Abstract
There exists a reciprocal relationship between the hypothalamic-pituitary-adrenal (HPA) and the hypothalamic-pituitary-gonadal (HPG) axes wherein the activation of one affects the function of the other and vice versa. For instance, both testosterone and oestrogen modulate the response of the HPA axis, while activation of the stress axis, especially activation that is repeating or chronic, has an inhibitory effect upon oestrogen and testosterone secretion. Alterations in maternal care can produce significant effects on both HPG and HPA physiology and behaviour in the offspring at adulthood. For example, changes in reproductive behaviour induced by altered maternal care may alter the expression of sex hormone receptors like ERα that govern sexual behaviour, and may be particularly important in determining the sexual strategies utilized by females. Stress in adulthood continues to mediate HPG activity in females through activation of a sympathetic neural pathway originating in the hypothalamus and releasing norepinephrine (NE) into the ovary, which produces a non-cyclic anovulatory ovary that develops cysts. In the opposite direction, sex differences and sex steroid hormones regulate the HPA axis. For example, although serotonin (5-HT) has a stimulatory effect on the HPA axis in humans and rodents that is mediated by the 5-HT1A receptor, only male rodents respond to 5-HT1A antagonism to show increased corticosterone responses to stress. Furthermore, oestrogen appears to decrease 5-HT1A receptor function at presynaptic sites, yet increase 5-HT1A receptor expression at postsynaptic sites. These mechanisms could explain heightened stress HPA axis responses in females compared to males. Studies on female rhesus macaques show that chronic stress in socially subordinate female monkeys produces a distinct behavioral phenotype that is largely unaffected by oestrogen, a hypo-responsive HPA axis that is hypersensitive to the modulating effects of oestrogen, and changes in 5-HT1A receptor binding in the hippocampus and hypothalamus of social subordinate female monkeys that are restored or inverted by oestrogen replacement. This review summarizes all of the abovementioned studies, which underscore the profound effect that the interaction of the reproductive and stress axes may have on human reproductive health and emotional wellbeing.
Keywords: reproduction, stress, maternal care, estrogen, serotonin, ovary
1. Introduction
The purpose of the following review is to examine results from four laboratories in both North and South America that are studying the interaction of the stress and reproductive axes on several levels.
It has been shown that the adult HPA axis reactivity can be altered early in life by differences in maternal care. In laboratory rats, the neuroendocrine and behavioural effects of postnatal environmental manipulations of the infant-mother relationship have been studied experimentally for more than 50 years. Among these, the most frequently applied postnatal manipulations are Neonatal Handling (NH), which consists of brief periods of daily separation of mothers and offspring (usually less than 15 min) taking place any time before weaning, and Maternal Separation (MS) which includes repeated removal of either pups or mother from the nest for periods ranging from 3 to 8 h per day during the first two postnatal weeks (1–3). While the effects of early-life manipulations on the HPA axis have been extensively characterised, few investigators have examined reproductive markers in rats following the MS stress paradigm, and there is evidence that NH can alter reproductive behaviour in various ways. Thus in many instances, early maternal care can set the stage for the interaction of the HPG and HPA axes in adult life.
There is, in fact, ample evidence that gonadal steroids, the end product of the HPG axis, actively modulate the function of the HPA axis in adults. Studies on female rats have found higher adrenocorticotropin (ACTH) levels subsequent to acute stress at proestrus or following treatment with proestrus levels of estrogen, and longer lasting post-stress elevations of corticosterone in female rats treated with estradiol or estradiol and progesterone (4). E2 has been shown to increase ACTH secretion in female baboons (5) and increase ACTH and cortisol by decreasing glucocorticoid negative feedback in female monkeys (6), and in women exercise stress enhances ACTH and AVP only in the mid-luteal stage when ovarian hormones are rising (7). In male rats testosterone decreases glucocorticoid and adrenocorticotrophin responses to stress (8, 9). Furthermore, gonadectomy increases both corticosterone and ACTH in male rats and this can be normalized by replacement with testosterone or dihydro-testosterone (10). These studies suggest that gonadal steroids modulate the HPA axis in both sexes.
In contrast, activation of the stress axis, especially activation that is repeating or chronic, has an inhibitory effect upon gonadal hormone secretion. For example, stress and stress hormones inhibit the release of gonadotropin releasing hormone from the hypothalamus, and glucocorticoids inhibit the release of luteinizing hormone from the pituitary and E2 and progesterone secretion by the ovary (11, 12) as well as testosterone from the testes (12, 13). One way that stress acts to mediate HPG activity in females is through activation of a sympathetic neural pathway originating in the hypothalamus and releasing norepinephrine into the ovary (14, 15). The deleterious effect that this sympathetic pathway can have on the ovary is likely a main contributor to the effect of stress on the HPG axis.
Data garnered from these substantially different experimental paradigms emphasize that the interaction of the reproductive and stress axes has far-reaching implications for human health.
2. Maternal separation stress and reproductive function: Effects on male and female rats
Given that a substantial amount of brain development occurs after birth, it is consequently subject to environmental influences, which may negatively or positively affect brain maturation. Even natural variations in the quality or quantity of maternal care can have a long-term impact on offspring brain and behaviour. Human epidemiological and animal experimental studies show that early social experiences influence the functioning of physiological processes even into adulthood (3, 16,22).
In both genders, rat sexual behaviour can be divided into two components, appetitive and consummatory (23). In females, appetitive behaviours, also named proceptive behaviours, consist in anogenital investigation, solicitations, hops and darts, and ear wiggling, while males display anogenital investigation, chase the females and attempt to mount them. The consummatory/receptive phase in females consists in the expression of the lordotic posture which allows the male to mount, perform several intromissions and ejaculate, the three main copulatory behaviours shown by males (24). Although results are not consistent across the literature, MS induces sexually dimorphic outcomes. While reproductive physiology is not significantly affected in females, an MS protocol has been described as producing significant effects on male reproductive physiology such as longer mount latencies, longer intromission latencies and a reduction in the percentage of animal ejaculating, but it does not affect female reproductive function (25). On the other hand, Greisen et al. found that MS led to a male phenotype with heightened sexual performance, reflected in decreased mount latency, decreased intromission latency, decreased post-ejaculatory interval, while mating behaviour was not affected in females (26). The discrepancies observed between these two studies may be explained because they employed different MS protocols and different control groups. However, although results may differ depending upon the experimental conditions, MS is a good animal model of early life stress that has been extensively used over the past decades. Further studies are still needed to elucidate the impact of early life stress on later life.
Interestingly, studies employing NH protocols, on the other hand, have found reduced sexual behaviour in males and females, reduced sexual receptivity, reduced lordosis quotient (LQ), increased frequency of anovulatory estrous cycles, and an altered hormonal profile of several hormones related to ovulation and sexual behaviour (27–29). This effect relates nicely with the effects of natural variations in maternal care, as the effects of early handling have been ascribed, at least in part, to the enhanced maternal care the pups receive upon their return to the dam. Upon the return of the mother NH increases maternal licking and grooming (LG) of the pups.
Findings suggest that the quality of parental care received during the early postnatal period programs the HPG axis in rats, subsequently influencing adult sexual behaviour, especially in female rats, in which offspring of high LG showed reduced LQ, higher percentages of mounts without intromission (reflecting a decreased quality of lordosis), received fewer ejaculations and were less likely to achieve pregnancy (30, 31). Also in the brain areas involved in the control of the hypothalamic-pituitary-gonadal axis and sexual behaviour (the VMH and AVPv), high LG female offspring show less oestrogen receptor-α (ERα) expression which correlates with the reproductive strategy displayed by these animals (32).
It is proposed that maternal care induces internal modifications that can “program” reproductive strategies in the female rat. Such neuroendocrine programming biases towards increased fecundity (i.e., the offspring of Low LG mothers) or increased investment in the offspring (the offspring of High LG mothers), adapting female offspring to respond to subtle variations in parental care in order to adapt to the everyday environmental condition they will face. Under high-risk environmental conditions, when the probability of survival is low, the optimal strategy is to maximise the number of offspring through accelerated mating. In contrast, a more propitious environmental favours greater investment in individual offspring at the cost of mating (31, 33).
In conclusion, early life experience affects adult sexual behaviour. Unfortunately, parental influences on progeny are, to date, not entirely understood. However, as researchers steadily gather more information about this system, it is becoming clear that, as in the rat, human parental programming of the reproductive system is likely to involve gene-environment interactions.
3. Sympathetic stress and ovarian function
3.1 Sympathetic nerves affect ovarian function
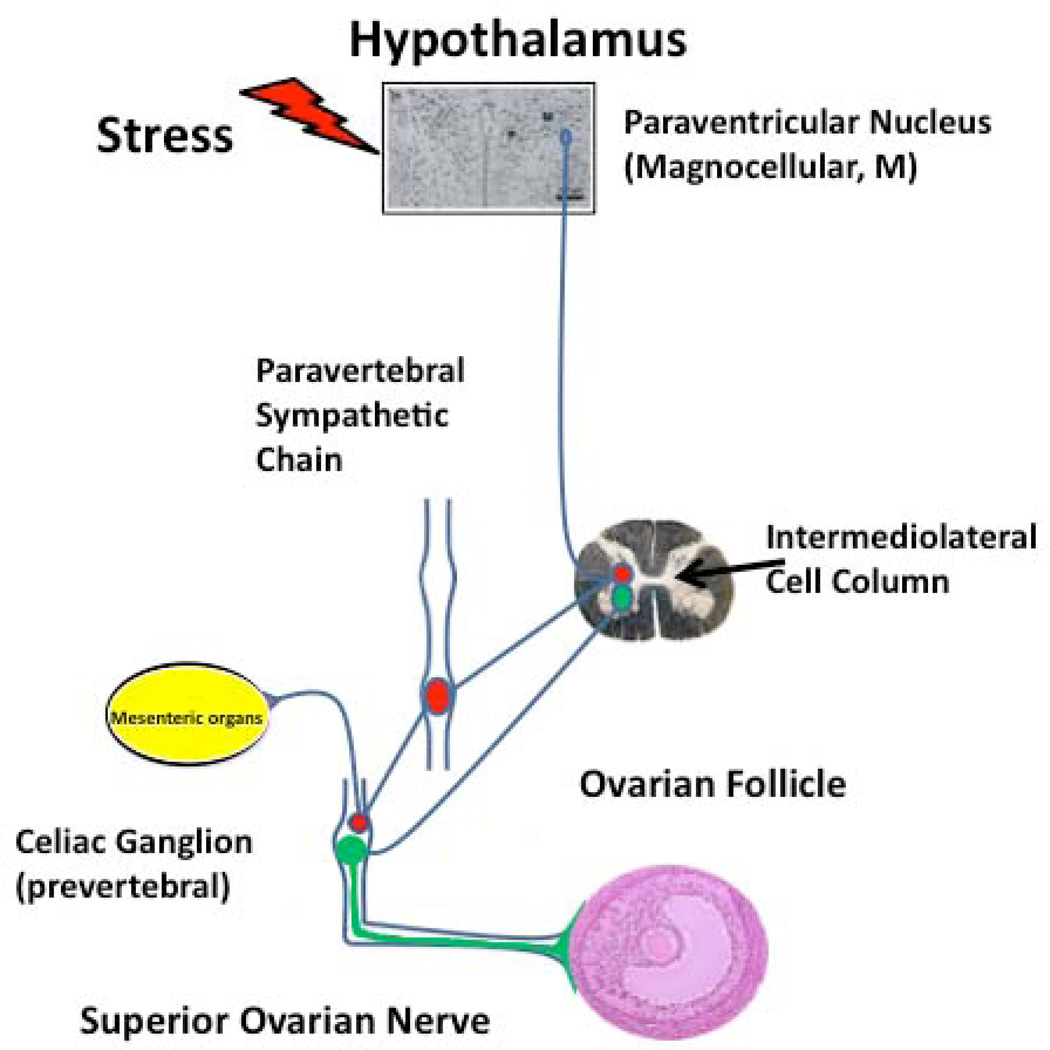
Sympathetic nerves arrive at the ovary originate from two sources (34, 35); (a) the ovarian plexus nerve, which travels along the ovarian artery, and (b) the superior ovarian nerve, which is associated with the suspensory ligament. Superior ovarian nerve fibers innervate the secretory components of the ovary, i.e., interstitial glands and follicles (36). A detailed tracing study by Gerendai et al. (37) demonstrated that the sympathetic pathway to the ovary originates in the paraventricular (PVN) region of the hypothalamus, results that have been confirmed by functional studies (37–39), leading us to propose the following neuroanatomical organization (Figure 1).
