Abstract
Aim
To determine the association between 10 min Apgar scores and 6–7-year outcomes in children with perinatal hypoxic-ischaemic encephalopathy (HIE) enrolled in the National Institute of Child Health and Human Development Neonatal Research Network (NICHD NRN) whole body cooling randomised controlled trial (RCT).
Methods
Evaluations at 6–7 years included the Wechsler Preschool and Primary Scale of Intelligence III or Wechsler Intelligence Scale for Children IV and Gross Motor Functional Classification Scale. Primary outcome was death/moderate or severe disability. Logistic regression was used to examine the association between 10 min Apgar scores and outcomes after adjusting for birth weight, gestational age, gender, outborn status, hypothermia treatment and centre.
Results
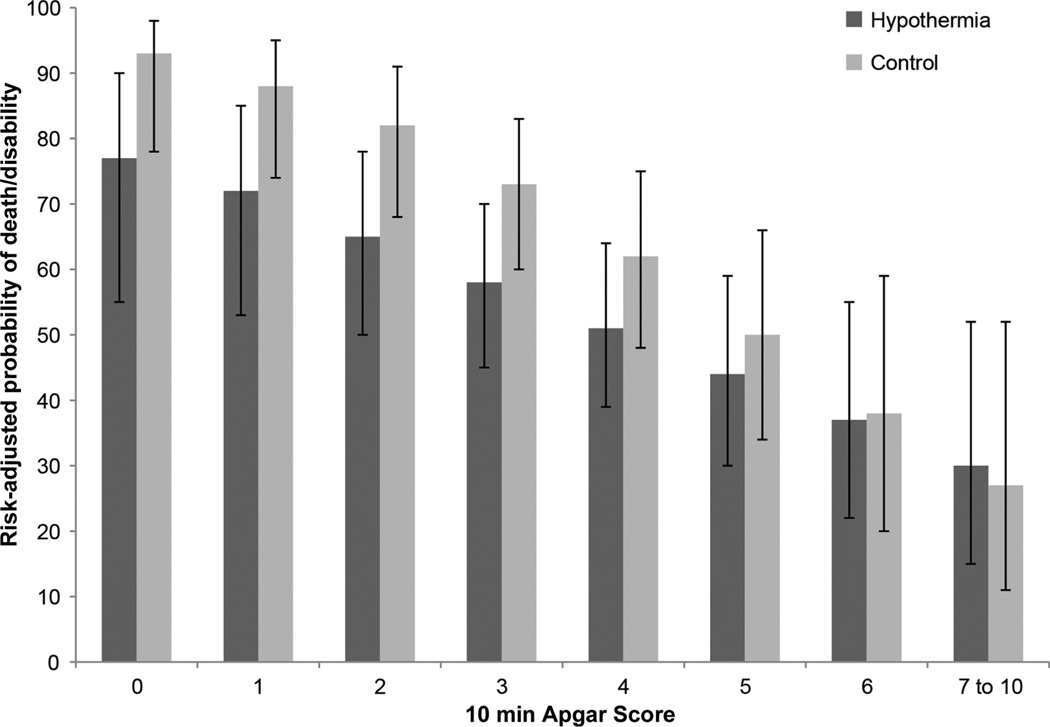
In the study cohort (n=174), 64/85 (75%) of those with 10 min Apgar score of 0–3 had death/disability compared with 40/89 (45%) of those with scores >3. Each point increase in 10 min Apgar scores was associated with a significantly lower adjusted risk of death/disability, death, death/IQ <70, death/cerebral palsy (CP) and disability, IQ<70 and CP among survivors (all p<0.05). Among the 24 children with a 10 min Apgar score of 0, five (20.8%) survived without disability. The risk-adjusted probabilities of death/disability were significantly lower in cooled infants with Apgar scores of 0–3; there was no significant interaction between cooling and Apgar scores (p=0.26).
Conclusions
Among children with perinatal HIE enrolled in the NICHD cooling RCT, 10 min Apgar scores were significantly associated with school-age outcomes. A fifth of infants with 10 min Apgar score of 0 survived without disability to school age, suggesting the need for caution in limiting resuscitation to a specified duration.
INTRODUCTION
The Apgar score is extensively used to evaluate an infant’s condition shortly after delivery.1 Although some studies have shown an association between low 5 min Apgar scores and death or cerebral palsy (CP), the correlation with future neurological outcome in term infants is poor.1–4 The risk of poor outcome increases when the Apgar score remains low at 10 min and beyond.1 The relationship between 10 min Apgar scores and neurodevelopmental outcomes has been investigated in a few studies.5,6 Of the 49 000 infants in the Collaborative Perinatal Project delivered from 1959 to 1966, 122 term infants had 10 min Apgar scores of 0–3.5 Of these infants, 34% died in the first year and 17% of survivors developed CP.5 At school age, 12/99 (12%) surviving children had CP, 11 of whom had mental retardation and 6 had seizure disorders; 80% were free of major handicap.5 Apgar scores were not important antecedents of seizure disorders in children without CP.3 Casalaz and colleagues reported that, among 14 infants with 10 min Apgar scores below 4, only one infant survived without impairment.6 Given the conflicting outcomes for infants with low 10 min Apgar scores, we previously examined the relationship between Apgar scores and neurodevelopmental outcomes at 18–22 months of age using data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NICHD NRN) randomised controlled trial (RCT) of whole body cooling for perinatal hypoxic-ischaemic encephalopathy (HIE). Each point decrease in 10 min Apgar scores was associated with a 45% increase in the odds of death or disability at 18 months, after adjustment for covariates.7,8 However, there are few data on school-age outcomes in relation to Apgar scores in the era of therapeutic cooling for HIE.
