Abstract
Iron deficiency chlorosis (IDC) is a yield limiting problem in soybean (Glycine max (L.) Merr) production regions with calcareous soils. Genome-wide association study (GWAS) was performed using a high density SNP map to discover significant markers, QTL and candidate genes associated with IDC trait variation. A stepwise regression model included eight markers after considering LD between markers, and identified seven major effect QTL on seven chromosomes. Twelve candidate genes known to be associated with iron metabolism mapped near these QTL supporting the polygenic nature of IDC. A non-synonymous substitution with the highest significance in a major QTL region suggests soybean orthologs of FRE1 on Gm03 is a major gene responsible for trait variation. NAS3, a gene that encodes the enzyme nicotianamine synthase which synthesizes the iron chelator nicotianamine also maps to the same QTL region. Disease resistant genes also map to the major QTL, supporting the hypothesis that pathogens compete with the plant for Fe and increase iron deficiency. The markers and the allelic combinations identified here can be further used for marker assisted selection.
Introduction
Iron (Fe) is an essential element for multiple plant functions including photosynthesis, respiration, chlorophyll biosynthesis, and redox reactions in plants, and is a structural component in heme, the Fe-sulfur cluster, and Fe-binding sites. To avoid toxicity, plants control the uptake, utilization, and storage in response to Fe availability [1]. Despite its abundance in the soil, Fe is only slightly soluble under aerobic conditions, especially in high-pH and calcareous soils [2]. Under these conditions, iron deficiency leads to developmental defects, including chlorosis, growth retardation, and reduced crop productivity [3]. Iron deficiency chlorosis (IDC) is an important yield-limiting factor for soybeans (G. max) grown on calcareous soils that have a high percentage of calcium carbonate and soluble salts. This soil type is common in the north-central regions of the United States [4] where soybean is widely grown. In these soils, interactions of high pH, carbonate and high field moisture content at planting leads to early IDC symptoms that effect yield. It is estimated that the current revenue losses due to IDC in soybean are $260 million [5].
IDC results from the inability of susceptible genotypes to efficiently mobilize iron into the plant when it is growing in high pH calcareous soils. In these soils, ferrous iron is not readily released from soil particles, and subsequently iron availability is limited. Based on its response to Fe availability, soybean is considered a Strategy I plant [6] that reduces ferric chelates at the root surface and absorbs ferrous ions across the plasma membrane of the root [2]. Other processes involved in Strategy I include excretion of proton and phenolic compounds from the roots to the rhizosphere, which increases the solubility of ferric ions and support the reducing capacity of ferric Fe on the root surface [2]. Strategy I plants also increase root hair formation, thereby increasing the surface area available for iron uptake [7]. Once iron enters the root, membrane transporters move Fe into the xylem where it chelates with citrate. The chelated form of iron then moves through the xylem stream to growing leaves. Iron is then remobilized from the leaves, forms a complex with nicotianamine, and is transported via the phloem to younger leaves and seeds. Excess water leads to an elevated concentration of bicarbonates in the root apoplast that impedes the Fe3+– chelate reductase activity necessary for the conversion of Fe3+ to Fe2+. Bicarbonates also immobilize the movement of iron to young leaves once it is absorbed at the root level [8].
Given the many physiological mechanisms associated with iron metabolism, it is not surprising that IDC is a complex quantitative trait with multiple genetic factors [9]. In addition to the genes involved in Fe acquisition, translocation and compartmentalization within the organelles, there are other genes involved in Fe transcriptional and post transcriptional changes that are expressed/repressed under deficient and excess conditions or affect iron homeostasis pathways [10]–[17].
Genetic experiments using bi-parental mapping populations have identified multiple quantitative trait loci (QTL) associated with IDC in soybean [11]–[12]. O’Rourke et al. [13] identified several genes induced under iron-limiting conditions using a microarray study. Severin et al. [14] discovered multiple introgressed genomic regions responsible for IDC in near isogenic lines, and Stec et al. [15] further refined these introgression regions. Recently, Peiffer et al. [5] evaluated candidate genes on Gm03.
A genome-wide association study (GWAS) is an excellent approach to discover genetic factors in a population because of the high number of recombinant events the population represents. GWAS uses the linkage disequilibrium (LD) pattern in a large population of unrelated individuals [16] to map significant effect loci. Since IDC is a complex trait, utilizing GWAS can identify major factors controlling the IDC response in soybean. Previously, Wang et al. [17] used GWAS to identify simple sequence repeat (SSR) markers associated with IDC in two independent populations. Later, nine shared SNP were discovered and confirmed by Mamidi et al. [9] in two separate populations. The objective of this study was to identify QTL and genes involved in IDC of soybean using a GWAS population and a new high density SNP map obtained through genotype-by-sequencing (GBS). Those results were then translated into SNP markers that can be used for marker assisted selection (MAS).
Materials and Methods
Phenotype and genotyping
Two plant populations consisting of unique set of advanced soybean breeding lines developed for the north-central region of the US were grown in years 2005 (n = 132) and 2006 (n = 138). IDC screening protocols and DNA isolation procedures were described previously [9]. The trait was rated on a scale of 1 to 5, where 1 indicates no chlorosis and a normal green plant, 2 indicates slight yellowing of upper leaves and the leaf veins, 3 indicates interveinal chlorosis in the upper leaves with no stunning of growth or death of tissue (necrosis), 4 indicates interveinal chlorosis of the upper leaves along with some apparent stunning of growth or necrosis of tissue, and 5 indicates severe chlorosis plus stunned growth and necrosis in the youngest leaves and growing points [9]. Phenotypic data was adjusted based on least squares means for each population independently.
GBS libraries were prepared and analyzed at the Institute for Genomic Diversity (IGD), Cornell University, according to Elshire et al. [18], using the enzyme ApeKI. The GBS analysis pipeline [3.0.128], an extension of the Java program TASSEL [19], was used to call SNPs from the sequenced GBS library. Reads were aligned to the soybean reference genome v1.1 [20] using BWA 0.6.1 [21]. VCFtools (v0.1.8) [22] was used to summarize the SNP data.
The data was analyzed by combining the two populations (which are of similar size) to account for unique alleles that are at a low frequency in one or both populations. These low frequency alleles can have a significant phenotypic effect in a small set of genotypes, but that effect cannot be detected in a normal statistical framework [16].
Genome-wide association study
Imputation
fastPHASE 1.3 [23], a likelihood-based imputation software, was used with default settings to impute missing data for the combined population. Imputation analysis was performed to increase the power of the study and fine map the causal variant [24]. A minor allele frequency (MAF) of 0.05 was used as cutoff, since variants at low frequency have little power to detect association with the phenotype [16].
Linkage disequilibrium (LD)
The LD between markers was estimated as the partial squared allele frequency correlation (r2) using CORR procedure in SAS 9.3. Three principal components (PC) that explain about 25% of cumulative variation were used as cofactors. We used a partial correlation because unlinked loci can be in LD simply because of population structure [25]–[26]. The decay of r2 with physical and/or genetic distance between loci is often used to determine the density of markers to use in whole genome association scans [27] whereas local LD on chromosomes is used to account for genes/QTL associated with trait variation. Overestimation of LD can lead to misinterpretations either on the extent of LD decay or on the size of the QTL [26]. LD decay graphs were plotted with physical distance (Mbp) vs. r2 for all intra-chromosomal comparisons using nonlinear regression as described by Remington et al. [28]. The expected decay of LD was estimated as described by Pyhajarvi et al. [29]. We fit this equation into a nonlinear regression model using NLIN procedure in SAS 9.3.
Population structure, kinship, and model testing
A principal component analysis was utilized to control for population structure [30]. PCs were estimated using the PRINCOMP procedure in SAS 9.3. The PCs that explained 25% (PC25) and 50% (PC50) cumulative variation were selected for the analysis. In addition an identity-by-state matrix [31] was used to control for relatedness estimated as a centered relatedness matrix in Gemma 0.92 [32]. Gower’s similarity coefficient was calculated using the DISTANCE procedure in SAS 9.3 to measure the relatedness by state of the individuals.
To test for marker-trait associations, the MIXED procedure in SAS for the three linear models that do not have kinship (Naïve, PC25 and PC50) was used. Since approximate tests compromise the analysis of complex genetic architectures when one genetic factor masks another [33], we used the exact test implemented in Gemma 0.92 to estimate marker significance [32] for the other three models with kinship (Kinship, PC25+kinship, PC50+kinship). As the standard errors follow a uniform distribution [34], we used a rank based mean squared difference (MSD) for model selection as described in Mamidi et al [9].
