Abstract
Purpose
Despite most epidemiologic studies reporting that an increase in milk intake affects the growth of prostate cancer, the results of experimental studies are not consistent. In this study, we investigated the proliferation of prostate cancer cells treated with casein, the main protein in milk.
Materials and Methods
Prostate cancer cells (LNCaP and PC3), lung cancer cells (A459), stomach cancer cells (SNU484), breast cancer cells (MCF7), immortalized human embryonic kidney cells (HEK293), and immortalized normal prostate cells (RWPE1) were treated with either 0.1 or 1 mg/mL of α-casein and total casein extracted from bovine milk. Treatments were carried out in serum-free media for 72 hours. The proliferation of each cell line was evaluated by an 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay.
Results
α-Casein and total casein did not affect the proliferations of RWPE1, HEK293, A459, SNU484, MCF7, HEK293, or RWPE1 cells. However, PC3 cells treated with 1 mg/mL of α-casein and casein showed increased proliferation (228% and 166%, respectively), and the proliferation of LNCaP cells was also enhanced by 134% and 142%, respectively. The proliferation mechanism of α-casein in PC3 and LNCaP cells did not appear to be related to the induction of Insulin-like growth factor-1 (IGF-1), since the level of IGF-1 did not change upon the supplementation of casein.
Conclusions
The milk protein, casein, promotes the proliferation of prostate cancer cells such as PC3 and LNCaP.
Keywords: Caseins, Cell proliferation, Milk, Neoplasms, Prostate
INTRODUCTION
Nutritional support or restrictions have been important issues for cancer control in recent decades. Milk is a very important food, and there have been many confusing and parochial reports on the relationship between milk intake and diverse cancers. Most epidemiologic studies [1,2,3,4,5,6], but not all [7,8], have reported an increase in prostate cancer risk with an increased milk intake. To elucidate the effect of milk on prostate cancer, numerous experimental studies have tried to identify hazardous ingredients in milk, such as calcium [6,9], estrogen [10], and insulin-like growth factor-1 (IGF-1) [11]. However, among the components of milk, protein has been enigmatic. A favorable effect of milk protein was the inhibition of mutation [12,13,14,15]. Several cell culture studies showed that milk protein could contribute to cancer prevention. The opposite results have appeared in recent epidemiologic studies [16,17]. The growth of prostate cancer cells was stimulated by milk protein [16,17]. Owing to these conflicting results, the potential role of milk protein in prostate cancer is still controversial.
Milk protein consists of 80% casein and 20% whey [18]. Casein has four subtypes: αs1-, αs2-, β-, and κ-casein [19]. α-Casein, a mix of αs1- and αs2-casein, is the main fraction of milk protein. Casein has potent antimutagenic effects proven through mutagen models [12,13,14]. Several animal experiments also showed an inhibitory effect of casein against mutagens [20,21]. Mice fed 20% casein diets had a significantly lower 1,2-dimethylhydrazine-induced colon cancer incidence than the control group [21]. The literature states that casein might prevent colon and breast cancer [20,21,22]. To date, however, the effect of casein itself on prostate cancer cells has never been investigated.
To establish the relationship between casein and prostate cancer, we evaluated the proliferation of immortalized prostate cells and diverse cancer cells including prostate cancer cells after treatment with casein and α-casein. Prior to a comparison between the casein-treated group and the control group, appropriate experimental conditions were established in prostate cancer cells PC3 and LNCaP.
MATERIALS AND METHODS
1. Cell lines and cultures
Human prostate cancer cell lines, androgen-independent PC-3 (ATCC, Manassas, VA, USA) and androgen-dependent LNCaP cells (ATCC, Manassas), were maintained in a Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 mg/mL). Human A549 (ATCC, Manassas) lung cancer cells, SNU-484 (KCLB, Seoul, Korea) stomach cancer cells, and HEK293 (ATCC, Rockville, MD, USA) human embryonic kidney cells were maintained in the same manner. Human breast cancer cells (MCF7; ATCC, Rockville) were maintained in Dulbecco's modified Eagle's minimal essential medium (Invitrogen) with the aforementioned supplements. Immortalized RWPE-1 (ATCC, Manassas) normal human prostate cells were grown in bovine pituitary extracts (50 µg/mL), keratinocyte, and epidermal growth factor (5 ng/mL) under the same incubation conditions. The cells were cultured at 37℃ in a humidified atmosphere under 5% CO2 in air. The cells were plated first in 10% FBS; the growth medium was removed after 24 hours and replaced with a serum-free medium supplemented with NaOH, α-casein, and casein from bovine milk at concentrations of 0.1 or 1 mg/mL for 72 hours. The cells were diluted in an appropriate medium before each experiment.
