Abstract
Purpose
Passive anterior knee laxity has been linked to non-contact ACL injury risk. High deceleration movements have been implicated in the non-contact ACL injury mechanism, and evidence suggests that greater anterior tibial translations (ATT) may occur in healthy knees that are lax compared to a tight knee. The purpose of this study was to determine the relationship between anterior knee laxity scores and ATT during drop landings using biplane fluoroscopy.
Methods
Sixteen healthy adults (10 women; 6 men) performed stiff drop landings (40 cm) while being filmed using a high-speed, biplane fluoroscopy system. Initial, peak and excursions for rotations and translations were calculated and regression analysis used to determine the 6DoF kinematic relationships with KT1000 scores with peak ATT occurring during the landing.
Results
KT1000 values were (+) correlated with peak ATT values for group (r = 0.89; P < 0.0001) and both genders (males, r = 0.97; P = 0.0003; females, r = 0.93; P = < 0.0001). Regression analysis yielded a significant linear fit for the group (r2 = 0.80; YATT-group = −0.516 + 1.2 × XKT1000-group) and for each gender (females: r2 = 0.86; YATT-females = 0.074 + 1.2 × XKT1000-females and males: r2 = 0.94; YATT-males = −0.79 + 1.2 × XKT1000-males).
Conclusion
A strong relationship was observed between passive anterior knee laxity measured via KT1000 and peak ATT experienced during dynamic activity in otherwise healthy persons performing a stiff drop-landing motion.
Keywords: ACL, Landing, Anterior tibial translation, Biomechanics
Introduction
Biomechanical injury risk assessments collected in the laboratory and neuromuscular training programs have shown promise in identifying individuals who are at a high risk of sustaining a non-contact ACL injury [12, 23]. However, using sophisticated motion capture techniques for screening potential “at-risk” athletes can be expensive and time consuming. Thus, research has been initiated to identify potential risk factors that can be easily obtained without expensive equipment or staff time [11, 32, 36].
Previous studies have suggested anterior knee laxity, as measured by the KT 1000/2000 devices, is predictive of ACL injuries [32, 36]. Woodford-Rogers et al. [36] found greater measures of knee laxity in the non-injured limb of ACL injured patients. Uhorchak et al. [32] noted athletes with higher knee laxity scores are more likely to injure their ACL. Due to technical limitations of traditional motion capture techniques, no study has been able to directly link passive anterior knee laxity to knee translations occurring in vivo during a high-demand activity such as the drop landing.
Given that the knee is exposed to considerable anterior shear forces during functional exercises such as the drop landing [16, 24, 25] and large quadriceps moments have been implicated in the non-contact ACL injury mechanisms [4, 7, 37], it is reasonable to hypothesize that greater ATT may occur in the otherwise healthy knee that exhibits greater passive anterior laxity compared to a knee that is more “tight”. Clinically, understanding the relationship of passive knee laxity to dynamic knee function, and knee translations in particular, is important if our goal is to understand and efficiently select appropriate parameters to examine risk of ACL injury in any population. Thus, this study aims to bridge the current gap between clinical measures of passive, anterior knee laxity and dynamic knee translation occurring in vivo.
The purposes of this study were to utilize high-speed, biplane fluoroscopy to: 1) determine the relationship between anterior knee laxity measured via KT 1000 and 3D knee rotations and translations measured in vivo during a drop-landing motion in healthy male and female adults. We hypothesized that individuals who posses higher anterior knee laxity scores as measured via KT 1000 will also exhibit greater ATT during the drop landing.
Materials and methods
Sixteen recreational athletes (10 women; mean age: 26.1 ± 5.5 years, mean height 1.6 ± 0.04 m, mean weight 65.4 ± 14.3 kg; and 6 men: mean age 32.1 ± 7.9 years; mean height 1.9 m ± 0.1, mean weight 85.1 kg ± 7.3) volunteered to participate in this study. These participants had no history of lower extremity injury. All subjects provided written informed consent approved in accordance with the National Institutes of Health’s guidelines.
For all testing, all subjects wore spandex-like shorts and women wore a tightly fit top. All subjects wore the same standardized court shoe (Turntec, model no. TM08061). Passive anterior knee laxity of each subject was measured using the KT 1000™ knee ligament arthrometer (MEDmetric® Corporation, San Diego, California, USA). All measures were made by a single examiner using methods as previously described [27] with an anterior force of 177 N with translation (in mm) recorded. The anterior knee laxity examination was conducted three times, and the translation values were averaged to produce a single value for each subject.
Subjects performed a drop-landing motion by stepping off a 40-cm high platform onto a force plate. The subjects were verbally instructed to land in a “stiff” manner by “trying not to bend at the hip, knee or ankle during the fall nor at or after ground impact”. Stiff landings were selected as the landing style for analysis because: (1) this landing style has often been cited as the way “not to land” to avoid ACL non-contact injuries [23]; (2) larger external ground reaction forces, joint reaction forces and ACL loads have been associated stiff landings [25]; and, (3) stiff landings allow for more consistent landing patterns with reduced variability across subjects [5]. After a 5-min warm-up, the subjects completed 10 stiff landing trials in which a single trial for each subject was collected by fluoroscopy for analysis. The foot of the dominant limb landed directly onto a force plate (Bertec Corp., Columbus, Ohio) secured to the laboratory floor. The force plate data were collected at 1200 Hz and synchronized with the fluoroscopy video images such that the specific fluoroscopy frame could be determined.
The subjects also completed a slow (2 s), unloaded knee extension motion from a seated position (hip angle at 90) starting at a knee flexion of 90 degrees to the fully extended position. The location of the tibia and femur at full extension was used to define the zero reference position of the bones of the knee [1].
