Abstract
Purpose
Nicotine, the major component in cigarette smoke, can promote tumor growth and angiogenesis in various cancers, including lung cancer. Hypoxia-inducible factor-1α (HIF-1α) is overexpressed in human lung cancers, particularly in non – small cell lung cancers (NSCLC), and is closely associated with an advanced tumor grade, increased angiogenesis, and resistance to chemotherapy and radiotherapy. The purpose of this study was to investigate the effects of nicotine on the expression of HIF-1α and its downstream target gene, vascular endothelial growth factor (VEGF), in human lung cancer cells.
Experimental Design
Human NSCLC cell lines A549 and H157 were treated with nicotine and examined for expression of HIF-1α and VEGF using Western blot or ELISA. Loss of HIF-1α function using specific small interfering RNA was used to determine whether HIF-1α is directly involved in nicotine-induced tumor angiogenic activities, including VEGF expression, cancer cell migration, and invasion.
Results
Nicotine increased HIF-1α and VEGF expression in NSCLC cells. Pharmacologically blocking nicotinic acetylcholine receptor – mediated signaling cascades, including the Ca2+/calmodulin, c-Src, protein kinase C, phosphatidylinositol 3-kinase, mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2, and the mammalian target of rapamycin pathways, significantly attenuated nicotine-induced up-regulation of HIF-1α protein. Functionally, nicotine potently stimulated in vitro tumor angiogenesis by promoting tumor cell migration and invasion. These proangiogenic and invasive effects were partially abrogated by treatment with small interfering RNA specific for HIF-1α.
Conclusion
These findings identify novel mechanisms by which nicotine promotes tumor angiogenesis and metastasis and provide further evidences that HIF-1αis a potential anticancer target in nicotine-associated lung cancer.
Worldwide, lung cancer remains the leading cause of cancer death in both men and women, with an estimated 1.2 million deaths annually, of which non–small cell lung cancer (NSCLC) accounts for 75% to 80% of deaths (1). Nicotine, a psychoactive/addictive compound in cigarette smoke and the major risk factor for lung cancer (2, 3), can promote cell proliferation in several tumor cell lines, including SCLC, NSCLC, gastric cancer, pancreatic cancer, and head and neck cancer, via multiple signaling pathways (4–8). Nicotine has been shown to protect cancer cells from apoptosis induced by diverse stimuli, such as opioid, tumor necrosis factor, UV light, chemotherapeutic drugs, and serum deprivation (5, 8–12). Moreover, several studies have reported that nicotine exerts proangiogenic activities in tumor xenografts and chick chorioallantoic membrane model of angiogenesis (6, 13–15). These studies indicate that nicotine possesses both tumor-promoting and angiogenic activities conducive to a more aggressive tumor phenotype. However, the mechanisms underlying nicotine-stimulated angiogenesis remain largely unknown.
Hypoxia-inducible factor-1 (HIF-1) activates the expression of a battery of genes involved in diverse aspects of cellular and integrative physiologic processes (16). This transcription factor consists of two subunits, HIF-1α and HIF-1β, of which HIF-1α function is tightly regulated by cellular oxygen concentration. Under hypoxic conditions, HIF-1α escapes ubiquitin degradation, forms a heterodimer with HIF-1β, binds to the cis-acting element (hypoxia-responsive elements), and activates downstream hypoxia-responsive genes (17). In addition to intratumoral hypoxia, HIF-1α activity is up-regulated by a variety of nonhypoxic signals, including the inactivation of several tumor suppressors, p53, pVHL, and PTEN (18, 19), the activation of several oncogenic pathways, Src, HER/2, and Ha-Ras (20–22), and the stimulation by certain cytokines, hormones, and growth factors (23, 24), suggesting a more ample role of HIF-1 in tumor biology.
Cumulative evidences have implied an essential role of HIF-1α pathway in tumorigenesis in several cancer types (25, 26). Clinically, high levels of HIF-1α have been detected in many solid tumors and their metastases and are closely correlated to an advanced tumor grade, an increased angiogenesis, and a resistance to chemotherapy and radiotherapy (27, 28). Similarly, increased expression of HIF-1α has been reported in NSCLC tissues with poor disease prognosis (29–31). A recent study has shown that HIF-1α activation and increased tumor metastasis were induced by a dysregulated signal transduction through the phosphatidylinositol 3-kinase (PI3K) pathway in NSCLC cells (32). These findings suggest that HIF-1α pathway is critical in tumor promotion and metastasis of NSCLC. However, the detailed molecular mechanisms underlying the overexpression of HIF-1α in NSCLC, specifically in the context of carcinogen-exposed microenvironment, such as cigarette smoke, and its contribution to human lung cancer are still poorly understood.
In this study, we have shown that nicotine, a major risk factor in lung cancer and others, significantly stimulates HIF-1α protein accumulation and VEGF expression in human NSCLC, and HIF-1α contributes, at least in part, to nicotine-enhanced in vitro tumor angiogenesis and invasion. Both proangiogenic and tumor-invasive effects induced by nicotine can be partially abrogated by treatment with small interfering RNA (siRNA) specific for HIF-1α. The mechanisms behind nicotine-induced HIF-1α expression seem to involve the nicotinic acetylcholine receptor (nAChR)-mediated activation of multiple signaling pathways.
Materials and Methods
Cell culture
Human NSCLC cell lines A427, A549, H2122 (adenocarcinoma), and H157 (squamous cell carcinoma cells) were obtained from the American Type Culture Collection and maintained in RPMI 1640 supplemented with 10% fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 Ag/mL; Invitrogen Corp.) at 37°C in a humidified atmosphere with 5% CO2.