2. Casein and experimental conditions
α-Casein and whole casein from bovine milk were purchased from Sigma-Aldrich (St. Louis, MO, USA). Each cell line (PC-3, LNCaP, MCF7, SNU484, A549, RWPE-1, or HEK293) was seeded in 12-well plates at a density of 1×105 cells/well under serum-free conditions. Each cell was treated with NaOH, α-casein, or casein from bovine milk at concentrations of 0.1 or 1 mg/mL on the first day only. After 3 days, proliferations of each cell line growth were measured by using an 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Prior to the establishment of the aforementioned experimental conditions, each cell line was cultured under various conditions. The number of treatments (first day only vs. everyday), duration of experiments (2~7 days), conditions with or without serum supplement, and concentration of casein (α-casein) were tested. Because serum is also a nutrient like casein, we chose a serum-free condition as the final experimental condition. To ascertain the results, experiments were repeated at least three times.
3. Measurement of cell proliferation and morphological change
After treatment, cell viability was assessed by incubating cells with 0.5 mg/mL of MTT for another 4 hours. Formazan produced by viable cells was prepared in dimethyl sulfoxide. Colorimetric analysis was performed at 570 nm in a Multiskan enzyme-linked immunosorbent assay (ELISA) reader (Thermo, Vantaa, Finland). Cell viability was presented as a relative percentage of controls. In addition, cells were photographed using light microscopy.
4. Statistical analysis
A two-way analysis of variance (ANOVA) test was employed to compare the experimental groups with the control group, while results before and after treatments were compared using Tukey's comparison test. Statistical significance was determined at p<0.05. All statistical calculations were computed using PASW Statistics ver. 18 (IBM Co., Armonk, NY, USA).
RESULTS
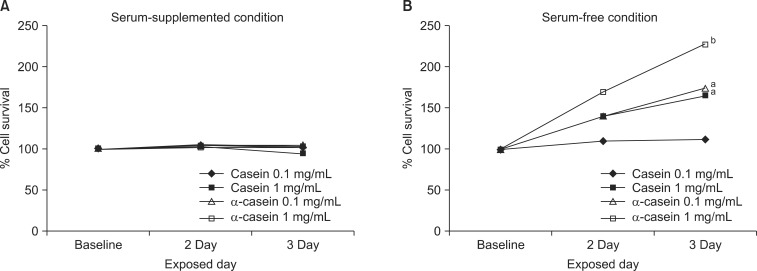
The changes in proliferation were evaluated after treatments with casein (or α-casein) for 2 to 3 days. There was no significant change in the proliferation of PC3 cells with FBS supplementation (Fig. 1A). The results in other cells were similar to the PC3 cell growth. Next, as FBS sometimes provides an artificial milieu, each cell was cultured in a serum-free medium and the changes in cell proliferation were investigated. Interestingly, casein induced a marked dose-dependent enhancement of cell proliferation in the PC3 and LNCaP cells (Fig. 1B). With the supplementation of 1 mg/mL of α-casein, the changes in the growths of PC-3 and LNCaP cells were 228% (p<0.001) and 134% (p<0.05), respectively. Similarly, the addition of 1 mg/mL of casein enhanced the growth to 166% (p<0.05) and 142% (p<0.05) proliferation in the PC-3 and LNCaP cells, respectively. However, there was no significant change in the growth of the other cells under the same experimental conditions.
Fig. 1.
Growth induction of casein and α-casein on proliferation of PC-3 cells. Cell survival was determined using 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. (A) Percentage of surviving cells versus control group under serum conditions for 2 to 3 days. (B) Percentage of surviving cells versus control group under serum-free conditions for 2 to 3 days. Data are presented as the mean value (n=36 in each cell). ap<0.05, bp<0.001 versus control responses.
