Abstract
Objective:
To evaluate the course and predictors of neuropsychiatric symptoms (NPS) and cognition in patients with de novo Parkinson disease (PD).
Methods:
Cross-sectional study of the cohort of de novo, untreated (at enrollment) patients with PD and healthy controls (HCs) from the Parkinson's Progression Markers Initiative. Participants have serial assessments of global cognition and symptoms of depression, anxiety, psychosis, impulse control disorders (ICDs), sleep and wakefulness, apathy, and fatigue. Available data up to 24 months of follow-up were included.
Results:
The available sample size was as follows: baseline (PD = 423, HCs = 196), 12 months (PD = 261, HCs = 145), and 24 months (PD = 96, HCs = 83). Patients with PD experienced more depression, fatigue, apathy, and anxiety than HCs at all time points, and apathy (p = 0.001) and psychosis (p = 0.003) increased over time in patients with PD. Approximately two-thirds of patients with PD who screened positive for depression at any given visit were not taking an antidepressant. The Montreal Cognitive Assessment score decreased significantly over time in patients with PD (p < 0.001), but the change was comparable to that in HCs. At the 24-month visit, 44% of patients had been on dopamine replacement therapy (DRT) for at least 1 year, and this group reported more incident ICDs (p = 0.009) and excessive daytime sleepiness (p = 0.03).
Conclusion:
Multiple NPS are more common in de novo, untreated patients with PD compared with the general population, but they also remain relatively stable in early disease, while global cognition slightly deteriorates. In contrast, initiation of DRT is associated with increasing frequency of several other NPS.
Neuropsychiatric symptoms (NPS) are frequent in patients with Parkinson disease (PD).1 Depression, anxiety, sleep disturbances, and apathy are reported to be the most prevalent NPS.2,3 Cognitive impairment is also common, with approximately 25% of PD patients without dementia having mild cognitive impairment (MCI)4 and up to 80% of patients progressing to dementia eventually.5 Other NPS include psychosis, impulse control disorders (ICDs), fatigue, and excessive daytime sleepiness (EDS).
It is unclear to what extent NPS are due to the neurodegenerative process of PD itself, psychosocial factors, or a complication of dopamine replacement therapy (DRT), and the relative contributions of these factors may differ across disease stages.
In early, untreated PD, NPS are more common compared with healthy controls (HCs).2,6 Regarding cognition, early, untreated patients with PD are more likely to be diagnosed with MCI than HCs.7–9 However, relatively little is known about the clinical course and prognostic significance of early NPS and cognitive impairment in PD.
The Parkinson's Progression Markers Initiative (PPMI) is a prospective, longitudinal study designed to identify PD progression biomarkers.10 Here we present the neuropsychiatric and cognitive data from baseline through the first 24 months of follow-up. We hypothesized that NPS would be common and relatively stable in severity in early PD and that initiation of DRT might modify the natural history of NPS progression.
METHODS
Participants.
The PPMI is an observational, international, multicenter (16 US and 5 European sites) cohort study of early, untreated (at enrollment) patients with PD and demographically comparable HCs.10 A public-private partnership, it is funded by the Michael J. Fox Foundation for Parkinson's Research and funding partners (a list of PPMI funding partners can be found at www.ppmi-info.org/fundingpartners). The aims and methodology of the study have been published elsewhere10 and are on the PPMI Web site (http://www.ppmi-info.org/study-design). At enrollment, patients with PD were required to meet established diagnostic criteria for PD, have a dopamine transporter imaging deficit, be untreated for PD, and be free from dementia based on the clinical assessment of the site investigator.
Data regarding motor, NPS, and cognitive performance included herein were obtained at the baseline, 6-month, 12-month, and 24-month visits. Data were obtained from the PPMI database (www.ppmi-info.org/data). Demographic information and clinical data were accessed according to the directions provided (accessed October 17, 2013). At this time, enrollment was complete and a total of 423 patients with PD and 196 HCs provided baseline data, 281 patients with PD completed the 6-month visit, 261 patients with PD and 145 HCs completed the 12-month visit, and 96 patients with PD and 83 HCs completed the 24-month visit (figure 1).
Figure 1. Number of participants at different time points.