Figure 1.
Sympathetic nerve control of the ovary. Retrovirus tracing mapped the anatomical nerve connection between the brain and the ovary (37). Functional studies either changing the activity of neurons of the paraventricular nucleus or pharmacological blocking of the stress-activated sympathetic nerve pathway (39, 44, 171), has permitted to verify the relevance of the sympathetic innervation at the ovary function.
In this diagram we propose that stimulation originating from the paraventricular area of the hypothalamus travels by a multisynaptic pathway arriving at the celiac ganglion that then projects to the ovary by postganglionic sympathetic fibers where it regulates steroidogenesis and early follicular development (15). It has also been demonstrated that norepinephrine (NE) facilitates follicular development, as seen by the inhibition of follicular growth following the ovarian denervation (40). Chronic changes (either decreases or increases) in the sympathetic input to the ovary can cause profound changes in ovarian function.
3.2 The sympathetic nerve participates in the development of the polycystic ovary
Polycystic ovary (PCO) syndrome, the most common cause of infertility in women during their reproductive years, is a complex disease characterized by anovulatory failure and the presence of ovarian cysts, amenorrhea, hyperandrogenemia, and variable levels of circulating gonadotropins (41). Because sympathetic nerves stimulate androgen secretion from the ovary, the possibility exists that a hyperactivation of sympathetic nerves could participate in the development and maintenance of ovarian cysts in the rat. In accordance with this hypothesis, sympathetic nerve activation induced by estradiol valerate administration to rats is causally related with both the development and maintenance of PCO and surgical ablation of the sympathetic nerves at the level of the supra optic nucleus of the hypothalamus results in the reversal of the anovulatory PCO and diminishes ovarian androgen secretion (42, 43). In addition, it has been shown that the hyperandrogenic condition is causally related with enhanced ovarian steroidal responsiveness to β-adrenoceptor stimulation, a condition also prevented surgical elimination of SON projections to the ovary (42, 43). This recovery of the ovulation was confirmed by the presence of corpus luteum in the denervated ovary and by the recovery of the oestrous cycling in rats.
Polycystic ovary syndrome (PCOS) is also characterized by metabolic abnormalities that are consistent with the metabolic syndrome. Enhanced sympathetic and adrenal medullar activities are important links between defects in insulin action and the development of hypertension. Despite extensive research seeking the pathogenesis of PCOS, there is still disagreement on the underlying mechanisms. The potential contribution of the sympathetic nervous system to the syndrome has been suggested in several studies, especially because of the role of NE to enhance androgens and progesterone secretion from the mammalian ovary (44, 45). Some believe that androgen excess early in life may provide a hormonal “insult” that results in manifestation of PCOS in adulthood (46), especially because PCOS is highly associated with conditions in which the fetus was exposed to high amounts of sex steroids during pregnancy. We have data demonstrating that mothers with PCOS maintain their hyperandrogenic condition during pregnancy, although their HPA axis has been suppressed (47). Hence, if chronically increased androgens reach the placental tissue in which the fetus is developing, the internal milieu can “program” its reproductive axis to be disturbed at the onset of puberty and adulthood. Therefore, one possibility to consider is that increased superior ovarian nerve input may contribute toward the etiology of PCOS through a stimulatory action on androgen secretion. This would explain the effectiveness of ovarian wedge resection or laparoscopic laser cauterization to increase ovulatory response in women with PCOS as procedures are likely to disrupt superior ovarian innervation.
3.3 Sympathetic stress and β-Adrenergic system spur the development of the polycystic ovary
The fact that the ovary communicates with the hypothalamus through a multisynaptic pathway implies that a centrally-originated stimulus could affect the ovary function independent from the well-known ovarian control mediated by gonadotropins. It has been demonstrated that cold stress, either acutely or chronically, selectively activates the sympathetic nerves without altering the ACTH response. Cold stress has been described as stressor that activates the sympathetic nervous system and alters ovarian function (44). When the cold stress procedure is chronic enough to affect a group of ovarian follicles (more than 4 weeks), it modifies follicular development by accelerating the transition from antral follicles to a group of preovulatory follicles that are not able to be released at ovulation, and therefore, moves follicles towards a precystic appearance in which there was a hypertrophied theca cells compartment in parallel with an increase in ovarian NE concentration (44).
The stress response is a multifactorial event that involves orchestrated neuroendocrine responses required to maintain homeostasis, but when stress becomes chronic it may induce pathology. To focus in the sympathetic nerve activity as one of the multiple factors involved in the chronic stress response we have recently applied a method to directly stimulate β-adrenoceptors by in vivo administration of the β-adrenoceptor agonist isoproterenol (48). We administered isoproterenol (125 µg/kg/d) for 10 d, to study the changes induced by β-adrenoceptor overstimulation in ovarian follicular development. Thirty days after isoproterenol withdrawal, there was a clear increase in the number of follicular cysts. The direct relation between the β- adrenergic receptor activation and follicular cyst development was demonstrated by the capacity of propranolol (a β-adrenergic antagonist) to reverse both the isoproterenol-induced hyperandrogenic condition and the ovarian cyst formation (48).
We can conclude that the neural axis originating at the hypothalamic paraventricular nucleus, controls ovary function and changes in the activity of this neural network regulate ovulation. Therefore stress, if chronic, could be harmful to reproduction. Experimental procedures aimed to attenuate the sympathetic activity could be a method to treat women with PCOS.
4. Afferent Mediators of Gonadal Status on the Paraventricular Nucleus of the Hypothalamus
4.1 There are sex differences in HPA axis function
The hypothalamic-pituitary-adrenal (HPA) axis involves the sequential release of a chain of hormones from the brain to the periphery, ultimately regulating the release of glucocorticoid steroids from the adrenal gland. Acute elevations in circulating glucocorticoids are adaptive, as they provide sources of energy to meet the metabolic demands of homeostatic threat. On the other hand, chronic elevations in glucocorticoids are pathological and linked to several types of disorders, including anxiety and depression. Thus, the HPA axis must be both tightly regulated and equally responsive to the demands of stress (49). Our research focuses on sex differences and sex steroid hormone regulation of the paraventricular nucleus of the hypothalamus (PVH), the final common pathway regulating adaptive neuroendocrine responses. The hypophysiotropic zone of the PVH houses corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) expressing neurons that synergize on the synthesis and release of adrenocorticotropin (ACTH) from the anterior pituitary, which then stimulates the release of glucocorticoids from the adrenals (cortisol in humans, corticosterone [CORT] in rodents).
Rodent studies have shown that females secrete higher levels of CRH than males, and higher levels of CORT in response to various challenges (50–52). The gonadal hormones are at least partly responsible for these sex differences in the rat, as androgen administration decreases ACTH and CORT secretion, whereas estrogens increase these measures (4, 53). In humans without psychiatric illness, the sex difference in stress HPA axis function is not so apparent on the surface. Thus, men often show similar, if not higher levels of cortisol than women in response to various acute challenges (54, 55). However, this does not discount an underlying influence for androgens and estrogens to regulate the HPA axis in humans, as manipulations of gonadal status in women and men often provoke changes in CRH and cortisol release similar to the results in rodents (56–60). Several disorders associated with chronic stress are more prevalent in women than in men, including depression and such anxiety-related disorders as posttraumatic stress disorder (61–63). Depression is frequently associated with abnormalities of the HPA axis, including hypercortisolemia (64), and cortisol levels have been reported to be higher in depressed women compared to men (65). Large variations in individual cortisol release patterns feature prominently in humans exposed to acute and repeated challenges (66), and the biological determinants for this variation are not understood. Thus, the neurobiological basis for the gender disparity in stress-related disorders remains unresolved, and as argued elsewhere, extensive phenotyping of HPA axis function remains essential (58, 67).
4.2 Serotonin modulates the HPA axis
Several lines of evidence support a stimulatory influence of serotonin (5-hydroxytryptamine; 5-HT) on the HPA axis in humans and rodents (68, 69), mediated, in part, by the 5-HT 1A receptor subtype (70–72). Sexual dimorphisms in HPA axis function and in the 5-HT system provide evidence to suggest that the brain 5-HT system has a higher potential for stimulating the HPA axis in females. Thus, females express higher levels of 5-HT and/or metabolites than males in brainstem, limbic forebrain and cortex under basal conditions (73, 74), and in response to various challenges (75–77).
Reported sex differences in 5-HT 1A receptor binding and/or expression have not been consistent (78, 79). However, estrogen has been shown to desensitize 5-HT 1A receptor coupling at both pre- and postsynaptic sites in unstressed animals. Presynaptic 5-HT 1A (somatodendritic) receptors diminish neuronal excitability of raphe neurons to reduce serotonin synthesis and release, whereas postsynaptic 5-HT 1A (heteroreceptors) receptors mediate signal transfer to non-serotonergic, forebrain neurons (80, 81). Taken together, the stimulatory effect of the 5-HT system on the HPA axis could reflect the net of 5-HT 1A receptor’s inhibitory and stimulatory influences on the PVH and it’s extended circuitries.
