Abstract
Background
Circadian variation of joint stiffness (morning stiffness) and its impact on functional ability are widely recognised in rheumatoid arthritis. Subsequent within-day variation of walking ability is important due to the increased availability of instrumented gait analysis. This study aimed to quantify diurnal variation of gait in patients with rheumatoid arthritis, and explore associations with disease characteristics.
Methods
Thirty one inpatients with rheumatoid arthritis walked at a self-selected speed along a GAITRite instrumented walkway 5 times during a single day.
Findings
Participants showed marked diurnal variation in gait, leading to a systematic variation throughout the day (F = 19.56, P = < 0.001). Gait velocity and stride length both increased, whereas the proportion of each gait cycle spent in stance phase or double support decreased, consistent with improving function throughout the day. Although absolute gait velocity correlated with disease characteristics, the magnitude of diurnal variation appeared to be independent of disease activity (rho = 0.26, P = 0.15), disease duration (rho = − 0.19, P = 0.324), and underlying functional ability (rho = 0.09, P = 0.65).
Interpretation
Although morning stiffness is well recognised in rheumatoid arthritis, this is the first time that its effect on gait has been quantified. Patients with rheumatoid arthritis exhibited a systematic change in walking ability throughout the day, which was independent of disease characteristics. These findings have important implications for the interpretation of existing data and the design of future studies. Repeat measures should be conducted at the same time of day to exclude the effects of diurnal variation.
Keywords: Rheumatoid arthritis, Gait analysis, Diurnal variation
Highlights
-
•
Patients with rheumatoid arthritis exhibited a systematic change in walking ability throughout the day.
-
•
The magnitude of within day change appeared independent of disease characteristics.
-
•
In future, repeat measures should be conducted at the same time of day to exclude the effects of diurnal variation.
1. Introduction
The impact of rheumatoid arthritis (RA) on the lower limb has been widely documented (Grondal et al., 2008, Helliwell et al., 2005, Michelson et al., 1994). Joint destruction and deformity are commonplace in the lower limb resulting in marked gait abnormalities and functional impairment, and an associated reduction in quality of life (Turner and Woodburn, 2008, Turner et al., 2003, Turner et al., 2006, Turner et al., 2008).
The restricted locomotor ability in patients with RA is typically manifest as reduced gait velocity, an increased double support period, reduced stride length, and reduced cadence (Fransen et al., 1994, Turner and Woodburn, 2008, Turner et al., 2003). These temporal and spatial gait parameters, particularly gait velocity, are widely reported in the literature as objective measures of physical function and have been shown to correlate with disease activity (Turner et al., 2008, Van Der Leeden et al., 2008). In addition to being used as an objective outcome measure in research, gait analysis is increasingly used in clinical practice to provide an objective assessment of functional ability, to plan and optimise clinical interventions, and evaluate response (Barkham et al., 2010, Van Der Leeden et al., 2008).
The pathophysiological processes in RA are modulated by a complex array of pro and anti inflammatory cytokines which show marked circadian variation (change over a 24 hour period) (Cutolo et al., 2008, Gibbs and Ray, 2013, Straub and Cutolo, 2007). Clinical symptoms also follow this changing pattern and there is a clear temporal relationship between elevated levels of pro-inflammatory cytokines and clinical symptoms such as morning stiffness (Cutolo et al., 2003, Cutolo et al., 2008, Gibbs and Ray, 2013). For some time there has been objective evidence of diurnal variation (within day change) in upper limb function (Helliwell et al., 1988, Herold and Günther, 1987) but to-date there has been no systematic study of the within day variation of gait parameters.
This study aimed to quantify any diurnal variation in temporal and spatial gait parameters in patients with RA, and to explore associations between the magnitude of variation and perceived stiffness, functional ability, and disease activity.