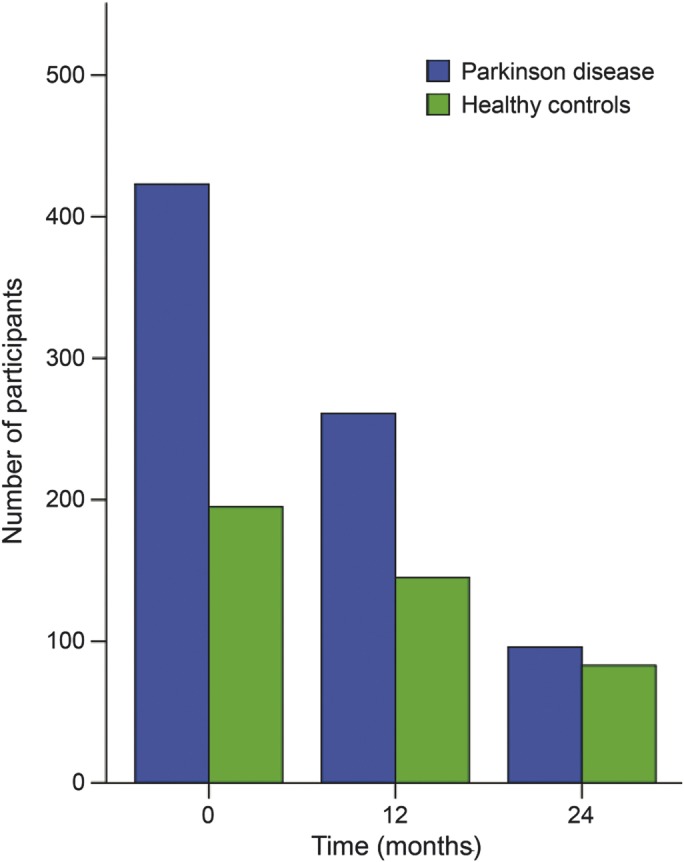
A total of 423 patients with Parkinson disease (PD) and 196 healthy controls (HCs) provided baseline data, 281 patients with PD completed the 6-month visit, 261 patients with PD and 145 HCs completed the 12-month visit, and 96 patients with PD and 83 HCs completed the 24-month visit.
Assessments.
Depression was assessed using the 15-item Geriatric Depression Scale (GDS), with a recommended cutoff score of ≥5 to indicate the presence of clinically significant depressive symptoms.11
Global cognitive abilities were assessed with the Montreal Cognitive Assessment (MoCA), and MCI for patients with PD was defined at the recommended cutoff value of <26.12,13 Cognitive comparisons between patients with PD and HCs are not possible, as HCs with a MoCA score <27 were excluded from the study.
The short version of the validated Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease (QUIP) was used to screen for ICDs (gambling, sexual, buying, and eating behaviors), related behaviors (punding [stereotyped, repetitive, purposeless behaviors], hobbyism [excessive engagement in hobbies], and walkabout [excessive wandering]), and compulsive medication use, and recommended cutoff scores were applied.14 All these behavioral disturbances are characterized by poorly controlled repetitive behaviors and have been associated with dopaminergic therapies in PD.
The Epworth Sleepiness Scale (ESS) was used to evaluate EDS. Outcomes are shown both as means and as a dichotomous variable, with a score ≥10 indicating EDS.15
Other measures include the Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) Part I apathy, fatigue, and psychosis items (any nonzero score was considered positive for these items); the State-Trait Anxiety Inventory (STAI) to assess severity of state and trait anxiety symptoms16; the University of Pennsylvania Smell Identification Test (UPSIT)17 to assess olfaction; the REM sleep behavior disorder (RBD) screening questionnaire,18 with a screening cutoff value of ≥5 indicating RBD; and the MDS-UPDRS motor score19 as a measure of disease severity. Patients were classified as having the tremor dominant, postural instability and gait disturbance (PIGD), or intermediate phenotypes, as previously described20; this variable was included given the association between PIGD subtype and cognitive impairment in previous research.21
Initiation of treatment for PD.
Study participants could start DRT at any point after baseline as part of routine clinical care. A participant was considered to have received DRT if (1) one of the following medications or medication classes was initiated: dopamine agonists, levodopa, monoamine oxidase type B (MAO-B) inhibitors, or amantadine; (2) it had been prescribed for at least 1 year; and (3) it was still active at the 24-month visit. Information about medication dosages was not readily ascertained in the database and is not included.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review board at each participating site and written informed consent was obtained from all study participants prior to enrollment.
Statistical analysis.
To evaluate the change over time in NPS within the PD group and between patients with PD and HCs over the 24-month period and to evaluate between-group comparisons at each time point, generalized estimating equation (GEE)22 analysis was used for dichotomous variables and linear mixed-effects models23 analysis was used for continuous variables, adjusted by the variable baseline score in the entire sample. Due to non-normality, logarithmic transformation was applied to GDS scores for the mixed-effects models.
Linear mixed-effects models analysis was also used to determine which demographic and clinical variables at baseline predicted cognitive decline (i.e., change in MoCA score) over the 24-month period among the group of patients who had already completed this assessment. In the implementation of the mixed-effects models, individual models were conducted for each predictor of interest, with repeated measures of MoCA score as dependent variable and predictor of interest, visit time, and predictor of interest x visit time interaction as the primary independent variables, as well as baseline MoCA score and other baseline characteristics listed in table 2 as covariates. In each model, the intercept was treated as a random effect and the above independent variables and covariates were treated as fixed effects. The predictive ability of each predictor on cognitive decline was examined through their interactions with visit time.
