Abstract
Background
Prolonged ethanol (EtOH) intake may perturb function of the hypothalamic–pituitary–adrenal axis in a manner that promotes dependence and influences EtOH withdrawal severity. Prior in vivo and in vitro studies suggest that corticosteroids, in particular, may be elevated during EtOH intoxication and withdrawal, suggesting that intracellular glucocorticoid receptors (GRs) may promote the development of EtOH dependence.
Methods
Adult male Sprague-Dawley rats were subjected to a 4-day binge-like EtOH administration regimen (3 to 5 g/kg/i.g. every 8 hours designed to produce peak blood EtOH levels (BELs) of <300 mg/dl). Subgroups of animals received s.c. injection of the GR antagonist mifepristone (20 or 40 mg/kg in peanut oil at 0800 hours on each of the 4 days prior to withdrawal). BELs were assessed at 0900 and 1500 hours on Days 2 (D2) and 4 (D4) of the regimen. BEL, blood corticosterone levels (BCLs), and EtOH withdrawal–associated behavioral abnormalities were assessed 10 to 12 hours after the final EtOH administration.
Results
Daily mean EtOH doses for D1 to D4 of the regimen were 14.4, 9.9, 7.1, and 8.6 g/kg, respectively. The EtOH gavage regimen produced mean BELs of 255 mg/dl at 0900 on D2 and 156.2 mg/dl at 0900 on D4 of the regimen. Withdrawal from the EtOH exposure regimen, beginning 10 hours after the last EtOH administration, produced significant elevations in BCL and behavioral abnormalities including tremors, stereotypy, and “wet dog shakes.” Mifepristone administration did not alter food intake or weight during the 4-day regimen, nor were there drug-dependent differences in BEL or BCL on withdrawal day. Although mifepristone produced no significant changes in behavior of EtOH-naïve animals, pretreatment with mifepristone (40 mg/kg) significantly reduced the severity of EtOH withdrawal.
Conclusions
Findings suggest that activation of GRs promotes neuroadaptation to binge-like EtOH exposure, contributing to the development of EtOH dependence. Further, GRs may represent therapeutic targets to be exploited in reducing the severity of EtOH withdrawal.
Keywords: Hypothalamic–Pituitary–Adrenal Axis, Stress, Alcoholism, Dependence, Hormone
Stress Often Precedes the development of alcohol dependence and is also a consequence of dependence, thereby perpetuating the cycle of dependence, withdrawal, and relapse to alcohol use (Heilig et al., 2010; Koob and Kreek, 2007). Indeed, many individuals report drinking to overcome negative effect (Addolorato et al., 2005), and it has long been appreciated that ethanol (EtOH) itself can act as a physiological stressor (Ellis, 1966). Work with clinical samples has demonstrated strong correlations between hypothalamic–pituitary–adrenal (HPA) axis disruption and alcohol dependence, craving and relapse to drinking in dependent individuals (Adinoff et al., 2005; O’Malley et al., 2002).
Acute alcohol exposure stimulates the release of corticotrophin-releasing factor (CRF), thereby initiating HPA axis activity and subsequently stimulating the synthesis and release of adreno-corticotropin releasing hormone and corticosterone (CORT) (Rivier et al., 1984). Plasma CORT levels have been reported to be either elevated (Rasmussen et al., 2000; Sinha et al., 2009) or reduced (Richardson et al., 2008; Weiss et al., 2001) following prolonged alcohol exposure. Similarly, HPA axis reactivity to subsequent stressors can be either dampened (Adinoff et al., 1998) or elevated (Fox et al., 2009) following a period of alcohol dependence. Factors contributing to these discrepancies in HPA axis activity and CORT concentration following acute or chronic EtOH administration likely include age of the subjects (Klimes-Dougan et al., 2001), sex of the subjects (Handa et al., 1994; Klimes-Dougan et al., 2001; Sinha et al., 2009), differences in diurnal timing of measurement (Klimes-Dougan et al., 2001), prior drug exposure (Sinha et al., 2009), rodent strain (Roberts et al., 1992), alcohol exposure paradigms (Ogilvie et al., 1997; Rasmussen et al., 2000), and the level of alcohol reactivity in individual animals (Richardson et al., 2008). In related findings, it was reported that polymorphisms in glucocorticoid receptors (GRs) (Rosmond et al., 2000) or CRF-1 receptor activity (Clarke et al., 2008) may influence basal CORT, and potentially, EtOH-stimulated CORT secretion. Interestingly, Huizink and colleagues (2006) reported that either elevated or reduced HPA axis activity may be associated with substance abuse disorders.