Treatment of cells with pharmacologic inhibitors
NSCLC cells at ~80% confluence were pretreated for 1 h with increasing concentrations of a-bungarotoxin (α-BTX), LY294002, U0126, rapamycin, GF109203X (bisindolylmaleimide I), PP2, BAPTA/AM, and W7 followed by incubation in the presence or absence of 5 μmol/L nicotine (Sigma) for 16 h. All inhibitors were purchased from Calbiochem and dissolved in DMSO (except α-BTX). The final DMSO concentration did not exceed 0.1% throughout the study. Concentrations of all inhibitors were titrated to effect without cellular toxicity as determined by trypan blue exclusion assay.
siRNA transfection assay
Chemically synthesized double-stranded siRNA specific for HIF-1α (siRNAHIF-1α), 5’-AGAGGUGGAUAUGUGUGGGdTdT-3’ and 5’-CCCACACAUAUCCACCUCUdTdT-3’, was purchased from Dharmacon Research, Inc. as described previously (33). The siRNA was transfected (200 nmol/L) using Oligofectamine reagent according to the manufacturer's instructions (Invitrogen). A non-targeting siRNA sequence (Dharmacon Research) was used as nonspecific control.
Immunofluorescence studies
NSCLC cells were exposed to 0.5 or 5.0 μmol/L of nicotine for 16 h, fixed with 2% paraformaldehyde, permeabilized with 1% Triton X-100 in PBS, and blocked with PBS containing 3% bovine serum albumin. Cells were incubated overnight with a mouse monoclonal anti-human HIF-1α antibody (1:100; BD Transduction Laboratories) at 4°C followed by incubation with Alexa Fluor 488–conjugated goat anti-mouse IgG (1:20,000; Molecular Probes) for 1 h at room temperature. Images were photographed using a fluorescence microscope. Cells incubated with Alexa Fluor 488– conjugated goat anti-mouse IgG in the absence of primary antibodies served as negative controls.
Western blot analysis
Whole-cell lysates were prepared as described previously (33). Protein concentrations were determined by bicinchoninic acid methods. Equal amounts of protein samples (100 Ag) were separated on 8% to 10% polyacrylamide-SDS gel, electroblotted onto nitrocellulose membranes (Hybond ECL, Amersham Pharmacia), blocked with TBS/5% nonfat dry milk for 2 h, and incubated with specific primary antibodies against HIF-1α or HIF-1β, total or phosphorylated p42/p44 mitogen-activated protein kinases (Thr202/Tyr204) or Akt (Ser473; New England Biolabs), phosphorylated p70S6K1 (Thr421/Ser424), phosphorylated 4E-BP1 (Ser65/Thr70), or α7-nAChR (Santa Cruz Biotechnology). The membranes were incubated with horseradish peroxidase–conjugated secondary antibody (1:2,000; Pierce), and signals were visualized by enhanced chemiluminescence detection system. To standardize for loading controls, the blots were reprobed with specific antibody against human β-actin (1:4,000; Sigma).
ELISA assay for VEGF production
VEGF secretion in the conditioned medium was assayed using the Human VEGF ELISA kit (PeproTech) according to the manufacturer's protocols. Results were normalized to cell counts (1 × 105).
In vitro angiogenesis assay
The in vitro angiogenesis assay kit was used according to the manufacturer's protocols (Chemicon). Human umbilical vascular endothelial cells (HUVEC; 5 × 103 per well) were seeded onto a 96-well cell culture plate coated with ECMatrix and incubated at 37°C with conditioned medium derived from siRNA-treated or nontreated A549 cells after culture in the presence or absence of 5 μmol/L nicotine for 24 h. Following 6-h incubation, capillary or tubule formation was observed under a phase-contrast microscope, and the average capillary tube branch points were enumerated in six random view fields per well.
Cell migration and invasion assay
The QCM Collagen-based Cell Invasion Assay kit was used to assess tumor cell invasion according to the manufacturer's protocol (Chemicon). Cells that migrated through the gel insert to the lower surface of the membrane were stained and photographed under a microscope. The migrated stained cells were solubilized and quantified by colorimetric measurement at 560 nm.
Statistical analysis
Data are presented as the mean ± SD for three separate experiments. One-way ANOVA and Bonferroni were used for statistical analysis using Statistical Package for the Social Sciences 11.0 for Windows software. P < 0.05 was considered to be statistically significant.
Results
Nicotine induces HIF-1α expression in human NSCLC cells
To explore the effects of nicotine on the expression of HIF-1α, A549 cells were treated with increasing concentration of nicotine for 16 h and whole-cell lysates were analyzed with Western blot. Treatment with nicotine increased HIF-1α protein expression in a concentration-dependent manner (Fig. 1A and B). Densitometric analysis reveals a 12-fold induction of HIF-1α protein accumulation at 5 to 10 μmol/L nicotine compared with nontreatment control (P < 0.01; Fig. 1B). Parallel immunofluorescence studies showed that treatment with nicotine led to an increased accumulation of HIF-1α protein in both cytoplasm and nucleus of A549 cells, a similar effect observed on exposure to hypoxia or hypoxia mimetics, such as cobalt chloride (CoCl2; Fig. 1B). Likewise, treatment with nicotine also resulted in a concentration-dependent increase in HIF-1α protein expression in H157 cell line, a squamous cell carcinoma of lung, whereas exposure to 5 μmol/L nicotine led to 7-fold induction of HIF-1α protein accumulation (P < 0.01; Supplementary Fig. S1A).
Fig. 1.
Nicotine induces HIF-1αprotein accumulation andVEGF production in A549 cells. A, top,Western blot analysis of HIF-1αprotein levels in A549 cells treated with increasing concentrations of nicotine for 16 h; bottom, densitometric analysis of the relative density of HIF-1αas in (A), wherein the density of the control was arbitrarily set as 1.0. B, immunofluorescence studies on HIF-1αprotein expression in A549 cells after treatment with 100 μmol/L CoCl2 and 0.5 and 5 μmol/L of nicotine for 16 h, respectively. Magnification, ×20. C, ELISA assay ofVEGF protein concentration in the conditioned media, which were normalized to cell numbers (1 ×105). Columns, mean; bars, SD. *, P < 0.05; **, P < 0.01, compared with control without nicotine treatment. Data are representative of three independent experiments.