Table 2.
Clinical and demographic predictors of cognitive decline over time in participants with PD
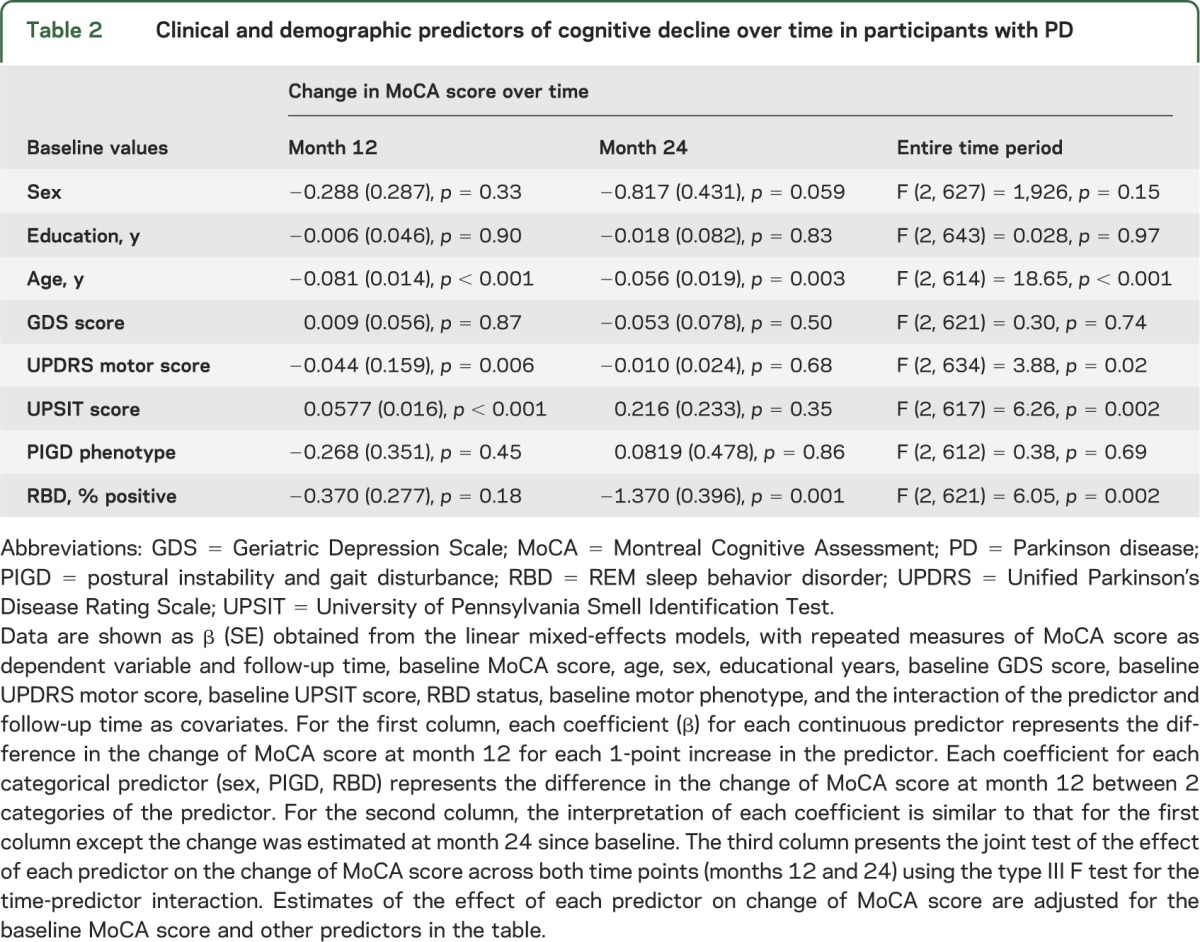
Baseline demographic characteristics were compared using t test or χ2 test. Fisher exact test, χ2 test, or Mann–Whitney U test was used to examine the impact of initiating DRT among the patients who completed the 24-month visit.
Normality assumptions were checked whenever the statistical procedures required normality assumption. Additional sensitivity analysis to evaluate the impact of missing data was conducted using GEE and linear mixed-effects models including only participants who completed the 24-month visit. All statistical tests were 2-sided. Statistical significance was set at p < 0.05. Analyses were conducted with PASW Statistics (version 18.0.0). GEE analyses were conducted with PASW statistics and SAS (version 9.2, SAS Institute Inc., Cary, NC).
RESULTS
Participant characteristics.