EtOH withdrawal was reported to produce elevations in cortisol secretion during the early withdrawal phase, reaching micromolar concentrations (Esel et al., 2001); these levels moderate with prolonged abstinence in humans (Adinoff et al., 1998; von Bardeleben et al., 1989). In fact, a subset of alcohol-dependent individuals display Cushing’s-like syndrome during active drinking and early withdrawal (Besemer et al., 2011). Interestingly, there may be a correlation between the magnitude of the rise in circulating cortisol and increased severity of alcohol withdrawal (Risher-Flowers et al., 1988). Rasmussen and colleagues (2000) showed chronic EtOH intake, which yielded moderate blood EtOH levels (BELs), is sufficient to produce high concentrations of free CORT during the early stage of withdrawal in rodents. Vendruscolo and colleagues (2012) reported a reduction in GR mRNA during early withdrawal from intermittent EtOH vapor exposure. Similarly, Alele and Devaud (2007) demonstrated that withdrawal from 14 days of exposure to a liquid EtOH diet produced marked elevations in plasma CORT in male, but not female, rats. In vitro studies of rodent, hippocampal explants are consistent with the hypothesis that CORT has a role in promoting neuronal excitation and, possibly, neuronal injury during EtOH withdrawal (Mulholland et al., 2005). As a whole, these findings suggest that hypercortisolemia may contribute to both the development of EtOH dependence and, hence, the severity of EtOH withdrawal. The present studies examined the hypothesis that GR antagonism would attenuate the development of alcohol dependence by administering the GR antagonist mifepristone daily during exposure to a modified 4-day EtOH gavage paradigm, modified after Majchrowicz (1975).
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN), weighing between 224 and 245 g upon arrival, were allowed 1 week to acclimate to single housing within the colony prior to handling. Animals were handled 2 minutes daily for 1 week, followed by 1 week of once daily exposure to intragastric gavage (i.g.) procedure using tap water. To prevent pica, animals were placed on wire mesh grates measuring the length and width of the cage floor 2 days prior to alcohol exposure regimen. Throughout all procedures, animals were allowed ad libitum access to standard rat chow and water.
EtOH Diet and Administration Procedure
EtOH and an isocaloric control diet solution contained 30% v/v Vanilla Ensure® (Abbott Nutrition, Columbus, OH) and an equal quantity of double distilled water. Additionally, EtOH diet contained 40%v/v 200 proof EtOH (Sigma-Aldrich, San Diego, CA). Isocaloric control diet was prepared by substituting EtOH with an isocaloric quantity of maltose (Sigma-Aldrich) dissolved in double distilled water. Animals received EtOH or isocaloric control diet 3 times daily (i.g.: 0800, 1600, 0000) for 4 days, as follows: Day 1 (D1): 5.0, 4.8, 4.6 g/kg; Day 2 (D2): 4.0, 3.2, 2.7 g/kg; Day 3 (D3): 2.7, 2.0, 2.4 g/kg; Day 4 (D4): 2.9, 3.1, 2.6 g/kg. These doses are equivalent to the weighted mean dose delivered in 2 previously conducted binge experiments (data not shown) using the traditional Majchrowicz (1975) model. This approach was adopted to avoid the subjectivity inherent in the Majchrowicz model, which requires a subjective intoxication rating to determine EtOH dose for each animal. Additionally, this modification allowed for equivalent dosing in all animals to achieve the desired BEL range (~200 mg/dl). To prevent overdosing, each animal was observed for righting and eye-blink reflexes prior to administration of EtOH diet; loss of either reflex resulted in no administration of EtOH or vehicle at that time point. During the experiments, loss of righting reflex was observed in 7 of 50 animals. No individual animal missed more than 1 EtOH dose and loss of reflex was observed in each EtOH treatment groups (EtOH + vehicle, n = 2; EtOH + 20 mg/kg, n = 3; EtOH + 40 mg/kg, n = 2). The dosing procedure used in these experiments resulted in fewer withheld EtOH doses than were reported by Majchrowicz in his seminal 1975 publication (17 observations in 63 animals). Animal and food weights were recorded daily at 0800.