Marker-trait associations
Markers are defined as significant at two levels, within 0.01 percentile, and 0.1 percentile tails of the empirical p-value distribution of 10,000 bootstraps. This method of using an empirical distribution of data as a replacement for a population with an unknown distribution has the advantage of simplicity and provides an efficient and precise estimation [35]–[36]. This approach is similar to choosing an arbitrary value but instead it is based on choosing a predefined percentile tail from an empirical distribution. Here, the confidence intervals for an empirical distribution were obtained from 10,000 bootstraps over ∼35,000 markers distributed throughout the genome. We chose this approach over a cutoff because p-value is dependent on the distribution of phenotype, the variation explained by the marker, structure and relatedness of the population, and heritability of the trait.
The means for the alleles were calculated using MEANS procedure in SAS. The percent of variation explained by the marker is calculated using a simple regression (GLM procedure) in SAS. The additive effect of the variant allele is calculated as half the difference between IDC mean of the variant allele and IDC mean of the reference allele.
To determine the minimal number of SNPs independently associated with IDC, stepwise regression analysis was applied to all significant markers from a single-SNP analysis at a significance level of 0.1 percentile [37]. In addition, a multiple R2 value for all these loci was also estimated [9]. A significant p-value of 0.05 was necessary for both marker and model for stepwise inclusion of the marker in REG procedure in SAS 9.3.
Annotation of SNP
To characterize SNPs as intergenic, UTR, intron, or coding site, snpEFF 3.3 [38] was used. Coding sites were further differentiated into synonymous or non-synonymous substitutions based on amino acid changes. Coding regions were also differentiated into transitions and transversions based on changes between or among purines and pyrimidines.
Significant QTL and genes
A QTL region is defined as the region around significant stepwise markers that has a partial LD (r2) >0.6 with the adjacent marker (corresponds to partial correlation of 0.77). Adjacent blocks were combined if the distance is less than 10 kb and includes up to four markers that are not in LD with either of the block. Candidate genes for iron homeostasis were identified from the research literature, and gene symbols are used from the annotation file available at phytozome (http://www.phytozome.net/soybean.php). A candidate iron gene is considered to be significant in our population if the gene is within the QTL region.
Epistatic interactions
To detect epistatic interactions between significant markers, we used a general linear model with both significant markers and their interaction term in the model. We used PC25 as covariates in the regression model and an interaction term was considered significant at a p-value of≤1E-03. Genes that are in LD with these significant markers are used to identify the gene-by-gene interactions.
Significant allelic combinations
We compared the allelic combinations of the significant markers (0.1 percentile) that were included in stepwise regression. Allelic combinations with a mean IDC<2.5 were termed tolerant, and those with a mean IDC>3.5 were considered susceptible. We chose these values based on standard genotypes grown in the field instead of a statistical approach of mean differentiation, because not all allelic combinations are present in the population. With this approach, the phenotypic distribution does not need to be continuous, where statistically two allelic combinations can be considered significantly different in the absence of intermediate phenotypes.
Results
Phenotype and genotyping
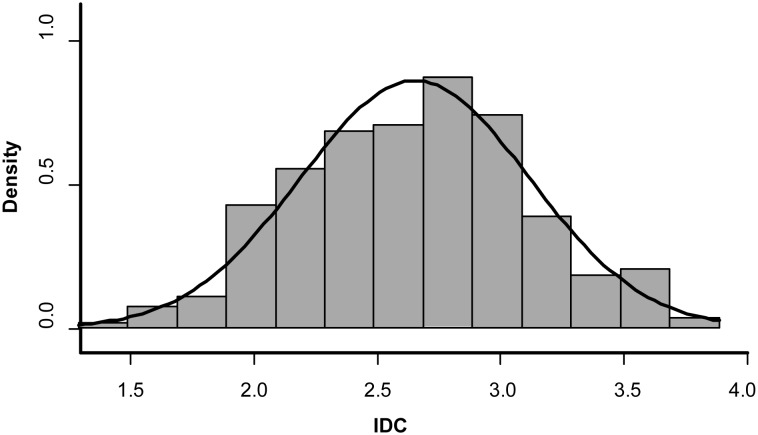
For the combined population, the range of IDC is 1.46 to 3.84 with an average of 2.76. IDC scores were normally distributed with a p-value of 0.15 for Kolmogorov–Smirnov test (Figure 1). An average of 1,645,563 reads was obtained for each genotype, and ∼92% of reads mapped to the reference genome. A total of 79,000 putative SNPs that are bi-allelic were identified in the population using the TASSEL-GBS analysis pipeline after removing SNPs that have greater than 50% missing data. The mean coverage depth for each identified SNP was 6.85±2.72.
Figure 1. Phenotypic distribution of iron deficiency chlorosis (IDC).
The rating of IDC (1–5) is on x-axis and the number of genotypes having the IDC score (represented as density) are presented as y-axis. The curve over the histogram represents the normal distribution of the IDC scores in the population.
Association Mapping
Imputation
Missing data (37.5%) was estimated using imputation. Of the 79,000 SNPs, 34,428 had a MAF>0.05 (Table S1). Based on the genome size of Glycine max, a polymorphic marker was located, on average, every 31 kbp. In addition, the maximum distance between two adjacent markers did not exceed 500 Kbp providing a good coverage of the SNPs. Of these polymorphic SNPs, 6,520 mapped to a coding region, 6,898 are located in the non-coding region of a gene model and 21,010 are in an intergenic region. The transition to transversions ratio was 1.67.
Linkage Disequilibrium Decay
A nonlinear regression model that fits the partial r2 of pairwise intra-chromosomal comparisons to physical distance was developed. The average decay of LD (r2) in terms of physical distance declined to r2 = 0.7 at ∼500 kb (Figure S1). The physical distance at which r2 = 0.2 is 3.2 Mbp and at r2 = 0.1 was 8.0 Mbp.
Population structure, Kinship and model selection
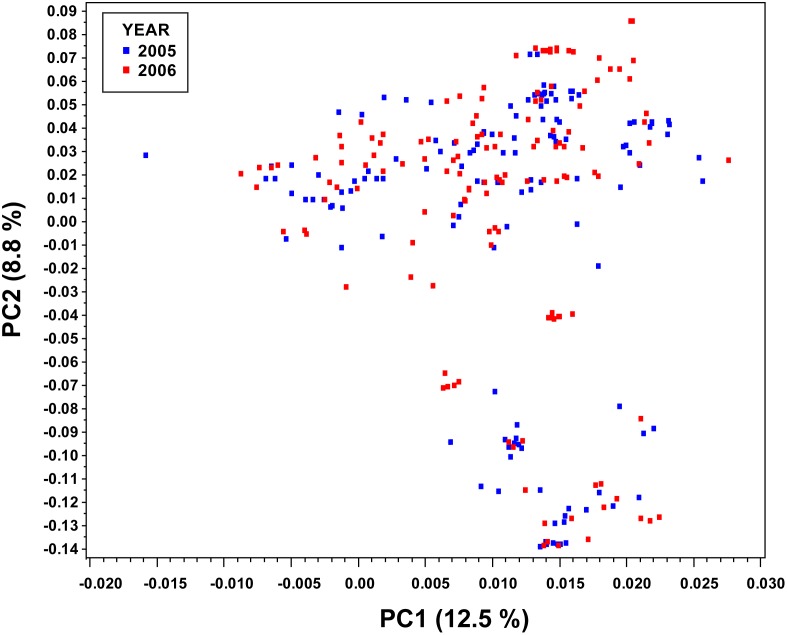
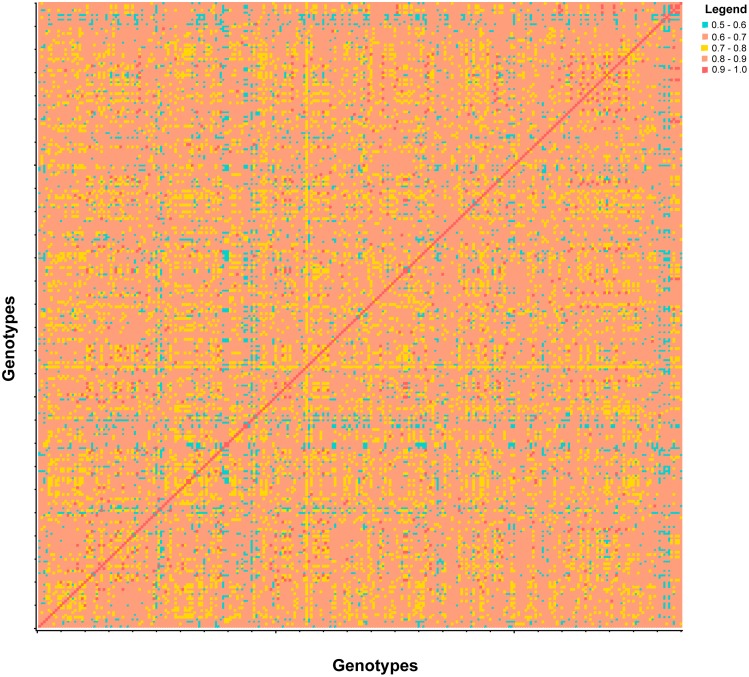
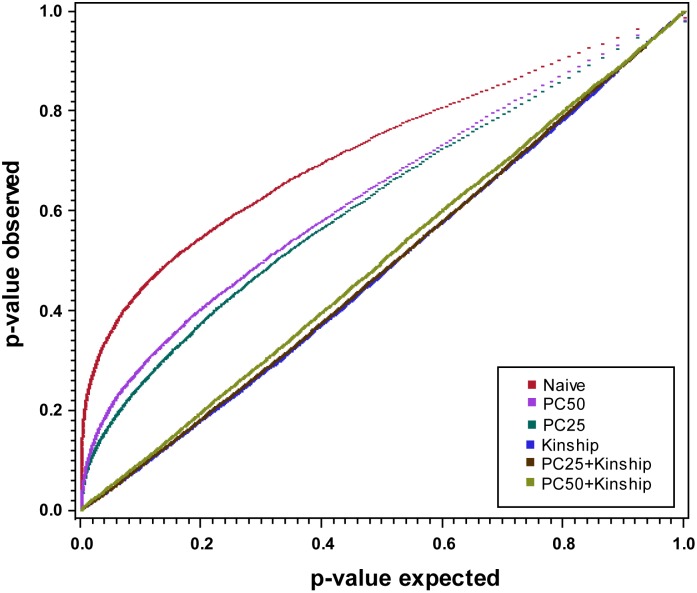
For the population, three and 13 PC explained 26.8% and 50.5% of the cumulative genotypic variation. Based on the first two PCs, two distinct clusters of populations were observed which indicated the presence of a subpopulation structure (Figure 2). In the population, 80% of the Gowers similarity coefficients are within the range of 0.6 to 0.7 (Figure 3) indicating the presence of relatedness among individuals. This is expected given the narrow genetic base of the breeding population. For the six models tested, the best linear model for the population included 13 PCs (PC50) and kinship matrix (MSD = 6.06E-06; Figure 4).