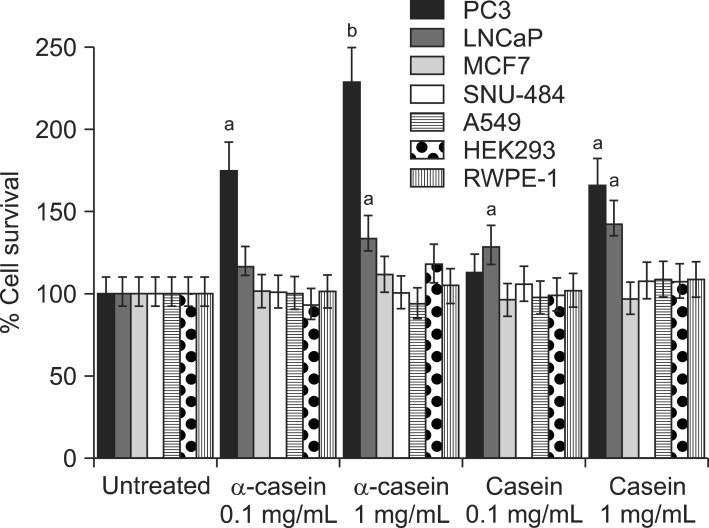
Fig. 2 shows the changes in the proliferation of each cell after 72 hours of serum-free culture. Treatment with α-casein induced marked dose-dependent increases in cell proliferation in the PC-3 and LNCaP cells. However, there was no change in the proliferation of the other cancer cells or immortalized prostate cells.
Fig. 2.
Casein can promote proliferation of prostate cancer cells. Prostate cancer cells (PC-3 and LNCaP) proliferated at an increased rate with α-casein supplementation under serum-free conditions. However, other cancer cells did not show any differences as compared to an untreated group. ap<0.05, bp<0.001 versus control responses.
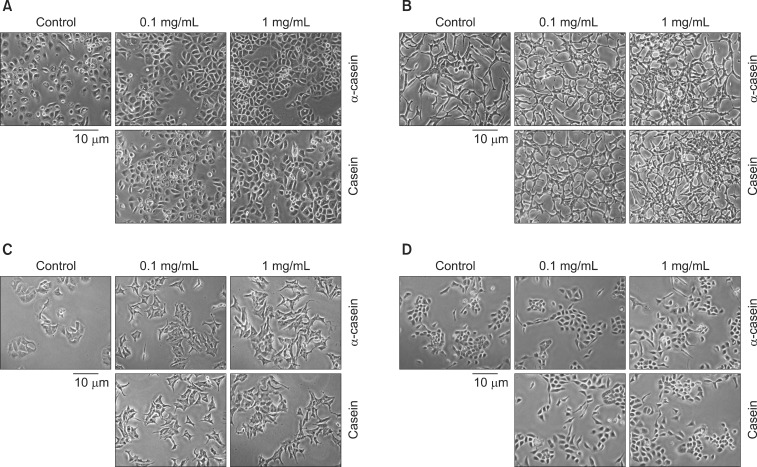
Fig. 3 shows representative morphological changes in each cell after exposure to α-casein and casein (0.1 and 1 mg/mL) for 72 hours. Under control conditions (NaOH alone), PC-3 cells appeared to have a typical phenotype, with round nuclei and homogeneous cytoplasm. PC-3 cells treated with casein or α-casein showed distinct morphological changes, increases in cell volume, cellular adhesion, and cell numbers. Likewise, LNCaP cells treated with casein or α-casein showed increased cellular adhesion. MCF7, SNU-484, and A549 cells treated with casein or α-casein showed no morphological changes compared with the untreated cells. RWPE-1 and HEK293 cells treated with casein or α-casein also showed an increase in cellular adhesion without any changes in cell volume or numbers.
Fig. 3.
The effects of α-casein and casein on morphology. The morphological changes of various cells were observed by light microscopy after treatment with α-casein and casein under serum-free conditions for 72 hours. Under control conditions (vehicle: NaOH), PC-3 cells appeared to have a typical phenotype, featured with round nuclei and homogeneity. (A) After treatment with casein (or α-casein), PC-3 cells showed the following different morphological changes: 1) increase in cell volume, 2) more cohesion, and 3) marked increase in cell number. (B) Likewise, LNCaP cells showed increased cellular adhesion. (C) Treated MCF7 cells showed no morphological changes as compared to the untreated cells. (D) RWPE-1 cells also showed an increase in cellular adhesion without the changes in cell volume and number.
DISCUSSION
Most epidemiologic studies have reported an unfavorable effect of milk on prostate cancer risk [1,2,3,5,8,23]. However, experimental results are diverse and complicated by several factors. First, the effects of the numerous components of milk remain unproven. Second, most components of milk interact with other components including hormones, growth factors, and minerals. Third, the results obtained in vitro should be replicable in animals or an in vivo study. Fourth, to understand the effect of dietary compounds on specific cells in a living body, digestion and their metabolites must also be considered.
