Abstract
Chronic graft-versus-host disease (cGvHD) is a major complication of allogeneic hematopoietic stem cell transplantation. Post-transplant thrombocytopenia in patients with cGvHD has been associated with poor outcome and its etiology is unclear. We investigated whether thrombopoiesis, assessed via measurement of the absolute immature platelet number (AIPN) in the blood, is impaired in cGVHD, and whether the level of thrombopoiesis correlates with the severity and activity of cGvHD as assessed via the NIH organ scoring system. We utilized a cohort of 110 well-characterized cGVHD patients, including 83 (75%) with severe cGVHD per NIH global score. Higher AIPN was associated with active therapeutic intent (p=0.026), lower Karnofsky score (p=0.0013), worse joint/fascia cGVHD (p=0.0005) and worse skin cGVHD (p=0.0044). AIPN correlated with platelet counts and was not correlated with ANC, WBC, CRP, ALC, albumin, total and average NIH scores, or number of prior systemic therapies. AIPN values for cGvHD patients substantially overlapped those of the normal population. Higher AIPN, as marker of active thrombopoiesisis, was associated with worse severity and activity of cGVHD, especially skin and joints/fascia manifestations. Among patients with stable moderate or severe cGVHD, there was no evidence of hypo production of platelets. Future studies should further investigate the role of thrombopoiesis in cGVHD.
Keywords: Chronic GVHD, NIH score, platelets, AIPN
Introduction
The etiology of thrombocytopenia in patients following transplantation, particularly in those with chronic Graft-versus-Host-Disease (cGvHD), is likely multifactorial and is of unclear etiology.1 Decreased productions could theoretically result from insufficient engrafted hematopoietic stem cell (HSC) numbers due to a low transplanted dose or destruction via rejection or other HSC damaging processes, marrow suppression from drugs or viral infection, or graft-versus-host attack on the marrow microenvironment, as recently demonstrated in a murine model.2 Increased destruction could be related to consumption due to transplant-associated microangiopathy or to an autoimmune ITP-like syndrome. There is some evidence for both impaired thrombopoiesis and a shortened half-life of platelets in patients with GvHD.3, 4 However, it is difficult in individual patients to assess the role of impaired thrombopoiesis versus platelet destruction in patients with thrombocytopenia, and study the impact of cGvHD on the bone marrow, because assessment of megakaryocyte number on bone marrow biopsy is only roughly quantitative and repeated sampling is not feasible, and there are few accurate or quantitative measures of ongoing platelet destruction. An understanding of the etiology of thrombocytopenia in these patients could have therapeutic relevance, as well as inherent biologic interest.
In 2006, Yamazaki et al. evaluated prolonged thrombocytopenia in alloHSCT recipients using the glycocalicin index (GCI), thrombopoietin (TPO) levels, and anti-GPIIb-IIIanti-platelet antibodies as measures designed to assess platelet turnover, platelet production and antiplatelet antibody responses respectively. 3 They compared thrombocytopenic alloHSCT recipients with immune thrombocytopenic purpura (ITP) and aplastic anemia patients. The GCI and TPO status in alloHSCT recipients with thrombocytopenia had a pattern similar to that seen in aplastic anemia, whereas GCI in alloHSCT recipients with thrombocytopenia was significantly lower than in patients with ITP, suggesting a major role for impaired thrombopoiesis in alloHSCT recipients. The patients in this study were diverse, analyzed early following transplantation, and not fully characterized regarding cGVHD. GCI as a measure of platelet destruction has low sensitivity and can give falsely elevated results in hypoplastic anemias4, circulating TPO levels have a complex relationship to thrombopoiesis and platelet destruction5, and the detection of specific antiplatelet antibodies is challenging, not routinely available in most hospitals, and of unclear significance post-transplantation.
As an alternative approach to assessing post-transplant thrombopoiesis we have focused on a simple peripheral blood test as a sensitive and practical measurement of ongoing thrombopoeisis. The Sysmex XE-2100 blood cell counter has been developed to measure the fraction of newly- released immature platelets containing high amounts of cytoplasmic RNA in the peripheral blood, analogous to measurement of reticulocytes in the erythroid lineage. The absolute immature platelet number (AIPN) has been utilized to predict marrow recovery after hematopoietic transplantation and to assess the level of thrombopoesis in patients with ITP. 6 In our own recent study, AIPN has been shown to correlate with ongoing megakaryocyte production of platelets, even in the setting of recent platelet transfusion.6
In the current study we asked whether thrombopoiesis as assessed via AIPN is impaired in patients with cGvHD, long-term following allogeneic stem cell transplantation, and to investigate any positive or negative correlations between AIPN and the severity and activity of cGvHD, as assessed via the NIH scoring system.