2. Methods
2.1. Participants
Assenting hospital in-patients with a diagnosis of RA and with ≥ 30 minute morning stiffness were recruited from the rheumatology ward. Patients were excluded if their primary lower limb/gait pathology was not due to RA or if they were unable to walk. Patients were allowed to continue their normal medication but were excluded from the study if they commenced or were scheduled to commence a new biologic or steroid (including intra-articular injection) during the current admission and prior to the completion of gait analysis. Walking sticks and elbow crutches were permitted where required. Ethical approval was obtained from the Leeds West Research Ethics Committee (08/H1307/65) and all participants provided written informed consent. All aspects of the study were conducted in accordance with the Declaration of Helsinki.
2.2. Data collection
The GAITRite instrumented walkway (CIR Systems Inc, USA) was used to measure function objectively and this has been validated previously for the measurement of temporal and spatial gait parameters (Menz et al., 2004, Rome, 2005). Patients were asked to walk at a self-selected speed at five time points throughout the day: at waking (0 h); + 1 h; + 3 h; + 6 h; and + 12 h. The study was undertaken alongside normal ward activities so a window of +/− 1 h was permitted for each time point at + 3 h and beyond. Preferred waking time was determined from each patient prior to entering the study but was usually between 6 am and 8 am. All patients were met on the ward at the agreed waking time and immediately transported to the gait laboratory by wheelchair for the 0 hour assessment, before beginning normal daily activities. GAITRite data were processed and analysed using the GAITRite v3.8 software (CIR Systems Inc, Sparta, New Jersey, USA).
The patient's own perception of severity of their general stiffness was captured at each time point using a 100 mm visual analogue scale (VAS) anchored with “no stiffness” and “worst stiffness imaginable”. Disease activity was measured using a composite index of disease activity Disease Activity Score (DAS28) (Smolen et al., 1995), and the UK version of Health Assessment Questionnaire Disability Index HAQ-DI provided a measure of self reported functional ability (Kirwan and Reeback, 1986).
2.3. Statistical analysis
Analysis was conducted on a per protocol basis, with patients failing to complete follow-up excluded from all analysis. Summary statistics were used to describe the demographic and clinical features of the participants.
Diurnal variation of gait velocity, stride length, duration of stance phase and double support (as a percentage of gait cycle) were described using summary statistics and 95% confidence intervals (95%CI). Gait velocity has been shown previously to be the most sensitive temporal and spatial gait parameter, so was explored further (Helliwell et al., 2007). Systematic within-day changes in gait velocity were examined using a general linear model repeated-measures ANOVA approach having checked the residuals for normality and heteroscedasticity using Kolmogorov–Smirnov tests and visual inspection of residuals plotted against fitted values. The threshold for statistical significance was set at alpha = 0.05; the Bonferroni method was used to adjust for multiple comparisons between time-points and adjusted P-values have been presented. To confirm the findings of the parametric test, a supplementary non-parametric Friedman test was performed with post-hoc Dunn–Bonferroni pairwise comparisons between time-points.
Associations between the magnitude of change in gait velocity and disease factors (HAQ, DAS28, and disease duration) were assessed using Spearman's rank correlation coefficient. Statistical analyses were conducted using SPSS 21 (IBM, Armonk, New York, USA)
3. Results
3.1. Participant profile
A total of 41 patients were screened for eligibility and 35 entered the study, of whom 31 completed the final follow-up assessment. Fig. 1 illustrates the flow of patients through the study and provides reasons for exclusions or non-completion. The 31 patients included in the analysis (11 male, 20 female) had a median age of 67 (range 35 to 87) and their mean BMI was 25.9 (SD = 5.46). The mean disease duration was 10.5 years (range 1 to 50), and median DAS28 and HAQ were 5.39 (IQR = 3.28 to 7.50) and 2.25 (IQR = 1.46 to 3.04) respectively.
Fig. 1.