Recent studies revealed that milk protein plays a key role in the development and modulation of prostate cancer cell proliferation [16,17,23]. Tate et al [16] reported that cow's milk stimulated the growth of prostate cancer cells (LNCaP) almost as much as digested whole milk. Like our results, neither casein nor digested milk increased the growth of breast cancer cells (MCF-7). Nielsen et al [17] evaluated the effect of whey protein, a second fraction of milk protein, on prostate cancer cell (PC-3) growth. While whey had an anti-proliferative effect on breast cancer cells (MCF-7), this protein had a stimulatory effect on prostate cancer cell (PC-3) growth under the control of the estrogen concentration. Our study also showed the proliferative effect of casein and α-casein on prostate cancer cells (PC-3). However, there is no significant change in the growth rate of breast cancer cells (MCF-7). Nevertheless, milk protein increases the proliferation of prostate cancer cells.
Milk includes multiple nutritional factors, carbohydrates, lipids, amino acids, and minerals [24]. Naturally, most milk proteins including casein can promote the growth of both normal cells and cancer cells. However, this study showed no proliferation of immortalized normal human prostate cells (RWPE-1). In addition, the growth of other cancer cells, human lung cancer (A549) and stomach cancer cells (SNU484), were not stimulated. We can speculate on the basis of these results that casein provided cancer-specific proliferating factors, not simple nutritional support.
The difference in the growth rate between PC3 and LNCaP is an intriguing finding. The growth of the PC3 cells was promoted more than that of the LNCaP cells by casein (or α-casein). However, no definite reason could be identified due to a lack of previous work. Note that androgen-sensitive LNCaP cells and -independent PC3 cells have different genetics, apoptotic behaviors, cell viability, and signaling pathways [25,26].
Unlike that of prostate cancer, the growth of breast cancer cells was reported to be suppressed by casein in the literature. Bonuccelli et al [22] showed that α-casein could significantly inhibit the growth and metastasis of one murine mammary tumor cell line (Met-1 cells) and two human breast cancer cell lines (MCF10A-H-Ras and MDA-MB-231 cells). In addition, α-casein mediated its tumor suppressor effects through the activation of STAT1 signaling. They speculated that α-casein could provide a 'differentiation' therapy for breast cancer.
Several authors have evaluated the effects of various states of casein on colon cancer. However, these results were discordant. Corpet and Chatelin-Pirot [27] reported that cooked casein could promote colon cancer in rats, perhaps because of mucosal abrasion. However, in an in vitro study, casein hydrolysates had an inhibitory effect on the viability and growth of colon cancer cell lines [28]. Recently, casein phosphopeptides, a family of bioactive peptides derived from the digestion of casein, were shown to modulate proliferation and apoptosis in intestinal adenocarcinoma cell lines [29].
To date, the theory that circulating IGF-1 concentrations are positively associated with an increased prostate cancer risk in humans has been the commonly accepted. Kimura et al [30] found that 9% of the IGF-1 fed to mice survived digestion and could be recovered intact out of the bloodstream; this figure increased to 67% when IGF-1 was fed together with casein. These observations provide very strong circumstantial evidence of increased levels of serum IGF-1 through excessive milk intake. Consequently, we understand that dietary casein might boost IGF-1 levels or prostate cancer cell proliferation. For this reason, the present study compared the effects of casein on the proliferation rates of various cancer cells. Despite greater proliferation in prostate cancer cells, there was no significant difference in the level of IGF-1 (data not shown). The addition of IGF-1 did not result in a further enhancement of prostate cancer cell growth [16]. These results suggest that casein promotes the proliferation of prostate cancer cells without any effect on IGF-1.
Casein cannot be absorbed directly from the digestive system. However, casein and α-casein have been detected in various conditions and tissues, even serum. No obvious mechanism of how casein might be transported from the intestines to the body tissues or cancer cells has yet been identified. Although casein promoted the growth of cancer cells under serum-free conditions in this study, it is not clear whether dietary casein could have an effect on prostate cancer cells in vivo. Further experiments on the molecular mechanisms of casein induced proliferation in prostate cancer cells and in vivo studies should be conducted.
CONCLUSIONS
Major milk proteins, α-casein and total casein, promoted the proliferation of PC-3 and LNCaP prostate cancer cells under serum-free conditions, but did not elicit any changes in the proliferation of other cancer cells or immortalized normal prostate cells. Furthermore, casein and α-casein showed dose-dependent proliferating properties. These effects of casein were not associated with IGF-1. To understand the relationship between proliferating prostate cancer cells and casein, a study on the molecular mechanisms of casein induced proliferation in prostate cancer cells as well as in vivo studies should be conducted.