Figure 2. A graph of the first two principal components obtained from all the 34,428 polymorphic SNPs.
This graph explains the similarities between the two independent populations and the overall population structure.
Figure 3. Heatmap of Gower’s similarity distance among the individuals of the population.
Based on the colors distribution, it is evident that relatedness can have a major effect on identifying associations to IDC in the population.
Figure 4. QQ plot for the six models tested.
P-value observed is plotted on the y-axis and P-expected is plotted on the x-axis. Each color represents a model of regression used. The best model is the one that is close to the diagonal line.
Marker Trait associations
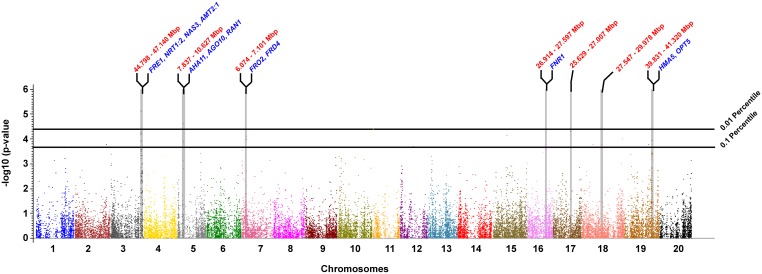
Five markers were significant at the 0.01 percentile level (p≤4.0E-05). One located on Gm03 at 45.03 Mbp, one on Gm05 at 8.82 Mbp and three on Gm11 at 0.53 Mbp (Table 1, Fig. 5). The variation explained by these markers ranged from 5% to 16%, and the mean allelic difference is between 0.31 and 0.38. Stepwise regression included three markers (one from each of the chromosome). Together these three markers explained 23.2% of phenotypic variation.
Table 1. Significant markers associated with IDC in the population of soybean.
| Chromosome | Position | –log10 (p-value) | Reference Allele | Variant Allele | R2 § | MAF§§ | Mean Difference | Additive Effect (Variant allele) | Included in stepwise regression§§§ |
| Gm02 | 46,939,135 | 3.784** | A | T | 5.20 | 21.48 | 0.26 | −0.13 | |
| Gm03 | 44,927,455 | 4.165** | A | G | 15.20 | 38.89 | 0.37 | −0.19 | |
| Gm03 | 44,930,187 | 4.165** | A | T | 15.20 | 38.89 | 0.37 | −0.19 | |
| Gm03 | 44,970,860 | 4.275** | A | G | 14.42 | 39.26 | 0.36 | −0.18 | |
| Gm03 | 44,985,022 | 4.165** | G | A | 15.20 | 38.89 | 0.37 | −0.19 | |
| Gm03 | 45,031,929 | 5.127*** | T | C | 15.77 | 38.89 | 0.38 | −0.19 | x |
| Gm03 | 45,038,912 | 3.684** | C | T | 14.71 | 39.26 | 0.36 | −0.18 | |
| Gm03 | 45,107,141 | 3.905** | C | A | 13.87 | 45.56 | 0.35 | −0.17 | |
| Gm03 | 45,343,317 | 3.689** | G | T | 17.28 | 42.59 | 0.39 | −0.19 | |
| Gm03 | 45,343,612 | 4.231** | A | T | 18.85 | 41.48 | 0.41 | −0.20 | |
| Gm05 | 8,817,714 | 3.912** | G | A | 10.81 | 49.26 | 0.30 | 0.15 | |
| Gm05 | 8,823,135 | 4.617*** | G | A | 11.43 | 49.63 | 0.31 | 0.16 | |
| Gm05 | 8,877,264 | 3.855** | G | T | 8.30 | 45.93 | 0.27 | 0.13 | x |
| Gm05 | 17,397,118 | 3.704** | G | A | 0.08 | 39.63 | 0.03 | 0.01 | |
| Gm05 | 17,397,130 | 3.704** | T | C | 0.08 | 39.63 | 0.03 | 0.01 | |
| Gm05 | 17,397,135 | 3.704** | C | T | 0.08 | 39.63 | 0.03 | 0.01 | |
| Gm07 | 6,397,319 | 3.961** | C | T | 8.80 | 25.56 | 0.32 | −0.16 | x |
| Gm07 | 6,405,421 | 3.961** | T | C | 8.80 | 25.56 | 0.32 | −0.16 | |
| Gm07 | 6,540,541 | 4.128** | G | A | 8.10 | 26.30 | 0.30 | −0.15 | |
| Gm07 | 6,575,306 | 3.961** | A | G | 8.80 | 25.56 | 0.32 | −0.16 | |
| Gm11 | 530,116 | 4.401*** | A | G | 4.73 | 8.89 | 0.35 | 0.18 | x |
| Gm11 | 530,117 | 4.401*** | A | C | 4.73 | 8.89 | 0.35 | 0.18 | |
| Gm11 | 530,118 | 4.401*** | G | T | 4.73 | 8.89 | 0.35 | 0.18 | |
| Gm12 | 20,406,669 | 3.697** | T | C | 0.02 | 20.74 | 0.01 | 0.01 | |
| Gm15 | 21,422,057 | 4.152** | A | G | 2.10 | 20.37 | 0.17 | 0.08 | |
| Gm16 | 27,300,116 | 3.779** | T | G | 4.80 | 8.52 | 0.36 | 0.18 | x |
| Gm17 | 25,859,992 | 4.322** | T | C | 0.66 | 6.67 | 0.15 | 0.08 | x |
| Gm17 | 25,859,995 | 4.322** | T | C | 0.66 | 6.67 | 0.15 | 0.08 | |
| Gm18 | 15,905,372 | 3.781** | T | C | 3.25 | 28.52 | 0.18 | 0.09 | |
| Gm18 | 28,141,888 | 3.786** | G | A | 3.78 | 22.59 | 0.22 | 0.11 | x |
| Gm18 | 59,284,729 | 4.006** | C | T | 0.70 | 45.93 | 0.08 | 0.04 | |
| Gm19 | 34,914,546 | 3.769** | A | G | 0.81 | 5.19 | 0.19 | 0.09 | |
| Gm19 | 40,193,564 | 3.955** | C | A | 15.62 | 44.07 | 0.37 | −0.18 | x |
***Significant at 0.01 percentile (4.0E-05).
**Significant at 0.1 percentile (2.1E-04).
R2– Calculated using a simple linear regression and is represented as percentage.
MAF = Minor Allele Frequency.
The markers that have an ‘x’ are included in stepwise regression. All other significant markers are in LD with one of these eight markers. This is the subset of markers around which QTLs were identified, and allelic combinations that have applications for MAS are characterized.
Figure 5. Manhattan plots with significant QTL that are associated with IDC.