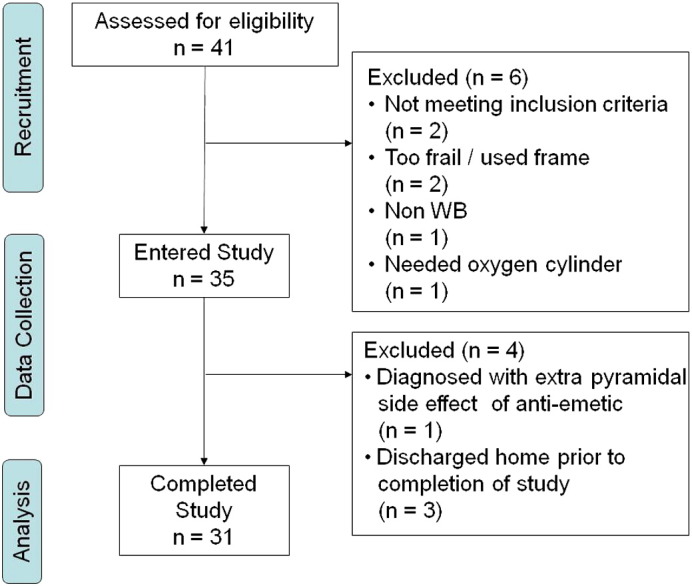
Study flow diagram.
3.2. Diurnal variation
Temporal and spatial gait parameters showed systematic changes consistent with improving physical function throughout the day (Table 1). Gait velocity and stride length both increased, whereas the proportion of each gait cycle spent in stance phase or double support decreased. Gait velocity, as the most sensitive parameter, exhibited a large relative improvement from baseline (Fig. 2).
Table 1.
Diurnal variation of temporal and spatial parameters.
| Waking | + 1 h | + 3 h | + 6 h | + 12 h | ||
|---|---|---|---|---|---|---|
| Gait velocity (cm/sec) | Mean | 37.64 | 43.75 | 45.52 | 48.01 | 53.03 |
| SD | 24.88 | 28.73 | 26.94 | 28.98 | 32.21 | |
| Stride length (cm) | Mean | 62.06 | 66.23 | 68.80 | 71.35 | 73.13 |
| SD | 26.64 | 28.28 | 26.96 | 28.49 | 30.11 | |
| Stance phase (% of gait cycle) | Mean | 74.28 | 73.08 | 71.98 | 72.19 | 71.12 |
| SD | 9.44 | 9.24 | 8.74 | 8.75 | 8.72 | |
| Double support (% of gait cycle) | Mean | 49.22 | 46.75 | 44.52 | 43.77 | 42.66 |
| SD | 18.65 | 18.25 | 16.61 | 16.79 | 17.14 |
Fig. 2.
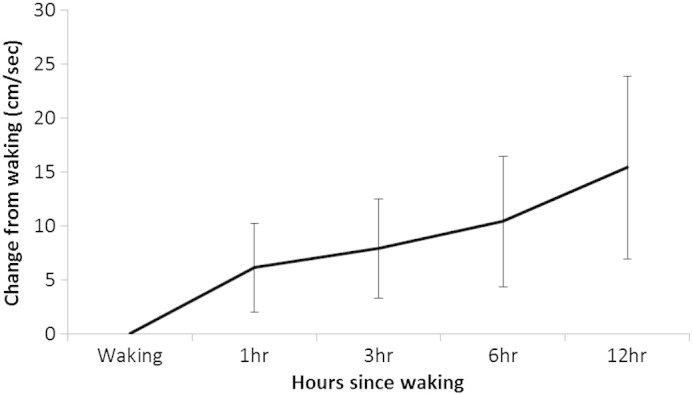
Variation in gait velocity from baseline.
A repeated measures ANOVA demonstrated a systematic increase in gait velocity throughout the day (F = 19.56, P < 0.001). Bonferroni-adjusted post-hoc pairwise comparisons identified significant differences between baseline and all subsequent time points (Table 2). Similarly, significant differences were also found between + 12 h and all other time points. Gait velocity was more stable between + 3 and + 6 h and the difference between these time points was not significant. Inspection of the model residuals indicated that there were borderline-significant departures from normality for the waking (P = 0.049) and + 12 hour measurements (P = 0.051); therefore, we repeated the analysis using a non-parametric Friedman test. This confirmed that gait velocity differed between the time-points (P < 0.001). Pairwise tests showed differences in gait velocity between baseline and all subsequent time-points; using the less powerful non-parametric test, there were significant differences between the + 12 hour measurements and those taken at baseline and + 1 hour; but following correction for multiple comparisons the + 12 hour measurements did not differ from those at + 3 or + 6 hours (supplementary data 1).