To examine whether nicotine enhances expression of VEGF, an immediate downstream target gene of HIF-1α, A549 cells were exposed to different concentrations of nicotine for 16 h and VEGF secretion in the conditioned medium was analyzed by ELISA. As shown in Fig. 1C, treatment with 5 μmol/L nicotine resulted in >2-fold increase in VEGF production (P < 0.05). Similar nicotine-induced up-regulation of VEGF was observed in H157 cells (Supplementary Fig. S1B). To further determine whether HIF-1α is directly involved in nicotine-induced VEGF expression, A549 cells were transfected with siRNAHIF-1α or nonspecific siRNA and subsequently exposed to 5 μmol/L nicotine for 16 h. The results showed that transfection with siRNAHIF-1α caused ~82% inhibition of HIF-1α protein accumulation (P < 0.01; Fig. 2A) but only 47% inhibition of VEGF protein secretion (P < 0.05; Fig. 2B). Taken together, these results suggest that HIF-1α contributes, at least in part, to nicotine-induced VEGF expression in A549 cells.
Fig. 2.
Induction ofVEGF expression by nicotine is HIF-1αdependent. A549 cells were transfected with siRNAHIF-1αor nonspecific siRNA and then exposed to 5 μmol/L nicotine for 16 h. A, top,Western blot analysis of HIF-1αprotein levels; bottom, densitometric analysis of the relative density of HIF-1αas in (A), wherein the density of the nontransfection control without nicotine treatment was arbitrarily set as 1.0. B, ELISA assay ofVEGF protein concentration in the conditioned media, which were normalized to cell numbers (1 × 105). Columns, mean; bars, SD. **, P < 0.01, compared with nontransfection control without nicotine treatment; #, P < 0.05; ##, P < 0.01, compared with nontransfected cells exposed to 5 Amol/L nicotine for 16 h. Data represent three independent experiments.
Signaling pathways activated by nicotine in human NSCLCs
Specific binding of nicotine to nAChRs leads to the activation of several signaling pathways, including extracellular signal-regulated kinase 1/2 (ERK1/2; refs. 6, 7, 34) and PI3K/ Akt, in several human cancer cells (5, 35, 36). Among all nAChR subunits, α7-nAChR is the major functional subunit (35, 37), commonly expressed in several NSCLC cell lines, including A549 (5, 11, 38). In this study, we also showed that α7-nAChR protein was expressed in A549 and three other NSCLC cell lines (Supplementary Fig. S2A). Further, similar to previous studies (5, 34, 35), our results showed that exposure of A549 cells to 5 μmol/L nicotine for 15 min led to ~4-fold increase in the phosphorylated ERK1/2 compared with untreated cells (P < 0.05); treatment with 5 μmol/L nicotine for 45 min increased the phosphorylated levels of Akt, p70S6K, and 4E-BP1 by 4.2-, 3.5-, and 3.8-fold over untreated cells, respectively (P < 0.05; Fig. 3A and B; Table 1). Similar nicotine activation of ERK1/2 and PI3K/Akt pathways was observed in H157 cells (Supplementary Fig. S2).
Fig. 3.
Activation of signaling pathways by nicotine in lung cancer cells. A549 cells were serum starved for 24 h followed by incubation with 5 μmol/L nicotine for different times. A, Western blot analysis of the phosphorylated levels of Akt (p-Akt), ERK1/2 (p-ERK1/2), p70S6K (p-p70S6K), and 4E-BP1 (p-4E-BP1).B, densitometric analysis of results in (A). Results are representative of three independent experiments. Columns, mean; bars, SD.
Table 1.
Fold induction of signaling components by nicotine
| Maximum fold |
||
|---|---|---|
| 15 min | 60 min | |
| Phosphorylated Akt | 4.23 ± 0.39* | |
| Phosphorylated ERK1/2 | 3.86 ± 0.42* | |
| Phosphorylated p70S6K | 3.46 ± 0.33* | |
| Phosphorylated 4E-BP1 | 3.83 ± 0.38* | |
P < 0.05, compared with control at the time point “0” min.
nAChR-mediated signaling pathways are involved in nicotine-induced HIF-α and VEGF expression in NSCLCs
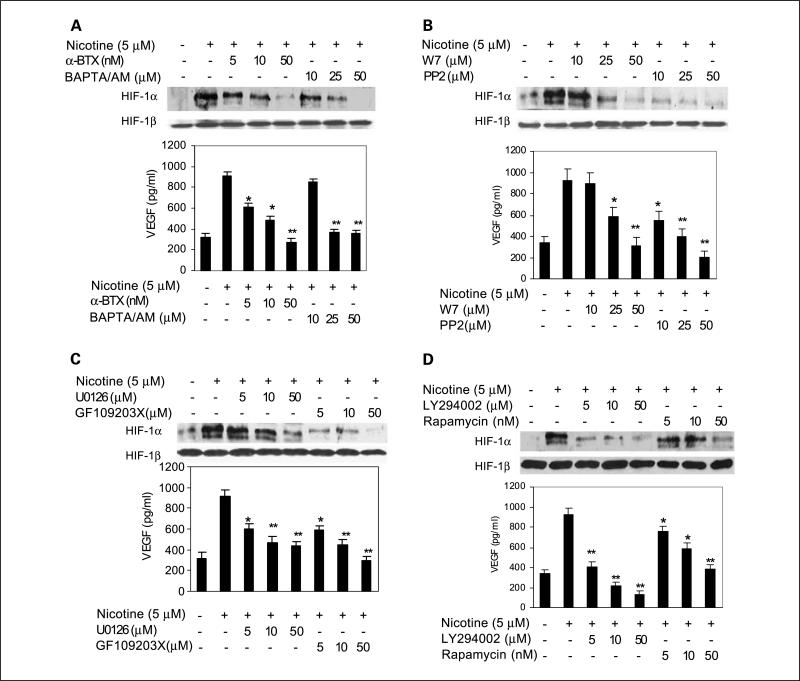
Next, we explored whether nicotine-activated signaling pathways are involved in nicotine-induced HIF-1α and VEGF expression. A549 cells were pretreated with increasing concentrations of α-BTX, an antagonist of nAChR (33, 35, 39), followed by incubation with 5 μmol/L nicotine for 16 h. Our results indicated that α-BTX specifically inhibited nicotine-induced HIF-1α and VEGF protein expression in a concentration-dependent manner (P < 0.01; Fig. 4A). At 50 nmol/L, α-BTX abrogated nicotine up-regulated HIF-1α and VEGF levels by 86.3 ± 13.6% (P < 0.01) and 69 ± 9.6% (P < 0.05), respectively (Supplementary Fig. S3A). To determine whether Ca2+/calmodulin is also essential for nicotine-induced HIF-1α and VEGF expression, A549 cells were pretreated with BAPTA/ AM, an intracellular calcium chelator, or W7, a specific antagonist of calmodulin. As shown in Fig. 4A and B, pretreatment with BAPTA/AM or W7 led to a dose-dependent decrease in nicotine-induced HIF-1α and VEGF expression. At 50 μmol/L, BAPTA/AM blocked nicotine-induced HIF-1α and VEGF levels by 92.1 ± 14.3% (P < 0.01) and 59.1 ± 10.3% (P < 0.05), respectively (Supplementary Fig. S3A), whereas W7 decreased nicotine-induced HIF-1α and VEGF levels by 95 ± 12.7% (P < 0.01) and 65.8 ± 9.3% (P < 0.05), respectively (Supplementary Fig. S3B).