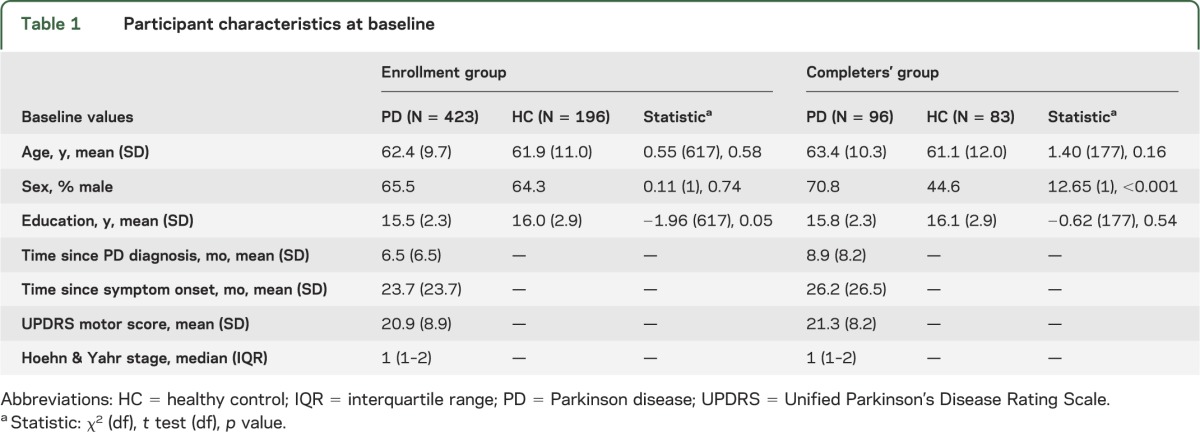
A total of 423 patients and 196 HCs were included in the study (the enrollment group). Of those, 96 patients with PD and 83 HCs had reached and participated in the 24-month follow-up visit (the completers' group) (table 1). There were no statistical differences in baseline NPS or MoCA scores between completers and noncompleters, except a marginal difference in ESS score in the PD group (scores for completers and noncompleters were 6.4 [3.6] and 5.6 [3.4], p = 0.048). There were no differences between patients with PD and HCs in age, sex, or education in the enrollment group; in the completers' group, patients with PD were more likely to be male compared with HCs (p < 0.001).
Table 1.
Participant characteristics at baseline
PD medication had been initiated at the 6-month visit in 9.6% (27/281) of patients, and the cumulative percentages increased to 58.8% (26/153) at the 12-month visit and 81.1% (77/95) at the 24-month visit (figure e-1 on the Neurology® Web site at Neurology.org). At the 24-month visit, 32.5% of treated patients were taking a dopamine agonist, 48.1% were taking levodopa, 36.4% a MAO-B inhibitor, and 22.1% amantadine.
Depression.
At baseline, 13.9% of patients with PD and 6.6% of HCs screened positive for depression. During the follow-up period, there was a nonsignificant increase in depression frequency to 18.7% in the PD group at the 24-month visit (p = 0.41). In contrast, a decrease to 2.4% at the 24-month visit was seen in the HC group. The between-group difference remained significant at each time point and widened over time (p = 0.18) (figure 2).
Figure 2. Depression over time in patients with PD and HCs.
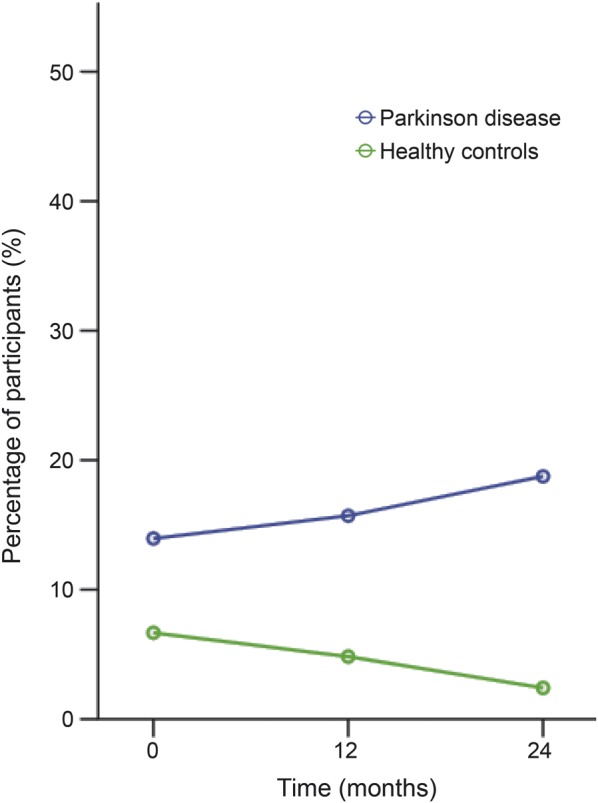
The prevalence of depression in patients with Parkinson disease (PD) and the difference between PD and healthy control (HC) increase over time.
At baseline, 16.1% of patients with PD were taking an antidepressant, and the proportion increased significantly to 25.0% at the 24-month visit (p = 0.002). In spite of increased antidepressant use over time, 65%–72% of patients with PD who screened positive for depression at a given visit were not being treated with an antidepressant.
