866–1868. [PubMed] [Google Scholar]
- 14.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65(4):1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 15.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 16.Grubb AO. Cystatin C: properties and use as diagnostic marker. Adv Clin Chem. 2000;35:63–99. doi: 10.1016/S0065-2423(01)35015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman DJ. Cystatin C. Ann Clin Biochem. 2002;39(2):89–104. doi: 10.1258/0004563021901847. [DOI] [PubMed] [Google Scholar]
- 18.Pei XH, Bao LH, Xu ZQ, et al. Diagnostic value of cystatin C and glomerular filtration rate formulae in Chinese nonelderly and elderly populations. J Nephrol. 2012;26(3):476–484. doi: 10.5301/jn.5000181. [DOI] [PubMed] [Google Scholar]
- 19.Pei XH, Liu Q, He J, et al. Are cystatin C-based equations superior to creatinine-based equations for estimating GFR in Chinese elderly population? Int Urol Nephrol. 2012;44:1877–1884. doi: 10.1007/s11255-012-0278-x. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo S, Imai E, Horio M, et al. Revised equations for estimating glomerular filtration rate (GFR) from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 23.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis. 2012;61(2):197–203. doi: 10.1053/j.ajkd.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157(7):471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Wang YN, Wang C, et al. A new equation to estimate glomerular filtration rate in Chinese elderly population. PLoS One. 2013;8(11):e79675. doi: 10.1371/journal.pone.0079675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng JF, Qiu L, Zhang L, et al. Multicenter study of creatinine- and/or cystatin C-based equations for estimation of glomerular filtration rates in Chinese patients with chronic kidney disease. PLoS One. 2013;8(3):e57240. doi: 10.1371/journal.pone.0057240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 28.Roos JF, Doust J, Tett SE, Kilpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children: a meta-analysis. Clin Biochem. 2007;40(5–6):383–391. doi: 10.1016/j.clinbiochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Ye XS, Zhu B, et al. Comparisons between the 2012 new CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equations and other four approved equations. PLoS One. 2014;9(1):e84688. doi: 10.1371/journal.pone.0084688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilbride HS, Stevens PE, Eaglestone G, et al. Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis. 2013;61(1):57–66. doi: 10.1053/j.ajkd.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Ma HJ, Huang H, et al. Is the Chronic Kidney Disease Epidemiology Collaboration creatinine–cystatin C equation useful for glomerular filtration rate estimation in the elderly? Clin Interv in Aging. 2013;8:1387–1391. doi: 10.2147/CIA.S52774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koppe L, Klich A, Dubourg L, Ecochard R, Hadj-Aissa A. Performance of creatinine-based equations compared in older patients. J Nephrol. 2013;26(4):716–723. doi: 10.5301/jn.5000297. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Chen JX, Wang C, et al. Assessment of glomerular filtration rate in elderly patients with chronic kidney disease. Int Urol Nephrol. 2013;45(5):1475–1482. doi: 10.1007/s11255-013-0498-8. [DOI] [PubMed] [Google Scholar]
- 34.O’Hare AM. The management of older adults with a low eGFR: moving toward an individualized approach. Am J Kidney Dis. 2009;53(6):925–927. doi: 10.1053/j.ajkd.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roderick PJ, Atkins RJ, Smeeth L, et al. CKD and mortality risk in older people: a community-based population study in the United Kingdom. Am J Kidney Dis. 2009;53(6):950–960. doi: 10.1053/j.ajkd.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 36.Raymond NT, Zehnder D, Smith SC, Stinson JA, Lehnert H, Higgins RM. Elevated relative mortality risk with mild-to-moderate chronic kidney disease decreases with age. Nephrol Dial Transplant. 2007;22(11):3214–3220. doi: 10.1093/ndt/gfm396. [DOI] [PubMed] [Google Scholar]
- 37.O’Hare AM, Bertenthal D, Covinsky KE, et al. Mortality risk stratification in chronic kidney disease: one size for all ages? J Am Soc Nephrol. 2006;17(3):846–853. doi: 10.1681/ASN.2005090986. [DOI] [PubMed] [Google Scholar]
- 38.Sjostrom P, Tidman M, Jones I. Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Invest. 2005;65(2):111–124. doi: 10.1080/00365510510013523. [DOI] [PubMed] [Google Scholar]
- 39.Grubb A, Nyman U, Bjork J, et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the Modification of Diet in Renal Disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51(8):1420–1431. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 40.Ma YC, Zuo L, Chen JH, et al. Improved GFR estimation by combined creatinine and cystatin C measurements. Kidney Int. 2007;72(12):1535–1542. doi: 10.1038/sj.ki.5002566. [DOI] [PubMed] [Google Scholar]
- 41.Wang T, Tziviskou E, Chu M, et al. Differences in survival on peritoneal dialysis between oriental Asians and Caucasians: one center’s experience. Int Urol Nephrol. 2003;35(2):267–274. doi: 10.1023/b:urol.0000020286.83411.d1. [DOI] [PubMed] [Google Scholar]
- 42.Macdonald J, Marcora S, Jibani M, et al. GFR estimation using cystatin C is not independent of body composition. Am J Kidney Dis. 2006;48(5):712–719. doi: 10.1053/j.ajkd.2006.07.001. [DOI] [PubMed] [Google Scholar]