ACKNOWLEDGEMENTS
This study was supported by the Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea.
References
- 1.Chan JM, Stampfer MJ, Ma J, Gann PH, Gaziano JM, Giovannucci EL. Dairy products, calcium, and prostate cancer risk in the Physicians' Health Study. Am J Clin Nutr. 2001;74:549–554. doi: 10.1093/ajcn/74.4.549. [DOI] [PubMed] [Google Scholar]
- 2.Rohrmann S, Platz EA, Kavanaugh CJ, Thuita L, Hoffman SC, Helzlsouer KJ. Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer Causes Control. 2007;18:41–50. doi: 10.1007/s10552-006-0082-y. [DOI] [PubMed] [Google Scholar]
- 3.Raimondi S, Mabrouk JB, Shatenstein B, Maisonneuve P, Ghadirian P. Diet and prostate cancer risk with specific focus on dairy products and dietary calcium: a case-control study. Prostate. 2010;70:1054–1065. doi: 10.1002/pros.21139. [DOI] [PubMed] [Google Scholar]
- 4.Kurahashi N, Inoue M, Iwasaki M, Sasazuki S, Tsugane AS Japan Public Health Center-Based Prospective Study Group. Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men. Cancer Epidemiol Biomarkers Prev. 2008;17:930–937. doi: 10.1158/1055-9965.EPI-07-2681. [DOI] [PubMed] [Google Scholar]
- 5.Tseng M, Breslow RA, Graubard BI, Ziegler RG. Dairy, calcium, and vitamin D intakes and prostate cancer risk in the National Health and Nutrition Examination Epidemiologic follow-up study cohort. Am J Clin Nutr. 2005;81:1147–1154. doi: 10.1093/ajcn/81.5.1147. [DOI] [PubMed] [Google Scholar]
- 6.Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst. 2005;97:1768–1777. doi: 10.1093/jnci/dji402. [DOI] [PubMed] [Google Scholar]
- 7.Huncharek M, Muscat J, Kupelnick B. Dairy products, dietary calcium and vitamin D intake as risk factors for prostate cancer: a meta-analysis of 26,769 cases from 45 observational studies. Nutr Cancer. 2008;60:421–441. doi: 10.1080/01635580801911779. [DOI] [PubMed] [Google Scholar]
- 8.Park SY, Murphy SP, Wilkens LR, Stram DO, Henderson BE, Kolonel LN. Calcium, vitamin D, and dairy product intake and prostate cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;166:1259–1269. doi: 10.1093/aje/kwm269. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez C, McCullough ML, Mondul AM, Jacobs EJ, Fakhrabadi-Shokoohi D, Giovannucci EL, et al. Calcium, dairy products, and risk of prostate cancer in a prospective cohort of United States men. Cancer Epidemiol Biomarkers Prev. 2003;12:597–603. [PubMed] [Google Scholar]
- 10.Qin LQ, Wang PY, Kaneko T, Hoshi K, Sato A. Estrogen: one of the risk factors in milk for prostate cancer. Med Hypotheses. 2004;62:133–142. doi: 10.1016/s0306-9877(03)00295-0. [DOI] [PubMed] [Google Scholar]
- 11.Saikali Z, Setya H, Singh G, Persad S. Role of IGF-1/IGF-1R in regulation of invasion in DU145 prostate cancer cells. Cancer Cell Int. 2008;8:10. doi: 10.1186/1475-2867-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parodi PW. A role for milk proteins and their peptides in cancer prevention. Curr Pharm Des. 2007;13:813–828. doi: 10.2174/138161207780363059. [DOI] [PubMed] [Google Scholar]
- 13.van Boekel MA, Weerens CN, Holstra A, Scheidtweiler CE, Alink GM. Antimutagenic effects of casein and its digestion products. Food Chem Toxicol. 1993;31:731–737. doi: 10.1016/0278-6915(93)90144-n. [DOI] [PubMed] [Google Scholar]
- 14.Jongen WM, van Boekel MA, van Broekhoven LW. Inhibitory effect of cheese and some food constituents on mutagenicity generated in Vicia faba after treatment with nitrite. Food Chem Toxicol. 1987;25:141–145. doi: 10.1016/0278-6915(87)90147-5. [DOI] [PubMed] [Google Scholar]
- 15.Phelan M, Aisling Aherne S, O'Sullivan D, FitzGerald RJ, O'Brien NM. Growth inhibitory effects of casein hydrolysates on human cancer cell lines. J Dairy Res. 2010;77:176–182. doi: 10.1017/S0022029909990471. [DOI] [PubMed] [Google Scholar]