Our primary aim in the current study was to determine the association between 10 min Apgar scores and 6–7-year outcomes in children with a history of perinatal HIE, enrolled in the NICHD NRN whole body cooling trial, some of whom had undergone cooling.7,9 Our secondary objectives were (a) to describe childhood outcomes of infants with 10 min Apgar score of 0 and (b) among infants with 10 min Apgar scores of 0–3 to identify characteristics in the first hours after birth that differed significantly between groups of infants who survived without disability compared with those who died or had moderate/severe disability at 6–7 years.
METHODS
This was a secondary analysis of data from the NICHD NRN whole body cooling RCT and the 6–7 -year follow-up outcomes study.7,9 Eligibility criteria for the trial included gestational age ≥36 weeks, age at admission <6 h and fulfilment of biochemical and clinical criteria such as severe acidosis in cord blood or postnatal blood gases or history of an acute perinatal event and need for resuscitation.7 All infants had moderate or severe encephalopathy or seizures. Infants with major congenital anomalies, severe growth restriction or moribund condition were excluded. Approval for the study was obtained from the institutional review boards of the human investigation committee of all participating sites of the NICHD NRN and written parental consent was obtained for all study subjects. Infants were randomly assigned to whole body cooling to 33.5°C for 72 h within 6 h of birth followed by rewarming over 6 h or conventional care.7 For the current secondary analysis, infants who had data available for 10 min Apgar scores and 6–7 -year outcomes were included. Data on Apgar scores, delivery room (DR) resuscitation interventions and cord blood gases were collected as part of the original trial.
Outcomes at 6–7 years
Evaluations by trained certified examiners included a detailed neurological examination, the Wechsler Preschool and Primary Scale of Intelligence III (WPPSI-III) for children below 7 years and 3 months or Wechsler Intelligence Scale for Children IV for older children.9 The neurological examination included assessment of cranial nerves and gross and fine motor functions. CP was classified according to the Surveillance of Cerebral Palsy in Europe and severity was categorised using the Gross Motor Function Classification Scale (GMFCS) levels I–V .9,10,11 The WPPSI involved seven core subtests designed to measure verbal, performance and full scale IQ. The WPPSI has been standardised in children with disabilities and has both a floor and a ceiling that can be assessed in low-functioning children. Attention and executive function and visuospatial processing were evaluated by the Developmental Neuropsychological Assessment (NEPSY) in a subset of surviving children, with normative scores of 100±15. Severe disability was defined as IQ score <55, GMFCS level IV or V or bilateral blindness. Moderate disability was defined as IQ scores 55–69, GMFCS level III, bilateral deafness, with or without amplification or refractory epilepsy.9 Mild disability was defined as IQ scores of 70–84 or GMFCS I or II and no disability as IQ>84 without CP, hearing or visual impairment or epilepsy.9
The primary outcome for this study was death/moderate or severe disability; secondary outcomes were death; moderate/ severe disability, death/IQ <70; death or moderate/severe CP; IQ<70 and moderate/severe CP.9
Statistical analysis
Characteristics of children with follow-up data were compared with those who were lost to follow-up or had missing data using χ2 tests and t tests. Mixed effects logistic regression models were conducted using SAS PROC GLIMMIX to determine associations between 10 min Apgar scores and 6–7-year outcomes to yield an OR and 95% CIs after controlling for treatment group (hypothermia vs conventional care), birth weight, gestational age, gender and outborn status. NRN centre was included as a random effect. The models were conducted for the primary outcome (death/disability) and secondary outcomes separately. Among those with a 10 min Apgar score of 0–3, we compared perinatal prerandomisation neonatal variables between subgroups of children who died or had disability and those who survived without disability, using t tests and χ2 tests as appropriate. The interaction between cooling and Apgar score was tested after controlling for confounders and risk-adjusted probabilities for the primary outcome for cooled and control infants by Apgar scores were calculated. A p value<0.05 was considered significant.
RESULTS
The 208 RCT participants were derived from 239 eligible infants; in 31 cases, parental consent was not requested or was refused.7 Of the 208 RCT participants, 191 had data on 10 min Apgar scores and of those, 174 had data on primary outcome (90 hypothermia and 84 controls). A comparison of the study subjects (n=174) and those excluded (n=34) showed that the study subjects and those excluded differed in their Apgar scores, cord pH and receipt of resuscitative interventions (see online supplementary table S1).