Chromosomes (1–20) ordered on x-axis and each chromosome is represented by a different color. The –log10(p-value) is presented on the y-axis. The cutoff horizontal lines indicate 0.01 and 0.1 percentile tails of the empirical distribution obtained using 10,000 bootstraps. Vertical grey blocks indicate QTL regions that have major effect on IDC trait identified by stepwise regression. The boundaries of the QTL region (based on the position of markers) and significant candidate genes are represented on the top of QTL.
At a significance level of 0.1 percentile (p≤2.12E-04), 33 significant markers were distributed on 10 of the 20 chromosomes (Table 1, Figure 5). Eight of these markers included in stepwise regression are on Gm03 (45.03 Mbp), Gm05 (8.87 Mbp), Gm07 (6.39 Mbp), Gm11 (0.53 Mbp), Gm16 (27.30 Mbp), Gm17 (25.85 Mbp), Gm18 (28.14 Mbp), and Gm19 (40.19 Mbp). These markers together explained 46.3% variation.
Annotation of SNP
Of the significant markers at 0.1 percentile, five SNP are present in a coding region (three are transitions and two are transversions), 11 in introns, two in 3′UTR regions, and 15 in intergenic regions. Of the five SNPs from a coding region, two are non-synonymous substitutions. For FRE1 (Glyma03G38620), one of these amino acid changes is associated with both a polarity and a charge change of the protein. This SNP at position 44,927,455 bp of Gm03 changes an asparagine to aspartic acid. The R-square explained by this marker alone is 15.2% for the population. The variant allele ‘A’ is more tolerant than reference allele. The other non-synonymous substitution is located in the gene model (Glyma03g39151) close to the above non-synonymous substitution at 45,343,612 bp. This SNP explains about 18.85% of the phenotypic variation in the population and has a partial r2 of 0.80 with the above marker. These two markers together explain 19.0% of phenotypic variation.
Significant QTL and genes
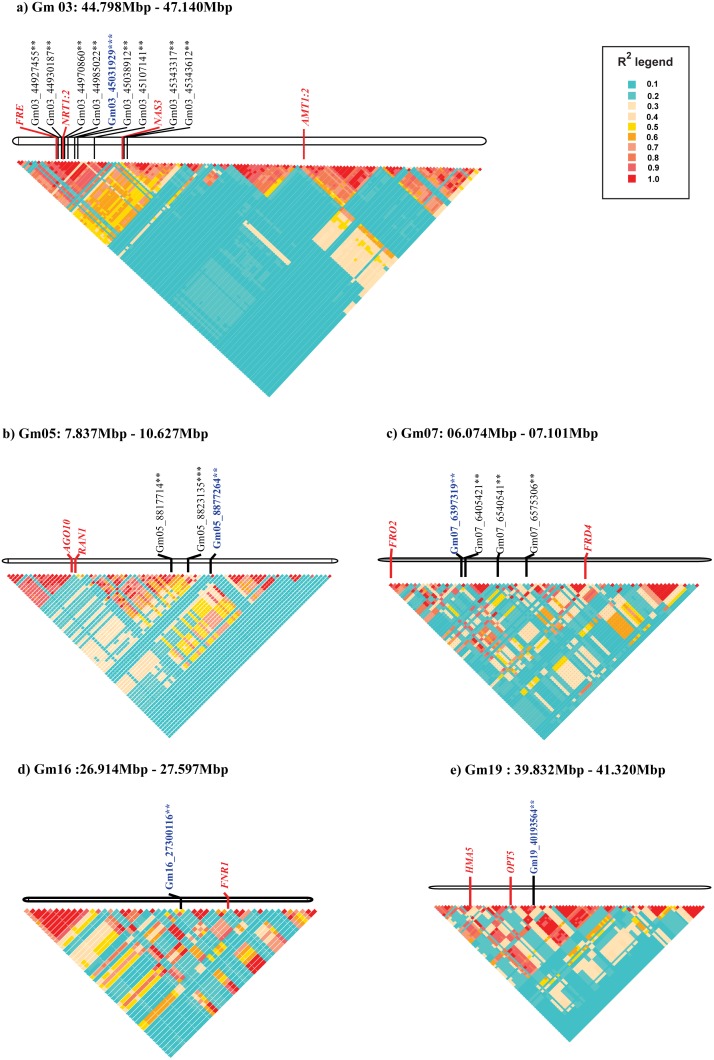
At the significance level of 0.01 percentile, three markers were included in the stepwise regression. One of these marker is in LD (r2≥0.6) with the adjacent markers, and the QTL is located on Gm03 between 44.798 Mbp and 47.140 Mbp (Table 2; Figure 5; Figure 6). The two major candidate genes in this QTL are phytochrome A (FRE1; Glyma03g38620) and nicotianamine synthase 3 (NAS3; Glyma03g39050). In addition to these genes, nitrate transporter 1∶2 (NRT1∶2; Glyma03g38640) and ammonium transporter 2 (AMT2; Glyma03g40700), each with a role in iron homeostasis, are also located within the QTL region. The QTL around the significant marker on Gm05 is between 7.837 and 10.627 Mbp (Table 2; Figure 5; Figure 6). The candidate genes in this QTL include stabilizer of iron transporter (AGO10, PNH, ZLL; Glyma05g08170), RAS-related nuclear protein-1 (RAN1; Glyma05g08260) and H (+)-ATPase 11 (AHA11; Glyma05g01460). The third significant marker on Gm11 is located in the 5′ UTR region of the CVP2 like 1 gene (CVL1; Glyma11g00990). The marker is not in LD with any of the adjacent markers.
Table 2. Significant QTL regions associated with IDC that have Candidate iron genes.
| Gene Model* | Chrom | Start | End | Genesymbol* | Annotation* | Function |
| Gm03: 44.798–47.140 | ||||||
| Glyma03g38620 | Gm03 | 44,925,730 | 44,930,360 | FRE1 | Phytochrome A | Increased reductase activity and better tolerance in high pH soils [47]–[48]. |
| Glyma03g38640 | Gm03 | 44,943,759 | 44,948,193 | NRT1∶2 | Nitrate Transporter 1∶2 | Increase concentration of nitrate in plants and lead to increased growth and root extension [71]–[72]. |
| Glyma03g39050 | Gm03 | 45,279,743 | 45,281,178 | NAS3 | Nicotianamine Synthase 3 | Synthesis of iron chelator, nicotiamine [67]–[68]. |
| Glyma03g40700 | Gm03 | 46,372,699 | 46,374,428 | AMT2;1 | Ammonium Transporter 2 | Increase concentration of nitrate in plants and lead to increased growth and root extension [71]–[72]. |
| Gm05: 7.837–10.627 | ||||||
| Glyma05g01460 | Gm05 | 960,779 | 967,307 | AHA11 | H(+)-ATPase 11 | Limits transport of iron in plants [60]. |
| Glyma05g08170 | Gm05 | 8,130,422 | 8,137,850 | AGO10 | Stabilizer of iron transporter SufD/Polynucleotidyl transferase | Stress response [82]. |
| Glyma05g08260 | Gm05 | 8,219,986 | 8,222,377 | RAN1 | RAS-related nuclear protein-1 | Involved in iron transport [76]. |
| Gm07: 6.074–7.101 | ||||||
| Glyma07g07380 | Gm07 | 6,059,979 | 6,064,461 | FRO2 | Ferric reduction oxidase 2 | Reduce iron at cell surface before transport [61] |
| Glyma07g08240 | Gm07 | 6,837,072 | 6,844,618 | FRD4 | Signal recognition particle receptor protein, chloroplast (FTSY) | Reduces iron in response to iron deficiency [56]. |
| Gm16: 26.914–27.597 | ||||||
| Glyma16g23710 | Gm16 | 27,560,086 | 27,563,676 | FNR1 | Ferrodoxin NADP (+) oxidoreductase 1 | Decreased chlorophyll biosynthesis [64]. |
| Gm19: 39.831–41.320 | ||||||
| Glyma19g32190 | Gm19 | 39,950,268 | 39,955,547 | HMA5 | Heavy Metal Atpase 5 | Maintain metal balance under Fe deficiency [78]–[80]. |
| Glyma19g32401 | Gm19 | 40,141,923 | 40,144,157 | OPT5 | Oligopeptide transporter 5 | Oligopeptide transporter [77]. |
*Gene Model, gene symbol and annotation obtained from phytozome (www.phytozome.net/soybean, V8.0).
Figure 6. Heatmap of major QTL regions.