Fig. 4.
Involvement of α7-nAChR – mediated activation of signaling cascades in nicotine-induced HIF-1αprotein accumulation andVEGF production. A549 cells were pretreated for 1h with increasing concentrations of various pharmacologic inhibitors, including α-BTX and BAPTA/AM (A),W7 and PP2 (B), U0126 and GF109203 (C), and LY294002 and rapamycin (D). Cells were subsequently incubated in the presence or absence of 5 μmol/L nicotine for 16 h. HIF-1αprotein levels in cell lysates and VEGF production in the conditioned medium were determined by Western blot and ELISA, respectively. *, P < 0.05; **, P < 0.01, compared with cells treated with 5 μmol/L nicotine for 16 h. Data are representative of results from three independent experiments. Columns, mean; bars, SD.
Previous studies have shown that activation of α7-nAChR by nicotine also leads to the activation of Src (11, 38–40) and the conventional isoforms of protein kinase C (PKC; ref. 40). To examine whether c-Src and PKC were involved in nicotine-induced HIF-1α and VEGF expression, A549 cells were pre-treated with PP2, a specific inhibitor of c-Src, or GF109203X, specific inhibitor of PKC isoforms. We found that both PP2 and GF109203X, even at low concentrations, drastically decreased nicotine-induced HIF-1α and VEGF expression (Fig. 4B and C). At 50 μmol/L, PP2 inhibited nicotine up-regulated HIF-1α and VEGF levels by 94.7 ± 12.2% (P < 0.01) and 77.7 ± 7.6% (P < 0.05), respectively (Supplementary Fig. S3B), whereas GF109203X attenuated nicotine up-regulated HIF-1α and VEGF levels by 93.3 ± 8.3% (P < 0.01) and 65.8 ± 7.3% (P < 0.05), respectively (Supplementary Fig. S3C).
To investigate the role of the activated ERK1/2 and PI3K/Akt pathways in nicotine-induced HIF-1α and VEGF expression, A549 cells were incubated with U0126, LY294002, and rapamycin, which are selective pharmacologic inhibitors of PI3K, mitogen-activated protein kinase/ERK kinase/ERK1/2, and mammalian target of rapamycin, respectively, before nicotine treatment. As expected, pretreatment with U0126, LY294002, and rapamycin significantly inhibited HIF-1α protein accumulation and VEGF production induced by nicotine (Fig. 4C and D). Further analyses indicated that, at 50 μmol/L, U0126 inhibited nicotine up-regulated HIF-1α and VEGF levels by 78.7 ± 10.6% (P < 0.01) and 55.0 ± 8.6% (P < 0.05), respectively (Supplementary Fig. S3C), whereas LY294002 abrogated nicotine up-regulated HIF-1α and VEGF levels by 89.2 ± 11.6% and 85.2 ± 12.6%, respectively (P < 0.01; Supplementary Fig. S3D). In parallel studies, in the presence of 50 nmol/L of rapamycin, nicotine up-regulated HIF-1α and VEGF levels were inhibited by 85.1 ± 10.3% (P < 0.01) and 61 ± 11.2% (P < 0.05), respectively (Supplementary Fig. S3D). To rule out the possibility that the inhibitory effects were secondary to cellular toxicity, A549 cells were treated with the highest concentration of each inhibitor for 24 h and cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Our results showed no obvious changes in cell morphology and viability (data not shown). Further, we tested whether these pharmacologic inhibitors had any effects on the basal level of HIF-1α protein in the absence of nicotine. Our results showed that only PP2 at 50 μmol/L and rapamycin at 50 nmol/L led to a moderate decrease in the basal level of HIF-1α (P < 0.05; Supplementary Fig. S4B and D), whereas other inhibitors have no effects (P > 0.05; Supplementary Fig. S4A-D). Taken together, these findings suggest that nAChR-mediated activation of signaling cascades is involved in nicotine-induced HIF-1α protein accumulation and VEGF production in lung cancer cells.