Description and calibration of the biplane fluoroscopy system
The biplane fluoroscopy system utilized in this study has been described in detail previously [2, 31]. In brief, the biplane fluoroscopy system comprised of two commercially available BV Pulsera c-arms with 30-cm image intensifiers (Philips Medical Systems, Best, Holland) which were modified and coupled to two high-speed, high-resolution (1,024 × 1,024) digital cameras (Phantom V5.1, Vision Research, Wayne, NJ) that were interfaced with the image intensifiers (Fig. 1).
Fig. 1.
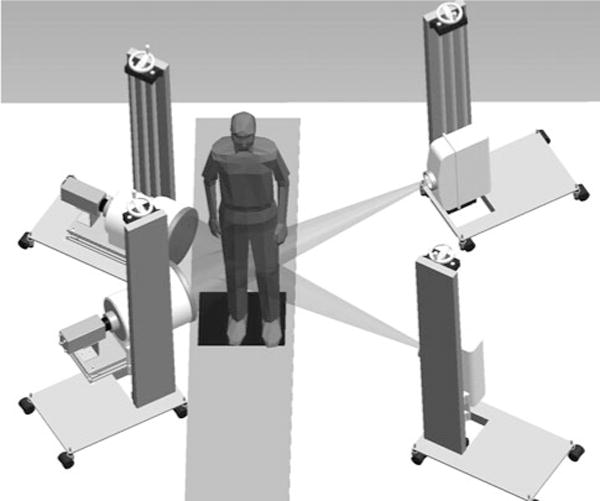
Computer rendering of the configuration of the biplane fluoroscopy system
Collection of in vivo landing data using biplane fluoroscopy
In vivo biplane fluoroscopy data collection consisted of two parts: (1) obtaining a static CT of the knee joint for bone pose estimations; and, (2) collecting the biplane fluoroscopy data during the drop landing.
Tracking the tibia and the femur in the fluoroscopy images requires 3D models of the bones. For each subject, a supine, high-resolution (voxels: 0.7 × 0.7 × 0.5 mm), static bone CT scan of the knee (12 cm above and below the joint line) utilizing an Aquilion 64 (Toshiba America Medical Systems, Tustin, CA) was obtained [31]. For each landing, biplane fluoroscopy data were collected for 1.0 s at 500 frames/s with a shutter speed of 1/2,000 of a second [31]. The x-ray generators were operated in radiographic mode at 60 mA and approximately 60 kV.
Data reduction
Data processing consisted of four steps: (1) 3D bone geometry reconstruction of the femur and tibia/fibula from CT data; (2) coordinate system assignment and geometry transformation; (3) bone pose determination in the biplane fluoroscopy data; and, (4) post-processing to extract the knee kinematics. The 3D geometries of the femur and tibia/fibula were extracted from the CT data using commercial software (Mimics, Materialize, Inc, Ann Harbor, MI). Custom software written in Matlab (The Mathworks, Natick, MA) was used to assign anatomical coordinate systems to the bones and to transform the bones to positions suitable for pose reconstruction.
The origin of the femoral coordinate system was placed at the midpoint between the medial and lateral femoral condyles on the center line of a cylinder fitted to the medial and lateral posterior condyles. The medio-lateral (ML) axis of the femur was assigned as the line through the long axis of this cylinder. The superior–inferior (SI) axis was placed along the posterior line of the femur, and an anterior–posterior (AP) axis was determined as the cross product of the ML and SI axes [1]. The femoral coordinate system was assigned to the tibia at full extension (i.e., 0 deg) in the knee extension trial representing the neutral or ‘zero’ position.
Determination of the bone poses from the biplane fluoroscopy data was performed using MBRSA (Medis Specials, Leiden, The Netherlands). A fully automatic 6 degree-of-freedom optimization algorithm was used to determine the pose (position and orientation), which optimally matched the detected contours with the projected contours from the imported bone geometries (Fig. 2). Using the bone pose sequences, knee kinematics were calculated using methods described by Grood and Suntay [9] where knee rotations and translations indicate motions of the tibia with respect to the femur (Fig. 3).
Fig. 2.
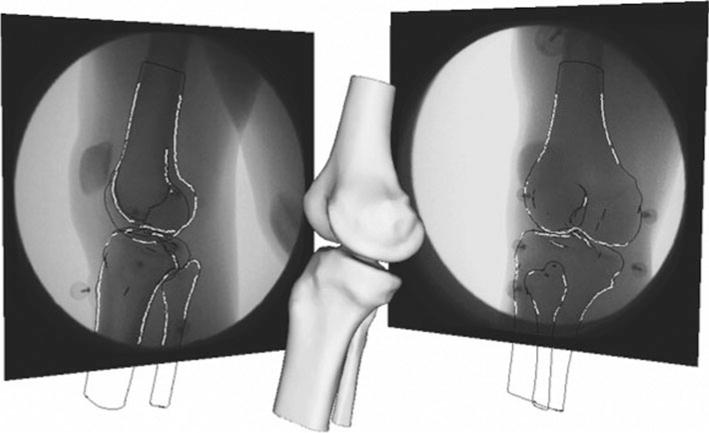
Example frame of the model-based Roentgen stereophotogrammetric analysis with the 3D model a subject’s knee being matched to the two fluoroscopic views
Fig. 3.