QTL that has known candidate gene are only represented. Only significant markers at the 0.1 percentile bootstrapped empirical distribution were included in the figure. Significance of the markers is indicated as *** if fall in the 0.01 percentile tail and ** if fall in the 0.1 percentile tail of the bootstrapped empirical distribution. The stepwise included markers are marked in blue. r2 values are represented in different colors. Marker pairs with r2 values>0.6 are considered to be in linkage disequilibrium. a) is the QTL on Gm03 (44.798 Mbp–47.140 Mbp), b) is the QTL on Gm05 (7.837 Mbp–10.627 Mbp), c) is the QTL on Gm07 (6.074 Mbp–7.101 Mbp), d) is the QTL on Gm16 (26.914 Mbp–27.597 Mbp) and e) is QTL region on Gm19 (39.832 Mbp–41.320 Mbp).
At a significance level of 0.1 percentile, eight markers are included in the stepwise regression model. These eight markers are located in eight QTL regions. The QTL on Gm03, Gm05 and Gm11 are the same as the ones identified at the 0.01 percentile level. The other QTL at this significance level include Gm07 (6.074 Mbp–7.101 Mbp), Gm16 (26.914–27.597 Mbp), Gm17 (25.629–27.007 Mbp), Gm18 (27.547–29.978 Mbp), and Gm19 (39.831–41.320 Mbp) (Table 2; Figure 5; Figure 6). On Gm07, the significant candidate genes include ferric reduction oxidase 2 (FRO2; Glyma07g07380), and signal recognition particle receptor protein on chloroplast (FRD4; Glyma07g08240). On Gm16, the significant candidate gene ferrodoxin NADP (+) oxidoreductase 1 (FNR1, Glyma16g23710) is within the QTL region. On Gm19, heavy metal atpase-5 (HMA5; Glyma19g32190), and oligopeptide transporter-5 (OPT5; Glyma19g32401) are significant.
Other than the genes involved in iron metabolism, several genes involved in disease resistance are also identified in the QTL regions. For example, QTL on Gm03 has two RING/U-box superfamily proteins that have a role in plant defense. Similarly on Gm05, two CC-NBS-LRR, one NBS and one LRR genes are present. On Gm07 within the QTL region, three NBS genes are present. On Gm16, seven LRR proteins, and on Gm19, six NBS proteins, two LRR and one RING/U-box superfamily proteins are present.
Epistatic interactions
With 33 significant markers at 0.1 percentile, 528 two way interactions were tested with a population structure component, and five interactions were significant at p-value<0.001. Three of these interactions are between Gm07 (around 6.4 Mbp) and Gm18 (59.28 Mbp). The other significant interactions are between Gm16 (27.30 Mbp) and Gm18 (59.28 Mbp), Gm02 (46.939) and Gm15 (21.42 Mbp). The genes within LD regions include cytochrome P450 like protein (Glyma18g50051) on Gm18 with FRO2 (Glyma07g07380) on Gm07 and FNR1 (Glyma16g23710) on Gm16. The genes on Gm02 include AUX1 (Glyma02g42290) and FER4 (Glyma02g43040) which interact with CCC1 (Glyma15g22520) on Gm15.
Significant Marker Allelic combinations
Allelic combinations of SNPs can be used to infer the tolerant/susceptible phenotype of a genotype. For the significant markers (0.1 percentile) that were included in stepwise regression in the population, 14 allelic combinations are predicted as tolerant (IDC mean≤2.5) and 6 allelic combinations indicate susceptibility (IDC mean≥3.5) (Table 3).
Table 3. Allelic combinations and IDC means for the allele combinations that have a mean<2.5 (Tolerant) and>3.5 (Susceptible).
| Allelic combination§ | # of genotypes | IDC | |||
| Minimum | Maximum | Mean | Standard Deviation | ||
| CTTATTGA | 3 | 1.63 | 2.23 | 1.88 | 0.31 |
| TGTATTGA | 7 | 1.46 | 2.58 | 2.17 | 0.37 |
| CGTAGTGA | 2 | 2.18 | 2.34 | 2.26 | 0.11 |
| CTTATTGC | 6 | 1.96 | 2.86 | 2.29 | 0.33 |
| TTTATTGA | 11 | 1.95 | 3.25 | 2.32 | 0.40 |
| CGCATTGA | 9 | 2.08 | 2.83 | 2.38 | 0.26 |
| CTCGTTAA | 1 | 2.4 | 2.4 | 2.40 | |
| CTTATTAA | 2 | 2.16 | 2.66 | 2.41 | 0.35 |
| CTCATTGA | 26 | 1.8 | 3.01 | 2.41 | 0.33 |
| CTTGTTGC | 2 | 2.16 | 2.69 | 2.43 | 0.37 |
| CTCATTGC | 9 | 2.11 | 3.03 | 2.44 | 0.33 |
| CGTATTGA | 5 | 2.31 | 2.57 | 2.47 | 0.13 |
| CTTATCGC | 2 | 2.42 | 2.52 | 2.47 | 0.07 |
| CGTATTGC | 1 | 2.5 | 2.5 | 2.50 | |
| TTCGTTAC | 1 | 3.56 | 3.56 | 3.56 | |
| TTCAGTAC | 1 | 3.58 | 3.58 | 3.58 | |
| TGCAGCGC | 1 | 3.68 | 3.68 | 3.68 | |
| TGCGTTGC | 1 | 3.7 | 3.7 | 3.70 | |
| TGCGTTAA | 1 | 3.72 | 3.72 | 3.72 | |
| TGCGTTAC | 1 | 3.79 | 3.79 | 3.79 | |
The order of the markers for the allelic combination are Gm03_45031929, Gm05_8877264, Gm07_6397319, Gm11_530116, Gm16_27300116, Gm17_25859992, Gm18_28141888, and Gm19_40193564.
Discussion
A higher marker density facilitates identification of high resolution QTL and gene discovery [39], [40]. One method of generating a high frequency of SNPs is next generation sequencing [41], [42]. Using GBS, we identified ∼35,000 polymorphic markers with a minor allele frequency>0.05. This provided good coverage, one marker at every 31 kb. This density is useful for characterizing most genetic factors responsible for soybean IDC given that LD decayed here to r2 = 0.7 at 500 kbp.
LD decay assesses the depth of markers required for GWAS. Partial LD was calculated using population structure as a cofactor, and as expected we observed a variation in LD decay when including this as a cofactor. For example, LD decay for the population is at 10 Mbp at r2 = 0.1 whereas adjustments for confounding effect of population structure reduced LD decay to 7 Mbp. The use of partial LD (r2) helps overcome the bias between adjacent markers and leads to smaller QTL regions.
The population consisted of breeding lines developed by public and private programs targeting the north central states of the US. The germplasm is expected to be narrow given the limited range of maturity group (0–1), the short history of soybean breeding in USA, and the limited number of traits selected in the breeding programs. Here we used multiple mixed models [31], [34], [43]–[45] that control for confounding effect of population structure and population relatedness [16], [46].
The percentile p-value cutoffs derived from empirical distribution were all within the p = 0.001 error level, and the significant markers were distributed on eleven of the twenty chromosomes. This is expected given the complex nature of iron homeostasis in plants. At a significance level of one percentile (p≤2.12E-04), many more markers were significant (345 markers) and explained the majority of the phenotypic variation in the population (R2 = 85%). However, owing to the complexity of the trait, we limited the interpretations to the 0.1 percentile to find the major QTL effects.
To determine if any significant SNP is a potential causal variant, we searched for non-synonymous substitutions. One was discovered in Far red elongated 1 (FRE1) which encodes a protein that increase reductase activity and provides a higher level of tolerance in high pH calcareous soils [47]–[49]. The other non-synonymous substitution was discovered in a gene with no known function related to iron homeostasis. This can be expected because the functions of many genes are still unknown [50]–[52].
Stepwise regression facilitates the selection of markers that have a major effect in a QTL region and simultaneously masks the effects of other minor QTL. As an example, the QTL regions on Gm02 and Gm15 that have a significant interaction were not included in the stepwise regression given these QTL have a minor effect. Additionally, the partial LD between the 33 significant markers is 0.09 (±0.247). But for the eight markers included in stepwise regression, partial LD (r2) is 0.008 (±0.021). This subset of markers included in stepwise regression explains 23.2% and 46.3% of the 0.01 and 0.1 percentile, respectively. This is similar to the variation explained by all significant markers (23.2% and 48.6% at the 0.01 and 0.1 percentile, respectively).
Comparing with independent populations, the combined population analysis was able to identify markers from eight addition QTL regions that are not discovered in independent populations at significance level of 0.1 percentile. Only six QTL regions are common with either one of the population. Further using a stepwise regression to identify major effect QTL, the combined analysis was able to identify three unique QTL regions (On Gm05, Gm17, and Gm18) that were not identified in the independent populations. Of the other five QTL regions, two are common with 2005 population (on Gm03, Gm11) and three are common with 2006 population (Gm07, Gm16, Gm19).