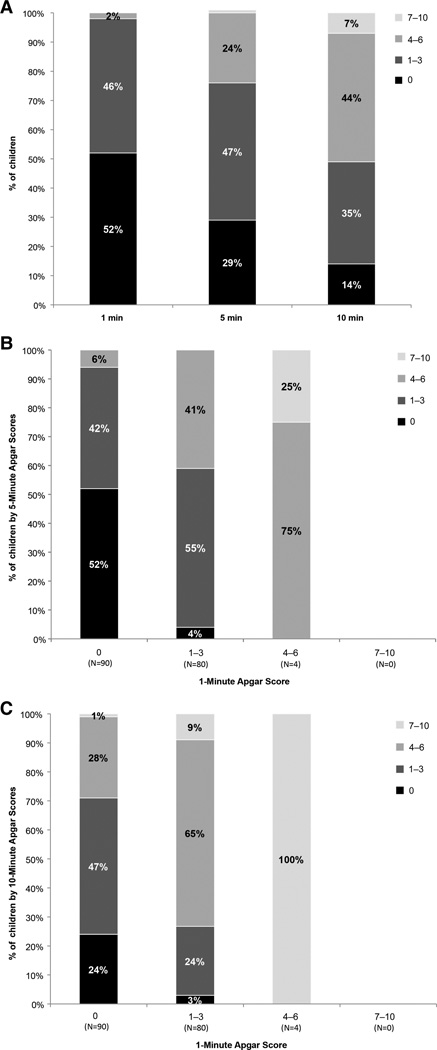
Figure 1A shows the distribution of Apgar scores at 1, 5 and 10 min. Online supplementary figures S1B,C depict the distributions of 5 min and 10 min Apgar scores in relation to the 1 min Apgar score. The 6–7-year outcomes in relation to 10 min Apgar scores are shown in table 1. Rates of adverse outcomes were generally higher with lower 10 min Apgar scores and lower in cooled (n=90) infants compared with controls (n=84), especially at lower Apgar scores, although the numbers were small (table 1). Mortality rate of cooled infants was 26/90 (28.9%) compared with 39/84 (46.4%) for those receiving standard care. Table 2 includes the adjusted and unadjusted ORs of 6–7-year outcomes with increasing 10 min Apgar scores. Each point increase in 10 min Apgar scores was associated with a significantly lower risk of death/disability, death/IQ <70, death, death/CP and among survivors, moderate/severe disability, IQ <70 and CP, after adjustment for birth weight, gestational age, gender, treatment group and outborn status. Figure 2 depicts the risk-adjusted probability of death or moderate/severe disability by treatment group and Apgar scores; these probabilities were significantly lower in cooled infants with Apgar scores of 0–3 (p<0.05). The interaction between cooling and Apgar scores was not statistically significant {F(1151)=1.13, p=0.259}.
Figure 1.
A: A bar graph showing the distribution of Apgar scores at 1, 5 and 10 min.
Table 1.
Outcomes of children at 6–7 years according to 10 min Apgar scores
| Apgar score N (%) |
Death |
Death/moderate/severe disability |
Death/IQ <70 |
Death/CP |
||||
|---|---|---|---|---|---|---|---|---|
| Hypothermia | Control | Hypothermia | Control | Hypothermia | Control | Hypothermia | Control | |
| Cohort (N=174) | 26 (29) | 39 (46) | 48 (53) | 56 (67) | 43 (48) | 55 (65) | 37 (41) | 53 (63) |
| 0 (n=24) | 6 (46) | 7 (64) | 10 (11) | 9 (82) | 10 (77) | 9 (82) | 9 (69) | 9 (82) |
| 1 (n=11) | 2 (67) | 5 (63) | 3 (100) | 7 (88) | 2 (67) | 6 (75) | 2 (67) | 6 (75) |
| 2 (n=14) | 3 (43) | 4 (57) | 6 (86) | 6 (86) | 6 (86) | 6 (86) | 5 (83) | 6 (86) |
| 3 (n=36) | 4 (27) | 13 (62) | 5 (33) | 18 (86) | 5 (33) | 18 (86) | 5 (33) | 18 (86) |
| 4 (n=38) | 8 (36) | 5 (31) | 12 (55) | 9 (56) | 10 (45) | 9 (56) | 10 (45) | 8 (50) |
| 5 (n=19) | 2 (13) | 1 (33) | 7 (44) | 1 (33) | 6 (38) | 1 (33) | 5 (31) | 1 (33) |
| 6 (n=20) | 1 (20) | 4 (27) | 1 (20) | 5 (33) | 1 (20) | 5 (33) | 1 (20) | 5 (33) |
| 7–10 (n=12) | 0 (0) | 0 (0) | 4 (44) | 1 (33) | 3 (33) | 1 (33) | 0 (0) | 0 (0) |
| Apgar score | Moderate/severe disability Hypothermia N (%) |
Control N (%) |
IQ <70 Hypothermia N (%) |
Control N (%) |
CP Hypothermia N (%) |
Control N (%) |
|---|---|---|---|---|---|---|
| Survivors to 6–7 years (N=109) | 22 (34) | 17 (40) | 17 (27) | 16 (36) | 11 (17) | 14 (31) |
| 0 (n=11) | 4 (57) | 2 (50) | 4 (57) | 2 (50) | 3 (43) | 2 (50) |
| 1 (n=4) | 1 (100) | 2 (67) | 0 (0) | 1 (33) | 0 (0) | 1 (33) |
| 2 (n=7) | 3 (75) | 2 (67) | 3 (75) | 2 (67) | 2 (67) | 2 (67) |
| 3 (n=19) | 1 (9) | 5 (71) | 1 (9) | 5 (63) | 1 (9) | 5 (63) |
| 4 (n=25) | 4 (29) | 4 (36) | 2 (14) | 4 (36) | 2 (14) | 3 (27) |
| 5 (n=16) | 5 (36) | 0 (0) | 4 (29) | 0 (0) | 3 (21) | 0 (0) |
| 6 (n=15) | 0 (0) | 1 (10) | 0 (0) | 1 (9) | 0 (0) | 1 (9) |
| 7–10 (n=12) | 4 (44) | 1 (33) | 3 (33) | 1 (33) | 0 (0) | 0 (0) |
One child is missing data for CP and two are missing data for moderate/severe disability. Five children were adjudicated as IQ <70 but had components of moderate/severe disability. CP, cerebral palsy.