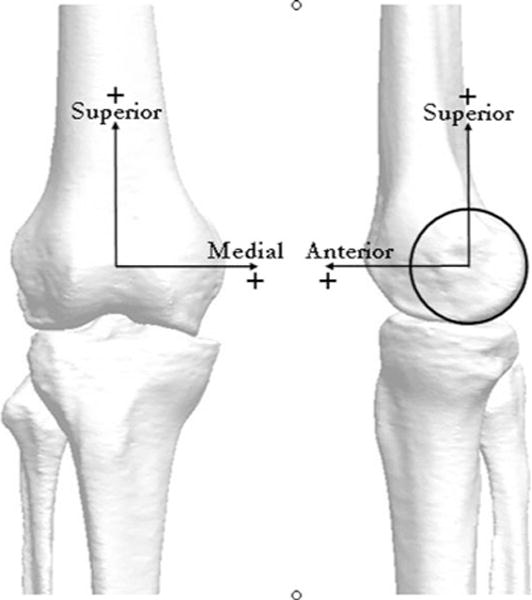
Representative bone geometry model derived from the CT scan depicting coordinate axes used to define the reference position of the tibia and femur: medial–lateral (ML), superior–inferior (SI), and anterior–posterior (AP)
Kinematic accuracies for tracking tantalum beads and bones using this biplane fluoroscopy system were determined. The accuracy and precision (mean and SD) of tracking 1.0 mm diameter tantalum markers were −5.5 μm and 32.5 μm, respectively. Based on the high accuracy of tracking tantalum beads, bead tracking were used as the gold standard for bone tracking methods. Tantalum beads were placed in a cadaveric knee specimen (3 beads per bone), and the specimen was manually ranged and cycled through full flexion–extension, varus–valgus and internal–external rotation. The mean and ±1 standard deviation of the differences in joint kinematics between those determined by tantalum beads and those by bone tracking were 0.2 ± 0.3 mm, −0.1 ± 0.1 mm, −0.05 ± 0.1 mm in translations; and 0.1 ± 0.1°, 0.3 ± 0.2°, 0.1 ± 0.3° in rotations. Additionally, tantalum beads were also placed in a cadaveric knee specimen (3 beads per bone), and the specimen was mounted on a vertical slide bar such that the tibial portion of the cadaver was rigidly fixed to the slide bar, and the femur was allowed to flex upon force plate (ground) impact. The specimen was allowed to drop from a height of 40 cm and impacted the force platform (with a peak impact force of 25 N/Kg). Seventeen frames taken at a 125 Hz (every fourth frame) sample from the 500-Hz sampling frequency including several frames before, at and after the impact and encompassed a knee excursion of ~70 degrees (from the free-fall angle of ~15 degrees, a contact angle of ~17 degrees to ~85 degrees of flexion) were tracked using the same bone tracking methods as described in this and a previous study [31]. The mean and ±1 standard deviation of the differences in joint kinematics between those determined by tracking the tantalum beads and those by bone tracking were 0.07 ± 0.7 mm, 0.1 ± 0.6 mm in medial–lateral and anterior–posterior translations; and 0.09 ± 0.4°, 0.1 ± 0.05°, 0.1 ± 0.8° in flexion, varus–valgus, internal–external rotations, respectively. These values are consistent with previous work by others using the MBRSA software [13, 29].
Following the example of precision motion measurements taken from cadaver knees [14], tibial translations and rotations measured from each subject during landing were referenced to the unloaded knee extension task. This was achieved by subtracting the translation and rotation of the tibia during the knee extension from data collected during the landing task at the same corresponding knee flexion angle.
Statistical analysis
Individual and group means ± 1 standard deviation for the initial, peak and excursions for knee flexion angle, knee internal–external rotation and knee varus–valgus angles as well as ATT and knee lateral translations (LT) were calculated for each subject’s trial. Gender comparisons between KT 1000 and kinematic datasets were conducted using ANOVA (alpha = 0.05). Prior to investigating the relationship between maximal ATT and laxity scores, the data describing these parameters were analyzed for normality. Pearson product moment correlation coefficients were then calculated to identify the relationship between passive knee laxity scores and maximal ATT occurring during the landing motion. Following the correlation analysis, linear regression was used to establish a prediction equation of passive anterior knee laxity score and maximal ATT during the landing.
Results
Figure 4 shows the vertical ground reaction force for each subject as well as the time period for which biplane fluoroscopy data was collected and analyzed. Peak ground reaction force was 2.99 ± 0.63 BW. There was no significant difference between genders with respect to peak ground reaction force (P = 0.39).
Fig. 4.
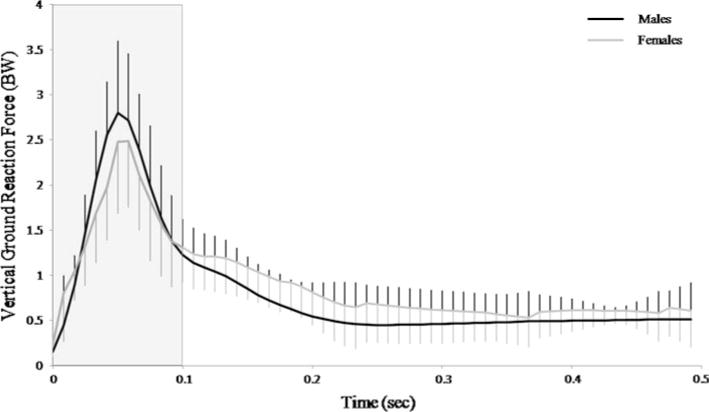
Time series plot of the individual trials of the vertical ground reaction force for each of the subjects. Shaded area represents the time period for biplane fluoroscopy data collection and analysis
Group- and gender-specific mean ± 1 standard deviation for KT 1000 values are provided in Table 1. No significant gender differences were observed in KT 1000 laxity scores (P = 0.09).
Table 1.