4.3 Sex differences modulate stress and 5-HT 1A receptor interactions
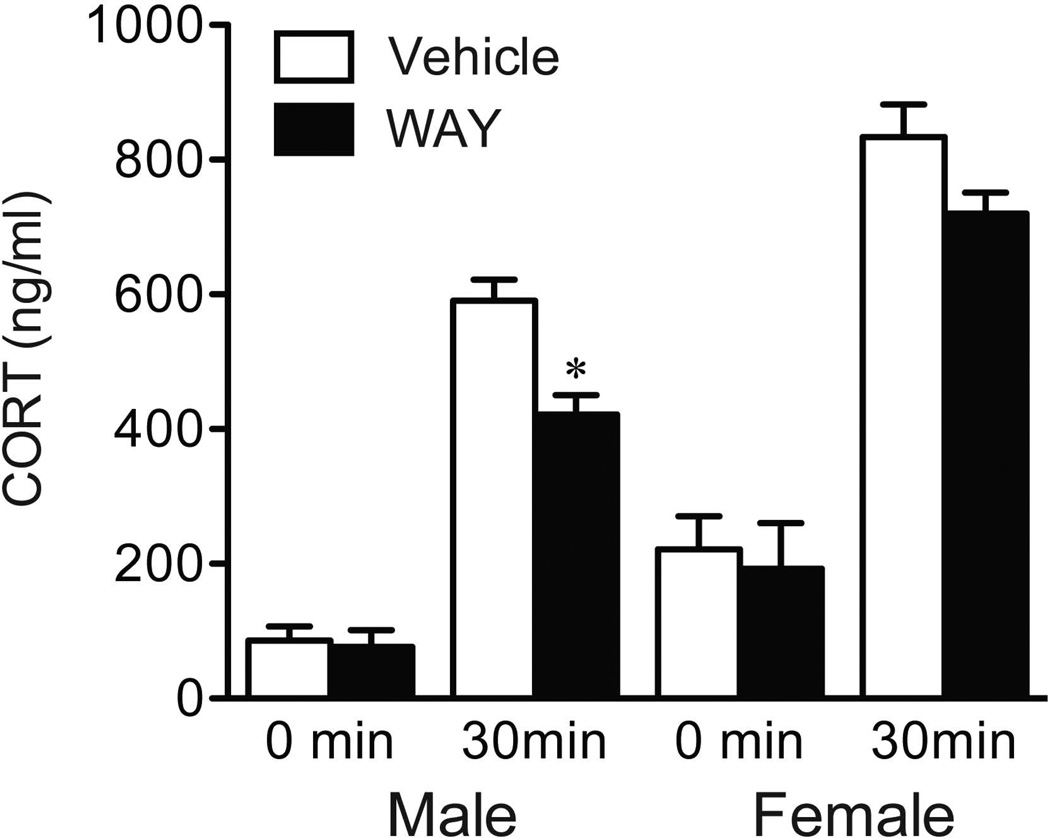
In humans and rodents, females show higher neuroendocrine responses to a systemic injection of the 5-HT 1A receptor agonist, 8-OH DPAT. We suspect that the endogenous requirements for 5-HT 1A receptors to regulate the HPA axis may also be sexually dimorphic under stressful conditions. Previous studies in the male rodent have shown that 8-OH-DPAT decreases the number of raphe neurons recruited to express Fos protein in responses to immobilization, whereas the 5-HT 1A receptor antagonist, WAY 100635, counteracts this effect (82). Building on the utility of this antagonist to unmask how 5-HT 1A receptors participate in HPA axis control circuitry, we recently examined neuroendocrine and Fos responses in male and female rats bearing systemic injections of vehicle or WAY 30 min in advance of restraint exposure (83). In line with a stimulatory role for the 5-HT 1A receptor on the HPA axis, WAY administration decreased the CORT response to restraint in males, but not in females (Figure 2). This sex difference in HPA output was not recapitulated at the level of the PVH, where males and females showed similar decrements in Fos protein induction in response to WAY. This result warrants further exploration on connectional and phenotypic grounds, given the heterogeneity of cell types localized to the hypophysiotropic zone of the PVH.
Figure 2.
Mean ± SEM relative numbers of double-labeled (Fos + TPH) neurons within the dorsal subdivision of dorsal raphe nucleus (DRD) in males and females (B). Scatterplot showing a significant negative correlation between plasma estradiol concentrations and Fos + TPH-labeled cells in the DRD of WAY females (C). **P < 0.01 vs. vehicle counterpart (n = 7–8 per group). Adapted with permission from Goel et al (83).
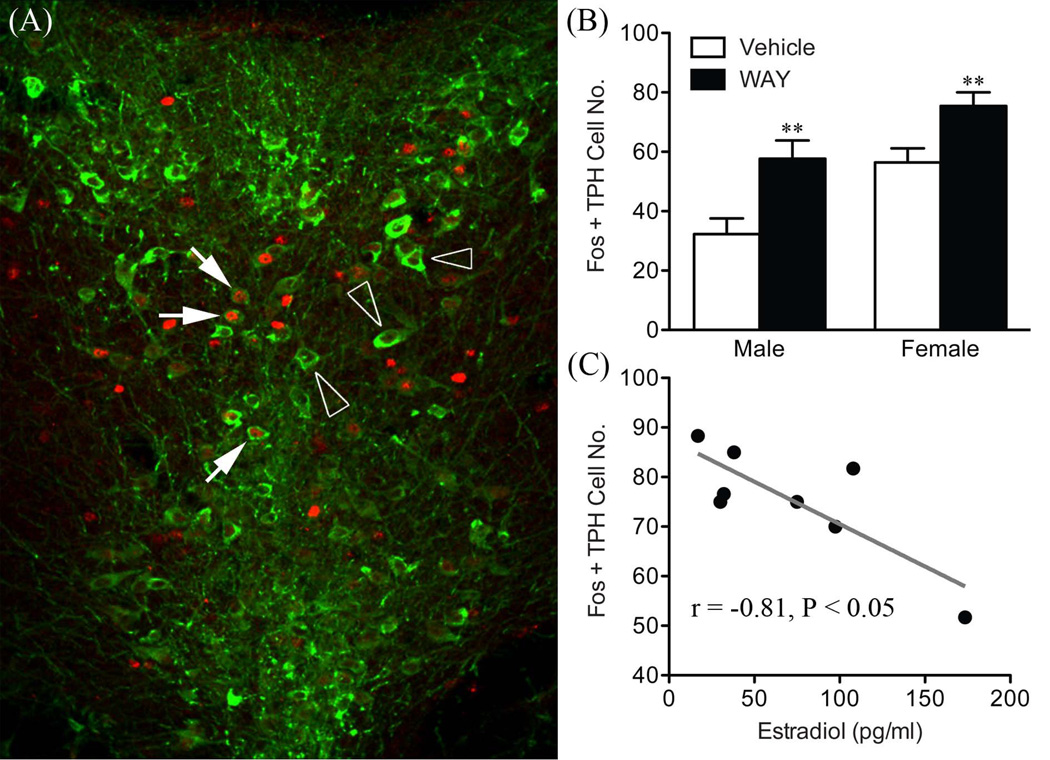
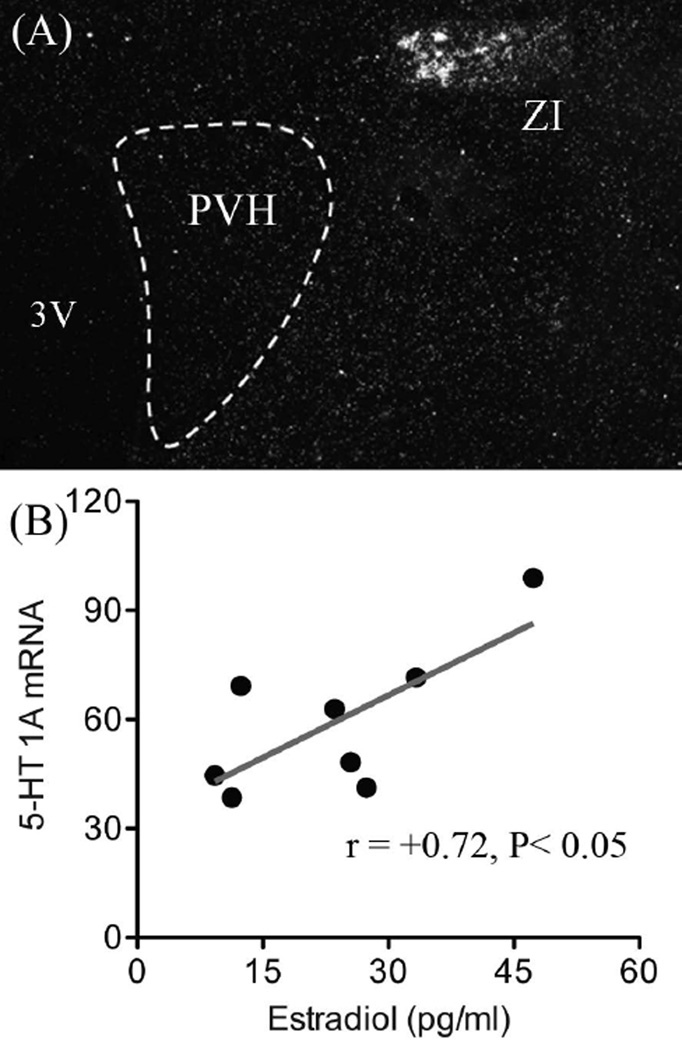
In contrast to the PVH, WAY administration had the opposite effect to potentiate the stress-induced activation of dorsal raphe nuclei identified as serotonergic (tryptophan hydroxylase expressing), in both males and females (Figure 3). However, a negative correlation between estrogen and Fos responses was identified in WAY treated females, to underscore a role for estrogen to decrease 5-HT 1A autoreceptor function. This could provide mechanisms for increasing 5-HT release in projecting structures and heightened HPA axis responses in females. Analysis of the relative levels of 5-HT 1A mRNA revealed no sex differences in the size or distribution of the transcript within various forebrain nuclei or the dorsal raphe nucleus. However, a positive relationship was found between estrogen and 5-HT 1A mRNA expression in females that was unique to the area of the zona incerta (Figure 4). Based on previous connectivity experiments, the zona incerta represents a key relay for 5-HT raphe projections to the hypophysiotropic zone of the PVH, as wells as for several limbic related structures (84–86). Thus, the organization of zona incerta projections implies that this region may be in a position to integrate neocortical and emotionally relevant information to changes in estrogen, as well as to coordinate central 5-HT and neuroendocrine responses.
Figure 3.
Dual fluorescence confocal image to show overlap in nuclear Fos-ir (red) and cytoplasmic tryptophan hydroxylase (TPH; green) staining within the dorsal raphe nucleus (A). Solid arrows show doubly labeled neurons and open arrowheads mark Fos-positive, TPH-negative cells. Mean ± SEM relative numbers of double-labeled (Fos + TPH) neurons within the dorsal subdivision of the dorsal raphe nucleus in males and females (B). Scatterplot showing a significant negative correlation between plasma estradiol concentrations and Fos + TPH-labeled cells in the DRD of WAY females (C). **P < 0.01 vs. vehicle counterpart (n = 7–8 per group). Adapted with permission from Goel et al (83).