Enhanced in vitro capillary and tubule formation stimulated by nicotine-treated lung cancer cells was abrogated by HIF-1α siRNA
To investigate whether nicotine induces angiogenic activity in lung cancer, an in vitro angiogenesis model was used. Our results showed that conditioned medium obtained from A549 cells after treatment with 5 μmol/L nicotine for 24 h induced a 3.41 ± 0.33–fold increase in capillary and tubule formation by HUVECs compared with control medium from untreated cells (Fig. 5A, d versus a; Fig. 5B, P < 0.01). To rule out the possibility that the increased tubule formation was due to nicotine itself, the controlled or untreated conditioned medium was supplemented with 5 μmol/L nicotine. Our result indicated that exposure of HUVECs to 5 μmol/L nicotine up to 6 h had no obvious effect on tubule formation (Supplementary Fig. S5, b versus a).
Fig. 5.
In vitro capillary tube formation stimulated by nicotine-treated lung cancer cells was attenuated by transfection with specific HIF-1αsiRNA. HUVECs (5 × 103 per well) were seeded onto the surface of 96-well cell culture plates precoated with polymerized ECMatrix and then incubated in the conditioned medium for 6 h at 37°C. A, images of capillary tubes photographed under a phase-contrast microscope. Magnification, ×10. a and d, HUVECs were incubated in the conditioned medium derived from nontransfected A549 cells after cultured in the presence (d) or absence (a) of nicotine for 24 h; b and e, HUVECs were incubated in the conditioned medium derived from A549 cells transfected with nonspecific siRNA (NS-siRNA) after cultured in the presence (e) or absence (b) of nicotine for 24 h; c and f, HUVECs were incubated in the conditioned medium derived from A549 cells transfected with specific HIF-1αsiRNA after cultured in the presence (f) or absence (c) of nicotine for 24 h. B, quantification of capillary tube formation. The averaged values of branch points formed were calculated by counting the capillary tube branch points in six random view fields per well. **, P < 0.01 (d compared with a); *, P < 0.05 (f compared with d); #, P < 0.05 (c compared with a). Data are representative of three separate experiments. Columns, mean; bars, SD.
Next, we determine whether HIF-1α is directly involved in nicotine-stimulated in vitro angiogenesis. A549 cells were transfected with siRNAHIF-1α or nonspecific siRNA followed by incubation in the presence or absence of 5 μmol/L nicotine for 24 h, and conditioned media were collected. As shown in Fig. 5, transfection with nonspecific siRNA had no obvious effect on baseline tubule formation (Fig. 5A, b versus a; Fig. 5B, P > 0.05) or that stimulated by nicotine (Fig. 5A, e versus d; Fig. 5B, P > 0.05). On the contrary, transfection with siRNAHIF-1α inhibited both baseline tubule formation (Fig. 5A, c versus a) and that stimulated by nicotine (Fig. 5A, f versus d), with an estimated capillary branch-point reduction of 35.0% and 37.8%, respectively (P < 0.05; Fig. 5B). Collectively, these results suggested that the enhanced in vitro angiogenesis stimulated by nicotine-treated A549 cells was, at least in part, dependent on HIF-1α expression.
Nicotine-stimulated invasion and migration of lung cancer cells was inhibited by transfection with HIF-1α siRNA
Previous studies have shown that nicotine can potently stimulate migration and invasion of human lung cancer cells (40). Consistently, our results showed that exposure to nicotine significantly promoted invasion of A549 cell ( ~ 10-fold) compared with control (Fig. 6A, d versus a; Fig. 6B, P < 0.01). We then explored whether HIF-1α is involved in nicotine-stimulated cell invasion and migration. A549 cells transfected with siRNAHIF-1α or nonspecific siRNA were cultured in the collagen-based cell invasion assay system and incubated in the presence or absence of 5 μmol/L nicotine for 24 h. As shown in Fig. 6, transfection with nonspecific siRNA had no obvious effect on invasion of cancer cells cultured in the absence of nicotine (Fig. 6A, b versus a; Fig. 6B, P > 0.05) or that stimulated by nicotine (Fig. 6A, e versus d; Fig. 6B, P > 0.05). On the contrary, transfection with siRNAHIF-1α inhibited nicotine-stimulated invasion of A549 cells by f36.6% (Fig. 6A, f versus d; Fig. 6B, P < 0.01). Similar results were observed using the monolayer wounding or scratch assay, wherein transfection with siRNAHIF-1α partially inhibited nicotine-stimulated migration of A549 cells (Supplementary Fig. S6). These results suggested that the enhanced invasion of A549 cells stimulated by nicotine-treated A549 cells was, at least in part, dependent on HIF-1α expression.
Fig. 6.
Involvement of HIF-1αin nicotine-stimulated invasion of A549 cells. A, images photographed under a phase-contrast microscope. Magnification, × 10. A549 cells transfected with nonspecific siRNA (b and e) or specific HIF-1αsiRNA (c and f) were seeded onto the QCM Collagen-based Cell Invasion Assay system and cultured the presence or absence of 5 μmol/L nicotine for 48 h, and cell migration from the upper to the lower surface of the membrane was stained and photographed using a computer imaging system, wherein nontransfected cells cultured in the presence or absence of nicotine were used as negative (a) and positive (d) controls. B, quantification of cell invasion by colorimetric measurement at 560 nm. The graph shows the relative A560 values, wherein the A560 value from negative control (a) was arbitrarily set as 1.0. **, P < 0.01 (d compared with a); *, P < 0.05 (f compared with d). Results are representative of three independent experiments. Columns, mean; bars, SD.
Discussion
Cumulative evidence indicates that nicotine, asides from its psychoactive and addictive effects, can promote tumor cell proliferation, survival, migration/invasion, and tumor angiogenesis and thus serves as a potent carcinogen (5, 6, 13, 35, 40). To date, the detailed molecular mechanisms whereby nicotine enhances tumor growth and progression remain largely unknown. A growing body of evidence implies activation of HIF-1α pathway as a critical step in carcinogenesis (41, 42) due to its linkage to several oncogenic and tumor suppressor gene pathways in cancer (43). In this study, to our knowledge, we showed for the first time that treatment with nicotine promotes HIF-1α protein accumulation (Fig. 1), which contributes, at least in part, to nicotine-induced cell invasion and in vitro tumor angiogenesis in human NSCLC cells (Figs. 5 and 6). Therefore, these findings have provided novel mechanisms of biological functions of nicotine in tumor carcinogenesis.