Mean and ± 1 standard deviation for passive anterior knee laxity measured via KT-1000 for the Group and each gender (M, male; F, female)
| Mean | SD | Minimum | Maximum | *P-value | |
|---|---|---|---|---|---|
| KT 1000 (mm) Group | 5.4 | 1.2 | 3.5 | 8.0 | ns |
| KT 1000, F | 5.8 | 1.2 | 4.0 | 8.0 | |
| KT 1000, M | 4.8 | 1.0 | 3.5 | 6.5 |
ns non-significance, P > 0.05
P-value is the comparison between gender
Data for initial, maximal, minimal and range of knee flexion, internal/external and varus/valgus rotations between the first frame after ground contact to maximal knee flexion are presented in Table 2 and time series in (Fig. 5a). There were no significant differences between genders in any of knee joint rotation variables (all P ≥ 0.14).
Table 2.
Mean and ± 1 standard deviation (SD), minima and maxima values for knee kinematic parameters for the Group and each gender (M, male; F, female)
| Mean | SD | Minimum | Maximum | *P-value | |
|---|---|---|---|---|---|
| Max knee flexion (deg) Group | 51.3 | 15.2 | 19.7 | 82.7 | ns |
| Max knee flexion, F | 55.7 | 12.7 | 32.6 | 82.7 | |
| Max knee flexion, M | 44.0 | 17.3 | 19.7 | 60.9 | |
| Min knee flexion (deg) Group | 17.5 | 11.0 | 2.6 | 37.5 | ns |
| Min knee flexion, F | 19.7 | 11.8 | 8.4 | 37.5 | |
| Min knee flexion, M | 13.8 | 9.4 | 2.6 | 26.4 | |
| Initial knee flexion (deg) Group | 18.0 | 13.9 | 0.2 | 50.8 | ns |
| Initial knee flexion, F | 21.5 | 14.9 | 8.3 | 50.8 | |
| Initial knee flexion, M | 12.3 | 10.9 | 0.2 | 26.4 | |
| Range knee flexion, (deg) Group | 33.8 | 9.8 | 17.1 | 49.2 | ns |
| Range knee flexion, F | 36.0 | 9.0 | 23.1 | 49.2 | |
| Range knee flexion, M | 30.2 | 11.0 | 17.1 | 45.7 | |
| Max ext rotation, (deg) Groupa | 0.7 | 4.2 | −7.1 | 7.5 | ns |
| Max ext rotation, F | 0.6 | 3.8 | −4.0 | 7.5 | |
| Max ext rotation, M | 1.0 | 5.2 | −7.1 | 6.4 | |
| Min ext rotation, (deg) Group | −5.1 | 4.7 | −16.0 | 4.8 | ns |
| Min ext rotation, F | −4.8 | 4.1 | −12.1 | 4.8 | |
| Min ext rotation, M | −5.5 | 6.0 | −16.0 | 0.4 | |
| Initial ext rotation, (deg) Group | −0.4 | 4.1 | −7.1 | 6.1 | ns |
| Initial ext rotation, F | −0.4 | 4.1 | −6.0 | 4.8 | |
| Initial ext rotation, M | −0.3 | 4.4 | −7.1 | 6.1 | |
| Range ext rotation, (deg) Group | 5.8 | 3.3 | 2.1 | 14.5 | ns |
| Range ext rotation, F | 5.4 | 3.9 | 2.1 | 14.5 | |
| Range ext rotation, M | 6.6 | 2.3 | 4.0 | 9.1 | |
| Max valgus angle, (deg) Groupa | 1.6 | 0.9 | 0.3 | 3.4 | ns |
| Max valgus angle, F | 1.6 | 0.9 | 0.5 | 3.4 | |
| Max valgus angle, M | 1.6 | 0.9 | 0.3 | 3.1 | |
| Min valgus angle, (deg) Group | −1.5 | 1.6 | −4.6 | 1.3 | ns |
| Min valgus angle, F | −1.8 | 1.7 | −4.6 | 1.3 | |
| Min valgus angle, M | −0.9 | 1.3 | −2.9 | 0.8 | |
| Initial valgus angle, (deg) Group | −0.4 | 2.3 | −4.6 | 2.8 | ns |
| Initial valgus angle, F | −0.6 | 2.6 | −4.6 | 2.8 | |
| Initial valgus angle, M | −0.1 | 1.7 | −2.2 | 2.6 | |
| Range valgus angle, (deg) Group | 3.1 | 1.3 | 0.9 | 5.6 | ns |
| Range valgus angle, F | 3.4 | 1.4 | 0.9 | 5.6 | |
| Range valgus angle, M | 2.5 | 0.9 | 1.9 | 4.3 |
ns non-significance, P > 0.05
P-value is the comparison between gender
For varus–valgus and internal and external rotation angles, negative values represent varus and internal rotation, respectively
Fig. 5.