Figure 4.
Dark-field photomicrograph to show the distribution of 5-HT 1A receptor mRNA expression in the vicinity of the paraventricular nucleus of the hypothalamus (PVH) (A). Dashed line defines the nuclear border of the PVH to emphasize the absence of the transcript relative to the distinct cluster of 5-HT 1A receptor expressing cells within the zona incerta (ZI). Scatterplot (B) showing a significant positive correlation between plasma estradiol concentrations and 5-HT 1A mRNA levels in the ZI of females. 3V: 3rd ventricle. Adapted with permission from Goel et al (83).
The results underscore important sex differences in 5-HT 1A receptor regulation of the acute HPA axis response at both pre- and postsynaptic sites. The nature by which functional changes in 5-HT 1A receptors underlie a sex difference in HPA axis responses to chronic or repeated forms of stress remains to be seen. The 5-HT 1A receptor not only drives the stimulatory effect of serotonin on the HPA axis, but is also a critical determinant of the antidepressant response (87). Thus, our current findings provide several new starting points for understanding the connectivity of 5-HT 1A sensitive projections to the HPA axis and how these may contribute to the sex disparity in affective disease.
5. Social subordination disrupts estrogen’s effects on behavior and physiology in female Rhesus monkeys
5.1 Social stress modulates estrogen's effects in female Rhesus macaques
As emphasized above, rodents represent an appropriate model for studying interactions between stress and short-term changes in reproductive function. Of note, the human reproductive cycle is radically different to that of the female rat (88). Indeed, the magnitude and duration of endogenous estrogen exposure, and consequently the reactivity of brain systems responding to estrogen, may not be entirely the same between female humans and rats. Similar to women, however, female rhesus monkeys display changes in ovarian hormones over a comparable 28-day cycle during the breeding season (89–92). Thus, the female rhesus monkey is perhaps more suitable for modeling psychopathologies in women attributed to major changes in ovarian hormone secretion (93–102).
Although there is utility in studying the effects of chronic psychogenic stress in the rodent, this can never approach the inherent complexities of psychosocial stress experienced by humans. By comparison, female macaques naturally form social hierarchies, in which subordinate (SUB) females are constantly harassed both physically and psychologically by their dominant (DOM) counterparts (103). This social organization provides an advantageous and translatable model for characterizing the effects of psychosocial stress on a multitude of physiological and psychological endpoints. Thus, chronic psychogenic stress exposure in SUB female macaques (104, 105) induces a number of phenotypes (106–112) that are similar to patients suffering from mood, metabolic and immune disorders (113–121). Moreover, female macaques also display remarkable similarities with women in other physiological domains, including central nervous system mediators of neuroendocrine and emotional responses to stress (122–127).
In a series of experiments completed over the last several years, Donna Toufexis and her colleagues at Emory University; Mark E. Wilson, Kim Wallen, and Mar Sanchez, along with Emory University graduate students; Vasiliki Michopoulos and Katherine Reding, have utilized this animal model to examine the effects of chronic psychosocial stress on the physiology and behavior of ovariectomized (OVX) SUB female monkeys, and to determine how these are modulated by the replacement of the major ovarian hormone 17β-estradiol (E2). To control for previous life-experiences and any possible genetic propensity that may predispose a female towards a particular social rank, we selected middle-ranking, unrelated adult females from large social groups to form 10 new groups of five females and one male. Females were randomly selected and sequentially added to the new group following which the dominance hierarchy quickly emerged (128). These small social groups functioned to exacerbate the social subordination stress that is usually dispersed throughout the normally large social groups favored by this species. In addition, since it has been shown that short promoter polymorphism of the serotonin transporter gene (SERT) interacts with stress to increase the occurrence of affective disorders in people (129–132) and also increases both behavioral and HPA reactivity in rhesus monkeys (128, 133–136), we evaluated the effect of the SERT polymorphism in our female monkey studies and reported these findings in experiments in which there was a statistically significant effect.
Results form our studies demonstrate that social subordination has profound effects on many aspects of behavior and physiology, of which some are enhanced, blunted or unaffected by E2 replacement (see(107, 108, 111, 112, 137–145) for some published results from these studies). In this mini-review we will elaborate on three of these findings.
5.2 Social subordination results in increased anxiety behavior and a disruption of socio-sexual behavior, which are not consistently modulated by E2
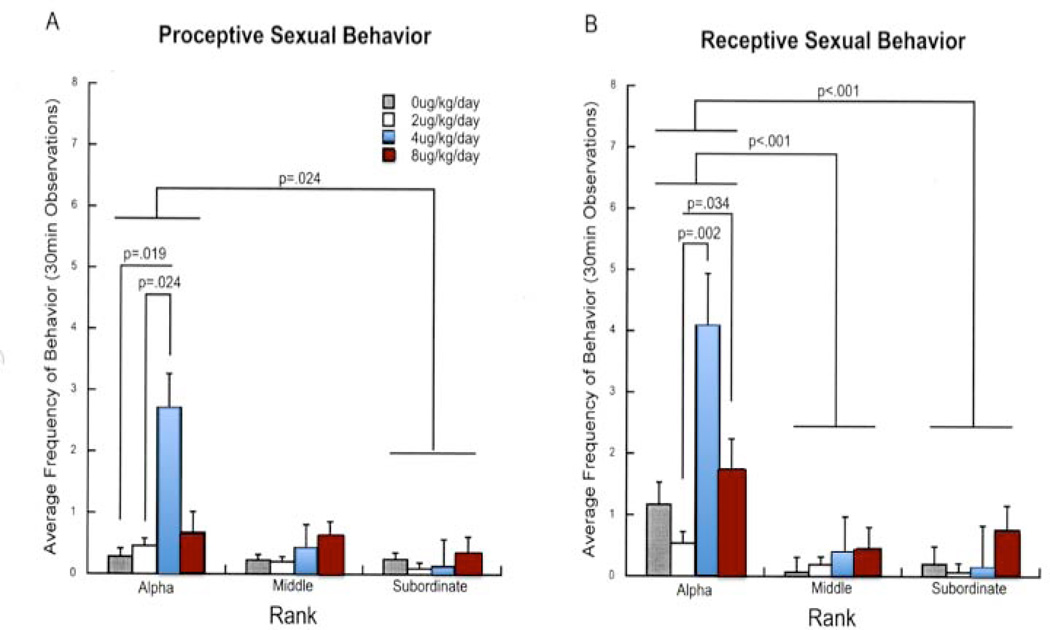
It had previously been shown that social subordination in female macaque monkeys increases depressive- (110, 146) and anxiety-like behaviors (116). In order to evaluate whether the well-established anxiolytic effects of E2 in rodents (147–155) were significantly affected by social status as well as by SERT polymorphism in female monkeys, a study led by Vasiliki Michopoulos (156) evaluated the effects of E2 on behavior in females prior to the addition of males to the group. The data showed that E2 reduced rates of anxiety in DOM females with the short promoter length SERT variant and SUB females with the long SERT variant. DOM females with the long SERT genotype already showed the lowest levels of anxiety behavior. In contrast, SUB females short SERT variant which showed high levels of anxiety like behavior were unaffected by E2. Thus, E2’s ability to attenuate anxiety is affected by both social subordination and SERT genotype in female macaques, as E2 is ineffective in modulating the high anxiety rates in SUB monkeys with the short SERT genotype. To determine the interaction between psychosocial stress and E2 on socio-emotional behaviors when males were present, a study lead by Katherine Reding (140) evaluated the effect of social status on reproduction, affiliation, aggression, submission, and anxiety-like behaviors in these small groups. Data (Figure 5) showed that E2 dose-dependently increased sexual motivation in DOM females, but was without effect in SUB females at any dose. E2 replacement also increased male affiliation behavior in DOM but not SUB females. Contact and non-contact aggression were also attenuated in DOM females. Overall, these results suggest that chronic social subordination stress attenuates E2's anxiolytic effects and reduces E2’s activational effects on sexual behavior and affiliation with males, and that these latter effects cannot be overcome in SUB monkeys even with higher doses of E2. Thus, the behavioral effects of E2 are significantly blunted by social subordination in female macaque monkeys.
Figure 5.
The interaction between social rank and E2 dose on sexual behavior toward males. Both (A) proceptive and (B) receptive behavior showed a main effect of social rank, a main effect of E2 dose, as well as an interaction effect between the two. Post-hoc analysis showed activational effects only in DOM Alpha females. Data are presented as average frequencies ± standard error. (140)
5.3 Social subordination results in altered hypothalamic-pituitary-adrenal (HPA) axis reactivity that is significantly modulated by E2
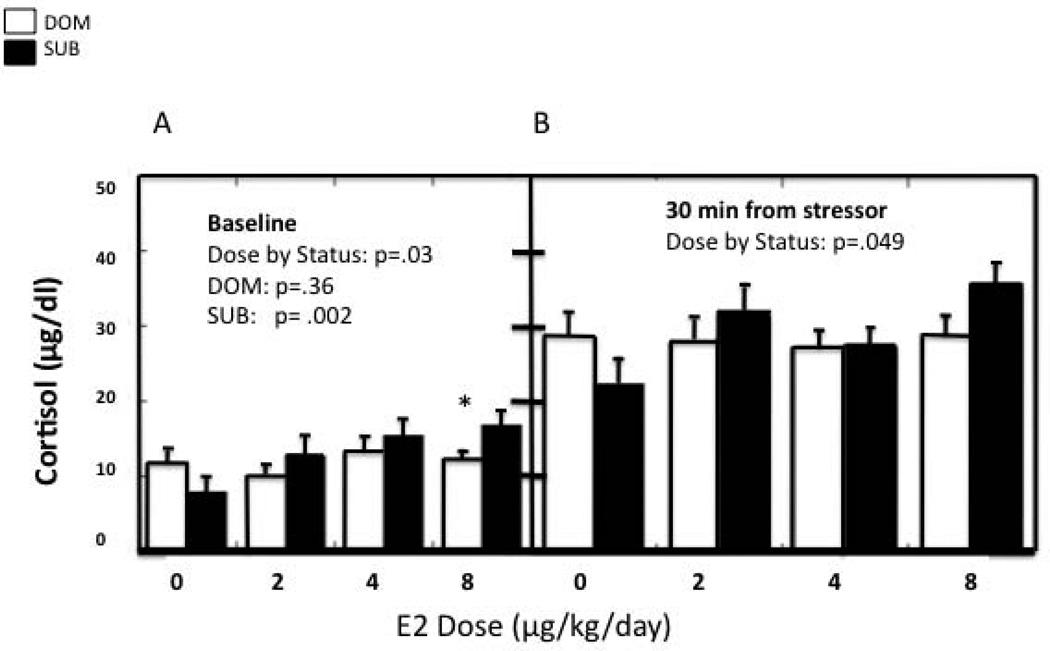
Although SUB female monkeys appear to suffer from many conditions that are related to chronic stress (146, 157–160), it has been difficult to establish differences in HPA axis activity due to social status. Previously the only consistent findings that indicate HPA dysregulation in SUB female monkeys are increased adrenal size (127, 161) and decreased glucocorticoid negative feedback following dexamethasone (Dex) injection (127, 128, 146, 162). It has been shown that sex steroids modulate adrenal morphology and function (163–165), and that E2 alters the diurnal release of cortisol (166) and glucocorticoid-induced negative feedback on the HPA axis (6), the use of naturally cycling female macaques in many previous studies (146, 157, 167) may have confounded some of these outcomes. Therefore, as with the studies described above, we first examined several features of HPA activity in OVX females and then determined the effect of E2-replacement on some of these endpoints. Our results showed that compared to OVX DOM females, OVX SUB females had flattened morning cortisol secretion, reduced dexamethasone-induced glucocorticoid negative feedback, and a decreased adrenal cortisol response to an ACTH challenge (168). These results indicate that the ability to initiate and curtail glucocorticoid release is significantly reduced in OVX SUB female monkeys. Interestingly, this suggests that SUB females have a hyporesponsive HPA phenotype resembling that observed in several human psychopathologies, including post-traumatic stress disorder. Because previous work by our group had shown that SUB females were hypersensitive to the effect of E2 on HPA activation (6), we next examined both basal and stress-induced cortisol levels in the same females during three different E2 replacement regimens. Results, depicted in Figure 6, showed that pre-stressor cortisol was dose-dependently increased by E2 in SUB but not DOM females. Furthermore, the increase in cortisol 30 min after the start of the stressor also showed a significant dose by status interaction, with non-replaced SUB females having a blunted increase compared to non-replaced DOM females and a greater increase than DOM females at the highest E2 dose. These data illustrate that DOM females exhibit a robust cortisol response irrespective of E2 dose while the CORT response of the SUB females is E2 dose-dependent. This suggests a reduced response to stress in SUB females lacking E2 and, as with the previous study, a hypersensitivity in E2-replaced SUB females. This hypersensitivity to E2 caused by chronic social stress may be very important when evaluating the stress response in women under chronic social stress who have experienced trauma or other adverse emotional events.