Table 2.
Pairwise comparison of gait velocity at different time points.
| Waking | + 1 h | + 3 h | + 6 h | ||
|---|---|---|---|---|---|
| + 12 h | Mean diff | 15.39 | 9.28 | 7.51 | 4.98 |
| Adj. sig. | < 0.001 | 0.003 | 0.010 | 0.015 | |
| + 6 h | Mean diff | 10.40 | 4.30 | 2.52 | |
| Adj. sig. | < 0.001 | 0.093 | 0.639 | ||
| + 3 h | Mean diff | 7.88 | 1.78 | ||
| Adj. sig. | < 0.001 | 1.000 | |||
| + 1 h | Mean diff | 6.10 | |||
| Adj. sig. | 0.001 |
3.3. Correlation with disease characteristics
Absolute gait velocity correlated with HAQ and disease duration at all time points but no correlation was found between DAS28 and absolute gait velocity. Similarly, patient reported stiffness only correlated with absolute gait velocity at waking and + 1 h whereas there was no evidence of a correlation between patient-perceived stiffness and gait velocity beyond the first hour (Supplementary data 2 and 3).
In contrast, the magnitude of within-day variation of gait velocity appeared to be independent of disease factors: There was no evidence of a correlation between HAQ (0.09, P = 0.65), DAS28 (0.26, P = 0.15), or disease duration (− 0.19, P = 0.32) and the magnitude of variation in gait velocity over the course of the day.
4. Discussion
This is the first study to quantify within-day variation of gait in patients with RA and has identified marked diurnal variation in a range of temporal and spatial gait parameters. The continued improvement led to a systematic difference in functional ability occurring over the course of the day. While the absolute gait parameters correlated with patient-reported functional ability, the magnitude of within-day variation appeared to be independent of disease activity, disease duration, and self-reported functional ability. This indicates that repeat measures of gait should be conducted at the same time of day to exclude the effects of diurnal variation and also that self-reported measures of functional ability are not adequate surrogates for evaluating diurnal variation clinically.
This study provides useful information on the measurement of gait in patients with RA at a time when instrumented gait analysis is becoming increasingly widely used in clinical and research applications. There was a clear improvement in temporal and spatial gait variables on the group level, but there was also notable between-patient variation. For example, the group mean improvement in gait velocity from baseline was 55%, but this ranged from a decrease of 41% through to an increase of 397% at 12 h. This observation further reinforces the conclusion that repeat measures should be taken at the same time of day to ensure that any changes arising from quantifying locomotor function reflect a true change in clinical status, rather than the natural diurnal variation occurring in people with RA as described here. This has important methodological implications for the design of future studies and where gait analysis is to be used to inform clinical decision making. These findings should also prompt a re-evaluation of existing studies which attribute a change in functional ability between time points to an intervention, without controlling for diurnal variation (Conrad et al., 1996, Locke et al., 1984, Macsween et al., 1999).
The classic pattern of stiffness in patients with RA, is one in which stiffness is worst first thing in the morning but then improves during the day before returning in the evening (Scott, 1960). More recently this pattern of symptoms and associated functional ability has been shown to correspond to circadian variation in pro-inflammatory cytokines (Cutolo et al., 2003, Cutolo et al., 2008, Gibbs and Ray, 2013, Helliwell et al., 1988). This study confirms that the improvement in function is sharpest during the first hour after waking, but walking ability continued to improve throughout the 12 hour study period. There was no evidence of a decrease in functional ability towards the end of the study day as has been suggested by other reports (Helliwell et al., 1988, Herold and Günther, 1987), although this may reflect the 12 hour study period whereby function was not evaluated through to patients’ bed time. This study quantified variation in gait during the day time (diurnal) as this is when most people conduct their daily activities and are most likely to undergo clinical gait analysis, rather than over the course of a 24 hour period (circadian).