Patients and Methods
Study Design
This study describes patients with cGvHD evaluated at the NIH between May 2009 and September 2012. The NIH cGVHD program has established a multidisciplinary clinic and research network to study the pathogenesis and natural history of cGVHD, enrolling patients in the National Cancer Institute Institutional Review Board-approved natural history protocol NCT00092235 emphasizing cross-sectional data and specimen collection. Patients were evaluated using multiple disease scales and outcome measures, including the 2005 National Institutes of Health (NIH) Consensus Project cGVHD severity scores. Between May 2009 and September 2012, a total of 123 consecutive patients were enrolled, 13 patients were ineligible owing to no sample sent for AIPN. Therefore, 110 patients meeting the diagnostic criteria specified in the NIH cGVHD Consensus Project and with available AIPN data were included in this analysis.7 (Table 1). All subjects signed National Cancer Institute Institutional Review Board approved informed consent. 48 of the 110 patients, enrolled between 2009 and 2010, were included in a prior analysis of inflammation and cGVHD, however, that study did not assess thrombopoiesis via measurement of AIPN in the patient cohort.8
Table 1.
Patient Characteristics
| Characteristics | n (%) |
|---|---|
| Sex | |
| Male | 67 (61) |
| Female | 43 (39) |
| Disease | |
| ALL/AML/MDS | 44 (40) |
| CML | 7 (6) |
| CLL | 10 (9) |
| Lymphoma | 22 (20) |
| Multiple myeloma | 9 (8) |
| Aplastic anemia/PNH | 3 (3) |
| Other | 15 (14) |
| Stem cell source | |
| Bone marrow | 18 (16) |
| Peripheral Blood | 89 (81) |
| Cord Blood | 3 (3) |
| Donor-recipient relationship | |
| Related | 53 (48) |
| Unrelated | 57 (52) |
| Classification | |
| Classic cGvHD | 85 (78) |
| Overlap cGvHD | 22 (20) |
| Late acute cGvHD | 2 (2) |
| cGvHD onset | |
| Progressive | 33 (30) |
| Quiescent | 38 (35) |
| De novo | 38 (35) |
| Therepeutic intent | |
| Active | 65 (64) |
| Not active | 37 (36) |
| TBI conditioning | |
| Yes | 46 (43) |
| No | 62 (57) |
| Karnofsky score | |
| 40–70 | 44 (40) |
| 80–100 | 66 (60) |
| Current systemic treatments | |
| Prednisone, dexamethasone, budesonide | 100 (90) |
| Azathioprine, mycophenolate mofetil | 62 (56) |
| mTOR inhibitors | 21 (20) |
| Calcineurin inhibitors | 37 (34) |
| mTOR inhibitors+ Calcineurin inhibitors | 7 (6) |
| Misc others* | 73 (66) |
| Platelet Count | |
| <100 × 109 | 5 (4) |
| <150 × 109 | 11 (10) |
| >150× 109 | 94 (86) |
| Intensity of immunosuppression+ | |
| None/mild | 22 (20) |
| Moderate | 40 (36) |
| High | 48 (44) |
| Prednisone dose per kg | |
| >0, <0.50 | 50 (45) |
| >0.50 | 19 (17) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; cGVHD, chronic graft-versus-host disease; CLL, chronic lymphocytic leukemia; MDS, myelodysplastic syndrome; PNH, paroxysmal nocturnal hemoglobinuria.
Rituximab, ECP, PUVA, Etanercept, Daclizumab, IVIG, Ontak, Thalidomide, Hydroxychloroquine, Imatinib.
Active cGvHD by therapeutic intent at the time of the visit: (1) increase systemic therapy because cGVHD is worse; (2) substitute systemic therapy due to lack of response; and (3) withdraw systemic therapy due to lack of response. Non-active: (1) decrease systemic therapy because cGVHD is better; (2) not change current systemic therapy because cGVHD is stable; (3) alter systemic therapy owing to its toxicity. Other: either did not receive any immunosuppressive therapy or did not meet any of the criteria.10
None/mild, single-agent prednisone 0.5 mg/kg per day; moderate, single-agent prednisone 0.5 mg/kg/day and/or any single agent/modality; high, 2 or more agents/modalities ± prednisone 0.5 mg/kg/day.