Figure 6.
Repeated measures analysis of variance was used to determine the effects of E2 dose and social status (dominant vs. subordinate) on cortisol levels at baseline and following stressor exposure. A. Basal cortisol is dose-dependently increased by E2 in SUB females only. B. E2-replacement increases plasma cortisol 30 minutes following an acute stressor in SUB females alone. Asterisk denotes a status difference in basal cortisol levels.
5.4 Social subordination results in differences in serotonin 1A (5-HT1A) receptor binding potential in brain regions implicated in emotional regulation and stress reactivity that is modified by E2 only in the hippocampus and hypothalamus
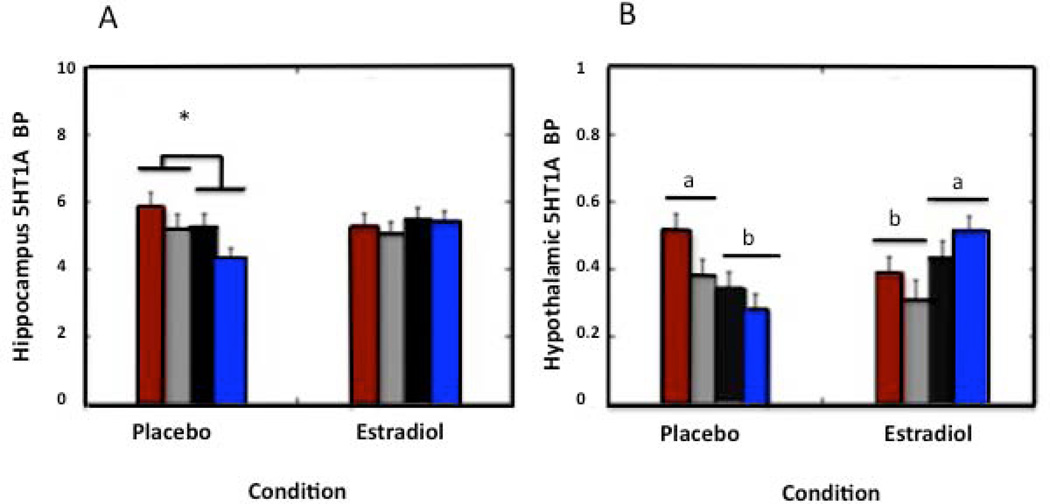
Since central reduction of the serotonin 5-HT1A receptor is associated with psychopathology in humans (169, 170), and has been related to behavioral depression in monkeys (110), we conducted a study to determine the effect of social status and SERT genotype on serotonin 5-HT1A receptor binding potential (5-HT1A BPND) in brain regions associated with emotional control and HPA activity in OVX female monkeys, and then assessed how these effects were modulated by E2 replacement. Positron emission tomography (PET) using a 5-HT1A receptor-specific ligand was performed to determine the levels of 1A receptor binding under a non-E2 condition and a 3 week E2 replacement condition in several brain regions including: anterior cingulate; medial prefrontal cortex; dorsolateral prefrontal cortex; orbitofrontal prefrontal cortex, amygdala, hippocampus, hypothalamus and raphe nucleus. Results show that female monkeys with the short SERT genotype have reduced 5-HT1A binding potential in the medial prefrontal cortex irrespective of social status, and that SUB females with the short SERT variant show a reduction in 5-HT1A binding potential within the anterior cingulate cortex (144). Moreover, 5-HT1A binding potential in these 2 regions was unaffected by E2 replacement. In contrast, as shown in Figure 7, hippocampal and hypothalamic 5-HT1A BPND was attenuated in subordinate females regardless of SERT genotype during the non-E2 condition, and this difference was normalized in the hippocampus and inverted in the hypothalamus with E2 (144). These data suggest that E2 can only alter central 5-HT1A BPND in brain regions that show no SERT genotype-linked control of -5HT1A binding.
Figure 7.
Mean ± SEM 5HT1A BPND during the placebo and E2-replacement for DOM, long SERT genotype Red bars, DOM, short SERT genotype(s-variant) Grey bars, SUB long SERT genotype Black bars, and SUB short SERT genotype (s-variant) females Blue bars. (A) Hippocampal 5HT1A BPND is attenuated in subordinate females during the placebo condition compared to dominant females (denoted by asterisk), an effect that is normalized upon E2 replacement. (B) Letters denote that hypothalamic 5HT1A BPND is attenuated in subordinate females during the placebo condition compared to dominant females, an effect that is reversed upon E2 replacement.(144)
Overall, these experiments show that social stress in OVX female macaque monkeys produces a distinct behavioral phenotype that is largely unaffected by E2, a hypo-responsive HPA axis that is hypersensitive to the modulating effects of E2, and changes in serotonin 1A receptor binding in the hippocampus and hypothalamus that are restored or inverted by E2 replacement. Results presented here elaborate the interaction between psychosocial stress and estrogen in the modulation of a range of emotional and social behavior, and begin to characterize the neurophysiology underlying these changes. This may be particularly relevant to women marginalized by low socio-economic status, who experience prolonged psychosocial stress, and are disproportionately affected by psychopathology.
6. Concluding remarks
The HPA and HPG endocrine axes function in a tandem, flexible, and bi-directional manner, to ensure both reproductive viability and survival. The development of stress responsivity as well as reproductive function is influenced by early environmental factors that alter maternal care. This, in turn, creates a framework onto which the imperative to reproduce is balanced against the need to maintain homeostasis. This balance is tested (or challenged) when environmental contingencies (stressors) acutely upset homeostasis, which may result in sex-specific modulation of neurotransmitter systems, as with 5-HT and stress HPA axis interactions. Intermittent or repeated stress exposure may place a greater load on HPA-HPG equilibrium, as signified by reduced ovarian function and pathologies associated with decrements in estrogen release. Finally, the actions of gonadal hormones to mediate adaptive neuroendocrine and behavioral responses may be completely impaired in the face of chronic stress exposure. As underscored here, where and how this breakpoint occurs to explain individual- and gender-based differences in stress related disease remains worthy of pursuit.
Acknowledgements
This work was funded by Fondecyt 1130049(HL), CONICET and SECyT-UNC (MAR) NIH grants: HD 046501 (MW), MH081816 (DT), F31MH085445 (VM), and RR00165, and the Canadian Institutes of Health Research CIHR MOP-42555 (VV). VV would like to thank Dr. Nirupa Goel and Leyla Innala for their technical expertise and contributions for the work described in section 4. DT would like to thank Mark E. Wilson, Kim Wallen, Mar Sanchez, Karherine Reding and Vasiliki Michopoulos for their essential and substantial contributions to the work described in section 5 of this article.