Table 2.
Association between each point increase in 10 min Apgar scores and outcomes
| Outcome | 10 min Apgar Unadjusted OR (95% CI) |
Area under the curve for unadjusted OR |
10 min Apgar Adjusted OR (95% CI) |
|---|---|---|---|
| All children (N=174) | |||
| Death | 0.71 (0.60 to 0.84)*** | 0.69 | 0.69 (0.58 to 0.83)*** |
| Death or moderate/severe disability | 0.68 (0.57 to 0.81)*** | 0.70 | 0.68 (0.57 to 0.82)*** |
| Death or IQ <70 | 0.69 (0.58 to 0.81)*** | 0.71 | 0.68 (0.57 to 0.82)*** |
| Death or CP | 0.63 (0.52 to 0.75)*** | 0.74 | 0.64 (0.52 to 0.77)*** |
| Survivors to 6–7 years (N=109) | |||
| Moderate/severe disability | 0.78 (0.64 to 0.96)* | 0.64 | 0.79 (0.63 to 0.98)* |
| IQ <70 | 0.78 (0.63 to 0.96)* | 0.64 | 0.79 (0.63 to 0.99)* |
| CP | 0.67 (0.52 to 0.85)** | 0.73 | 0.69 (0.53 to 0.89)** |
ORs are adjusted for centre, birth weight, gestational age, gender, treatment group and outborn status. Apgar scores of 7–10 are combined due to small numbers of children with these scores.
p<0.05,
p<0.01,
p<0.001.
CP, cerebral palsy.
Figure 2.
Risk-adjusted probability of death or moderate/severe disability by treatment group and Apgar scores.
Among the 24 children with a 10 min Apgar score of 0, there were 11 survivors of whom five (20.8%) survived without moderate/severe disability and 3 underwent therapeutic cooling. Their median IQ was 90 (range 77–99); three had moderate and two had severe HIE at randomisation. Their median (range) scores for attention/executive function were 92 (72–110) and their median (range) scores for visuospatial function were 90 (82–132). Two of the infants had mild disability (IQs 77 and 83) while the remaining three had no disability. Five other survivors with 10 min Apgar scores of 0 had moderate/severe disability at both 18 months and 6–7 years of age and one had missing CP data.
Among those with Apgar scores at 10 min of 0–3 (n=85), subgroups of children who died or had moderate/severe disability (n=64) and survivors without disability (n=21) differed in the proportion of severe, rather than moderate, HIE, acidosis and time of onset of respiration (table 3).
Table 3.