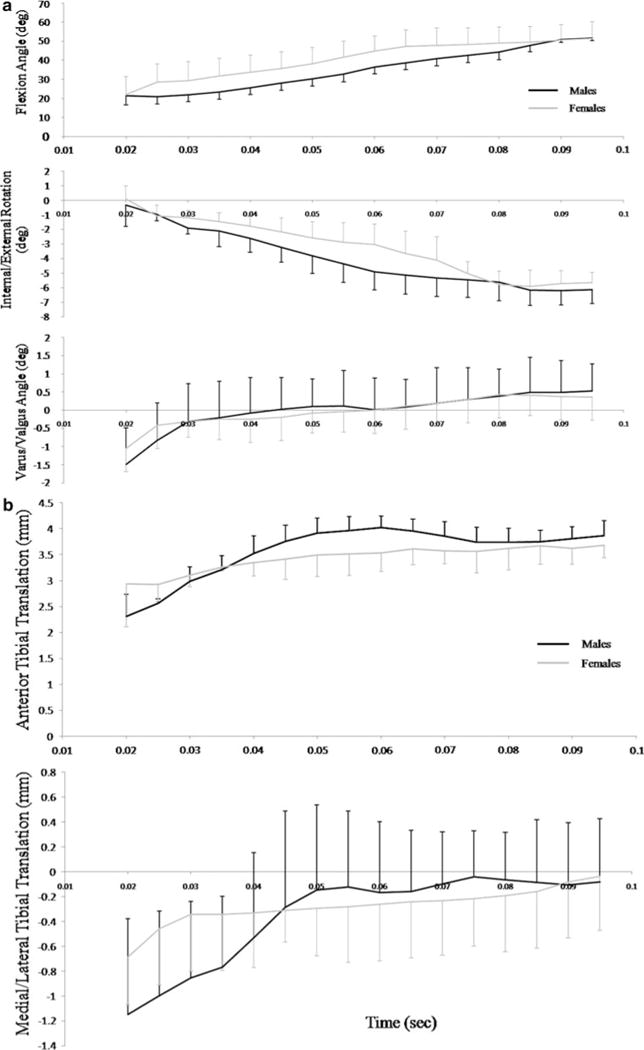
a Represents knee flexion, internal/external and varus/valgus rotations between the first frame after ground contact to maximal knee flexion angle. b Represents anterior tibial translation (ATT) and knee medial/lateral translations over the same time period. Values in both graphs are the group mean average with ± 1 standard error
Group- and gender-specific mean ± 1 standard deviations for initial, maximal, minimal and range of ATT, lateral translations (LT) and time to maximal ATT are presented in Table 3 and time series data are provided in Fig. 5b. There were no significant differences between genders in the initial, maximal, minimal or range of ATT or LT values (all P ≥ 0.22). There was no significant difference in the time to maximal ATT between genders (P = 0.81).
Table 3.
Mean and ±1 standard deviation (SD), minima and maxima values for knee kinematic parameters for the Group and each gender (M, male; F, female)
| Mean | SD | Minimum | Maximum | *P-value | |
|---|---|---|---|---|---|
| Max ATT N, (mm) Group | 4.4 | 0.8 | 3.0 | 6.1 | ns |
| Max ATT N, F | 4.5 | 0.9 | 3.0 | 6.1 | |
| Max ATT N, M | 4.3 | 0.8 | 3.4 | 5.4 | |
| Initial ATT N, (mm) Group | 1.2 | 2.8 | −6.2 | 4.3 | ns |
| Initial ATT N, F | 0.8 | 3.3 | −6.2 | 4.3 | |
| Initial ATT N, M | 2.1 | 1.1 | 1.1 | 4.1 | |
| Range ATT N, (mm) Group | 4.2 | 2.5 | 1.2 | 10.8 | ns |
| Range ATT N, F | 4.9 | 2.8 | 1.2 | 10.8 | |
| Range ATT N, M | 3.2 | 1.4 | 1.9 | 5.8 | |
| Max LT N, (mm) Group | 1.6 | 1.6 | −0.1 | 5.5 | ns |
| Max LT N, F | 1.4 | 1.8 | −0.1 | 5.5 | |
| Max LT N, M | 1.8 | 1.4 | 0.2 | 3.9 | |
| Initial LT N, (mm) Group | 0.4 | 1.9 | −1.6 | 5.5 | ns |
| Initial LT N, F | 0.5 | 2.1 | −1.6 | 5.4 | |
| Initial LT N, M | 0.3 | 1.4 | −1.2 | 2.3 | |
| Range LT N, (mm) Group | 3.8 | 1.8 | 1.6 | 7.4 | ns |
| Range LT N, F | 4.2 | 1.8 | 1.6 | 7.4 | |
| Range LT N, M | 3.3 | 1.7 | 1.7 | 6.2 | |
| Time to Max ATT, (msec) Group | 65.4 | 24.6 | 30.0 | 108.0 | ns |
| Time to Max ATT, F | 64.0 | 23.4 | 30.0 | 102.0 | |
| Time to Max ATT, M | 67.7 | 28.7 | 34.0 | 108.0 | |
| Peak VGRF (BW), Group | 3.0 | 0.6 | 2.2 | 4.6 | ns |
| Peak VGRF (BW), F | 3.1 | 0.7 | 2.2 | 4.6 | |
| Peak VGRF (BW), M | 2.8 | 0.6 | 2.3 | 3.7 |
ns non-significance, P > 0.05
P-value is the comparison between gender
Significant linear correlations were observed. Notably, when group correlations were made (men and women pooled together) KT 1000 values were positively correlated with maximal ATT values (r = 0.89; P < 0.0001) (Fig. 6). This correlation remained significant when the data were separated by gender (males, r = 0.97; P = 0.0003; females, r = 0.93; P = < 0.0001). Regression analysis yielded a significant linear fit for the group (r2 = 0.80; YATT-group = −0.516 + 1.2 × XKT1000-group) as well for each gender (females: r2 = 0.86; YATT-females = 0.074 + 1.2 × XKT1000-females; and males: r2 = 0.94; YATT-males = −0.79 + 1.2 × XKT1000-males).
Fig. 6.