For the population, we identified seven QTL on seven chromosomes. Given the complex quantitative nature of iron metabolism, multiple QTL distributed throughout the genome are expected. The region on Gm03 is in the same region identified earlier by Severin et al. [14] and Stec et al. [15] using an introgression analysis and is a significant QTL in many biparental studies [53]–[55]. In addition to this major QTL, other QTL regions were also identified earlier. For example, QTL region on Gm05 were identified earlier by Severin et al. [14]. The QTL on Gm16 is close to an introgression region identified earlier [14], [15]. Similarly Lin et al. [53] identified QTL on Gm18 and Gm19 and Lin et al.[56] identified QTL on Gm18. Most of these regions were also identified earlier using the same population with about 1000 polymorphic markers [9]. The major difference of this study from the previous one is the ability to narrow the QTL region and the ability to study the entire genome given a polymorphic marker every 31 kb. In addition we were able to identify significant SNP markers within the candidate genes.
Iron is a structural component of many important processes in plants, and multiple physiological steps are used by strategy-I plants, such as soybean, to manage plant growth and development in iron-deficient conditions [56]. Protons release results in the acidification of the rhizosphere which increases the solubility of iron. Ferric iron is reduced at the root surface, and ferrous iron uptake increases [57]–[59]. Here we discovered multiple genes located in major QTL associated with these major activities.
AHA11 (Gm05) is an abundant protein found in the seedlings and leaves [60] and limits the transport of iron to leaves resulting in chlorotic plants. Ferric-chelate reductase oxidase (FRO2; Gm07) is expressed in the epidermal cells of Fe-deficient roots and is one of the main Fe (III) chelate reductase proteins. It reduces iron at the cell surface before transport into roots [61]. FRO2 also interacts with IRT1 under Fe sufficiency conditions to increase iron uptake [62], [63]. FRE1 (Gm03) increases reductase activity and tolerance to high pH in calcareous soils [47], [48]. Based on transgenic lines in Oryza, Ishimaru et al. [47], [48] suggested that this gene is regulated by the IRT1 since transgenic lines have a higher level of Fe uptake and enhanced tolerance to low Fe availability in both hydroponic culture and calcareous soils.
Ferric chelate reductase defective (FRD4; Gm07) is a gene that acts post-translationally and reduces Fe (III) chelate reductase activity in response to iron deficiency [56]. In addition, frd4 mutants are chlorotic, grow more slowly, have smaller chloroplasts, and possess fewer thylakoid membranes and grana stacks. Map-based cloning revealed that this gene encodes a component that is responsible for the insertion of proteins into the thylakoid membranes of the chloroplast [56]. Ferredoxin NADP reductase 1 (FNR1; Gm16) is an enzyme that catalyzes reversible electron transfer in photosynthetic electron transport. In iron stress conditions, its activity is decreased resulting in reduced chlorophyll biosynthesis [64].
Nicotianamine (NA) is an iron chelator involved in iron (Fe) acquisition, transport, and homeostasis [65], [66], and NA is synthesized by nicotianamine synthase that is encoded by NAS3 (Gm03) gene. Overexpression of NAS genes in rice increased Fe and Zn uptake by at least twofold [67], [68]. Nicotianamine synthase genes are induced by nitrate levels [69], [70]. Ammonium and nitrate transporters (Gm03) increase the uptake of nitrate from rhizosphere and increase the concentration of nitrate in plants and lead to increased growth and extension of roots [71], [72]. In addition, nitrates enhance H+ extrusion and acidify the rhizosphere leading to increased bioavailability of Fe [71]–[75].
Several transporters such as RAN1 (Gm05), OPT5 (Gm19), and HMA (Gm19) are in the significant QTL regions. RAN1 is involved in copper trafficking and iron transport [76]. OPT5, an oligopeptide transporter is highly expressed in multiple organs of adult plants [77], and HMA sequesters Cd, Zn, and Ni in vacuoles under Fe deficiency to maintain metal balance and to detoxify heavy metals [78]–[80].
Argonaute (AGO) like proteins are down-regulated under conditions of low Fe and phosphorus [81]. Vaucheret [82] suggested that these genes have functions in both development and stress response by regulating gene expression at various levels and has additional functions including chromatin remodeling, DNA methylation, translational repression, and RNA cleavage. One such AGO gene, AGO10 (Gm05) is in a major QTL.
Disease resistant genes (R genes) are distributed throughout the G.max genome and are a key component of gene interactions between plants and pathogens [83]. We identified multiple R genes in IDC QTL regions. Iron is an important element for all living organisms and pathogens and hosts actively compete for available metals. Plants need metals for defensive generation of reactive oxygen species (ROS) and other plant defenses that limit pathogen growth whereas pathogens use low-metal conditions as a signal to recognize and respond to the host environment [84]. Pathogens have developed sophisticated strategies to acquire metal during plant growth that include production of multiple siderophores. Chen et al. [85] studied the interaction IDC and soybean cyst nematode (SCN) resistance and discovered SCN susceptible varieties had higher IDC rating. Since there exists a significant interaction between diseases and IDC, selecting for disease resistance may improve IDC tolerance.
For many traits, estimating epistatic interactions in plants [86], [87] has been restricted to bi-parental populations [50], [88]–[90]. For the population, FRO2, a proton with reductase activity interacts with Cytochrome P450 like proteins. Cytochrome P450 proteins accumulate in response to iron deficiency [91]. If iron uptake is limited by the function of FRO2, it would lead to iron deficiency and limit AUX1 and initiate cytochrome P450 activities. Similar to Cytochrome P450 proteins, activity of FNR is reduced under iron deficient conditions [64]. AUX1 is a gene that is involved in auxin transport, a plant hormone that accumulates in Fe-deficient Arabidopsis roots and acts upstream of nitric oxide [92]. FER4 is a gene responsible for cellular iron homeostasis and subcellular iron trafficking [93]. CCC is a transporter molecule that transports iron [94]. If the activity of the transporter is reduced, auxin accumulates more and the activity of FER4 is reduced.
Evaluating allelic combinations of markers can define appropriate groups of markers for MAS. It is well understood, that there is no single universal marker that is significant in all populations. This is because of the altered effect of the genes and the QTL. It is the combination of these markers that have a significant difference on the phenotype. Of the many allelic combinations possible for multiple SNP (2n, where n is the number of biallelic markers), only a few of them represent the extremes of phenotype. We provide allelic combination details for the extreme phenotypes for the significant markers that are included in the stepwise regression.
Conclusion
We used a high density GBS data set with a large plant GWAS population to map genes/QTL involved in IDC of soybean. Stepwise regression was used to select a subset of markers that have a major effect on IDC. We identified several significant QTL and candidate iron genes confirming the polygenic nature of the trait. Among these, a non-synonymous substitution identifies FRE1 as a candidate gene. Stepwise regression was effective for selecting the subset of markers that have major effect. This subset of markers can further be used for validation and testing in other populations. A high throughput study on expression differences of genes and the variants responsible for these differences would further help breeders identify varieties tolerant to IDC.
Supporting Information
Genome-wide linkage disequilibrium (LD) decay plot for the population. Linkage disequilibrium, measured as partial R2, between pairs of polymorphic marker loci (intra-chromosomal comparisons) is plotted against the physical distance (Mbp).
(PDF)
Detailed information of SNPs used in the study. SNP postions, alleles based on soybean reference genome V1.1 (phytozome.org) and minor allele frequency for this population (in %).
(XLSX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data necessary to replicate the findings of the paper are contained in Table S1.
Funding Statement
This work is supported by North Central Soybean Research Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pil JS, Jungmin P, Mi-Jeong P, Youn-Sung K, Sang-Gyu K, et al. (2012) A Golgi-localized MATE transporter mediates iron homoeostasis under osmotic stress in Arabidopsis. Biochemical Journal 442: 551–561. [DOI] [PubMed] [Google Scholar]
- 2. Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annual Review of Plant Biology 63: 131–152. [DOI] [PubMed] [Google Scholar]
- 3. Briat J-F, Curie C, Gaymard F (2007) Iron utilization and metabolism in plants. Current Opinion in Plant Biology 10: 276–282. [DOI] [PubMed] [Google Scholar]
- 4. Franzen DW, Richardson JL (2000) Soil factors affecting iron chlorosis of soybean in the Red River Valley of North Dakota and Minnesota. Journal of Plant Nutrition 23: 67–78. [Google Scholar]
- 5. Peiffer GA, King KE, Severin AJ, May GD, Cianzio SR, et al. (2012) Identification of candidate genes underlying an iron efficiency quantitative trait locus in soybean. Plant Physiology 158: 1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marschner H, Romheld V, Kissel M (1986) Different strategies in higher plants in mobilization and uptake of iron. Journal of Plant Nutrition 9: 695–713. [Google Scholar]
- 7. Schmidt W (1999) Mechanisms and regulation of reduction-based iron uptake in plants. New Phytologist 141: 1–26. [Google Scholar]
- 8.Barker AV, Pilbeam DJ (2010) Handbook of plant nutrition. CRC press.