Cognitive performance.
The mean MoCA score in patients with PD decreased from 27.1 (2.3) at baseline to 26.2 (2.9) at month 24 (p < 0.001). Using the recommended screening cutoff value, 21.5% of patients at baseline, 34.2% at month 12, and 35.5% at month 24 were cognitively impaired. Of the cognitively impaired patients at baseline, 34.6% reverted to cognitively normal at the 24-month visit, while 26.0% of cognitively normal patients at baseline became cognitively impaired at 24 months. The mean MoCA scores in the HC group also decreased over time (p < 0.001), from 28.5 (3.1) at baseline to 27.7 (2.0) at month 24.
In the mixed-effects model analysis, baseline higher age (p < 0.001), higher MDS-UPDRS motor score (p = 0.02), screening positive for RBD (p = 0.002), and lower UPSIT score (p = 0.002) predicted cognitive decline over 24 months in the PD group. Male sex predicted decline at a trend level (p = 0.06) (table 2).
Change over time in other NPS.
The proportion of patients with PD who screened positive for ICDs or related behavior symptoms at baseline was 21% and did not increase significantly over the 24-month follow-up period (table e-1). In addition, there was no significant difference between patients with PD and HCs in the frequency of ICD symptoms at any time point.
There was a trend for EDS symptoms to increase over time in patients with PD. However, there was no significant difference in EDS as a dichotomous variable between patients with PD and HCs at any time point or over time (p = 0.13).
Fifty percent of patients with PD had a positive score on the MDS-UPDRS Part I fatigue item at baseline, and there was a trend increase to 57.1% at month 12 and 61.5% at month 24 (p = 0.09). The percentage of patients with PD who screened positive for apathy on the MDS-UPDRS Part I item for apathy was 16.7% at baseline and increased significantly to 23.3% and 30.2% at the 12- and 24-month visits (p = 0.001). A higher percentage of patients with PD compared to HCs screened positive for fatigue and apathy at all time points during the follow-up period, although the between-group differences did not change over time (table e-1).
STAI total, state, and trait anxiety scores were significantly higher in the PD group compared with HCs at each time point but did not change significantly in patients with PD or between PD and HC groups over time (table e-1).
The prevalence of psychosis was significantly higher in the PD group compared with HCs at month 12 and increased significantly over time in patients with PD: 3.0%, 5.3%, and 10.0% at baseline, 12 months, and 24 months, respectively (p = 0.003) (table e-1).
Impact of initiation of DRT on new-onset NPS.
At the 24-month visit, 81% of patients had started DRT and 43.7% of patients had been taking DRT for a minimum of 1 year (hereafter named the DRT group) (figure e-1; table 3). While some NPS might be expected to improve with DRT (e.g., depression or apathy), others may begin or worsen (e.g., ICDs and psychosis).
Table 3.
Impact of initiating dopamine replacement therapy on neuropsychiatric symptoms
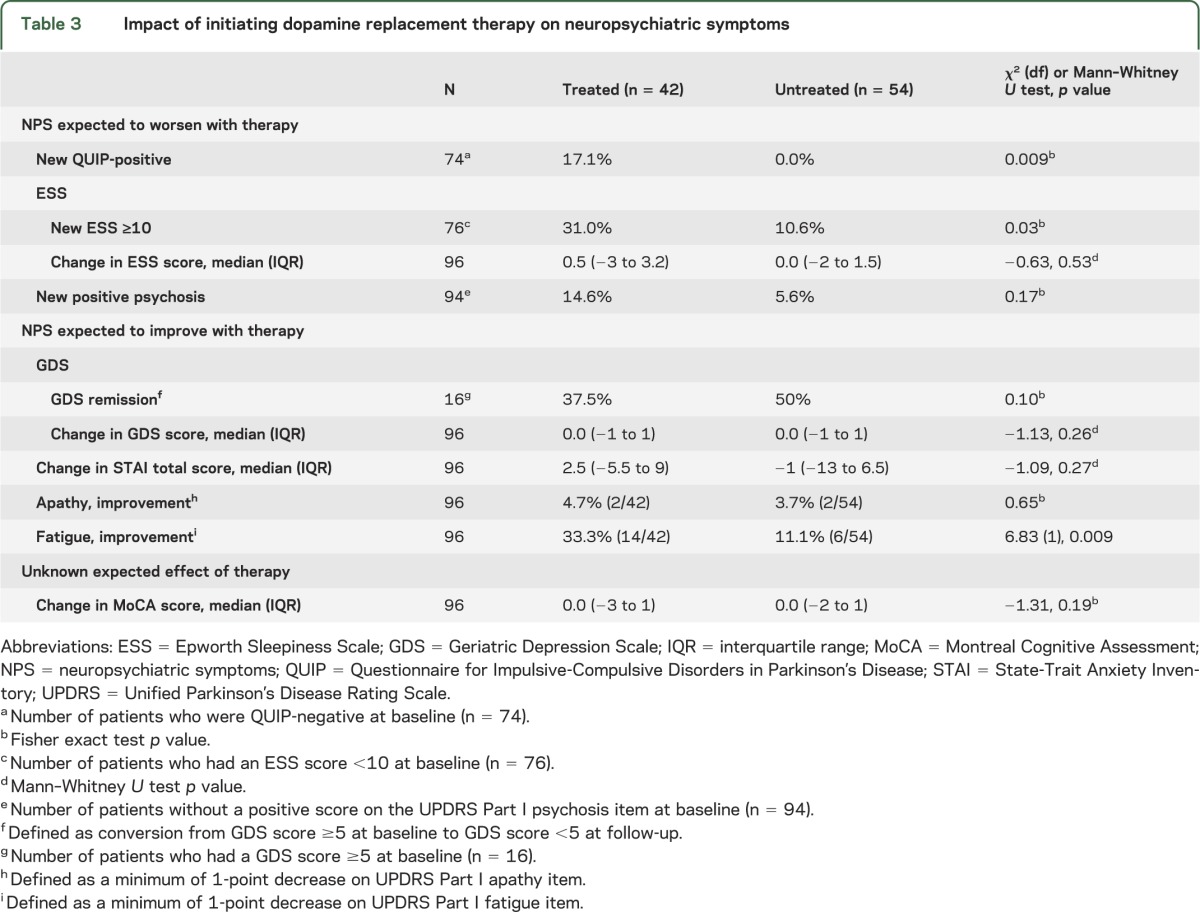