Patient- and transplant-related variables surveyed included recipient age, gender, time from transplant to analyzed visit, donor relationship (related or not), cord blood donor, total-body irradiation (TBI) conditioning, cGVHD onset, GVHD classification at time of analyzed visit, current systemic therapy for cGVHD, platelet count, AIPN, NIH global score, therapeutic intent, Karnofsky score, organ involvement (skin, eyes, oral, liver, GI tract, lungs, joints/fascia, female genital), maximum score for an organ, intensity and type of immunosupression, and treatment received prior to registration date.
Disease was defined as “active” if the practitioner decided to increase systemic therapy due to worsening disease, to substitute systemic therapy due to lack of response or withdraw systemic therapy due to lack of response. Disease was defined as “non-active” if the practitioner decided to decrease systemic therapy because the cGVHD was improving, not to change current systemic therapy because cGVHD was stable or to alter systemic therapy only because of toxicity. 8
AIPN
The AIPN was measured using a Sysmex XE-5000 automated hematology analyzer, with platelet (PLT) gating accomplished using forward light scatter and identification of immature PLTs achieved by staining with polymethine and oxazine fluorescent dyes (Sysmex America). 9 These fluorescent dyes penetrate cell membranes and bind to and fluorescently stain RNA, allowing separation of immature (RNA-rich) and mature PLTs. Immature platelet fraction (IPF) is expressed as a percentage, which represents the ratio of immature PLTs to the total number of PLTs×100. AIPN was calculated by multiplying the platelet count by the IPF and dividing by 100.
Statistical analysis
Comparisons between two groups of parameters were determined using a Wilcoxon rank sum test. Comparisons among three or more unordered categories were performed using a Kruskal-Wallis test. The association between AIPN and an ordered categorical parameter were determined by using the Jonckheere-Terpstra test for trend.10 Correlation between AIPN and continuous parameters was determined using Spearman rank correlation. The magnitude of the correlation coefficient (r) is of much greater importance than the p-value, which is a test of whether r=0. Spearman correlations are interpreted as follows; |r| > 0.70=strong correlation; 0.5 < |r| < 0.7 =moderately strong correlation; 0.3 < |r| < 0.5=weak to moderately strong correlation; |r| < 0.3=weak correlation. Results are presented without any formal correction for multiple comparisons. However, in view of the number of statistical tests performed, p < 0.005 is considered statistically significant while 0.005 < p < 0.05 is considered a strong trend.
Results
Patient Population
The epidemiologic, disease and transplant characteristics of the population are given in Table 1. The median age was 44 years (4-70 years). Median time from transplant to enrollment in the cGVHD study and assessment of clinical parameters and AIPN was 36 months (6-254).
cGVHD Characteristics
Overall, 83 of 110 patients (75%) were categorized as severe by the NIH global score (Table 2); with a median of five organs involved (1-8). 77 % were characterized with classic cGVHD and 20% were classified with overlap GVHD. The onset of cGVHD was progressive in 30%, de novo in 35%, and quiescent in 35% (Table 1). The most commonly involved organs were eyes (73%), skin (86%), lung (87%), joints and fascia (76%) and mouth (77%) (Table 2). Global organ severity was driven primarily by skin disease (44%) and lung (44%; moderate plus severe), followed by joint/fascia, eye, and gastrointestinal tract involvement (Table 2).
Table 2.