References
- 1.Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Reviews in the neurosciences. 2000;11(4):383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- 2.Cirulli F, Berry A, Alleva E. Early disruption of the mother-infant relationship: effects on brain plasticity and implications for psychopathology. Neuroscience and biobehavioral reviews. 2003;27(1–2):73–82. doi: 10.1016/s0149-7634(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 3.Claessens SE, Daskalakis NP, van der Veen R, Oitzl MS, de Kloet ER, Champagne DL. Development of individual differences in stress responsiveness: an overview of factors mediating the outcome of early life experiences. Psychopharmacology. 2011;214(1):141–154. doi: 10.1007/s00213-010-2118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129(5):2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 5.Giussani DA, Farber DM, Jenkins SL, Yen A, Winter JA, Tame JD, Nathanielsz PW. Opposing effects of androgen and estrogen on pituitary-adrenal function in nonpregnant primates. Biol Reprod. 2000;62(5):1445–1451. doi: 10.1095/biolreprod62.5.1445. [DOI] [PubMed] [Google Scholar]
- 6.Wilson ME, Legendre A, Pazol K, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic-hypothalamic- pituitary-adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26(2):89–97. doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- 7.Altemus M, Roca C, Galliven E, Romanos C, Deuster P. Increased vasopressin and adrenocorticotropin responses to stress in the midluteal phase of the menstrual cycle. The Journal of clinical endocrinology and metabolism. 2001;86(6):2525–2530. doi: 10.1210/jcem.86.6.7596. [DOI] [PubMed] [Google Scholar]
- 8.Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16(5):1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. Journal of neuroendocrinology. 2002;14(6):506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 10.Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. Journal of neuroendocrinology. 2004;16(12):989–998. doi: 10.1111/j.1365-2826.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- 11.Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129(3):229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen A. Environment, human reproduction, menopause, and andropause. Environmental health perspectives. 1993;101(Suppl):291–100. doi: 10.1289/ehp.93101s291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norman RL, Smith CJ. Restraint inhibits luteinizing hormone and testosterone secretion in intact male rhesus macaques: effects of concurrent naloxone administration. Neuroendocrinology. 1992;55(4):405–415. doi: 10.1159/000126151. [DOI] [PubMed] [Google Scholar]
- 14.Lara HE, Hill DF, Katz KH, Ojeda SR. The gene encoding nerve growth factor is expressed in the immature rat ovary: effect of denervation and hormonal treatment. Endocrinology. 1990;126(1):357–363. doi: 10.1210/endo-126-1-357. [DOI] [PubMed] [Google Scholar]
- 15.Mayerhofer A, Dissen GA, Costa ME, Ojeda SR. A role for neurotransmitters in early follicular development: induction of functional follicle-stimulating hormone receptors in newly formed follicles of the rat ovary. Endocrinology. 1997;138(8):3320–3329. doi: 10.1210/endo.138.8.5335. [DOI] [PubMed] [Google Scholar]
- 16.Horii-Hayashi N, Sasagawa T, Matsunaga W, Matsusue Y, Azuma C, Nishi M. Developmental changes in desensitisation of c-Fos expression induced by repeated maternal separation in pre-weaned mice. Journal of neuroendocrinology. 2013;25(2):158–167. doi: 10.1111/j.1365-2826.2012.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McEwen BS. Early life influences on life-long patterns of behavior and health. Mental retardation and developmental disabilities research reviews. 2003;9(3):149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- 18.Nishi M, Horii-Hayashi N, Sasagawa T, Matsunaga W. Effects of early life stress on brain activity: implications from maternal separation model in rodents. General and comparative endocrinology. 2013:181306–181309. doi: 10.1016/j.ygcen.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 19.McEwen BS. Effects of Stress on the Developing Brain. Cerebrum : the Dana forum on brain science. 2011:201114. [PMC free article] [PubMed] [Google Scholar]
- 20.Murgatroyd C, Spengler D. Epigenetics of early child development. Frontiers in psychiatry. 2011:216. doi: 10.3389/fpsyt.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucion AB, Pereira FM, Winkelman EC, Sanvitto GL, Anselmo-Franci JA. Neonatal handling reduces the number of cells in the locus coeruleus of rats. Behavioral neuroscience. 2003;117(5):894–903. doi: 10.1037/0735-7044.117.5.894. [DOI] [PubMed] [Google Scholar]
- 22.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 23.Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Hormones and behavior. 1976;7(1):105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- 24.Veening JG, Coolen LM, Gerrits PO. Neural mechanisms of female sexual behavior in the rat; comparison with male ejaculatory control. Pharmacology, biochemistry, and behavior. 2013 doi: 10.1016/j.pbb.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Rhees RW, Lephart ED, Eliason D. Effects of maternal separation during early postnatal development on male sexual behavior and female reproductive function. Behavioural brain research. 2001;123(1):1–10. doi: 10.1016/s0166-4328(00)00381-8. [DOI] [PubMed] [Google Scholar]
- 26.Greisen MH, Bolwig TG, Husum H, Nedergaard P, Wortwein G. Maternal separation affects male rat copulatory behaviour and hypothalamic corticotropin releasing factor in concert. Behavioural brain research. 2005;158(2):367–375. doi: 10.1016/j.bbr.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Gomes CM, Raineki C, Ramos de Paula P, Severino GS, Helena CV, Anselmo-Franci JA, Franci CR, Sanvitto GL, Lucion AB. Neonatal handling and reproductive function in female rats. The Journal of endocrinology. 2005;184(2):435–445. doi: 10.1677/joe.1.05907. [DOI] [PubMed] [Google Scholar]
- 28.Gomes CM, Donadio MV, Anselmo-Franci J, Franci CR, Lucion AB, Sanvitto GL. Neonatal handling induces alteration in progesterone secretion after sexual behavior but not in angiotensin II receptor density in the medial amygdala: implications for reproductive success. Life sciences. 2006;78(25):2867–2871. doi: 10.1016/j.lfs.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Padoin MJ, Cadore LP, Gomes CM, Barros HM, Lucion AB. Long-lasting effects of neonatal stimulation on the behavior of rats. Behavioral neuroscience. 2001;115(6):1332–1340. doi: 10.1037//0735-7044.115.6.1332. [DOI] [PubMed] [Google Scholar]
- 30.Cameron NM, Fish EW, Meaney MJ. Maternal influences on the sexual behavior and reproductive success of the female rat. Hormones and behavior. 2008;54(1):178–184. doi: 10.1016/j.yhbeh.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Cameron NM. Maternal programming of reproductive function and behavior in the female rat. Frontiers in evolutionary neuroscience. 2011:310. doi: 10.3389/fnevo.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cameron NM, Soehngen E, Meaney MJ. Variation in maternal care influences ventromedial hypothalamus activation in the rat. Journal of neuroendocrinology. 2011;23(5):393–400. doi: 10.1111/j.1365-2826.2011.02124.x. [DOI] [PubMed] [Google Scholar]
- 33.Cameron NM, Shahrokh D, Del Corpo A, Dhir SK, Szyf M, Champagne FA, Meaney MJ. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. Journal of neuroendocrinology. 2008;20(6):795–801. doi: 10.1111/j.1365-2826.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- 34.Curry TE, Jr, Lawrence IE, Jr, Burden HW. Ovarian sympathectomy in the golden hamster: effects on estrous cyclicity and follicular development. Exp Clin Endocrinol. 1985;86(3):284–290. doi: 10.1055/s-0029-1210498. [DOI] [PubMed] [Google Scholar]
- 35.Burden HW, Lawrence IE, Jr, Louis TM. The adrenergic innervation of the guinea pig ovary during prenatal and postnatal periods. Acta Anat (Basel) 1985;122(3):193–196. doi: 10.1159/000146001. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence IE, Jr, Burden HW. The origin of the extrinsic adrenergic innervation to the rat ovary. Anat Rec. 1980;196(1):51–59. doi: 10.1002/ar.1091960106. [DOI] [PubMed] [Google Scholar]
- 37.Gerendai I, Kocsis K, Halasz B. Supraspinal connections of the ovary: structural and functional aspects. Microsc Res Tech. 2002;59(6):474–483. doi: 10.1002/jemt.10225. [DOI] [PubMed] [Google Scholar]
- 38.Fiedler J, Jara P, Luza S, Dorfman M, Grouselle D, Rage F, Lara HE, Arancibia S. Cold stress induces metabolic activation of thyrotrophin-releasing hormone-synthesising neurones in the magnocellular division of the hypothalamic paraventricular nucleus and concomitantly changes ovarian sympathetic activity parameters. J Neuroendocrinol. 2006;18(5):367–376. doi: 10.1111/j.1365-2826.2006.01427.x. [DOI] [PubMed] [Google Scholar]
- 39.Jara P, Rage F, Dorfman M, Grouselle D, Barra R, Arancibia S, Lara HE. Cold-induced glutamate release in vivo from the magnocellular region of the paraventricular nucleus is involved in ovarian sympathetic activation. J Neuroendocrinol. 2009;22(9):979–986. doi: 10.1111/j.1365-2826.2010.02040.x. [DOI] [PubMed] [Google Scholar]
- 40.Lara HE, McDonald JK, Ojeda SR. Involvement of nerve growth factor in female sexual development. Endocrinology. 1990;126(1):364–375. doi: 10.1210/endo-126-1-364. [DOI] [PubMed] [Google Scholar]
- 41.Greiner M, Paredes A, Araya V, Lara HE. Role of stress and sympathetic innervation in the development of polycystic ovary syndrome. Endocrine. 2005;28(3):319–324. doi: 10.1385/ENDO:28:3:319. [DOI] [PubMed] [Google Scholar]
- 42.Barria A, Leyton V, Ojeda SR, Lara HE. Ovarian steroidal response to gonadotropins and beta-adrenergic stimulation is enhanced in polycystic ovary syndrome: role of sympathetic innervation. Endocrinology. 1993;133(6):2696–2703. doi: 10.1210/endo.133.6.8243293. [DOI] [PubMed] [Google Scholar]
- 43.Lara HE, et al. Activation of ovarian sympathetic nerves in polycystic ovary syndrome. Endocrinology. 1993;133(6):2690–2695. doi: 10.1210/endo.133.6.7902268. [DOI] [PubMed] [Google Scholar]
- 44.Dorfman M, Arancibia S, Fiedler JL, Lara HE. Chronic intermittent cold stress activates ovarian sympathetic nerves and modifies ovarian follicular development in the rat. Biol Reprod. 2003;68(6):2038–2043. doi: 10.1095/biolreprod.102.008318. [DOI] [PubMed] [Google Scholar]
- 45.Glover V, O'Connor TG, O'Donnell K. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev. 2010;35(1):17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Lara HE, Dorfman M, Venegas M, Luza SM, Luna SL, Mayerhofer A, Guimaraes MA, Rosa ESAA, Ramirez VD. Changes in sympathetic nerve activity of the mammalian ovary during a normal estrous cycle and in polycystic ovary syndrome: Studies on norepinephrine release. Microsc Res Tech. 2002;59(6):495–502. doi: 10.1002/jemt.10229. [DOI] [PubMed] [Google Scholar]
- 47.Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17(10):2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- 48.Luna SL, Neuman S, Aguilera J, Brown DI, Lara HE. In vivo beta-adrenergic blockade by propranolol prevents isoproterenol-induced polycystic ovary in adult rats. Horm Metab Res. 44(9):676–681. doi: 10.1055/s-0031-1301304. [DOI] [PubMed] [Google Scholar]
- 49.Williamson M, Bingham B, Viau V. Central organization of androgen-sensitive pathways to the hypothalamic-pituitary-adrenal axis: implications for individual differences in responses to homeostatic threat and predisposition to disease. Progress in neuro-psychopharmacology & biological psychiatry. 2005;29(8):1239–1248. doi: 10.1016/j.pnpbp.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones and behavior. 1994;28(4):464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 51.Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. 2002;52(4):318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- 52.Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146(1):137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- 53.Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004:16272–16278. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- 54.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Uhart M, Chong RY, Oswald L, Lin PI, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31(5):642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Kirschbaum C, Schommer N, Federenko I, Gaab J, Neumann O, Oellers M, Rohleder N, Untiedt A, Hanker J, Pirke KM, Hellhammer DH. Short-term estradiol treatment enhances pituitary-adrenal axis and sympathetic responses to psychosocial stress in healthy young men. J Clin Endocrinol Metab. 1996;81(10):3639–3643. doi: 10.1210/jcem.81.10.8855815. [DOI] [PubMed] [Google Scholar]