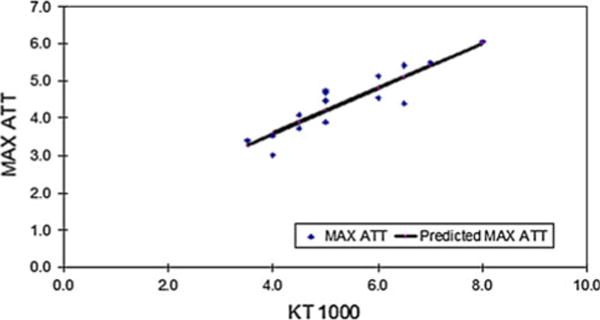
Linear regression plot for maximal ATT (Max ATT) versus KT 1000 values. Dots represent individual values with predicted regression line in solid black
Discussion
A major finding of this study was that peak ATT was positively correlated with KT 1000 in both healthy males and female adult knees when performing a stiff drop-landing motion. This finding is in agreement and generally supports previous work which has shown anterior knee laxity to be positively associated with an increased risk of ACL injury. Passive anterior knee laxity has been cited as risk factor for ACL injury but the mechanical relationship of passive knee laxity to knee translations during dynamic activities has not been described in vivo. In this study, the 6 DOF in vivo tibio-femoral motions during a stiff drop landing in healthy men and women was determined by means of a high-speed biplane fluoroscopy system. The results of this study show that passive knee laxity obtained by KT 1000 testing to be significantly correlated with ATT during the drop landing.
Both passive and active structures are responsible for maintaining knee stability during dynamic activities. How these structures interact to provide such stability is unknown at this time. It is plausible, however, that increased passive knee joint laxity may contribute to ACL injury as lax ligaments may allow excessive knee translations at the time of external loading and before muscle stabilizing forces can react or be otherwise applied. KT 1000 measures are easily obtained and show high intratester reliability with slightly lower but acceptable intertester reliability in the hands of experienced users [3, 10]. Previous studies have suggested anterior knee laxity, as measured by the KT 1000/2000 devices, is predictive of ACL injuries [32, 36]. Woodford-Rogers et al. [36] found greater measures of knee laxity in the non-injured limb of ACL injured patients. Uhorchak et al. [32] noted athletes with higher knee laxity scores are more likely to injure their ACL. In the present study, anterior knee laxity as measured by KT 1000 independently explained 79% of the variance in ATT measured during the drop landing across all subjects. This direct relationship has not been previously documented and suggests that anterior knee laxity taken during a passive examination can be predictive of ATT during a dynamic activity. Moreover, this relationship was observed in otherwise healthy individuals whose KT 1000 measures were (for both men and women) within normal limits as provided by previous reports [28, 36]. These observations serve to support previous retrospective studies which have noted increased risk ACL injury with increased anterior knee laxity [6, 32].
The magnitudes of ATT and lateral translation found in this study are also of interest and are comparable to the values in previous dynamic, in vivo reports listing ATT values of ~4–5 mm for walking and ~8 mm for running [19, 30]. It should be noted that during walking, jogging and running, the peak external forces range from 1 to 2 BW [15] which are lower than those during the drop landings performed here (2.9 BW). With higher external forces as with the stiff landing, one would expect higher knee translations to result. However, current knowledge regarding ACL stiffness and load mitigation are based primarily on in vitro investigations [34, 35], and these are limited in external and physiologic (muscular) load applications. Future experiments investigating increased demands on the knee using the presented fluoroscopy-based imaging technology are warranted to better understand the underlying neuromuscular control mechanics associated with increasing demand on knee function and how ACL stiffness and other soft tissues around the knee interact to help mitigate increased external loads in vivo, across gender and age ranges.
Controversy surrounds the causative event underlying the non-contact ACL injury. The fact that the ACL provides ~85% of the total restraint to ATT when the knee is at 20–30° of flexion [21] coupled with previous research [20, 22], which has shown combined ACL/MCL ligament injuries are rare (<30%), suggests that a primarily sagittal plane mechanism may be responsible for the non-contact ACL injury as discussed by Yu and Garrett [37]. This theory is also supported by in vitro data which shows that low flexion angles coupled with high quadriceps forces cause increases in ACL strain [21] and even ACL rupture [7]. The current study provides general support of the “anterior shear/quadriceps induced injury” theory as ATT was directly correlated with KT 1000 measures and not lateral translation values in healthy men and women performing a motion that is characterized by large quadriceps forces directed onto the tibia at relatively low knee flexion angles.
Another theory of the non-contact ACL injury is the high valgus knee angle or “valgus collapse” mechanism [12, 26]. Hewett et al.[12] reported individuals with greater than 8 degrees of knee valgus during a pre-screening test went on to tear their ACL during seasonal game play. In the controlled laboratory settings, researchers have investigated the knee valgus angle during landing using traditional motion capture techniques [5, 8, 12, 17, 24]. Given the differences in data recording, processing, landing heights, verbal cues and the inherent differences between motion capture techniques, there is ample variability in the knee valgus angles reported by these studies [8, 16, 17]. The knee valgus angle data reported herein are generally less than previously reported valgus knee angles for women and men landing in controlled laboratory settings. As such, this study contributes to the non-contact ACL injury body of literature as data from previous studies on landing are based on traditional motion capture or video-based technology [8, 16–18] which may be over-estimating the true valgus knee angle during the landing motion. Investigations to make comparisons between traditional motion capture techniques and biplane fluoroscopy techniques, particularly with regard to varus–valgus knee motions, are warranted and are currently underway.
The time required to reach maximal ATT following initial ground contact is also important for the basic hypothesis for neuromuscular training programs to prevent non-contact ACL injury mechanism. This study shows that the peak ATT is highly variable, occurring on average 65.4 ± 24.6 ms (range 30–108 ms) after ground contact in men and women performing a stiff style landing in a non-injurious, controlled laboratory setting. Video analyses from Krosshaug et al. [18] indicate that ACL rupture occurs very quickly after ground contact. Pflum et al. [25] using model and simulation techniques also estimated peak ACL load to occur at ~40 ms after ground contact. A longer time to peak ATT supports the implementation of neuromuscular training programs as this can allow for short (spinal) loop and potentially long (cortical) loop neuro-muscular responses times to be employed to prevent the injury.