NIH cGvHD Global and Organ Severity Scores
| Score | n (%) |
|---|---|
| NIH global severity score | |
| 1 = mild | 3 (3) |
| 2 = moderate | 24 (22) |
| 3 = severe | 83 (75) |
| NIH organ severity scores | |
| Skin | |
| 0 = none | 23 (21) |
| 1 = mild | 14 (13) |
| 2 = moderate | 24 (22) |
| 3 = severe | 48 (44) |
| Mouth | |
| 0 = none | 32 (30) |
| 1 = mild | 56 (51) |
| 2 = moderate | 14 (13) |
| 3 = severe | 7 (6) |
| Eyes | |
| 0 = none | 27 (25) |
| 1 = mild | 38 (35) |
| 2 = moderate | 34 (31) |
| 3 = severe | 10 (9) |
| Gastrointestinal tract | |
| 0 = none | 61 (55) |
| 1 = mild | 31 (28) |
| 2 = moderate | 6 (5) |
| 3 = severe | 11 (10) |
| Liver | |
| 0 = none | 52 (47) |
| 1 = mild | 36 (33) |
| 2 = moderate | 19 (17) |
| 3 = severe | 2 (2) |
| Lungs | |
| 0 = none | 22 (20) |
| 1 = mild | 39 (35) |
| 2 = moderate | 38 (34) |
| 3 = severe | 10 (9) |
| Joints and fascia | |
| 0 = none | 33 (31) |
| 1 = mild | 22 (20) |
| 2 = moderate | 38 (34) |
| 3 = severe | 16 (14) |
| Female genital | |
| 0 = none | 31 (28) |
| 1 = mild | 6 (5) |
| 2 = moderate | 12 (11) |
| 3 = severe | 5 (4) |
Abbreviations: Mild cGVHD involves only one or two organs or sites with no clinically significant functional impairment (max score 1). Moderate involves at least one organ or site with clinically significant but no major disability (max score 2) or three or more organs or sites with no clinically significant functional impairment (max score 1). A lung score of 1 is also moderate cGVHD. Severe cGVHD indicates major disability caused by cGVHD (score 3). A lung score of 2 or 3 is also classified as severe cGVHD.11