Infants (n=85) with Apgar scores of 0–3 at 10 min: comparison based on survival without moderate/severe disability
| Characteristic mean±SD or N (%) |
Survived without moderate/severe disability (N=21) |
Died and/or moderate/severe disability (N=64) |
p Value |
|---|---|---|---|
| Male | 14 (67) | 34 (53) | 0.28 |
| Birth weight (kg) | 3443.9±622.5 | 3384.0±564.1 | 0.69 |
| Gestational age (weeks) | 38.8±1.2 | 39.0±1.5 | 0.60 |
| Intrapartum complications | |||
| Fetal decelerations | 14 (67) | 49 (77) | 0.37 |
| Cord prolapse | 4 (19) | 12 (19) | 0.98 |
| Uterine rupture | 3 (14) | 7 (11) | 0.68 |
| Maternal pyrexia | 1 (5) | 4 (6) | 0.79 |
| Shoulder dystocia* | 4 (19) | 3 (5) | 0.04 |
| Maternal haemorrhage | 1 (5) | 7 (11) | 0.40 |
| Emergency C-section | 15 (71) | 51 (80) | 0.43 |
| DR interventions | |||
| Oxygen | 21 (100) | 64 (100) | — |
| Bag and mask ventilation | 21 (100) | 62 (97) | 0.41 |
| Endotracheal intubation | 20 (95) | 63 (98) | 0.40 |
| Chest compressions* | 13 (62) | 58 (91) | 0.002 |
| Medications | 14 (67) | 55 (86) | 0.05 |
| Continued resuscitation at 10 min | |||
| Oxygen* | 17 (81) | 64 (100) | <0.001 |
| Bag and mask ventilation | 11 (52) | 42 (66) | 0.28 |
| Endotracheal intubation | 18 (86) | 60 (94) | 0.25 |
| Chest compressions | 11 (52) | 37 (58) | 0.61 |
| Medications | 11 (52) | 39 (61) | 0.49 |
| Time to spontaneous respiration ≥10 min* | 16 (76) | 57 (89) | 0.005 |
| Cord blood gas | |||
| pH* | 6.9±0.2 | 6.8±0.2 | 0.012 |
| Base deficit (meq/L)* | 16.5±7.6 | 23.4±7.9 | 0.016 |
| pH* | |||
| <7.0 | 7 (64) | 35 (92) | 0.018 |
| 7.01–7.15 n (%) | 4 (36) | 3 (8) | |
| First postnatal blood gas | |||
| pH* | 7.1±0.1 | 6.9±0.2 | 0.001 |
| Base deficit* | 16.7±4.9 | 21.3±8.1 | 0.025 |
| Inborn | 10 (48) | 28 (44) | 0.76 |
| Severe HIE* | 4 (19) | 41 (64) | <0.001 |
Among those who survived without moderate/severe disability, 21 had data on time to spontaneous respiration and 11 had data on cord pH and base deficit. Among those who died or had moderate/severe disability, 59 had data on time to spontaneous respiration, 38 had data on cord pH and 32 had data on base deficit. First postnatal gases are noted for infants who did not have cord blood gases available. Percentages are computed out of the number of children with data on the corresponding variable. DR, delivery room; HIE, hypoxic-ischaemic encephalopathy.
DISCUSSION
Among infants with perinatal HIE enrolled in the NICHD NRN whole body cooling RCT, some of whom underwent cooling, we found an independent association between 10 min Apgar scores and childhood outcomes at 6–7 years. The risk-adjusted probabilities of death/disability were lower in cooled infants with Apgar scores of 0–3 and the interaction between cooling and Apgar scores was not statistically significant, although the numbers to assess the effects of cooling were small. In an earlier report of the same NICHD NRN whole body cooling RCT cohort at the 18 month follow-up, death or disability occurred in 76%, 82% and 80% of infants with 10 min Apgar scores of 0, 1 and 2, respectively.8 Classification and regression tree analysis indicated that 10 min Apgar scores were discriminators of 18 -month outcome.8 In a secondary analysis of the CoolCap study, a higher Apgar score was associated with better 18 -month outcomes in univariate, but not in multivariate analysis.12
A few previous population-based Scandinavian studies have found an association between 5 min Apgar scores and childhood outcomes.13–15 Lie and colleagues found that among 543 064 singleton children born in Norway over a 10-year period and alive at 1 year of age, 988 had CP by 5 years of age.13 In children with a birth weight of ≥2500 g, an Apgar score <4 was associated with a 125-fold (95% CI 91 to 170) risk of CP compared with scores >8.13 In a similar previous cohort from Norway, children with 5 min Apgar scores of 0–3 had a 386-fold increased risk for neonatal death (95% CI 270 to 552) and an 81-fold (95% CI 48 to 138) increased risk for CP at 8– 12 years compared with those who had 5 min Apgar scores of 7–10.14 In a Swedish study, low 5 min (3 vs 6) Apgar scores in term infants were associated with a high risk for CP.15 Although these population-based studies demonstrate an association between low 5 min Apgar scores and later adverse outcomes, the relatively low prevalence of low Apgar scores limits their prognostic value.
Outcomes of infants with a 10 min Apgar score of 0 have been reported in a few previous studies, many with short-term follow-up without formal neurodevelopmental assessments.16,17 In one study involving 58 infants with a 10 min Apgar score of 0, only one survived with CP.16 Harrington and colleagues reported death or severe handicap in 94%, moderate handicap in 2%, mild handicap in 1% and undetermined outcome in 3% of 94 identified infants with 10 min Apgar scores of 0 who were resuscitated successfully.17 The follow-up durations for the three surviving infants in the hospital-based cohort were 11 months, 2 years and 5 years, respectively.17 In another single centre study on 85 infants with perinatal HIE who underwent cooling, 10 min Apgar scores of 0 (noted in 12 infants) was significantly associated with death until 9 months (OR 51.7; 95% CI 9.9 to 269.5).18 Nine infants died, two had spastic quadriparesis and global delay at 18–24 months and one had extensive encephalo-malacia on MRI.18 In our previous report of 18-month follow-up, among 25 infants with a 10 min Apgar score of 0, 19 (76%) either died or had moderate or severe disability; the six (24%) survivors without moderate or severe disability had a mean Mental Developmental Index of 87 (range 73–100).8 The results from the current 6–7-year follow-up of the same cohort are consistent. Five (21%) children with 10 min Apgar scores of 0, three of whom underwent cooling, survived with IQs between 77 and 99, normal visuospatial and attention/executive functions and without CP. Our results are different from the previous literature, however, and it is not entirely clear why. Studies prior to the era of therapeutic hypothermia have suggested that infants who survive following severe asphyxia either have motor deficits or are non-disabled—an ‘all or none’ effect although they did not evaluate higher cognitive function.19,20 In our study, standardised neurodevelopmental assessments were performed by examiners trained to reliability at similar times and data were systematically collected with uniform definitions. Our study population was limited to infants who were enrolled in the whole body cooling RCT.