When considering systematic improvement it should be noted that some measures of functional ability, such as grip strength, are associated with a learning effect, whereby functional ability improves over time due to participants increasing familiarity with a previously unfamiliar task such as using a dynamometer (Bellace et al., 2000, Reddon et al., 1985). The possibility of a learning effect cannot be excluded from the current study but is considered unlikely as participants are used to walking and the GAITRite walkway does not require attachment of any equipment to participants or learning of any special protocols.
Steps were also taken to ensure that any observed change in functional ability was not the result of starting a new medication. No patients were recruited if they had, or were scheduled to commence a new biologic or steroid (including intra-articular injection) prior to or during the day and prior to completion of the study. This step was taken to reduce confounding and to attempt to ensure that any observed variation in gait was the result of diurnal variation rather than a response to any new medication. Patients were able to continue their normal medication as prescribed.
Temporal and spatial gait parameters, particularly gait velocity, are widely reported in the literature and have previously been shown to correlate with disease characteristics (Fransen and Edmonds, 1999, Turner and Woodburn, 2008, Turner et al., 2003). The current study explored this relationship further and found that although absolute gait velocity correlated with HAQ and disease duration, there was no correlation with measures of disease activity. Such findings are not unexpected in a cohort with established disease. The functional ability of patients with established disease is known to be linked more to accumulated damage over the course of their disease, rather than being driven purely by current disease activity (Aletaha et al., 2006, Smolen et al., 2007, Welsing et al., 2001).
A major strength of the study is that it is the first to measure temporal and spatial gait parameters throughout the course of a day in a cohort of patients with RA. The study design enabled the quantification of variation in gait throughout the day and at clinically meaningful time points, thereby increasing the clinical relevance of the findings. However, the practicalities of capturing gait at the earliest time points necessitated that patients were already on site: the sample therefore consisted of inpatients who were admitted as part of their ongoing care and were among the more severely affected in the RA population. Although this may limit the generalisability of the findings, it was notable that the magnitude of diurnal variation of gait was independent of all disease factors including disease activity and it is likely that a similar effect is present in out-patient populations also.
With such large within-day variation in walking ability, it would be helpful in the future to study community dwelling participants, potentially using activity monitors to record temporal and spatial gait parameters over a 24 hour period. Further development of the current generation of activity monitors appears to be required however, before they can be used reliably in patients with RA (Backhouse et al., 2013).
In conclusion, this study quantified, for the first time, marked diurnal variation in temporal and spatial gait parameters in patients with rheumatoid arthritis. Patients showed continued improvement in function throughout the day. Although the underlying walking ability was related to patient-reported functional ability and disease duration, the magnitude of the variation in measured gait parameters throughout the day was independent of disease activity, disease duration, and underlying functional ability. This study has important implications for the interpretation of existing data and should inform future data collection. This study indicates that repeat measures obtained on different days should be conducted at the same time of day to exclude the effects of diurnal variation.
Conflict of interest statement
All authors have declared that they have no financial interests that could create a conflict of interest or the appearance of a conflict of interest with regard to this work.
Footnotes
Funding and grant support: supported by grant ID 17866 and 18932 from Arthritis Research UK.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.clinbiomech.2014.05.009.
Appendix A. Supplementary data
Supplementary tables.