- 16.Tate PL, Bibb R, Larcom LL. Milk stimulates growth of prostate cancer cells in culture. Nutr Cancer. 2011;63:1361–1366. doi: 10.1080/01635581.2011.609306. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen TS, Höjer A, Gustavsson AM, Hansen-Møller J, Purup S. Proliferative effect of whey from cows' milk varying in phyto-oestrogens in human breast and prostate cancer cells. J Dairy Res. 2012;79:143–149. doi: 10.1017/S0022029911000902. [DOI] [PubMed] [Google Scholar]
- 18.Jenness R. Comparative aspects of milk proteins. J Dairy Res. 1979;46:197–210. doi: 10.1017/s0022029900017040. [DOI] [PubMed] [Google Scholar]
- 19.Swaisgood HE. Review and update of casein chemistry. J Dairy Sci. 1993;76:3054–3061. doi: 10.3168/jds.S0022-0302(93)77645-6. [DOI] [PubMed] [Google Scholar]
- 20.McIntosh GH, Regester GO, Le Leu RK, Royle PJ, Smithers GW. Dairy proteins protect against dimethylhydrazine-induced intestinal cancers in rats. J Nutr. 1995;125:809–816. doi: 10.1093/jn/125.4.809. [DOI] [PubMed] [Google Scholar]
- 21.Papenburg R, Bounous G, Fleiszer D, Gold P. Dietary milk proteins inhibit the development of dimethylhydrazine-induced malignancy. Tumour Biol. 1990;11:129–136. doi: 10.1159/000217647. [DOI] [PubMed] [Google Scholar]
- 22.Bonuccelli G, Castello-Cros R, Capozza F, Martinez-Outschoorn UE, Lin Z, Tsirigos A, et al. The milk protein α-casein functions as a tumor suppressor via activation of STAT1 signaling, effectively preventing breast cancer tumor growth and metastasis. Cell Cycle. 2012;11:3972–3982. doi: 10.4161/cc.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pettersson A, Kasperzyk JL, Kenfield SA, Richman EL, Chan JM, Willett WC, et al. Milk and dairy consumption among men with prostate cancer and risk of metastases and prostate cancer death. Cancer Epidemiol Biomarkers Prev. 2012;21:428–436. doi: 10.1158/1055-9965.EPI-11-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolb AF, Huber RC, Lillico SG, Carlisle A, Robinson CJ, Neil C, et al. Milk lacking α-casein leads to permanent reduction in body size in mice. PLoS One. 2011;6:e21775. doi: 10.1371/journal.pone.0021775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen KC, Peng CC, Peng RY, Su CH, Chiang HS, Yan JH, et al. Unique formosan mushroom Antrodia camphorata differentially inhibits androgen-responsive LNCaP and -independent PC-3 prostate cancer cells. Nutr Cancer. 2007;57:111–121. doi: 10.1080/01635580701268360. [DOI] [PubMed] [Google Scholar]
- 26.Dozmorov MG, Hurst RE, Culkin DJ, Kropp BP, Frank MB, Osban J, et al. Unique patterns of molecular profiling between human prostate cancer LNCaP and PC-3 cells. Prostate. 2009;69:1077–1090. doi: 10.1002/pros.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corpet DE, Chatelin-Pirot V. Cooked casein promotes colon cancer in rats, may be because of mucosal abrasion. Cancer Lett. 1997;114:89–90. doi: 10.1016/s0304-3835(97)04631-4. [DOI] [PubMed] [Google Scholar]
- 28.Phelan M, Aherne-Bruce A, O'Sullivan D, FitzGerald RJ, O'Brien NM. Potential bioactive effects of casein hydrolysates on human cultured cells. Int Dairy J. 2009;19:279–285. [Google Scholar]
- 29.Perego S, Cosentino S, Fiorilli A, Tettamanti G, Ferraretto A. Casein phosphopeptides modulate proliferation and apoptosis in HT-29 cell line through their interaction with voltage-operated L-type calcium channels. J Nutr Biochem. 2012;23:808–816. doi: 10.1016/j.jnutbio.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Kimura T, Murakawa Y, Ohno M, Ohtani S, Higaki K. Gastrointestinal absorption of recombinant human insulin-like growth factor-I in rats. J Pharmacol Exp Ther. 1997;283:611–618. [PubMed] [Google Scholar]