Within the scope of this study, limitations are acknowledged. This study utilized a convenience sample of healthy male and female adults in a controlled setting for experimentation; as such, can be considered a limitation in understanding knee motion during the actual non-contact ACL injury mechanism. The lack of gender differences in KT 1000 values and landing kinematics signifies that the women in this study were, according to popular ACL injury screening methods [12], not at risk for ACL injury, which makes it difficult to infer “risk” from the current data. Thus, the women in this study land “more like males” and also possessed knee laxity values within normal female datasets [28, 36]. Moreover, we did not control for female-specific issues such as menstrual cycle which can affect ligamentous laxity [33]; and, only stiff landings were evaluated. Stiff landings were selected a priori in an attempt to reduce landing variability and to maximize loading at the knee and thus increase our ability to detect ATT, and women tend to land less flexed [5]. The use of the stiff landings may have also limited the differences between genders by limiting the motor control parameters by which males and females land. Thus, the use of stiff landings and the current data may not be applicable to other landing styles.
Despite these limitations, the current results have valuable applications to better understanding knee function during stiff style landings with regard to the timing and magnitude of ATT and its relationship to passive anterior knee laxity measures. Clinically, knee laxity scores via the KT 1000 are often obtained. Since meaningful relationships between ATT and anterior knee laxity were noted in these otherwise healthy individuals (in both genders and in those not at risk for ACL injury); this data provide a valuable baseline for comparative investigations of men and women who may be identified as “at risk” for noncontact ACL injury using traditional motion capture screening techniques [12]. It is hoped that the direct link between passive ATT obtained via KT 1000 and dynamic ATT measured during drop landings will inspire new research aimed at pre-screening those at risk for ACL injury using passive knee laxity as a risk factor.
Conclusion
In conclusion, the major finding of this study was that peak ATT was positively correlated with KT 1000 in both healthy males and female adult knees when performing a stiff drop-landing motion. These findings are in agreement and generally support previous work which has shown that anterior knee laxity is associated with an increased risk of ACL injury and is a parameter that is easily obtained and should be considered in future, prospective studies concerning the non-contact ACL injury.
Acknowledgments
This study was funded in part by the Steadman Philippon Research Institute and a grant from the National Institutes of Health (AR39683 to PI: Savio L-Y. Woo). The Steadman Philippon Research Institute is a 501(c)3 non-profit institution supported financially by private donations and corporate support from the following entities: Smith & Nephew Endoscopy, Arthrex, Siemens Medical Solutions USA, Saucony, OrthoRehab, Ossur Americas, Alignmed LLC and Opedix. Thank you to Medis Specials for providing the MBRSA software.
Contributor Information
Michael R. Torry, Email: mtorry@ilstu.edu, School of Kinesiology and Recreation, Illinois State University, Normal, IL, USA.
C. Myers, Biomechanics Research Laboratory, Steadman Philippon Research Institute, Vail, CO, USA
W. W. Pennington, Biomechanics Research Laboratory, Steadman Philippon Research Institute, Vail, CO, USA
K. B. Shelburne, Department of Mechanical and Materials Engineering, The University of Denver, Denver, CO, USA
J. P. Krong, Biomechanics Research Laboratory, Steadman Philippon Research Institute, Vail, CO, USA
J. E. Giphart, Biomechanics Research Laboratory, Steadman Philippon Research Institute, Vail, CO, USA
J. R. Steadman, Biomechanics Research Laboratory, Steadman Philippon Research Institute, Vail, CO, USA
Savio L-Y Woo, Musculoskeletal Research Center, Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA.
References
- 1.Blankevoort L, Huiskes R, de Lange A. Helical axes of passive knee joint motions. J Biomech. 1990;23:1219–1229. doi: 10.1016/0021-9290(90)90379-h. [DOI] [PubMed] [Google Scholar]
- 2.Braun S, Millett PJ, Yongpravat C, et al. Biomechanical evaluation of shear force vectors leading to injury of the biceps reflection pulley: a biplane fluoroscopy study on cadaveric shoulders. Am J Sports Med. 2010;38:1015–1024. doi: 10.1177/0363546509355142. [DOI] [PubMed] [Google Scholar]
- 3.Daniel DM, Stone ML, Sachs R, et al. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13:401–407. doi: 10.1177/036354658501300607. [DOI] [PubMed] [Google Scholar]
- 4.Davies H, Tietjens B, Van Sterkenburg M, et al. Anterior cruciate ligament injuries in snowboarders: a quadriceps-induced injury. Knee Surg Sports Traumatol Arthrosc. 2009;17:1048–1051. doi: 10.1007/s00167-008-0695-7. [DOI] [PubMed] [Google Scholar]
- 5.Decker MJ, Torry MR, Wyland DJ, et al. Gender differences in lower extremity kinematics, kinetics and energy absorption during landing. Clin Biomech. 2003;18:662–669. doi: 10.1016/s0268-0033(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 6.Decoster LC, Bernier JN, Lindsay RH, et al. Generalized joint hypermobility and its relationship to injury patterns among NCAA Lacrosse players. J Athl Train. 1999;34:99–105. [PMC free article] [PubMed] [Google Scholar]
- 7.Demorat G, Weinhold P, Blackburn T, et al. Aggressive quadriceps loading can induce non-contact anterior cruciate ligament injury. Am J Sports Med. 2004;32:477–483. doi: 10.1177/0363546503258928. [DOI] [PubMed] [Google Scholar]