AIPN
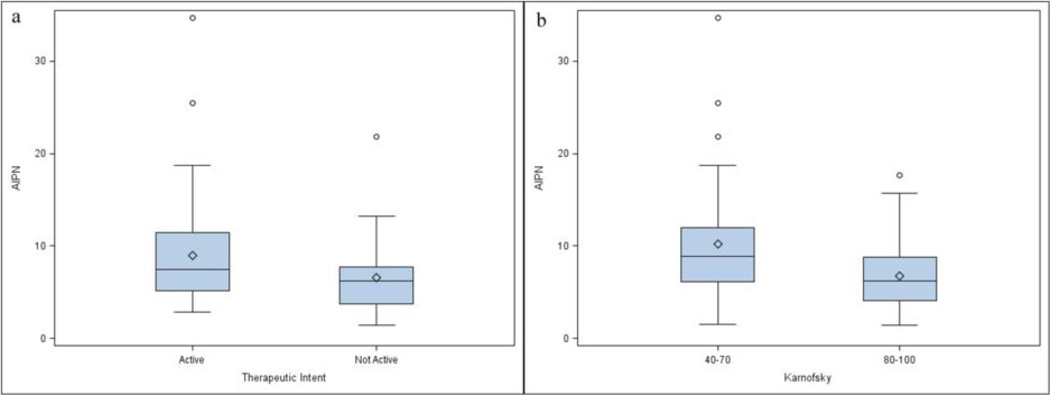
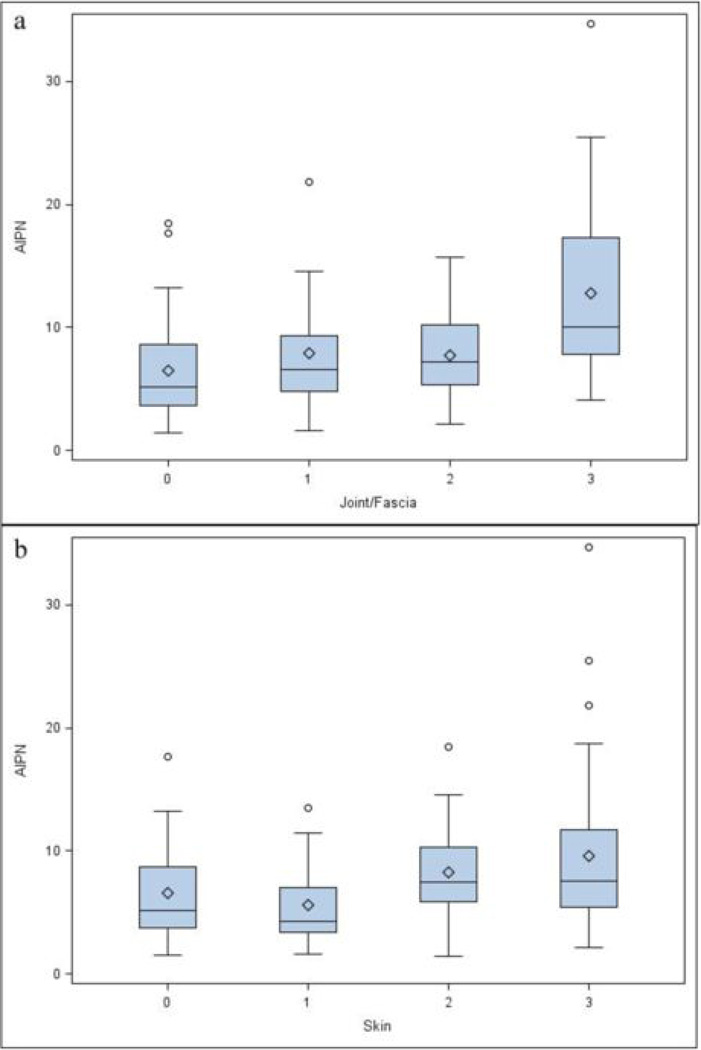
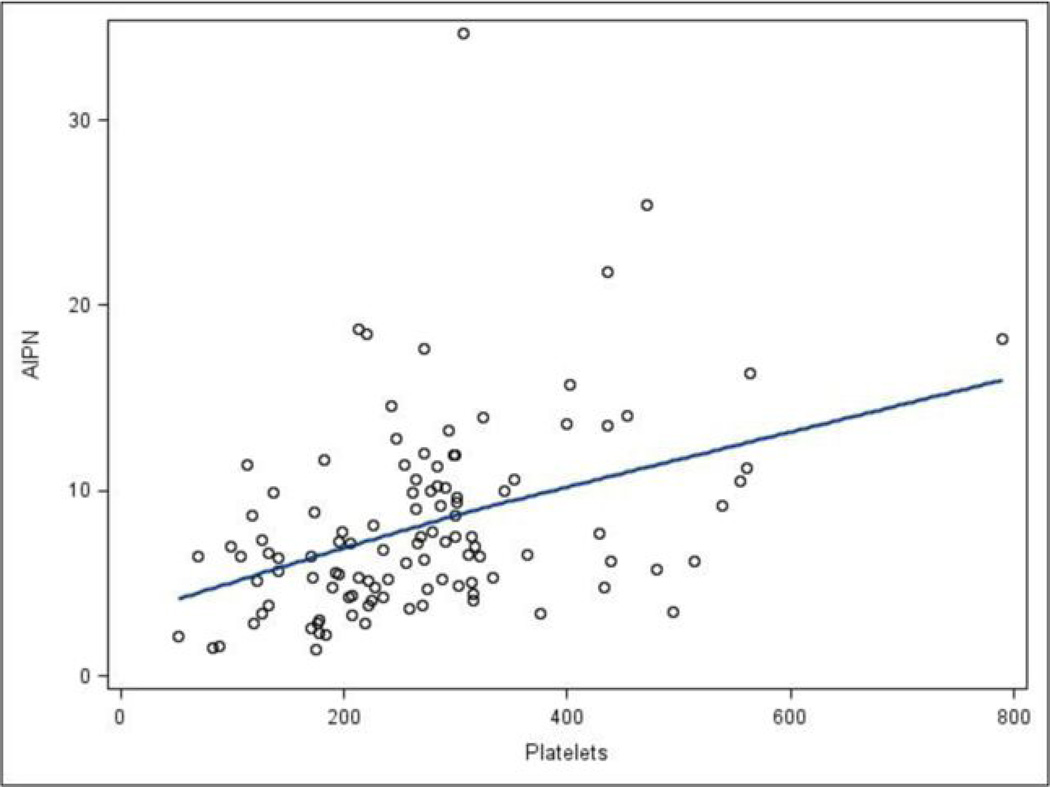
Higher AIPN was associated with active disease by therapeutic intent (p=0.026) (Figure 1a), lower Karnofsky (p=0.0013) (Figure 1b), worse NIH cGVHD joint/fascia (p=0.0005) (Figure 2a) and worse skin NIH cGVHD (p=0.0044) (Figure 2b), but not with NIH global score, type of transplant conditioning, donor relationship, cGVHD onset, cGVHD classification, genital NIH cGVHD, lung NIH cGVHD, liver NIH cGVHD, GI tract NIH cGVHD, eye NIH cGVHD, mouth NIH cGVHD, maximal overall organ score, ANC, WBC, CRP, LDH, lymphocyte count, albumin, or number of prior systemic therapies. Patients requiring a higher intensity of immunosupression were characterized by a higher AIPN (p=0.14), but without statistical significance, and specifically there was no association with patients being on or off calcineurin inhibitors, sirolimus, or both, or with steroid dosing. AIPN was moderately correlated with platelet counts (r = 0.42) (Figure 3), and Karnofsky (r = 0.37), but was not correlated with ANC, WBC, CRP, lymphocytes, albumin, total and average NIH scores, or number of prior systemic therapies.