Nicotine exerts its biological effects by binding to the nAChRs, thus leading to the activation of a cascade of signaling pathways (44), which include the influx of Ca2+ and activation of calmodulin, PKC (40, 45), c-Src, Janus-activated kinase 2/signal transducers and activators of transcription 3 (11, 38–40), PI3K/Akt/mammalian target of rapamycin (5, 35, 46), and Raf-1/mitogen-activated protein kinase/ERK1/2 (7, 34, 45). In the present study, we also showed that nicotine activates both PI3K/Akt and ERK1/2 signaling pathways (Fig. 3) and that pharmacologically blocking the activation of nAChR-mediated signaling cascades, including the Ca2+/calmodulin, c-Src, PKC, PI3K/Akt, mitogen-activated protein kinase/ERK kinase/ERK1/2, and the mammalian target of rapamycin, significantly attenuated nicotine up-regulated HIF-1α protein accumulation and VEGF protein expression in A549 cells (Fig. 4). These results suggest that nicotine promotes HIF-1α protein accumulation and VEGF expression in human NSCLC cells by activating various downstream nAChR-mediated signaling pathways.
nAChRs were once thought to be restricted to neuronal cells, but recently, the expression of nAChR subunits has also been shown in many nonneuronal cells, including normal human bronchial epithelial cells, human lung cancer cells (5, 11, 38, 45, 46), and oral keratinocytes (39). Previous studies have reported the expression of specific α7-nAChR in several NSCLC cell lines, including A549 cells (5, 11, 38). For instance, Dasgupta et al. (11) have recently shown expression of α7-nAChR protein in A549 cells and several other NSCLC cell lines. Consistent with these findings, we also showed the expression of α7-nAChR protein in four NSCLC cell lines, including A549 cell (Supplementary Fig. S2A). In another recent study, Carlisle et al. (45) reported that functional combinations of muscle-type and neuronal nAChR subunits were expressed at both mRNA and protein levels by three lung cancer cell lines, 201T, 273T, and A549; however, none of these cell lines examined expressed α2-nAChR, α4-nAChR, and α7-nAChR proteins. The apparent controversial findings about the expression of different subunits of nAChR in lung cancer cell lines may reflect the culture conditions, growth medium, passage numbers, or inherent heterogeneity of tumor cell lines. Further studies will examine the expression profile of nAChR in several established lung cancer cell lines in parallel with human lung tumor samples. α-BTX, a polypeptide composed of 74 amino acids containing five disulfide bridges, has been reported to block α7-nAChR–mediated downstream signaling cascades triggered by nicotine (35, 37). Reconstitution experiments in Xenopus oocytes have shown the effects of α-BTX on neuronal nAChR to be highly specific for the a7-subtype (IC50, 1.6 nmol/L) but not for the α3h4-subtype (IC50, >3 μmol/L; ref. 47). Recently, several studies have used α-BTX as a specific antagonist of α7-nAChR to block nicotine-stimulated downstream signaling pathways (35, 39, 40). However, the questions whether the muscle-type nAChR was expressed and whether α-BTX can block both α7- and muscle-type receptors were not addressed. Interestingly, Carlisle et al. (45) have recently shown that nicotine activates cell signaling pathways through both muscle-type and neuronal nAChRs in NSCLC cells, and α-BTX acts as an antagonist to both α7- and muscle-type receptors. In the present study, our results showed that pretreatment with α-BTX significantly inhibited nicotine-induced HIF-1α and VEGF expression in A549 cells (Fig. 4). However, based on recent findings by Carlisle et al. (45), more studies are needed to clarify whether α7-type, muscle-type, or both are functionally involved in nicotine-induced HIF-1α and VEGF expression in human lung cancer cells.
VEGF has been recognized as one of the principal initiators of tumor angiogenesis. VEGF expression is regulated by a plethora of external factors (48), of which hypoxia is the best-characterized mediator of VEGF secretion, whereas HIF-1 protein is stabilized and bound to the hypoxia-responsive elements on VEGF promoter, thus leading to the transcriptional activation of the VEGF gene (17, 20, 26). In addition to hypoxia-responsive elements, the promoters of VEGF share many consensus sites for Sp1/Sp3, AP-2, Egr-1, and signal transducers and activators of transcription 3 (48). Previous studies have reported that nicotine stimulates VEGF expression in cancer cells (6, 14, 49), but the underlying mechanisms remain largely unknown. In this study, we showed that nicotine significantly stimulated VEGF production, which can be partially inhibited (~46.8%) by disrupting HIF-1α expression using siRNA strategy (Figs. 1 and 3). Meanwhile, our results indicate that use of selective pharmacologic inhibitors to block nicotine-stimulated downstream signaling pathways had a stronger overall inhibitory effect on nicotine-induced HIF-1α expression than on the expression of VEGF gene (Fig. 4). Functionally, we found that nicotine-treated lung cancer cells significantly stimulated in vitro capillary-like tubule formation by HUVECs and such stimulatory effect was partially abolished (~37.8%) by transfection with specific HIF-1α siRNA (Fig. 5). Collectively, these findings suggest that HIF-1α contributes, at least in part, to the up-regulation of VEGF expression and the in vitro tumor angiogenesis enhanced by nicotine in lung cancer cells. Therefore, further studies are necessary to investigate whether other transcription factors, such as Sp1, AP-1, and signal transducers and activators of transcription 3, are also responsible for nicotine-stimulated VEGF expression.