Regarding new-onset ICD symptoms, 74 patients who completed the 24-month visit were QUIP-negative at baseline, and of this group, 6 incident cases of ICD or related behavior symptoms were reported in the DRT group, whereas there were no ICD incident symptoms in the untreated group (p = 0.009). Concerning duration of exposure, 23 patients who were QUIP-negative at baseline had been taking DRT medication for <1 year at the 24-month visit, and none of them reported new-onset ICD symptoms.
There were more incident cases of EDS in the DRT group compared with the untreated group (31.0% vs 10.6%, p = 0.03). Although not statistically significant, the frequency of new-onset psychosis was nearly 3 times as high in the DRT group compared with the untreated group (table 3).
Examining NPS that might be expected to improve with DRT, the DRT group showed a significant improvement in the MDS-UPDRS Part I fatigue item score over time, with 33.3% improving by at least 1 point compared with 11.1% of untreated patients (p = 0.009). There was no effect of initiation of DRT on depression (p = 0.26), cognitive performance (p = 0.19), anxiety (p = 0.27), or apathy (p = 0.65) scores (table 3).
DISCUSSION
We found that newly diagnosed, untreated patients with PD exhibit higher rates of depression, anxiety, fatigue, and apathy than HCs. The overall frequency of most NPS was stable over the first 2 years of the disease. In contrast, initiation of DRT was associated with an increase in several problematic NPS in PD, including ICD symptoms and EDS, and with a decrease in fatigue.
Our findings suggest that although the majority of NPS do not worsen substantially in the first years of the disease regardless of treatment, the initiation of DRT does not significantly improve most of these symptoms on average. This may in part reflect the fact that the psychiatric burden and cognitive deficits in a de novo PD cohort are not high overall compared with later disease stages.
The dopamine system is thought to play some role in many NPS in PD.24 While a hyperdopaminergic state may contribute to onset of ICDs, psychosis, and EDS,25,26 a hypodopaminergic state has been associated with apathy, depression and anxiety. “Hyperdopaminergic” symptoms might occur after initiation of DRT in untreated PD.27,28 We found that treatment initiation with DRT for at least 1 year, but not less than 1 year, was associated with a statistical increase in ICD symptoms and EDS and a nonsignificant increase in the frequency of psychosis. While fatigue significantly improved with DRT, there was no significant improvement in anxiety, apathy, depression, or cognition.
Prior analysis of a partial PPMI baseline dataset showed that the frequency of ICD symptoms was equally common in patients with early, untreated PD and HCs.29 Using the full baseline dataset plus longitudinal data, there still is no evidence for an increased frequency of ICD symptoms in patients with PD compared with controls out to 2 years. However, patients who were on DRT for at least 1 year exhibited more incident ICD and related behaviors than patients who either did not initiate DRT or were exposed for less than 1 year. These findings suggest that a minimum duration of exposure to DRT is needed to significantly increase the risk of developing ICD symptoms and would explain the relative stability and lack of differences seen between patients with PD and HCs in this study.