Figure 1.
Effect of variables on AIPN measurement. (a)Therapeutic intent (p=0.026). Active vs. Not Active (definitions in the Methods section), (b) Karnofsky score (p=0.0013). The upper and lower small line segments at the ends of the whiskers extend at most to +/− 1.5 times the interquartile range. The circles are values, which lie beyond the whiskers.
Figure 2.
Patients with cGVHD involvement per organ, effect on AIPN. 0= no involvement, 1= mild, 2= moderate, 3=severe involvement (a) Joint fascia involvement (p=0.0005), (b) Skin involvement (p=0.0044). The upper and lower small line segments at the ends of the whiskers extend at most to +/− 1.5 times the interquartile range. The circles are values, which lie beyond the whiskers.
Figure 3.
The scatter plot of platelets vs. AIPN provided along with a LOESS (locally fitted regression) line. AIPN was correlated with platelet counts (Spearman r = 0.42).
AIPN showed no association in our cohort with NIH global score, type of transplant conditioning, donor relationship, cGVHD onset, cGVHD classification, genital NIH cGVHD, lung NIH cGVHD, liver NIH cGVHD, GI tract NIH cGVHD, eye NIH cGVHD, mouth NIH cGVHD, or maximum overall organ score.
We recently reported that in 107 normal volunteers, values of AIPN ranged from 2.4 to 20.7 with a mean of 6.0 +/−2.7. 6 These values translate to an estimated 95% CI of 0.6 to 11.4. In the present data, AIPN ranged from 1.4 to 34.7. The majority of our cGVHD cohort had an AIPN value within the normal range. Only 22/110 AIPN values were greater than 11.4, and only 3 exceeded 20.7. All 3 patients with elevated AIPN had severe cGVHD. Six patient’s values were below 2.4 and 3 of these 6 with very low AIPN had less than 100x 109 /ul platelets.
Discussion
Prior studies evaluating the relationship of cGVHD to thrombopoiesis have reported seemingly contradictory results. Higher platelet counts, presumably reflecting higher ongoing thrombopoiesis, correlated with more active and more severe cGVHD in our prior study.8 This relationship was hypothesized to be related to ongoing inflammation and reactive thrombocytosis, potentially mediated by IL-6, which is a strong stimulator of platelet production. 12, 13 In addition, platelets may contribute to pathogenesis of fibrosis via release of fibrogenic cytokines, and in that study higher platelet counts were associated with more severe skin and joint/fascia cGVHD. Some reports have recognized thrombocytopenia at the time of cGVHD diagnosis as a poor prognostic factor for survival in cGVHD. 1, 14, 15, 16, 17,18.
In our current study of a large outpatient cohort of cGVHD patients, there was no evidence of absolute hypoproduction of platelets from the marrow, as the majority of patients had values for immature platelet levels within the normal range. All of our analyses linked a higher AIPN to worse cGVHD, as evidenced by significant correlations of AIPN with skin or joint/fascia scores, performance status, active therapy, and a trend towards a correlation with higher intensity of immunosupression. These data do not provide evidence for a general graft versus marrow microenvironmental process in cGVHD impairing platelet production. In fact, only 16 patients, 13% of this population, had a below normal platelet counts and even fewer (n = 3) had a platelet counts less than 100,000/ul and a low AIPN. So only in this subgroup is there evidence for platelet hypoproduction, potentially caused by a graft-versus-niche effect.