References
- Aletaha D., Smolen J., Ward M.M. Measuring function in rheumatoid arthritis: identifying reversible and irreversible components. Arthritis Rheum. 2006;54:2784–2792. doi: 10.1002/art.22052. [DOI] [PubMed] [Google Scholar]
- Backhouse M.R., Hensor E., White D., Keenan A.M., Helliwell P.S., Redmond A.C. Concurrent validation of activity monitors in patients with rheumatoid arthritis. Clinical Biomechanics. 2013;28(4):473–479. doi: 10.1016/j.clinbiomech.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkham N., Coates L.C., Keen H., Hensor E.M.A., Fraser A., Redmond A.C., Cawkwell L., Emery P. Double-blind placebo-controlled trial of etanercept in the prevention of work disability in ankylosing spondylitis. Ann. Rheum. Dis. 2010;69:1926–1928. doi: 10.1136/ard.2009.121327. [DOI] [PubMed] [Google Scholar]
- Bellace J.V., Healy D., Besser M.P., Byron T., Hohman L. Validity of the Dexter Evaluation System's Jamar dynamometer attachment for assessment of hand grip strength in a normal population. J. Hand Ther. 2000;13:46–51. doi: 10.1016/s0894-1130(00)80052-6. [DOI] [PubMed] [Google Scholar]
- Conrad K.J., Budiman-Mak E., Roach K.E., Hedeker D. Impacts of foot orthoses on pain and disability in rheumatoid arthritics. J. Clin. Epidemiol. 1996;49:1–7. doi: 10.1016/0895-4356(96)00534-3. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Seriolo B., Craviotto C., Pizzorni C., Sulli A. Circadian rhythms in RA. Ann. Rheum. Dis. 2003;62:593–596. doi: 10.1136/ard.62.7.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M., Straub R., Buttgereit F. Circadian rhythms of nocturnal hormones in rheumatoid arthritis: translation from bench to bedside. Ann. Rheum. Dis. 2008;67:905. doi: 10.1136/ard.2008.088955. [DOI] [PubMed] [Google Scholar]
- Fransen M., Edmonds J. Gait variables: appropriate objective outcome measures in rheumatoid arthritis. Rheumatology. 1999;38:663–667. doi: 10.1093/rheumatology/38.7.663. [DOI] [PubMed] [Google Scholar]
- Fransen M., Heussler J., Margiotta E., Edmonds J. Quantitative gait analysis—comparison of rheumatoid arthritic and non-arthritic subjects. Aust. J. Physiother. 1994;40:191–199. doi: 10.1016/S0004-9514(14)60578-X. [DOI] [PubMed] [Google Scholar]
- Gibbs J.E., Ray D.W. The role of the circadian clock in rheumatoid arthritis. Arthritis Res. Ther. 2013;15:205. doi: 10.1186/ar4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondal L., Tengstrand B., Nordmark B., Wretenberg P., Stark A. The foot: still the most important reason for walking incapacity in rheumatoid arthritis: distribution of symptomatic joints in 1,000 RA patients. Acta Orthop. 2008;79:257–261. doi: 10.1080/17453670710015067. [DOI] [PubMed] [Google Scholar]
- Helliwell P., Howe A., Wright V. An evaluation of the dynamic qualities of isometric grip strength. Ann. Rheum. Dis. 1988;47:934–939. doi: 10.1136/ard.47.11.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell P., Reay N., Gilworth G., Redmond A., Slade A., Tennant A., Woodburn J. Development of a foot impact scale for rheumatoid arthritis. Arthritis Rheum. 2005;53:418–422. doi: 10.1002/art.21176. [DOI] [PubMed] [Google Scholar]
- Helliwell P., Woodburn J., Redmond A., Turner D.E., Davies H. Churchill Livingstone; London: 2007. The Foot and Ankle in Rheumatoid Arthritis: A Comprehensive Guide. [Google Scholar]
- Herold M., Günther R. Circadian rhythm of C-reactive protein in patients with rheumatoid arthritis. Prog. Clin. Biol. Res. 1987;227:271. [PubMed] [Google Scholar]
- Kirwan J.R., Reeback J.S. Stanford Health Assessment Questionnaire modified to assess disability in British patients with rheumatoid arthritis. Rheumatology. 1986;25:206–209. doi: 10.1093/rheumatology/25.2.206. [DOI] [PubMed] [Google Scholar]