As expected, global cognition scores declined slightly over the follow-up period. Consistent with prior studies, age, greater motor severity, presence of RBD symptoms, and worse olfaction at baseline predicted future cognitive decline.30–35 These findings suggest that early diffuse brainstem pathology, widespread PD pathology, or comorbid pathology that occurs with aging might contribute to initial cognitive decline in PD.
This study also sheds light on the variability in NPS screening assessments in individuals over time. Although as a group patients with PD worsened significantly in MoCA score over time, one-third of patients initially classified as cognitively impaired at baseline were cognitively normal at the 24-month follow-up, perhaps due in part to learning effects, as there were no significant changes in other NPS over time in this subgroup of patients (data not shown). In addition, nearly half of patients with clinically significant depression at baseline screened negative at the 24-month follow-up. These findings highlight the limitations of performing single time point assessments and using screening instruments instead of more detailed diagnostic or assessment tools.
Consistent with prior studies,36,37 we found that the majority of PD patients with clinically significant depression were not treated with antidepressants during this 2-year period, suggesting that depression is undertreated in early PD. Since depression symptoms did worsen over time in this study and have been shown to influence the initiation of DRT in a previous study,38 it is important to appropriately screen for and adequately treat depression in this population starting at disease onset.
Several limitations of this study should be noted. First, at the time of data download, the 2-year follow-up assessment had not yet been completed by all patients in the study. In order to control for this, statistical tests that account for missing data were utilized (i.e., GEE and mixed-effects models). Also, there were no statistical differences in baseline NPS or MoCA scores between completers and noncompleters. In addition, analyses including only the completers group for change in the NPS over time and the predictors of cognitive decline were done as an additional sensitivity analysis, and the results did not differ from the primary analyses. Second, HCs were not matched to patients with PD on demographic characteristics; they were similar overall in the enrollment group, but the percentage of males was higher in PD in the completers group. We adjusted sex as a covariate in all GEE and mixed-effects model analyses to account for this imbalance. In addition, the consistency of results between the full sample analyses and the sensitivity analyses in the completers' group further strengthened our conclusions. Finally, information about dosages and duration of treatment was not included as it was not readily available in the database.
This study provides an initial glimpse at the prevalence, course, and predictors of NPS and global cognition in the first years after PD diagnosis, including the impact of initiating DRT. As more patients complete long-term follow-up assessments, future PPMI analyses are needed to confirm and expand these preliminary results regarding the frequency of, risk factors for, and prognostic role of NPS and cognitive impairment in early PD. Similarly, future analyses will help elucidate the effects of initiation of various DRT medications on NPS.
Supplementary Material
GLOSSARY
- DRT
dopamine replacement therapy
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- GDS
Geriatric Depression Scale
- GEE
generalized estimating equation
- HC
healthy control
- ICD
impulse control disorder
- IQR
interquartile range
- MAO-B
monoamine oxidase type B
- MCI
mild cognitive impairment
- MDS-UPDRS
Movement Disorders Society Unified Parkinson's Disease Rating Scale
- MoCA
Montreal Cognitive Assessment
- NPS
neuropsychiatric symptoms
- PD
Parkinson disease
- PIGD
postural instability and gait disturbance
- PPMI
Parkinson's Progression Markers Initiative
- QUIP
Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease
- RBD
REM sleep behavior disorder
- STAI
State-Trait Anxiety Inventory
- UPSIT
University of Pennsylvania Smell Identification Test
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Patricia de la Riva contributed to the study concept and design, statistical analysis, and interpretation of data. Kara Smith contributed to the study design and revision of the manuscript for content. Sharon X. Xie contributed to the interpretation of data, statistical analysis, and revision of the manuscript. Daniel Weintraub contributed to the study concept and design, analysis or interpretation of data, and revision of the manuscript for content, including medical writing for content.
STUDY FUNDING
Supported by the Michael J. Fox Foundation for Parkinson's Research and funding partners: Abbott, Avid Radiopharmaceuticals, Biogen Idec, Covance, Bristol-Myers Squibb, Meso Scale Discovery, Piramal, Eli Lilly and Co, F. Hoffman–La Roche Ltd, GE Healthcare, Genentech, GlaxoSmithKline, Merck and Co, Pfizer Inc, and UCB Pharma SA.