Our data instead support the hypothesis that ongoing inflammation in cGVHD stimulates increased thrombopoiesis in the most severe patients, most likely via higher levels of thrombopoietic cytokines. In this scenario higher platelet production may be an acute phase reactant and a biomarker of cGVHD associated inflammation, resulting from proinflammatory cytokines such as IL6, interferon and IL17 directly and indirectly stimulating thrombopoiesis.8, 19, 20. The other possibility is that increased thrombopoiesis plays an actual pathophysiologic role in the process of fibrogenesis in cGVHD, given that platelets and megakaryocytes release cytokines such as platelet-derived growth factor and transforming growth factor that stimulate fibrosis.21, 22. This hypothesis needs to be tested in further studies and may have potentially important implications in devising future treatment strategies, including anti-inflammatory and cytokine-specific drugs for some of the most devastating and disabling symptoms of cGVHD.8, 12, 13 It is possible that treatment with agents that increase platelet production to a supraphysiologic level, such as thrombopoietic agonists, might worsen the fibrotic complications of cGVHD.
How can we reconcile our finding that increased thrombopoiesis is associated with worse cGVHD with studies reporting that thrombocytopenia is associated with poorer outcomes in cGVHD? 14, 15, 16, 17, 18. It is important to note that these seminal studies examined thrombocytopenia as a prognostic factor only at the initial onset or diagnosis of cGVHD, usually within one year of transplantation, and did not study platelet counts specifically in association with organ-specific cGVHD manifestations or later in the course of cGVHD. As low platelet counts after allogeneic HSCT are not uncommon and are associated with worse survival, even without cGVHD, 23 it remains unsettled if low platelets at the onset of cGVHD is a specific process with poor prognosis, or is simply a manifestation of complex factors following allogeneic HSCT, some linked to acute GVHD, including ongoing infection, marrow suppressive drugs, endothelial damage, inflammation, and autoimmunity.8 Perhaps patients with early onset of severe cGVHD have enhanced platelet destruction but are unable to appropriately compensate by increasing marrow production due to concurrent drug therapies, infection or simply incomplete recovery of marrow stem cell reserve early post-transplantation. In contrast, our study included cGVHD outpatients a median of three years following transplantation, and enrolled very few patients in the first year post-transplant. In our cohort, it is also relevant to stress that we may not have captured patients with early onset thrombocytopenia and severe cGVHD, since these patients may not have survived long enough to enroll in our long-term cross-sectional natural history study.
There are some limitations of this study; this cohort did not include many patients with thrombocytopenia: only a few patients under 100,000 or even under 150,000/ul, so the question still remains if those patients with very low platelets and cGVHD have lost the ability of the marrow to make platelets, at least at a level sufficient to keep up with increased destruction. This study includes referral patients who are primarily late in the course of cGVHD with a particularly high prevalence of severe but stable cGVHD, especially skin and lung, and may not be representative of patient cohorts followed at transplant centers or earlier following transplantation. This study is by design a cross-sectional analysis of outpatients, which permits enrollment of a broad spectrum of the patients with the most severe forms of cGVHD but without intercurrent infectious complications or other acute confounding factors. Our observed association of higher AIPN and platelet counts with cGVHD activity and severity according to the NIH criteria should be explored in further studies focusing on longitudinal observation of patient cohorts after allogeneic HSCT. Future studies should also investigate any mechanistic connection between thrombopoiesis and cGVHD.
Footnotes
Conflict of Interest
There is no any competing financial interest in relation to the work described.
References
- 1.Pulanic D, Lozier JN, Pavletic SZ. Thrombocytopenia and hemostatic disorders in chronic graft versus host disease. Bone Marrow Transplant. 2009;44:393–403. doi: 10.1038/bmt.2009.196. [DOI] [PubMed] [Google Scholar]