- 8.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35:1745–1750. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- 9.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 10.Hantan W, Pace M. Reliability of measuring anterior laxity of the knee joint using a knee ligament arthrometer. Phys Ther. 1987;67(3):357–359. doi: 10.1093/ptj/67.3.357. [DOI] [PubMed] [Google Scholar]
- 11.Hertel J, Dorfman J, Braham R. Lower extremity malalignments and and anteriro cruciate ligment injury history. J Sports Sci Med. 2004;3:220–225. [PMC free article] [PubMed] [Google Scholar]
- 12.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33:492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 13.Hurschler C, Seehaus F, Emmerich J, et al. Accuracy of model-based RSA contour reduction in a typical clinical application. Clin Orthop Relat Res. 2008;466:1978–1986. doi: 10.1007/s11999-008-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishibashi Y, Rudy TW, Livesay GA, et al. The effect of anterior cruciate ligament graft fixation site at the tibia on knee stability: evaluation using a robotic testing system. Arthroscopy. 1997;13:177–182. doi: 10.1016/s0749-8063(97)90152-3. [DOI] [PubMed] [Google Scholar]
- 15.Keller TS, Weisberger AM, Ray JL, et al. Relationship between vertical ground reaction force and speed during walking, slow jogging, and running. Clin Biomech. 1996;11:253–259. doi: 10.1016/0268-0033(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 16.Kernozek TW, Ragan RJ. Estimation of anterior cruciate ligament tension from inverse dynamics data and electromyography in females during drop landing. Clin Biomech. 2008;23:1279–1286. doi: 10.1016/j.clinbiomech.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Kernozek TW, Torry MRHVANH, et al. Gender differences in frontal and sagittal plane biomechanics during drop landings. Med Sci Sports Exerc. 2005;37:1003–1012. [PubMed] [Google Scholar]
- 18.Krosshaug T, Slauterbeck JR, Engebretsen L, et al. Biomechanical analysis of anterior cruciate ligament injury mechanisms: three-dimensional motion reconstruction from video sequences. Scand J Med Sci Sports. 2007;17:508–519. doi: 10.1111/j.1600-0838.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Kozanek M, Hosseini A, et al. New fluoroscopic imaging techniques for investigation of 6DOF knee kinematics during treadmill walking. J Orthop Surg Res. 2009;4:1–5. doi: 10.1186/1749-1799x-1184-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: a 10-year study. Knee. 2006;13:184–188. doi: 10.1016/j.knee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee–the contributions of the supporting structures. A quantitative in vitro study. J Bone Joint Surg Am. 1976;58:583–594. [PubMed] [Google Scholar]
- 22.Miyasaka K, Daniel DM, Stone ML, et al. The incidence of knee ligament injuries in the general population. American J Knee Surg. 1991;4:3–8. [Google Scholar]
- 23.Myer GD, Ford KR, Khoury J, et al. Biomechanics laboratory-based prediction algorithm to identify female athletes with high knee loads that increase risk of ACL injury. Br J Sports Med. 2010 doi: 10.1136/bjsm.2009.069351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers CA, Hawkins D. Alterations to movement mechanics can greatly reduce anterior cruciate ligament loading without reducing performance. J Biomech. 2010;43:2657–2664. doi: 10.1016/j.jbiomech.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Pflum MA, Shelburne KB, Torry MR, et al. Model prediction of anterior cruciate ligament force during drop-landings. Med Sci Sports Exerc. 2004;36:1949–1958. doi: 10.1249/01.mss.0000145467.79916.46. [DOI] [PubMed] [Google Scholar]
- 26.Quatman CE, Hewett TE. The anterior cruciate ligament controversy: is valgus collapse a sex specific mechanism. Br J Sports Med. 2009;43(5):328–335. doi: 10.1136/bjsm.2009.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rangger C, Daniel DM, Stone ML, et al. Diagnosis of an ACL disruption with KT-1000 arthrometer measurements. Knee Surg Sports Traumatol Arthrosc. 1993;1:60–66. doi: 10.1007/BF01552161. [DOI] [PubMed] [Google Scholar]
- 28.Scerpella T, Stayer T, Makhuli B. Ligamentous laxity and non-contact ACL tears: a gender based comparison. Orthopedics. 1997:656–660. doi: 10.3928/0147-7447-20050701-12. [DOI] [PubMed] [Google Scholar]
- 29.Seehaus F, Emmerich J, Kaptein BL, et al. Experimental analysis of Model-Based Roentgen Stereophotogrammetric Analysis (MBRSA) on four typical prosthesis components. J Biomech Eng. 2009;131:041004–10. doi: 10.1115/1.3072892. [DOI] [PubMed] [Google Scholar]
- 30.Tashman S, Collon D, Anderson K, et al. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 31.Torry MR, Shelburne KB, Peterson DS, et al. Knee kinematic profiles during drop landings: a biplane fluoroscopy study. Med Sci Sports Exerc. 2010 doi: 10.1249/MSS.0b013e3181f1e491. [DOI] [PubMed] [Google Scholar]
- 32.Uhorchak JM, Scoville CR, Williams GN, et al. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31:831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 33.Wojtys EM, Huston LJ, Lindenfeld TN, et al. Association between the menstrual cycle and anterior cruciate ligament injuries in female athletes [see comments] Am J Sports Med. 1998;26:614–619. doi: 10.1177/03635465980260050301. [DOI] [PubMed] [Google Scholar]
- 34.Woo SL, Hollis JM, Adams DJ, et al. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19:217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- 35.Woo SL, Livesay GA, Engle C. Biomechanics of the human anterior cruciate ligament. ACL structure and role in knee motion. Orthop Rev. 1992;21:835–842. [PubMed] [Google Scholar]
- 36.Woodford-Rogers B, Cyphert L, Denegar CR. Risk factors for anterior cruciate ligament injury in high school and college athletes. J Athl Train. 1994;29:343–346. [PMC free article] [PubMed] [Google Scholar]
- 37.Yu B, Garrett WE. Mechanisms of non-contact ACL injuries. Br J Sports Med. 2007;41(Suppl):i47–i51. doi: 10.1136/bjsm.2007.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]