There is evidence that, in addition to its critical role in angiogenesis, HIF-1α plays an equally important role in tumor metastasis (32, 42, 50). Nicotine, besides its antiapoptotic (10–12) and proangiogenic (13–15) activities, is capable to promote tumor invasion and metastasis (14, 40) mediated by matrix metalloproteinases 2 and 9 and plasminogen activators (urokinase-type plasminogen activator and its receptor; ref. 14). In addition, Xu and Deng (40) have recently reported that PKCι promotes nicotine-induced migration and invasion of human lung cancer cells via phosphorylation of μ- and m-calpains. In this study, we found that migration and invasion of A549 cells stimulated by nicotine was suppressed by ~36.6% after disruption of HIF-1α by specific siRNA (Fig. 6), suggesting that HIF-1α contributes, at least in part, to nicotine-stimulated cell migration and invasion of lung cancer. Further studies are in progress to delineate the interaction between HIF-1α and the above-mentioned pathways involved in the biological actions of nicotine in the migration and invasion of lung cancer cells.
In summary, the study described here has shown for the first time to our knowledge that nicotine stimulates HIF-1α protein accumulation and VEGF expression in NSCLC cells. We also found that HIF-1α contributes, at least in part, to nicotine-promoted cell migration, invasion, and tumor angiogenesis by lung cancer cells. These unique findings have greatly extended our current knowledge about the molecular mechanisms underlying the tumorigenic activities of nicotine and provide potential targets for the development of novel anticancer therapy in the management of local and systemic diseases in tobacco-associated human lung cancer.
Supplementary Material
Acknowledgments
Grant support: NIH grants AR47359 (A.D. Le) and P50 CA90833 (Z-F. Zhang). The costs of publication of this article were defrayed in part by the payment of page charges.This article must therefore be hereby marked advertisement in accordance with18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Brognard J, Clark AS, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–97. [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Samuals A, Ghfoor A, Ward E, Thunn MJ. Cancer statistics 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Wu XH, Wang HF, Liu YF, Lu XY, Wang JJ, Li K. His-tone adduction with nicotine: a bio-AMS study. Radiocarbon. 1997;39:293–7. [Google Scholar]
- 4.Maneckjee R, Minna JD. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc Natl Acad Sci U S A. 1990;87:3294–8. doi: 10.1073/pnas.87.9.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsurutani J, Castillo SS, Brognard J, et al. Tobacco components stimulate Akt-dependent proliferation and NF-κB-dependent survival in lung cancer cells. Carcinogenesis. 2005;26:1182–95. doi: 10.1093/carcin/bgi072. [DOI] [PubMed] [Google Scholar]
- 6.Shin VY, Wu WKK, Ye YN, et al. Nicotine promotes gastric tumor growth and neovascularization by activating extracellular signal-regulated kinase and cyclooxygenase-2. Carcinogenesis. 2004;25:2487–95. doi: 10.1093/carcin/bgh266. [DOI] [PubMed] [Google Scholar]
- 7.Bose C, Zhang HL, Udupa KB, Chowdhury P. Activation of p-ERK1/2 by nicotine in pancreatic tumor cell line AR42J: effects on proliferation and secretion. Am J Physiol Gastrointest Liver Physiol. 2005;289:926–34. doi: 10.1152/ajpgi.00138.2005. [DOI] [PubMed] [Google Scholar]
- 8.Onoda N, Nehmi A, Weiner D, Mujumdar S, Christen R, Los G. Nicotine affects the signaling of the death pathway, reducing the response of head and neck cancer cell lines to DNA damage agents. Head Neck. 2001;23:860–70. doi: 10.1002/hed.1125. [DOI] [PubMed] [Google Scholar]
- 9.Maneckjee R, Minna JD. Opioids induce while nicotine suppresses apoptosis in human lung cancer cells. Cell Growth Differ. 1994;5:1033–40. [PubMed] [Google Scholar]
- 10.Zhang T, Lu H, Shang X, et al. Nicotine prevents the apoptosis induced by menadione in human lung cancer cells. Biochem Biophys Res Commun. 2006;242:928–34. doi: 10.1016/j.bbrc.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta P, Kinkade R, Joshi B, DeCook C, Haura E, Chellapan S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci U S A. 2006;103:6332–7. doi: 10.1073/pnas.0509313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heusch WL, Maneckjee R. Signaling pathways involved in nicotine regulation of apoptosis of human lung cancer cells. Carcinogenesis. 1998;19:551–6. doi: 10.1093/carcin/19.4.551. [DOI] [PubMed] [Google Scholar]
- 13.Heeschen C, Jang JJ, Weis M, et al. Nicotine stimulates angiogenesis and tumor growth and atherosclerosis. Nat Med. 2001;7:833–9. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 14.Shin VY, Wu WKK, Chu KM, et al. Nicotine induces cyclooxygenase-2 and vascular endothelial growth factor receptor-2 in association with tumor-associated invasion and angiogenesis in gastric cancer. Mol Cancer Res. 2005;3:607–15. doi: 10.1158/1541-7786.MCR-05-0106. [DOI] [PubMed] [Google Scholar]
- 15.Mousa S, Mousa SA. Cellular and molecular mechanisms of nicotine's pro-angiogenesis activity and its potential impact on cancer. J Cell Biochem. 2006;97:1370–8. doi: 10.1002/jcb.20741. [DOI] [PubMed] [Google Scholar]
- 16.López-Lázaro M. HIF-1: hypoxia-inducible factor or dysoxia-inducible factor? FASEB J. 2006;20:828–32. doi: 10.1096/fj.05-5168hyp. [DOI] [PubMed] [Google Scholar]
- 17.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor-1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamura T, Sato S, Iwai K, et al. Activation of HIF-1α ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci U S A. 2000;97:10430–5. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zundel W, Schindler C, Haas-Kogan D, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of a vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322–31. [PubMed] [Google Scholar]
- 21.Jiang BH, Agani F, Passaniti A, Semenza GL. V-SRC induces expression of hypoxia-inducible factor 1 (HIF) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF in tumor progression. Cancer Res. 1997;57:5328–35. [PubMed] [Google Scholar]