- 2.Shono Y, Ueha S, Wang Y, Abe J, Kurachi M, Matsuno Y, et al. Bone marrow graft-versus- host disease: early destruction of hematopoietic niche after MHC-mismatched hematopoietic stem cell transplantation. Blood. 2010;115(26):5401–5411. doi: 10.1182/blood-2009-11-253559. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki R, Kuwana M, Mori T, Okazaki Y, Kawakami Y, Ikeda Y, et al. Prolonged thrombocytopenia after allogeneic hematopoietic stem cell transplantation: associations with impaired platelet production and increased platelet turnover. Bone Marrow Transplant. 2006;38:377–384. doi: 10.1038/sj.bmt.1705444. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi S, Oshida M, Kiyoi T, Todokoro S, Kashiwagi H, Honda S, et al. Comparison of reticulated platelet count with plasma glycocalicin concentration as a marker of platelet turnover in patients with thrombocytopenic disorders. RinshoKetsueki. 2000;41:705–711. [PubMed] [Google Scholar]
- 5.Emmons RV, Reid DM, Cohen RL, Meng G, Young NS, Dunbar CE, et al. Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood. 1996;87:4068–4071. [PubMed] [Google Scholar]
- 6.Bat T, Leitman SF, Calvo KR, Chauvet D, Dunbar CE. Measurement of the absolute immature platelet number reflects marrow production and is not impacted by platelet transfusion. Transfusion. 2012 doi: 10.1111/j.1537-2995.2012.03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filipovich AH, Weisdorf D, Pavletic SZ, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft - versus - Host Disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Grkovic L, Baird K, Steinberg SM, Williams KM, Pulanic D, Cowen EW, et al. Clinical laboratory markers of inflammation as determinants of chronic graft-versus-host disease activity and NIH global severity. Leukemia. 2012;26:633–643. doi: 10.1038/leu.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walters J, Garrity P. Performance evaluation of the Sysmex XE-2100 hematology analyzer. Lab Hematol. 2000;6:83–92. [Google Scholar]
- 10.Hollander M, Wolfe DA. John Wiley & Sons, Inc. 2nd edition. New York, NY: 1999. Non-parametric Statistical Methods; pp. 189–269. [Google Scholar]
- 11.Cho BS, Min CK, Eom KS, Kim YJ, Kim HJ, Lee S, et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia. 2009;23:78–84. doi: 10.1038/leu.2008.276. [DOI] [PubMed] [Google Scholar]
- 12.Ito H. Anti-interleukin-6 therapy for Crohn's disease. Curr Pharm Des. 2003;9:295–305. doi: 10.2174/1381612033391900. [DOI] [PubMed] [Google Scholar]
- 13.Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 14.Akpek G, Zahurak ML, Piantadosi S, Margolis J, Doherty J, Davidson R, et al. Development of a prognostic model for grading chronic graft-versus-host disease. Blood. 2001 Mar 1;97(5):1219–1226. doi: 10.1182/blood.v97.5.1219. [DOI] [PubMed] [Google Scholar]
- 15.Akpek G, Lee SJ, Flowers ME, Pavletic SZ, Arora M, Lee S, et al. Performance of a new clinical grading system for chronic graft-versus-host disease: a multicenter study. Blood. 2003 Aug 1;102(3):802–809. doi: 10.1182/blood-2002-10-3141. [DOI] [PubMed] [Google Scholar]
- 16.Pavletic SZ, Smith LM, Bishop MR, Lynch JC, Tarantolo SR, Vose JM, et al. Prognostic factors of chronic graft-versus-host disease after allogeneic blood stem-cell transplantation. Am J Hematol. 2005 Apr;78(4):265–274. doi: 10.1002/ajh.20275. [DOI] [PubMed] [Google Scholar]
- 17.Kuzmina Z, Eder S, Böhm A, Pernicka E, Vormittag L, Kalh P, et al. Significantly worse survival of patients with NIH-defined chronic graft-versus-host disease and thrombocytopenia or progressive onset type: results of a prospective study. Leukemia. 2012 Apr;26(4):746–756. doi: 10.1038/leu.2011.257. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Simón JA, Afram G, Martino R, Piñana JL, Caballero-Velazquez T, Ringden O, et al. Evaluation of prognostic factors among patients with chronic graft-versus-host disease. Haematologica. 2012 Aug;97(8):1187–1195. doi: 10.3324/haematol.2011.055244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001 Nov 1;98(9):2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood. 2007 Nov 15 10;110:3804–3813. doi: 10.1182/blood-2007-05-091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol. 2007 Nov;139(3):351–362. doi: 10.1111/j.1365-2141.2007.06807.x. [DOI] [PubMed] [Google Scholar]
- 22.Terui T, Niitsu Y, Mahara K, Fujisaki Y, Urushizaki Y, Mogi Y, et al. The production of transforming growth factor-beta in acute megakaryoblastic leukemia and its possible implications in myelofibrosis. Blood. 1990 Apr 1;75(7):1540–1548. [PubMed] [Google Scholar]
- 23.Dominietto A, Raiola AM, Van Lint MT, Lamparelli T, Gualandi F, Berisso G, et al. Factors influencing haematological recovery after allogeneic haemopoietic stem cell transplants: graft-versus-host disease, donor type, cytomegalovirus infections and cell dose. British Journal of Haematology. 112:219–227. doi: 10.1046/j.1365-2141.2001.02468.x. [DOI] [PubMed] [Google Scholar]