- 9. Mamidi S, Chikara S, Goos RJ, Hyten DL, Annam D, et al. (2011) Genome-wide association analysis identifies candidate genes associated with iron deficiency chlorosis in soybean. The Plant Genome 4: 154–164. [Google Scholar]
- 10. Curie C, Briat J-F (2003) Iron transport and signaling in plants. Annual Review of Plant Biology 54: 183–206. [DOI] [PubMed] [Google Scholar]
- 11. Diers BW, Cianzio SR, Shoemaker RC (1992) Possible identification of quantitative trait loci affecting iron efficiency in soybean. Journal of Plant Nutrition 15: 2127–2136. [Google Scholar]
- 12. King KE, Peiffer GA, Reddy M, Lauter N, Lin SF, et al. (2013) Mapping of Iron and Zinc Quantitative Trait Loci in Soybean for Association to Iron Deficiency Chlorosis Resistance. Journal of Plant Nutrition 36: 2132–2153. [Google Scholar]
- 13. O’Rourke J, Charlson D, Gonzalez D, Vodkin L, Graham M, et al. (2007) Microarray analysis of iron deficiency chlorosis in near-isogenic soybean lines. BMC Genomics 8: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Severin AJ, Peiffer GA, Xu WW, Hyten DL, Bucciarelli B, et al. (2010) An integrative approach to genomic introgression mapping. Plant Physiology 154: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stec AO, Bhaskar PB, Bolon Y-T, Nolan R, Shoemaker RC, et al. (2013) Genomic heterogeneity and structural variation in soybean near isogenic lines. Frontiers in Plant Science 4: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Myles S, Peiffer J, Brown PJ, Ersoz ES, Zhang Z, et al. (2009) Association mapping: critical considerations shift from genotyping to experimental design. The Plant Cell Online 21: 2194–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J, McClean PE, Lee R, Goos RJ, Helms T (2008) Association mapping of iron deficiency chlorosis loci in soybean (Glycine max L. Merr.) advanced breeding lines. Theoretical and Applied Genetics 116: 777–787. [DOI] [PubMed] [Google Scholar]
- 18. Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, et al. (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PloS One 6: e19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, et al. (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23: 2633–2635. [DOI] [PubMed] [Google Scholar]
- 20. Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, et al. (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183. [DOI] [PubMed] [Google Scholar]
- 21. Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danecek P, Auton A, Abecasis G, Albers CA, Banks E, et al. (2011) The variant call format and VCFtools. Bioinformatics 27: 2156–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scheet P, Stephens M (2006) A fast and flexible statistical model for large-scale population genotype data: applications to inferring missing genotypes and haplotypic phase. The American Journal of Human Genetics 78: 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marchini J, Howie B (2010) Genotype imputation for genome-wide association studies. Nature Reviews Genetics 11: 499–511. [DOI] [PubMed] [Google Scholar]
- 25. Mangin B, Siberchicot A, Nicolas S, Doligez A, This P, et al. (2011) Novel measures of linkage disequilibrium that correct the bias due to population structure and relatedness. Heredity 108: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long Q, Rabanal FA, Meng D, Huber CD, Farlow A, et al. (2013) Massive genomic variation and strong selection in Arabidopsis thaliana lines from Sweden. Nature genetics 45: 884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stram DO (2004) Tag SNP selection for association studies. Genetic Epidemiology 27: 365–374. [DOI] [PubMed] [Google Scholar]
- 28. Remington DL, Thornsberry JM, Matsuoka Y, Wilson LM, Whitt SR, et al. (2001) Structure of linkage disequilibrium and phenotypic associations in the maize genome. Proceedings of the National Academy of Sciences 98: 11479–11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pyhäjärvi T, García-Gil MR, Knürr T, Mikkonen M, Wachowiak W, et al. (2007) Demographic history has influenced nucleotide diversity in European Pinus sylvestris populations. Genetics 177: 1713–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, et al. (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics 38: 904–909. [DOI] [PubMed] [Google Scholar]
- 31. Zhao K, Aranzana MJ, Kim S, Lister C, Shindo C, et al. (2007) An Arabidopsis example of association mapping in structured samples. PLoS Genetics 3: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou X, Stephens M (2012) Genome-wide efficient mixed-model analysis for association studies. Nature Genetics 44: 821–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rakitsch B, Lippert C, Stegle O, Borgwardt K (2013) A Lasso multi-marker mixed model for association mapping with population structure correction. Bioinformatics 29: 206–214. [DOI] [PubMed] [Google Scholar]
- 34. Yu J, Pressoir G, Briggs WH, Bi IV, Yamasaki M, et al. (2005) A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nature Genetics 38: 203–208. [DOI] [PubMed] [Google Scholar]
- 35. Hall P, Miller H (2010) Bootstrap confidence intervals and hypothesis tests for extrema of parameters. Biometrika 97: 881–892. [Google Scholar]
- 36. Li J, Tai BC, Nott DJ (2009) Confidence interval for the bootstrap P-value and sample size calculation of the bootstrap test. Journal of Nonparametric Statistics 21: 649–661. [Google Scholar]
- 37. Meyer KB, O’Reilly M, Michailidou K, Carlebur S, Edwards SL, et al. (2013) Fine-scale mapping of the FGFR2 breast cancer risk locus: putative functional variants differentially bind FOXA1 and E2F1. The American Journal of Human Genetics 93: 1046–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cingolani P, Platts A, Coon M, Nguyen T, Wang L, et al. (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6: 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davey JW, Hohenlohe PA, Etter PD, Boone JQ, Catchen JM, et al. (2011) Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nature Reviews Genetics 12: 499–510. [DOI] [PubMed] [Google Scholar]
- 40. Deschamps S, Campbell MA (2010) Utilization of next-generation sequencing platforms in plant genomics and genetic variant discovery. Molecular Breeding 25: 553–570. [Google Scholar]
- 41. Ganal MW, Durstewitz G, Polley A, Berard A, Buckler ES, et al. (2011) A large maize (Zea mays L.) SNP genotyping array: development and germplasm genotyping, and genetic mapping to compare with the B73 reference genome. PloS One 6: e28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mandel JR, Nambeesan S, Bowers JE, Marek LF, Ebert D, et al. (2013) Association Mapping and the Genomic Consequences of Selection in Sunflower. PLoS Genetics 9: e1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gurung S, Mamidi S, Bonman JM, Jackson EW, Del Rio LE, et al. (2011) Identification of novel genomic regions associated with resistance to Pyrenophora tritici-repentis races 1 and 5 in spring wheat landraces using association analysis. Theoretical and Applied Genetics 123: 1029–1041. [DOI] [PubMed] [Google Scholar]
- 44. Iqbal MJ, Mamidi S, Ahsan R, Kianian SF, Coyne CJ, et al. (2012) Population structure and linkage disequilibrium in Lupinus albus L. germplasm and its implication for association mapping. Theoretical and Applied Genetics 125: 517–530. [DOI] [PubMed] [Google Scholar]
- 45. Patel JS, Mamidi S, Bonman JM, Adhikari TB (2013) Genome-wide Association Analysis in Spring Wheat Accessions Identifies QTL Associated with Resistance to a 2 Novel Isolate of Pyrenophora tritici-repentis 3. Crop Science 53: 842–852. [Google Scholar]
- 46. Astle W, Balding DJ (2009) Population structure and cryptic relatedness in genetic association studies. Statistical Science 24: 451–471. [Google Scholar]
- 47. Ishimaru Y, Kim S, Tsukamoto T, Oki H, Kobayashi T, et al. (2007) Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency in calcareous soil. Proceedings of the National Academy of Sciences 104: 7373–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oki H, Kim S, Nakanishi H, Takahashi M, Yamaguchi H, et al. (2004) Directed evolution of yeast ferric reductase to produce plants with tolerance to iron deficiency in alkaline soils. Soil Science and Plant Nutrition 50: 1159–1165. [Google Scholar]
- 49. Samuelsen AI, Martin RC, Mok DWS, Mok MC (1998) Expression of the yeast FRE genes in transgenic tobacco. Plant Physiology 118: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grillo MA, Li C, Hammond M, Wang L, Schemske DW (2013) Genetic architecture of flowering time differentiation between locally adapted populations of Arabidopsis thaliana . New Phytologist 197: 1321–1331. [DOI] [PubMed] [Google Scholar]
- 51. Pineau C, Loubet S, Lefoulon C, Chalies C, Fizames C, et al. (2012) Natural Variation at the FRD3 MATE Transporter Locus Reveals Cross-Talk between Fe Homeostasis and Zn Tolerance in Arabidopsis thaliana . PLoS Genetics 8: e1003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang N, Gur A, Gibon Y, Sulpice R, Flint-Garcia S, et al. (2010) Genetic analysis of central carbon metabolism unveils an amino acid substitution that alters maize NAD-dependent isocitrate dehydrogenase activity. PloS One 5: e9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lin S, Cianzio S, Shoemaker R (1997) Mapping genetic loci for iron deficiency chlorosis in soybean. Molecular Breeding 3: 219–229. [Google Scholar]