- 57.Rubinow DR, Roca CA, Schmidt PJ, Danaceau MA, Putnam K, Cizza G, Chrousos G, Nieman L. Testosterone suppression of CRH-stimulated cortisol in men. Neuropsychopharmacology. 2005;30(10):1906–1912. doi: 10.1038/sj.npp.1300742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubinow DR, Schmidt PJ. Gonadal steroids, brain, and behavior: role of context. Dialogues in clinical neuroscience. 2002;4(2):123–137. doi: 10.31887/DCNS.2002.4.2/drubinow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young EA. Glucocorticoid cascade hypothesis revisited: Role of gonadal steroids. Depression. 1995;3(1–2):20–27. [Google Scholar]
- 60.Torpy DJ, Papanicolaou DA, Chrousos GP. Sexual dimorphism of the human stress response may be due to estradiol-mediated stimulation of hypothalamic corticotropin-releasing hormone synthesis. J Clin Endocrinol Metab. 1997;82(3):982–984. doi: 10.1210/jcem.82.3.3824-1. [DOI] [PubMed] [Google Scholar]
- 61.Kessler RC. Epidemiology of women and depression. Journal of affective disorders. 2003;74(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 62.Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma, violence & abuse. 2009;10(3):198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- 63.Bangasser DA. Sex differences in stress-related receptors: "micro" differences with "macro" implications for mood and anxiety disorders. Biology of sex differences. 2013;4(1):2. doi: 10.1186/2042-6410-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herbert J. Cortisol and depression: three questions for psychiatry. Psychological medicine. 2013;43(3):449–469. doi: 10.1017/S0033291712000955. [DOI] [PubMed] [Google Scholar]
- 65.Young EA, Haskett RF, Murphy-Weinberg V, Watson SJ, Akil H. Loss of glucocorticoid fast feedback in depression. Archives of general psychiatry. 1991;48(8):693–699. doi: 10.1001/archpsyc.1991.01810320017003. [DOI] [PubMed] [Google Scholar]
- 66.Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic medicine. 1995;57(5):468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34(1):2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 68.Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 69.Porter RJ, Gallagher P, Watson S, Young AH. Corticosteroid-serotonin interactions in depression: a review of the human evidence. Psychopharmacology (Berl) 2004;173(1–2):1–17. doi: 10.1007/s00213-004-1774-1. [DOI] [PubMed] [Google Scholar]
- 70.Hemrick-Luecke SK, Evans DC. Comparison of the potency of MDL 100,907 and SB 242084 in blocking the serotonin (5-HT)(2) receptor agonist-induced increases in rat serum corticosterone concentrations: evidence for 5-HT(2A) receptor mediation of the HPA axis. Neuropharmacology. 2002;42(2):162–169. doi: 10.1016/s0028-3908(01)00166-6. [DOI] [PubMed] [Google Scholar]
- 71.Jorgensen HS. Studies on the neuroendocrine role of serotonin. Dan Med Bull. 2007;54(4):266–288. [PubMed] [Google Scholar]
- 72.Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32(6):1174–1184. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 73.Carlsson M, Carlsson A. A regional study of sex differences in rat brain serotonin. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(1):53–61. doi: 10.1016/0278-5846(88)90061-9. [DOI] [PubMed] [Google Scholar]
- 74.Dominguez R, Cruz-Morales SE, Carvalho MC, Xavier M, Brandao ML. Sex differences in serotonergic activity in dorsal and median raphe nucleus. Physiol Behav. 2003;80(2–3):203–210. doi: 10.1016/j.physbeh.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 75.Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145(8):3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- 76.Heinsbroek RP, van Haaren F, Feenstra MG, van Galen H, Boer G, van de Poll NE. Sex differences in the effects of inescapable footshock on central catecholaminergic and serotonergic activity. Pharmacol Biochem Behav. 1990;37(3):539–550. doi: 10.1016/0091-3057(90)90025-d. [DOI] [PubMed] [Google Scholar]
- 77.Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. Eur J Neurosci. 2006;24(11):3245–3254. doi: 10.1111/j.1460-9568.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- 78.Mendelson SD, McEwen BS. Autoradiographic analyses of the effects of restraint-induced stress on 5-HT1A, 5-HT1C and 5-HT2 receptors in the dorsal hippocampus of male and female rats. Neuroendocrinology. 1991;54(5):454–461. doi: 10.1159/000125951. [DOI] [PubMed] [Google Scholar]
- 79.Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94(1):251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]
- 80.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999:381083–381152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 81.Polter AM, Li X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cellular signalling. 2010;22(10):1406–1412. doi: 10.1016/j.cellsig.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rioja J, Santin LJ, Dona A, de Pablos L, Minano FJ, Gonzalez-Baron S, Aguirre JA. 5-HT1A receptor activation counteracts c-Fos immunoreactivity induced in serotonin neurons of the raphe nuclei after immobilization stress in the male rat. Neurosci Lett. 2006;397(3):190–195. doi: 10.1016/j.neulet.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 83.Goel N, Innala L, Viau V. Sex differences in serotonin (5-HT) 1A receptor regulation of HPA axis and dorsal raphe responses to acute restraint. Psychoneuroendocrinology. 2014:40232–40241. doi: 10.1016/j.psyneuen.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 84.Roger M, Cadusseau J. Afferents to the zona incerta in the rat: a combined retrograde and anterograde study. J Comp Neurol. 1985;241(4):480–492. doi: 10.1002/cne.902410407. [DOI] [PubMed] [Google Scholar]
- 85.Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1993:332123–332143. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- 86.Sita LV, Elias CF, Bittencourt JC. Connectivity pattern suggests that incerto-hypothalamic area belongs to the medial hypothalamic system. Neuroscience. 2007;148(4):949–969. doi: 10.1016/j.neuroscience.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 87.Blier P. Altered function of the serotonin 1A autoreceptor and the antidepressant response. Neuron. 2010;65(1):1–2. doi: 10.1016/j.neuron.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 88.Lomniczi A, Wright H, Castellano JM, Sonmez K, Ojeda SR. A system biology approach to identify regulatory pathways underlying the neuroendocrine control of female puberty in rats and nonhuman primates. Hormones and behavior. 2013;64(2):175–186. doi: 10.1016/j.yhbeh.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wallen K, Winston LA, Gaventa S, Davis-DaSilva M, Collins DC. Periovulatory changes in female sexual behavior and patterns of ovarian steroid secretion in group-living rhesus monkeys. Hormones and behavior. 1984;18(4):431–450. doi: 10.1016/0018-506x(84)90028-x. [DOI] [PubMed] [Google Scholar]
- 90.Appt SE. Usefulness of the monkey model to investigate the role soy in postmenopausal women's health. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2004;45(2):200–211. doi: 10.1093/ilar.45.2.200. [DOI] [PubMed] [Google Scholar]
- 91.Ferin M, Warren M, Dyrenfurth I, Vande Wiele RL, White WF. Response of rhesus monkeys to LRH throughout the ovarian cycle. The Journal of clinical endocrinology and metabolism. 1974;38(2):231–237. doi: 10.1210/jcem-38-2-231. [DOI] [PubMed] [Google Scholar]
- 92.Weinbauer GF, Niehoff M, Niehaus M, Srivastav S, Fuchs A, Van Esch E, Cline JM. Physiology and Endocrinology of the Ovarian Cycle in Macaques. Toxicologic pathology. 2008;36(7S):7S–23S. doi: 10.1177/0192623308327412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. The American journal of psychiatry. 2004;161(2):195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 94.Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biology of mood & anxiety disorders. 2012;2(1):3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aloysi A, Van Dyk K, Sano M. Women's cognitive and affective health and neuropsychiatry. The Mount Sinai journal of medicine, New York. 2006;73(7):967–975. [PubMed] [Google Scholar]
- 96.Aoki M, Shimozuru M, Kikusui T, Takeuchi Y, Mori Y. Sex differences in behavioral and corticosterone responses to mild stressors in ICR mice are altered by ovariectomy in peripubertal period. Zoological science. 2010;27(10):783–789. doi: 10.2108/zsj.27.783. [DOI] [PubMed] [Google Scholar]
- 97.Backstrom T, Mattsson B. Correlation of symptoms in pre-menstrual tension to oestrogen and progesterone concentrations in blood plasma. A preliminary study. Neuropsychobiology. 1975;1(2):80–86. doi: 10.1159/000117480. [DOI] [PubMed] [Google Scholar]
- 98.Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biological psychiatry. 2013;73(4):371–378. doi: 10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological psychiatry. 2012;72(1):19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological psychiatry. 2011;70(10):920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, Norrholm SD, Bradley B, Ressler KJ. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49(7):1884–1891. doi: 10.1016/j.cortex.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lebron-Milad K, Graham BM, Milad MR. Low estradiol levels: a vulnerability factor for the development of posttraumatic stress disorder. Biological psychiatry. 2012;72(1):6–7. doi: 10.1016/j.biopsych.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 103.Bernstein IS, Gordon TP, Rose RM. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol (Basel) 1974;21(2):81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- 104.Bernstein IS. Dominance, aggression and reproduction in primate societies. J Theor Biol. 1976;60(2):459–472. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- 105.Bernstein IS, Gordon TP. The function of aggression in primate societies. Am Sci. 1974;62(3):304–311. [PubMed] [Google Scholar]
- 106.Michopoulos V, Checchi M, Sharpe D, Wilson ME. Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. Hormones and behavior. 2011;59(4):528–535. doi: 10.1016/j.yhbeh.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Michopoulos V, Higgins M, Toufexis D, Wilson ME. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology. 2012;37(7):1071–1085. doi: 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Michopoulos V, Toufexis D, Wilson ME. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology. 2012;37(9):1479–1490. doi: 10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reding K, Fair D, Wilson ME, Toufexis D, Sanchez M. Chronic psychosocial stress and estradiol alter amygdalo-cortical intrinsic functional connectivity. San Francisco, CA: Society for Biological Psychiatry; 2013. [Google Scholar]
- 110.Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, Henderson JA, Smith MA, Buchheimer N. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch Gen Psychiatry. 2006;63(4):396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- 111.Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(17):6490–6495. doi: 10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Asher J, Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social stress and the polymorphic region of the serotonin reuptake transporter gene modify oestradiol-induced changes on central monoamine concentrations in female rhesus monkeys. Journal of neuroendocrinology. 2013;25(4):321–328. doi: 10.1111/jne.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaplan JR, Chen H, Manuck SB. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. Am J Primatol. 2009;71(9):732–741. doi: 10.1002/ajp.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5(2):169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 115.Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: a primate continuum. ILAR. 2004;4589:115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- 116.Wilson ME, Kinkead B. Gene-environment interactions, not neonatal growth hormone deficiency, time puberty in female rhesus monkeys. Biology of reproduction. 2008;78(4):736–743. doi: 10.1095/biolreprod.107.065953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zehr JL, Van Meter PE, Wallen K. Factors regulating the timing of puberty onset in female rhesus monkeys (Macaca mulatta): role of prenatal androgens, social rank, and adolescent body weight. Biology of reproduction. 2005;72(5):1087–1094. doi: 10.1095/biolreprod.104.027755. [DOI] [PubMed] [Google Scholar]