- Locke M., Perry J., Campbell J., Thomas L. Ankle and subtalar motion during gait in arthritic patients. Phys. Ther. 1984;64:504–509. doi: 10.1093/ptj/64.4.504. [DOI] [PubMed] [Google Scholar]
- Macsween A., Brydson G., Hamilton J. The effect of custom moulded ethyl vinyl acetate foot orthoseson the gait of patients with rheumatoid arthritis. Foot. 1999;9:128–133. [Google Scholar]
- Menz H.B., Latt M.D., Tiedemann A., Mun San Kwan M., Lord S.R. Reliability of the GAITRite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture. 2004;20:20–25. doi: 10.1016/S0966-6362(03)00068-7. (see comment) [DOI] [PubMed] [Google Scholar]
- Michelson J., Easley M., Wigley F.M., Hellmann D. Foot and ankle problems in rheumatoid arthritis. Foot Ankle Int. 1994;15:608–613. doi: 10.1177/107110079401501106. [DOI] [PubMed] [Google Scholar]
- Reddon J., Stefanyk W., Gill D., Renney C. Hand dynamometer: effects of trials and sessions. Percept. Mot. Skills. 1985;61:1195. doi: 10.2466/pms.1985.61.3f.1195. [DOI] [PubMed] [Google Scholar]
- Rome K. Within-day reliability of temporal-spatial gait parameters associated with rheumatoid arthritic feet. Musculoskeletal Care. 2005;3:17–23. doi: 10.1002/msc.22. [DOI] [PubMed] [Google Scholar]
- Scott J.T. Morning stiffness in rheumatoid arthritis. Ann. Rheum. Dis. 1960;19:361–368. doi: 10.1136/ard.19.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J.S., Breedveld F.C., Eberl G., Jones I., Leeming M., Wylie G.L., Kirkpatrick J. Validity and reliability of the twenty-eight-joint count for the assessment of rheumatoid arthritis activity. Arthritis Rheum. 1995;38:38–43. doi: 10.1002/art.1780380106. [DOI] [PubMed] [Google Scholar]
- Smolen J.S., Aletaha D., Koeller M., Weisman M.H., Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- Straub R., Cutolo M. Circadian rhythms in rheumatoid arthritis: implications for pathophysiology and therapeutic management. Arthritis Rheum. 2007;56:399. doi: 10.1002/art.22368. [DOI] [PubMed] [Google Scholar]
- Turner D.E., Woodburn J. Characterising the clinical and biomechanical features of severely deformed feet in rheumatoid arthritis. Gait Posture. 2008;28:574–580. doi: 10.1016/j.gaitpost.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Turner D., Woodburn J., Helliwell P.S., Cornwall M.W., Emery P. Pes planovalgus in RA: a descriptive and analytical study of foot function determined by gait analysis. Musculoskeletal Care. 2003;1:21–33. doi: 10.1002/msc.36. [DOI] [PubMed] [Google Scholar]
- Turner D.E., Helliwell P., Emery P., Woodburn J. The impact of rheumatoid arthritis on foot function in the early stages of disease: a clinical case series. BMC Musculoskelet. Disord. 2006;7:102–110. doi: 10.1186/1471-2474-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D.E., Helliwell P.S., Siegel K.L., Woodburn J. Biomechanics of the foot in rheumatoid arthritis: identifying abnormal function and the factors associated with localised disease ‘impact’. Clin. Biomech. 2008;23:93–100. doi: 10.1016/j.clinbiomech.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Van Der Leeden M., Steultjens M.P.M., Terwee C.B., Rosenbaum D., Turner D.E., Woodburn J., Dekker J. A systematic review of instruments measuring foot function, foot pain, and foot-related disability in patients with rheumatoid arthritis. Arthritis Care Res. 2008;59:1257–1269. doi: 10.1002/art.24016. [DOI] [PubMed] [Google Scholar]
- Welsing P.M.J., Van Gestel A.M., Swinkels H.L., Kiemeney L.A.L.M., Van Riel P.L.C.M. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001;44:2009–2017. doi: 10.1002/1529-0131(200109)44:9<2009::AID-ART349>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.