- 22.Li YM, Zhou BP, Deng J, Pan Y, Hay N, Hung MC. A hypoxia-independent hypoxia-inducible factor-1 activation pathway induced by phosphatidylinositol-3 kinase/Akt in HER2 overexpression cells. Cancer Res. 2005;65:3257–63. doi: 10.1158/0008-5472.CAN-04-1284. [DOI] [PubMed] [Google Scholar]
- 23.Stiehl DP, Jelkmann W, Wenger RH, Hellwig-Bürgel T. Normoxic induction of the hypoxia-inducible factor 1α by insulin and interleukin-1β involves the phosphatidylinositol-3-kinase pathway. FEBS Lett. 2002;512:157–62. doi: 10.1016/s0014-5793(02)02247-0. [DOI] [PubMed] [Google Scholar]
- 24.Pagé EL, Robitaille GA, Pouysségur J, Richard DE. Induction of hypoxia-inducible factor-1α by transcriptional and translational mechanisms. J Biol Chem. 2002;277:48403–9. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- 25.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 26.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 27.Zagzag D, Zhong H, Scaizitti JM, et al. Expression of hypoxia-inducible factor 1α in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606–18. [PubMed] [Google Scholar]
- 28.Zhong H, Chiles K, Feldser D, et al. Modulation of hypoxia-inducible factor 1α expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–5. [PubMed] [Google Scholar]
- 29.Giatromanolak A, Koukourakis MI, Sivridis E, et al. Relation of hypoxia inducible factor 1α and 2α in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–90. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swinson DEB, Jones JL, Cox G, Richardson D, Harris AL, O'Byrne KJ. Hypoxia inducible factor 1α in non small cell lung cancer: relation to growth factor, protease and apoptosis pathways. Int J Cancer. 2004;111:43–50. doi: 10.1002/ijc.20052. [DOI] [PubMed] [Google Scholar]
- 31.Hirami Y, Aoe M, Tsukuda K, et al. Relation of epidermal growth factor receptor, phosphorylated-Akt, and hypoxia inducible factor 1α in non-small cell lung cancers. Cancer Lett. 2004;214:157–64. doi: 10.1016/j.canlet.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 32.Phillips RJ, Mestas J, Gharaee-Kermani M, et al. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor 1α. J Biol Chem. 2005;280:22473–81. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang QZ, Zhang ZF, Rao JY, et al. Treatment with siRNA and oligonucleotides targeted to HIF-1α induced apoptosis in human tongue squamous cell carcinomas. Int J Cancer. 2004;111:849–57. doi: 10.1002/ijc.20334. [DOI] [PubMed] [Google Scholar]
- 34.Cattaneo MG, D'Atri F, Vicentini LM. Mechanisms of mitogen-activated protein kinase activation by nicotine in small-cell lung carcinoma cells. Biochem J. 1997;328:499–503. doi: 10.1042/bj3280499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West KA, Brognard J, Clark AS, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HY, Oh SH, Woo JK, et al. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. J Natl Caner Inst. 2005;97:1695–9. doi: 10.1093/jnci/dji377. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Pereira EF, Maus AD, et al. Human bronchial epithelial and endothelial cells express α7 nicotinic acetylcholine receptors. Mol Pharmacol. 2001;60:1201–9. doi: 10.1124/mol.60.6.1201. [DOI] [PubMed] [Google Scholar]
- 38.Dasgupta P, Rastogi S, Pillai S, et al. Nicotine induces cell proliferation by β-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116:2208–17. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of α7 nicotinic receptor in oral keratinocytes. FASEB J. 2006;20:2093–101. doi: 10.1096/fj.06-6191com. [DOI] [PubMed] [Google Scholar]
- 40.Xu LJ, Deng XM. Protein kinase Cι promotes nicotine-induced migration and invasion of cancer cells via phosphorylation of μ- and m-calpains. J Biol Chem. 2006;281:4457–66. doi: 10.1074/jbc.M510721200. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka H, Yamamoto M, Hashimoto N, et al. Hypoxia-independent overexpression of hypoxia-inducible factor-1α as an early change in mouse hepatocarcinogenesis. Cancer Res. 2006;66:11263–70. doi: 10.1158/0008-5472.CAN-06-1699. [DOI] [PubMed] [Google Scholar]
- 42.Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1α is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 2007;67:563–72. doi: 10.1158/0008-5472.CAN-06-2701. [DOI] [PubMed] [Google Scholar]
- 43.Vogelstein B, Kinzler KW. Cancer genes and the pathway they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 44.Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signaling. Trends Pharmacol Sci. 2004;25:317–2446. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Carlisle DL, Liu XW, Hopkins TM, et al. Nicotine activates cell-signaling pathways through muscle-type and neuronal nicotinic acetylcholine receptors in non-small cell lung cancer cells. Pulm Pharmacol Ther. 2006 doi: 10.1016/j.pupt.2006.07.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Carlisle DL, Hopkins TM, Gaither-Davis A, et al. Nicotine signals through muscle-type and neuronal nicotinic acetylcholine receptors in both human bronchial epithelial cells and airway fibroblasts. Respir Res. 2004;5:27. doi: 10.1186/1465-9921-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez MG, Montiel C, Herrero CJ, et al. Unmasking the functions of the chromaffin cell α7 nicotinic receptor by using short pulses of acetylcholine and selective blockers. Proc Natl Acad Sci U S A. 1998;95:14184–9. doi: 10.1073/pnas.95.24.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Page's G, Pouysségur J. Transcriptional regulation of the vascular endothelial growth factor gene—a concert of activating factors. Cardiovasc Res. 2005;65:564–73. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 49.Lane D, Gray EA, Mathur RS, Mathur SP. Up-regulation of vascular endothelial growth factor-C by nicotine in cervical cancer cell lines. Am J Reprod Immunol. 2005;53:15853–60. doi: 10.1111/j.1600-0897.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 50.Krishnamachary B, Berg-Dixon S, Kelly B, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–43. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.