DISCLOSURE
P. de la Riva received financial support from the group of young neurologists of the Spanish Society of Neurology. K. Smith reports no disclosures. S. Xie is supported by Morris K. Udall Parkinson's Disease Research Center of Excellence NS-053488 and Alzheimer's Disease Core Center (AG 10124). D. Weintraub has received funding support from the Michael J. Fox Foundation for Parkinson's Research. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Weintraub D, Stern MB. Psychiatric complications in Parkinson's disease. Am J Geriatr Psychiatry 2005;13:844–851 [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Brønnick K, Alves G, et al. The spectrum of neuropsychiatric symptoms in patients with early untreated Parkinson's disease. J Neurol Neurosurg Psychiatry 2009;80:928–930 [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri K, Healy D, Schapira A. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol 2006;5:235–245 [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Bronnick K, Williams-Gray CH, et al. Mild cognitive impairment in Parkinson's disease: a multicenter pooled analysis. Neurology 2010;75:1062–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003;60:387–392 [DOI] [PubMed] [Google Scholar]
- 6.Khoo TK, Yarnall AJ, Duncan GW, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 2013;80:276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poletti M, Frosini D, Pagni C, et al. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;83:601–606 [DOI] [PubMed] [Google Scholar]
- 8.Broeders M, De Bie RMA, Velseboer DC, Speelman JD, Muslimovic D, Schmand B. Evolution of mild cognitive impairment in Parkinson disease. Neurology 2013;81:346–352 [DOI] [PubMed] [Google Scholar]
- 9.Yarnall A, Breen D, Duncan G, et al. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD Study. Neurology 2014;82:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marek K, Jennings D, Lasch S, et al. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011;95:629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weintraub D, Oehlberg KA, Katz IR, Stern MB. Test characteristics of the 15-Item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry 2006;14:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalrymple-Alford J, MacAskill M, Nakas C, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology 2010;75:1717–1725 [DOI] [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699 [DOI] [PubMed] [Google Scholar]
- 14.Weintraub D, Stewart S, Shea JA, et al. Validation of the Questionnaire for Impulsive-Compulsive Behaviors in Parkinson's Disease (QUIP). Mov Disord 2009;24:1461–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–545 [DOI] [PubMed] [Google Scholar]
- 16.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970 [Google Scholar]
- 17.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984;32:489–502 [DOI] [PubMed] [Google Scholar]
- 18.Stiasny-Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire—a new diagnostic instrument. Mov Disord 2007;22:2386–2393 [DOI] [PubMed] [Google Scholar]
- 19.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and climimetric testing results. Mov Disord 2008;23:2129–2170 [DOI] [PubMed] [Google Scholar]
- 20.Kang JH, Irwin DJ, Chen-Plotkin AS, et al. Association of cerebrospinal fluid β-amyloid 1-42, T-tau, P-tau182, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol 2013;70:1277–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burn D, Rowan E, Allan L, Molloy S, O'Brien J, McKeith I. Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006;77:585–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeger SL, Liang K-Y, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988;44:1049–1060 [PubMed] [Google Scholar]
- 23.Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics 1982;38:963–997 [PubMed] [Google Scholar]
- 24.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 2005;128:1314–1322 [DOI] [PubMed] [Google Scholar]
- 25.Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 2010;67:589–595 [DOI] [PubMed] [Google Scholar]
- 26.Burn DJ, Tröster AI. Neuropsychiatric complications of medical and surgical therapies for Parkinson's disease. J Geriatr Psychiatry Neurol 2004;17:172–180 [DOI] [PubMed] [Google Scholar]
- 27.Ardouin C, Chereau I, Llorca P, et al. Assessment of hyper- and hypodopaminergic behaviors in Parkinson's disease. Rev Neurol 2009;175:845–856 [DOI] [PubMed] [Google Scholar]
- 28.Barone P, Poewe W, Albrecht S, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:573–580 [DOI] [PubMed] [Google Scholar]
- 29.Weintraub D, Papay K, Siderowf A, Screening for impulse control disorder symptoms in patients with de novo Parkinson disease: a case-control study. Neurology 2013;80:176–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans JR, Mason SL, Williams-Gray CH, et al. The natural history of treated Parkinson's disease in an incident, community based cohort. J Neurol Neurosurg Psychiatry 2011;82:1112–1118 [DOI] [PubMed] [Google Scholar]
- 31.Vendette M, Gagnon J-F, Dècary A, et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology 2007;69:1843–1849 [DOI] [PubMed] [Google Scholar]
- 32.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord 2006;21:1123–1130 [DOI] [PubMed] [Google Scholar]
- 33.Baba T, Kikuchi A, Hirayama K, et al. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson's disease: a 3-year longitudinal study. Brain 2012;135:161–169 [DOI] [PubMed] [Google Scholar]
- 34.Boot B, Boeve B, Roberts R, et al. Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population-based study. Ann Neurol 2012;71:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams-Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain 2007;130:1787–1798 [DOI] [PubMed] [Google Scholar]
- 36.Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Recognition and treatment of depression in Parkinson's disease. J Geriatr Psychiatry Neurol 2003;16:178–183 [DOI] [PubMed] [Google Scholar]
- 37.Shulman LM, Taback RL, Rabinstein AA, et al. Non-recognition of depression and other non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord 2002;8:193–197 [DOI] [PubMed] [Google Scholar]
- 38.Ravina B, Camicioli R, Como PG, et al. The impact of depressive symptoms in early Parkinson disease. Neurology 2007;69:342–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