The decision of how long to continue resuscitations is difficult, especially when low Apgar scores occur unexpectedly in otherwise uncomplicated pregnancies. Prior resuscitation guidelines have suggested limiting the duration of resuscitation in infants born without a heart rate and in whom the heart rate has not returned until 10 min in view of the high mortality and adverse neurodevelopmental outcomes in survivors. Our results confirm that the risk of a poor outcome at school age is increased with progressively lower Apgar scores at 10 min. However, the better than expected outcomes of almost 50% of survivors with 10 min Apgar scores of 0, at both 18–22 months and 6–7 years of age, make the decision regarding limiting resuscitation to a certain duration even more difficult. The 2011 International Liason Committee on Resuscitation and Neonatal Resuscitation Program recommendations on the duration of resuscitation are nuanced and recognise that the decision to continue resuscitation may be influenced by the aetiology of the arrest, gestational age, time of initiation of resuscitation, the potential role of therapeutic hypothermia and parental views.21,22
Our exploratory data suggest that acidosis, need for and response to DR resuscitation interventions and HIE severity may be objective early prognostic indicators in this population. Cord blood acidosis and HIE severity are biologically plausible attributes, which have previously been shown to have predictive value in HIE.23–27
We recognise the limitations of our study. The RCT eligibility criteria included birth in or transport into a NRN site within 6 h of birth and excluded infants for whom no aggressive therapy was planned; DR deaths were not included. Although 86% of the original RCT cohort were part of the current analysis, those excluded differed in some key aspects from the study cohort. The ‘quality’ of resuscitation was not measured. The inherent subjectivity of Apgar scores was a limitation. The data analysis among infants with 10 min Apgar scores of 0–3 to delineate perinatal variables associated with favourable outcomes and the effects of cooling involved small numbers and should be considered exploratory. Because of the effects of cooling on mortality, cooled infants were over-represented (58.7%) among survivors at 6–7 years.24
Nonetheless, the study included a large cohort of infants with standardised school-age assessments. Detailed data on DR interventions and perinatal events were available. HIE was diagnosed by stringent clinical and biochemical criteria and managed according to a consistent protocol in tertiary level centres. The results from this study underscore the prognostic utility of a 10 min Apgar score and that about a fifth of infants with 10 min Apgar scores of 0 may survive unimpaired to school age.
Supplementary Material
What is already known on this topic.
The Apgar score is extensively used to evaluate an infant’s condition shortly after delivery. Population-based studies have demonstrated an association between low Apgar scores at 10 min and adverse neurodevelopmental outcomes. The National Institute of Child Health and Human Development whole body cooling randomised controlled trial cohort showed a 45% increase in odds of death or disability at 18 months with each point decrease in Apgar score at 10 min.
What this study adds.
This is the first evaluation of the association between school-aged outcomes and 10 min Apgar scores in the era of therapeutic cooling. Around a fifth of babies with no sign of life at 10 min may survive without moderate or severe disability. Among infants with perinatal hypoxic-ischaemic encephalopathy, 10 min Apgar scores correlate with childhood outcomes at 6–7 years of follow-up.
Acknowledgements
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study: NRN steering committee Chairs: Alan H. Jobe, MD PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–present). Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904)—Abbot R. Laptook, MD; William Oh, MD; Angelita M. Hensman, RN BSN; Lucy Noel; Victoria E. Watson, MS CAS. Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80)—Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Nancy Bass, MD; Harriet G. Friedman MA; Nancy S. Newman, BA RN; Bonnie S. Siner, RN. Cincinnati Children’s Hospital Medical Center and University of Cincinnati Hospital (U10 HD27853, M01 RR8084)—Kurt Schibler, MD; Edward F. Donovan, MD; Kate Bridges, MD; Jean J. Steichen, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Holly L. Mincey, RN BSN; Jody Hessling, RN; Teresa L. Gratton, PA. Duke University School of Medicine, University Hospital, Alamance Regional Medical Center and Durham Regional Hospital (U10 HD40492, M01 RR30)—Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Kathryn E. Gustafson, PhD; Kathy J. Auten, MSHS; Katherine A. Foy, RN; Kimberley A. Fisher, PhD FNP-BC IBCLC; Sandy Grimes, RN BSN; Melody B. Lohmeyer, RN MSN. Emory University, Grady Memorial Hospital and Emory University Hospital Midtown (U10 HD27851, M01 RR39)—Barbara J. Stoll, MD; David P. Carlton, MD; Lucky Jain, MD; Ann M. Blackwelder, RNC BS MS; Ellen C. Hale, RN BS CCRC; Sobha Fritz, PhD. Eunice Kennedy Shriver National Institute of Child Health and Human Development—Linda L. Wright, MD; Elizabeth M. McClure, MEd; Stephanie Wilson Archer, MA. Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children and Wishard Health Services (U10 HD27856, M01 RR750)—Brenda B. Poindexter, MD MS; James A. Lemons, MD; Diana D. Appel, RN BSN; Jessica Bissey, PsyD HSPP; Dianne E. Herron, RN; Lucy C. Miller, RN BSN CCRC; Leslie Richard, RN; Leslie Dawn Wilson, BSN CCRC. RTI International (U10 HD36790)—W. Kenneth Poole, PhD; Jeanette O’Donnell Auman, BS; Margaret Cunningham, BS; Betty K. Hastings; Jamie E. Newman, PhD MPH; Carolyn M. Petrie Huitema, MS; Scott E. Schaefer, MS; Kristin M. Zaterka-Baxter, RN BSN. Stanford University and Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70)—Krisa P. Van Meurs, MD; Susan R. Hintz, MD MS Epi; David K. Stevenson, MD; M. Bethany Ball, BS CCRC; Maria Elena DeAnda, PhD. University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32)—Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Kathleen G. Nelson, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Vivien A. Phillips, RN BSN; Laurie Lou Smith, EdS NCSP. University of California-San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461)—Martha G. Fuller, RN MSN; Radmila West PhD; Neil N. Finer, MD; David Kaegi, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Chris Henderson, RCP CRTT; Wade Rich, BSHS RRT. University of Miami Holtz Children’s Hospital (U10 HD21397, M01 RR16587)—Shahnaz Duara, MD; Sylvia Hiriart-Fajardo, MD; Mary Allison, RN; Maria Calejo, MS;Ruth Everett-Thomas, RN MSN; Silvia M. Frade Eguaras, MA; Susan Gauthier, BA. University of Rochester Medical Center, Golisano Children’s Hospital (U10 HD40521, M01 RR44)—Dale L. Phelps, MD; Gary J. Myers, MD; Diane Hust, MS RN CS; Linda J. Reubens, RN CCRC. University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System and Children’s Medical Center Dallas (U10 HD40689, M01 RR633)—Pablo J. Sánchez, MD; R. Sue Broyles, MD; Abbot R. Laptook, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Roy J. Heyne, MD; Cathy Boatman, MS CIMI; Cristin Dooley, PhD LSSP; Gaynelle Hensley, RN; Jackie F. Hickman, RN; Melissa H. Leps, RN; Susie Madison, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Lizette E. Torres, RN; Alicia Guzman. University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373, M01 RR2588)—Jon E. Tyson, MD MPH; Kathleen A. Kennedy, MD MPH; Esther G. Akpa, RN BSN; Patty A. Cluff, RN; Claudia I. Franco, RN BSN; Anna E. Lis, RN, BSN; Georgia E. McDavid, RN; Patti L. Pierce Tate, RCP; Nora I. Alaniz, BS; Pamela J. Bradt, MD MPH; Magda Cedillo; Susan Dieterich, PhD; Patricia W. Evans, MD; Charles Green, PhD; Margarita Jiminez, MD; Terri Major-Kincade, MD MPH; Brenda H. Morris, MD; M. Layne Poundstone, RN BSN; Stacey Reddoch, BA; Saba Siddiki, MD; Maegan C. Simmons, RN; Laura L. Whitely, MD; Sharon L. Wright, MT; Lourdes M. Valdés PhD. Wayne State University, Hutzel Women’s Hospital and Children’s Hospital of Michigan (U10 HD21385)—Yvette R. Johnson, MD MPH; Laura A. Goldston, MA; Geraldine Muran, RN BSN; Deborah Kennedy, RN BSN; Patrick J. Pruitt, BS. Yale University, Yale-New Haven Children’s Hospital (U10 HD27871, M01 RR125, UL1 RR24139)—Patricia Gettner, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Elaine Romano, MSN; Joanne Williams, RN BSN; Susan DeLancy, MA CAS.
Funding
The Neonatal Research Network’s Whole-Body Hypothermia Trial and its 6–7 Year School-age Follow-up were provided grant support by the National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Grant numbers are provided for each site above. Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating centre (DCC) for the network, which stored, managed and analysed the data for this study. On behalf of the NRN, Dr Abhik Das (DCC Principal Investigator) and Mr Scott A. McDonald (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
Contributors All the authors have seen and approved the manuscript in its present form and accept full responsibility for the contents of the reported research. All authors contributed to the concept, design, analysis, interpretation of data and provided critical input into revising the manuscript.
Competing interests None.
Ethics approval Investigational review board of the human investigational committee of all participating sites of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating centre (DCC) for the network, which stored, managed and analysed the data for this study.