- 54. Lin SF, Grant D, Cianzio S, Shoemaker R (2000) Molecular characterization of iron deficiency chlorosis in soybean. Journal of Plant Nutrition 23: 1929–1939. [Google Scholar]
- 55. Lin S-F, Baumer JS, Ivers D, de CianzoSR, Shoemaker RC (1998) Field and nutrient solution tests measure similar mechanisms controlling iron deficiency chlorosis in soybean. Crop Science 38: 254–259. [Google Scholar]
- 56. Durrett TP, Connolly EL, Rogers EE (2006) Arabidopsis cpFtsY mutants exhibit pleiotropic defects including an inability to increase iron deficiencyinducible root Fe (III) chelate reductase activity. The Plant Journal 47: 467–479. [DOI] [PubMed] [Google Scholar]
- 57. Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proceedings of the National Academy of Sciences 93: 5624–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397: 694–697. [DOI] [PubMed] [Google Scholar]
- 59. Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, et al. (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. The Plant Cell Online 14: 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Alsterfjord M, Sehnke PC, Arkell A, Larsson H, Svennelid F, et al. (2004) Plasma membrane H+-ATPase and 14-3-3 isoforms of Arabidopsis leaves: evidence for isoform specificity in the 14-3-3/H+-ATPase interaction. Plant and cell physiology 45: 1202–1210. [DOI] [PubMed] [Google Scholar]
- 61. Kim SA, Guerinot ML (2007) Mining iron: iron uptake and transport in plants. FEBS letters 581: 2273–2280. [DOI] [PubMed] [Google Scholar]
- 62. Connolly EL, Campbell NH, Grotz N, Prichard CL, Guerinot ML (2003) Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiology 133: 1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Connolly EL, Fett JP, Guerinot ML (2002) Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. The Plant Cell Online 14: 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Miller GW, Pushnik JC, Welkie GW (1984) Iron chlorosis, a world wide problem, the relation of chlorophyll biosynthesis to iron. Journal of Plant Nutrition 7: 1–22. [Google Scholar]
- 65. Pich A, Manteuffel R, Hillmer S, Scholz G, Schmidt W (2001) Fe homeostasis in plant cells: does nicotianamine play multiple roles in the regulation of cytoplasmic Fe concentration? Planta 213: 967–976. [DOI] [PubMed] [Google Scholar]
- 66. von Wiren N, Klair S, Bansal S, Briat J-F, Khodr H, et al. (1999) Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants. Plant Physiology 119: 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee S, Jeon US, Lee SJ, Kim Y-K, Persson DP, et al. (2009) Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proceedings of the National Academy of Sciences 106: 22014–22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Masuda H, Usuda K, Kobayashi T, Ishimaru Y, Kakei Y, et al. (2009) Overexpression of the barley nicotianamine synthase gene HvNAS1 increases iron and zinc concentrations in rice grains. Rice 2: 155–166. [Google Scholar]
- 69. Wang R, Okamoto M, Xing X, Crawford NM (2003) Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1,000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiology 132: 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang Y-H, Garvin DF, Kochian LV (2001) Nitrate-induced genes in tomato roots. Array analysis reveals novel genes that may play a role in nitrogen nutrition. Plant Physiology 127: 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bai H, Euring D, Volmer K, Janz D, Polle A (2013) The Nitrate Transporter (NRT) Gene Family in Poplar. PloS One 8: e72126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gu R, Duan F, An X, Zhang F, von Wiren N, et al. (2013) Characterization of AMT-Mediated High-Affinity Ammonium Uptake in Roots of Maize (Zea mays L.). Plant and Cell Physiology 54: 1515–1524. [DOI] [PubMed] [Google Scholar]
- 73. Bloom AJ, Meyerhoff PA, Taylor AR, Rost TL (2002) Root development and absorption of ammonium and nitrate from the rhizosphere. Journal of Plant Growth Regulation 21: 416–431. [Google Scholar]
- 74. Taylor AR, Bloom AJ (1998) Ammonium, nitrate, and proton fluxes along the maize root. Plant, Cell & Environment 21: 1255–1263. [Google Scholar]
- 75. Zou C, Shen J, Zhang F, Guo S, Rengel Z, et al. (2001) Impact of nitrogen form on iron uptake and distribution in maize seedlings in solution culture. Plant and Soil 235: 143–149. [Google Scholar]
- 76. Woeste KE, Kieber JJ (2000) A strong loss-of-function mutation in RAN1 results in constitutive activation of the ethylene response pathway as well as a rosette-lethal phenotype. The Plant Cell Online 12: 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stacey MG, Osawa H, Patel A, Gassmann W, Stacey G (2006) Expression analyses of Arabidopsis oligopeptide transporters during seed germination, vegetative growth and reproduction. Planta 223: 291–305. [DOI] [PubMed] [Google Scholar]
- 78. Arrivault S, Senger T, Kramer U (2006) The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. The Plant Journal 46: 861–879. [DOI] [PubMed] [Google Scholar]
- 79. Morel M, Crouzet J, Gravot A, Auroy P, Leonhardt N, et al. (2009) AtHMA3, a P1B–ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiology 149: 894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schaaf G, Honsbein A, Meda AR, Kirchner S, Wipf D, et al. (2006) AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. Journal of Biological Chemistry 281: 25532–25540. [DOI] [PubMed] [Google Scholar]
- 81. Zheng L, Huang F, Narsai R, Wu J, Giraud E, et al. (2009) Physiological and transcriptome analysis of iron and phosphorus interaction in rice seedlings. Plant Physiology 151: 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vaucheret H (2008) Plant argonautes. Trends in Plant Science 13: 350–358. [DOI] [PubMed] [Google Scholar]
- 83. Kang Y, Kim K, Shim S, Yoon M, Sun S, et al. (2012) Genome-wide mapping of NBS-LRR genes and their association with disease resistance in soybean. BMC Plant Biology 12: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fones H, Preston GM (2012) Reactive oxygen and oxidative stress tolerance in plant pathogenic Pseudomonas. FEMS microbiology letters 327: 1–8. [DOI] [PubMed] [Google Scholar]
- 85. Chen S, Kurle JE, Stetina SR, Miller DR, Klossner LD, et al. (2007) Interactions between iron-deficiency chlorosis and soybean cyst nematode in Minnesota soybean fields. Plant and Soil 299: 131–139. [Google Scholar]
- 86. Mackay TFC (2014) Epistasis and quantitative traits: using model organisms to study gene-gene interactions. Nature Reviews Genetics 15: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shang J, Zhang J, Sun Y, Liu D, Ye D, et al. (2011) Performance analysis of novel methods for detecting epistasis. BMC Bioinformatics 12: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Caicedo AL, Stinchcombe JR, Olsen KM, Schmitt J, Purugganan MD (2004) Epistatic interaction between Arabidopsis FRI and FLC flowering time genes generates a latitudinal cline in a life history trait. Proceedings of the National Academy of Sciences 101: 15670–15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jiang Z, Han Y, Teng W, Zhang Z, Sun D, et al. (2010) Identification of QTL underlying the filling rate of protein at different developmental stages of soybean seed. Euphytica 175: 227–236. [Google Scholar]
- 90. Lin H, Liang Z-W, Sasaki T, Yano M (2003) Fine mapping and characterization of quantitative trait loci Hd4 and Hd5 controlling heading date in rice. Breeding Science 53: 51–59. [Google Scholar]
- 91. Colangelo EP, Guerinot ML (2004) The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. The Plant Cell Online 16: 3400–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen WW, Yang JL, Qin C, Jin CW, Mo JH, et al. (2010) Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiology 154: 810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tarantino D, Santo N, Morandini P, Casagrande F, Braun H-P, et al. (2010) AtFer4 ferritin is a determinant of iron homeostasis in Arabidopsis thaliana heterotrophic cells. Journal of Plant Physiology 167: 1598–1605. [DOI] [PubMed] [Google Scholar]
- 94. Conte SS, Walker EL (2011) Transporters contributing to iron trafficking in plants. Molecular Plant 4: 464–476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genome-wide linkage disequilibrium (LD) decay plot for the population. Linkage disequilibrium, measured as partial R2, between pairs of polymorphic marker loci (intra-chromosomal comparisons) is plotted against the physical distance (Mbp).
(PDF)
Detailed information of SNPs used in the study. SNP postions, alleles based on soybean reference genome V1.1 (phytozome.org) and minor allele frequency for this population (in %).
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data necessary to replicate the findings of the paper are contained in Table S1.