- 118.Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain, behavior, and immunity. 1991;5(3):296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- 119.Paiardini M, Hoffman J, Cervasi B, Ortiz AM, Stroud F, Silvestri G, Wilson ME. T-cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys. Brain, behavior, and immunity. 2009;23(2):286–293. doi: 10.1016/j.bbi.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neuroscience and biobehavioral reviews. 2009;33(2):133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Yoav G. Social environment is associated with gene regulatory variation in rhesus macaque immune system. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1202734109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Willard SL, Shively CA. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis) American journal of primatology. 2012;74(6):528–542. doi: 10.1002/ajp.21013. [DOI] [PubMed] [Google Scholar]
- 123.Bellino FL, Wise PM. Nonhuman primate models of menopause workshop. Biology of reproduction. 2003;68(1):10–18. doi: 10.1095/biolreprod.102.005215. [DOI] [PubMed] [Google Scholar]
- 124.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(13):5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29(1):80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 126.Kaplan JR, Manuck SB, Anthony MS, Clarkson TB. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstetrics and gynecology. 2002;99(3):381–388. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- 127.Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biological psychiatry. 1998;44(9):882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- 128.Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiology & behavior. 2008;93(4–5):807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Veenstra-VanderWeele J, Anderson GM, Cook EH., Jr Pharmacogenetics and the serotonin system: initial studies and future directions. European journal of pharmacology. 2000;410(2–3):165–181. doi: 10.1016/s0014-2999(00)00814-1. [DOI] [PubMed] [Google Scholar]
- 130.Melke J, Landen M, Baghei F, Rosmond R, Holm G, Bjorntorp P, Westberg L, Hellstrand M, Eriksson E. Serotonin transporter gene polymorphisms are associated with anxiety-related personality traits in women. American journal of medical genetics. 2001;105(5):458–463. doi: 10.1002/ajmg.1434. [DOI] [PubMed] [Google Scholar]
- 131.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (New York, NY. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 132.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science (New York, NY. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 133.Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular psychiatry. 2002;7(10):1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- 134.Bethea CL, Streicher JM, Coleman K, Pau FK, Moessner R, Cameron JL. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism (5HTTLPR) Behav Genet. 2004;34(3):295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- 135.Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biological psychiatry. 2004;55(7):733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 136.Suomi SJ. Gene-environment interactions and the neurobiology of social conflict. Annals of the New York Academy of Sciences. 2003:1008132–1008139. doi: 10.1196/annals.1301.014. [DOI] [PubMed] [Google Scholar]
- 137.Johnson ZP, Lowe J, Michopoulos V, Moore CJ, Wilson ME, Toufexis D. Oestradiol differentially influences feeding behaviour depending on diet composition in female rhesus monkeys. Journal of neuroendocrinology. 2013;25(8):729–741. doi: 10.1111/jne.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Michopoulos V, Embree M, Reding K, Sanchez MM, Toufexis D, Votaw JR, Voll RJ, Goodman MM, Rivier J, Wilson ME, Berga SL. CRH receptor antagonism reverses the effect of social subordination upon central GABAA receptor binding in estradiol-treated ovariectomized female rhesus monkeys. Neuroscience. 2013:250300–250308. doi: 10.1016/j.neuroscience.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moore CJ, Lowe J, Michopoulos V, Ulam P, Toufexis D, Wilson ME, Johnson Z. Small changes in meal patterns lead to significant changes in total caloric intake. Effects of diet and social status on food intake in female rhesus monkeys. Appetite. 2012:62C60–62C69. doi: 10.1016/j.appet.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reding K, Michopoulos V, Wallen K, Sanchez M, Wilson ME, Toufexis D. Social status modifies estradiol activation of sociosexual behavior in female rhesus monkeys. Hormones and behavior. 2012;62(5):612–620. doi: 10.1016/j.yhbeh.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Toufexis DJ, Wilson ME. Dihydrotestosterone differentially modulates the cortisol response of the hypothalamic-pituitary-adrenal axis in male and female rhesus macaques, and restores circadian secretion of cortisol in females. Brain research. 2012:142943–142951. doi: 10.1016/j.brainres.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Michopoulos VP, M. Molly Embree M, Reding K, Wilson M, Toufexis D, Sanchez M. Social stress and estradiol synergistically diminish 5HT1A receptor binding potential in female rhesus monkeys. Absract for the annual meeting of the Society for Behavioral Neuroendocrinology. 2013 [Google Scholar]
- 143.Moore CJ, Michopoulos V, Johnson ZP, Toufexis D, Wilson ME. Dietary variety is associated with larger meals in female rhesus monkeys. Physiology & behavior. 2013:119190–119194. doi: 10.1016/j.physbeh.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Michopoulos V, Diaz MP, Embree M, Reding K, Votaw JR, Mun J, Voll RJ, Goodman MM, Wilson M, Sanchez M, Toufexis D. Oestradiol alters central 5HT1A receptor binding potential differences related to psychosocial stress but not differences related to 5HTTLPR genotype in female rhesus monkeys. Journal of neuroendocrinology. 2013 doi: 10.1111/jne.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moore CJ, Lowe J, Michopoulos V, Ulam P, Toufexis D, Wilson ME, Johnson Z. Small changes in meal patterns lead to significant changes in total caloric intake. Effects of diet and social status on food intake in female rhesus monkeys. Appetite. 2013:6260–6269. doi: 10.1016/j.appet.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biological psychiatry. 1997;41(8):871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 147.Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Hormones and behavior. 2001;40(4):472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- 148.Walf AA, Frye CA. Effects of two estradiol regimens on anxiety and depressive behaviors and trophic effects in peripheral tissues in a rodent model. Gender medicine. 2009;6(1):300–311. doi: 10.1016/j.genm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rodriguez-Sierra JF, Howard JL, Pollard GT, Hendricks SE. Effect of ovarian hormones on conflict behavior. Psychoneuroendocrinology. 1984;9(3):293–300. doi: 10.1016/0306-4530(84)90008-8. [DOI] [PubMed] [Google Scholar]
- 150.Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Differential effects of exogenous and endogenous estrogen on anxiety as measured by elevated T-maze in relation to the serotonergic system. Behavioural brain research. 2009;198(1):142–148. doi: 10.1016/j.bbr.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 151.Andrade TG, Nakamuta JS, Avanzi V, Graeff FG. Anxiolytic effect of estradiol in the median raphe nucleus mediated by 5-HT1A receptors. Behavioural brain research. 2005;163(1):18–25. doi: 10.1016/j.bbr.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 152.Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17 Beta-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology. 2005;179(3):637–643. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- 153.Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behavioural brain research. 2001;122(1):1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- 154.Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behavioral neuroscience. 2004;118(2):306–313. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- 155.Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Anxiolytic property of estrogen related to the changes of the monoamine levels in various brain regions of ovariectomized rats. Physiology & behavior. 2006;87(4):828–835. doi: 10.1016/j.physbeh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 156.Michopoulos V, Checchi M, Sharpe D, Wilson ME. Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. Hormones and behavior. 2011;59(4):528–535. doi: 10.1016/j.yhbeh.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, Pierre PJ, Bennett A, Garg PK, Garg S, Nader MA. Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience. 2009;158(4):1257–1265. doi: 10.1016/j.neuroscience.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kaplan JR, Adams MR, Anthony MS, Morgan TM, Manuck SB, Clarkson TB. Dominant social status and contraceptive hormone treatment inhibit atherogenesis in premenopausal monkeys. Arteriosclerosis, thrombosis, and vascular biology. 1995;15(12):2094–2100. doi: 10.1161/01.atv.15.12.2094. [DOI] [PubMed] [Google Scholar]
- 159.Kaplan JR, Chen H, Appt SE, Lees CJ, Franke AA, Berga SL, Wilson ME, Manuck SB, Clarkson TB. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Hum Reprod. 2010;25(12):3083–3094. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shively CA, Clarkson TB, Kaplan JR. Social deprivation and coronary artery atherosclerosis in female cynomolgus monkeys. Atherosclerosis. 1989;77(1):69–76. doi: 10.1016/0021-9150(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 161.Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology of Macaca fascicularis. Physiology & behavior. 1984;33(5):777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- 162.Collura LA, Hoffman JB, Wilson ME. Administration of human leptin differentially affects parameters of cortisol secretion in socially housed female rhesus monkeys. Endocrine. 2009;36(3):530–537. doi: 10.1007/s12020-009-9250-7. [DOI] [PubMed] [Google Scholar]
- 163.Kasprzak A, Lesniewska B, Malendowicz LK. Sex differences in adrenocortical structure and function. XXI. The effects of gonadectomy and testosterone or estradiol replacement on mitotic activity of the rat adrenal cortex. Experimental and clinical endocrinology. 1986;87(1):26–30. doi: 10.1055/s-0029-1210518. [DOI] [PubMed] [Google Scholar]
- 164.Malendowicz LK, Robba C, Nussdorfer GG. Sex differences in adrenocortical structure and function. XXII. Light- and electron-microscopic morphometric studies on the effects of gonadectomy and gonadal hormone replacement on the rat adrenal cortex. Cell and tissue research. 1986;244(1):141–145. doi: 10.1007/BF00218391. [DOI] [PubMed] [Google Scholar]
- 165.Malendowicz LK. Sex differences in adrenocortical structure and function. XXIII. Time-studies on the long-term effects of gonadectomy on rat adrenal cortex. Experimental and clinical endocrinology. 1986;88(1):6–12. [PubMed] [Google Scholar]
- 166.Smith CJ, Norman RL. Influence of the gonads on cortisol secretion in female rhesus macaques. Endocrinology. 1987;121(6):2192–2198. doi: 10.1210/endo-121-6-2192. [DOI] [PubMed] [Google Scholar]
- 167.Shively CA. Behavioral and neurobiological effects of estrogen replacement therapy and a history of triphasic oral contraceptive exposure. Psychoneuroendocrinology. 1998;23(7):713–732. doi: 10.1016/s0306-4530(98)00039-0. [DOI] [PubMed] [Google Scholar]
- 168.Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Hormones and behavior. 2012;62(4):389–399. doi: 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biological psychiatry. 1999;46(10):1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 170.Moses-Kolko EL, Price JC, Thase ME, Meltzer CC, Kupfer DJ, Mathis CA, Bogers WD, Berman SR, Houck PR, Schneider TN, Drevets WC. Measurement of 5-HT1A receptor binding in depressed adults before and after antidepressant drug treatment using positron emission tomography and [11C]WAY-100635. Synapse (New York, NY. 2007;61(7):523–530. doi: 10.1002/syn.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fiedler J, et al. Cold stress induces metabolic activation of thyrotrophin-releasing hormone-synthesising neurones in the magnocellular division of the hypothalamic paraventricular nucleus and concomitantly changes ovarian sympathetic activity parameters. J Neuroendocrinol. 2006;18(5):367–376. doi: 10.1111/j.1365-2826.2006.01427.x. [DOI] [PubMed] [Google Scholar]