REFERENCES
- 1.American Academy of Pediatrics, Committee on Fetus and Newborn; American College of Obstetricians and Gynecologists and Committee on Obstetric Practice. The Apgar score. Pediatrics. 2006;117:1444–1447. [Google Scholar]
- 2.Moster D, Lie RT, Markestad T. Joint association of Apgar scores and early neonatal symptoms with minor disabilities at school age. Arch Dis Child Fetal Neonatal Ed. 2002;86:F16–F21. doi: 10.1136/fn.86.1.F16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson KB, Ellenberg JH. Obstetric complications as risk factors for cerebral palsy or seizure disorders. JAMA. 1984;251:1843–1848. [PubMed] [Google Scholar]
- 4.Casey BM, McIntire DD, Leveno KJ. The continuing value of the Apgar score for the assessment of newborn infants. N Engl J Med. 2001;344:467–471. doi: 10.1056/NEJM200102153440701. [DOI] [PubMed] [Google Scholar]
- 5.Nelson KB, Ellenberg JH. Apgar scores as predictors of chronic neurologic disability. Pediatrics. 1981;68:36–44. [PubMed] [Google Scholar]
- 6.Casalaz DM, Marlow N, Speidel BD. Outcome of resuscitation following unexpected apparent stillbirth. Arch Dis Child Fetal Neonatal Ed. 1998;78:F112–F115. doi: 10.1136/fn.78.2.f112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankaran S, Laptook AR, Ehrenkranz RE, et al. Whole body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 8.Laptook AR, Shankaran S, Ambalavanan N, et al. Outcome of term infants using Apgar scores at 10 minutes following hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:1619–1626. doi: 10.1542/peds.2009-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankaran S, Pappas A, McDonald SA, et al. NICHD Neonatal Research Network Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cans C. Surveillance of Cerebral Palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816–824. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- 11.Palisano RJ, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 12.Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–921. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 13.Lie KK, Groholt Ek, Eskild A. Association of cerebral palsy with Apgar score in low and normal birthweight infants: population-based cohort study. BMJ. 2010;341:c4990. doi: 10.1136/bmj.c4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moster D, Lie RT, Irgens LM, et al. The association of Apgar score with subsequent death and cerebral palsy: a population-based study in term infants. J Pediatr. 2001;138:798–803. doi: 10.1067/mpd.2001.114694. [DOI] [PubMed] [Google Scholar]
- 15.Thorngren-Jerneck K, Herbst A. Perinatal factors associated with cerebral palsy in children born in Sweden. Obstet Gynecol. 2006;108:1499–505. doi: 10.1097/01.AOG.0000247174.27979.6b. [DOI] [PubMed] [Google Scholar]
- 16.Jain L, Ferre C, Vidyasagar D, et al. Cardiopulmonary resuscitation of apparently stillborn infants: survival and long-term outcome. J Pediatr. 1991;118:778–782. doi: 10.1016/s0022-3476(05)80046-0. [DOI] [PubMed] [Google Scholar]
- 17.Harrington DJ, Redman CW, Moulden M, et al. The long term outcome in surviving infants with Apgar zero at 10 minutes: a systematic review of the literature and hospital-based cohort. Am J Obstetr Gynecol. 2007;463:e1–e5. doi: 10.1016/j.ajog.2006.10.877. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar S, Bhagat I, Dechert RE, et al. Predicting death despite therapeutic hypothermia in infants with hypoxic-ischemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95:F423–F428. doi: 10.1136/adc.2010.182725. [DOI] [PubMed] [Google Scholar]
- 19.Thomson AJ, Searle M, Russell G. Quality of survival after severe birth asphyxia. Arch Dis Child. 1977;52:620–626. doi: 10.1136/adc.52.8.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dweck HS, Huggins W, Dorman LP, et al. Developmental sequelae in infants having severe perinatal asphyxia. Am J Obstet Gynecol. 1974;119:811–815. doi: 10.1016/0002-9378(74)90094-5. [DOI] [PubMed] [Google Scholar]
- 21.Perlman JM, Wyllie J, Kattwinkel J, et al. Neonatal Resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with treatment recommendations. Circulation. 2010;122(suppl2):S516–S538. doi: 10.1161/CIRCULATIONAHA.110.971127. [DOI] [PubMed] [Google Scholar]
- 22.Kattwinkel J, Perlman JM, Aziz K, et al. Neonatal Resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics. 2010;126:e1400. doi: 10.1542/peds.2010-2972E. [DOI] [PubMed] [Google Scholar]
- 23.Ambalavanan N, Carlo WA, Shankaran S, et al. National Institute of Child Health and Human Development Neonatal Research Network. Predicting outcomes of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics. 2006;118:2084–93. doi: 10.1542/peds.2006-1591. [DOI] [PubMed] [Google Scholar]
- 24.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunn AJ, Wyatt JS, Whitelaw A, et al. CoolCap Study Group Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr. 2008;152:55–58. doi: 10.1016/j.jpeds.2007.06.003. 58.e1. [DOI] [PubMed] [Google Scholar]
- 26.Shankaran S, Laptook AR, Tyson JE, et al. NICHD Neonatal Research Network. Evolution of encephalopathy during whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2012;160:567–572. doi: 10.1016/j.jpeds.2011.09.018. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodwin TM, Belai I, Hernandez P, et al. Asphyxial complications in the term newborn with severe umbilical academia. Am J Obster Gynecol. 1992;162:1506–1512. doi: 10.1016/0002-9378(92)91728-s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.