Mifepristone Treatment
Mifepristone (20 or 40 mg/kg) (Sigma-Aldrich) or vehicle (peanut oil) were administered subcutaneously (s.c.) once daily following the 0800 administration of EtOH or control diet. Drug was suspended in peanut oil and sonicated for 30 minutes at least 24 hours prior to injection, it was then stored at 4°C until needed. Suspension was vortexed for 10 to 15 minutes prior to and as needed throughout dosing.
Analysis of Blood EtOH and Corticosterone Concentrations
Tail blood was collected (approximately 140 µl/animal/time point) at 4 time points as follows: D2 at 0900, D2 at 1500, D4 at 0900, and D4 at 1500 hours. Following behavioral observation on Day 5 (D5), animals were sacrificed by rapid decapitation (no anesthesia was used) and trunk blood was collected (~1200 hours). Upon collection, blood was placed on ice and centrifuged (5 minutes at 21,890×g); blood plasma was collected and stored at −80°C. BELs were determined using an Analox AM1 instrument (Analox Instruments, Lunenburg, MA), which measures blood alcohol concentrations indirectly through measurement of molecular oxygen levels. In the presence of molecular oxygen, EtOH is oxidized by the enzyme alcohol oxidase to form acetaldehyde and hydrogen peroxide. Under the conditions of the assay, oxygen consumption is directly proportional to EtOH concentration in the plasma sample.
Blood CORT levels (BCLs) were determined from trunk plasma obtained ~12 hours after the final EtOH administration using a competitive EIA Corticosterone kit (IDS Limited, Fountain Hills, AZ). Briefly, 100 µl of each diluted sample (1:20), calibrator, and control, and 100 µl of enzyme (CORT labeled with horseradish peroxidase) were added to an antibody-coated plate (polyclonal rabbit anti-CORT) and incubated at 4°C for 24 hours. Following incubation, the plate was thrice washed and 200 µl of substrate (tetramethylbenzidine and hydrogen peroxide) was added. After 30 minutes, stop solution (0.5 Mhydrochloric acid) was added, and the microplate was read using a Beckman Coulter DTX 880 Mulitmodal Detector (Lagerhausstrasse, Austria) with Beckman Coulter Multimode Detection Software (v.20.0.12).Mean absorbance values for samples, controls, and calibrators were measured at 450 nm 3 times producing a mean value; this measure was used to calculate percent binding (B/B0% = [(mean absorbance)/(mean absorbance for “0” calibrator)]×100). Mean concentration of CORT for each sample (ng/ml) was determined based upon the calibration curve.
Assessment of EtOH Withdrawal Signs
Approximately 10 hours following the final dose of EtOH, animals were individually placed in a square plexiglass chamber and observed for 2 minutes each. Using a behavioral scale adapted from Majchrowicz (1975), after Self and colleagues (2009), animals were evaluated blindly for signs of withdrawal. A sign was included in final analysis if observed by 2 of the 3 raters. Signs included rigidity or tremor in tail or body, stereotypy, splayed paws, motor abnormalities (e.g., retropulsion, abnormalities in gate), head shakes, duration of immobility, and convulsions.
Statistical Analysis
Statistical analyses were performed using analysis of variance (ANOVA). When appropriate, post hoc comparisons were conducted using Tukey’s HSD test. Weight loss, food consumption, and BEL data were analyzed using a mixed factors 3-way ANOVA. Weight loss and food consumption were analyzed with day (T, W, TH, F) as the within-subjects factor and diet (isocaloric diet [ID], EtOH diet [ED]) and drug (vehicle, 20, 40 mg/kg) as the between-subject factors. Because EtOH dosing varied between D2, D4, and D5 (4.0 g/kg on D2 vs. 2.9 g/kg on D4 vs. 0.0 g/kg on D5), BEL data were analyzed within each day. Data collected on D2 and D4 were analyzed separately in a mixed 3-way ANOVA, with time of day (0900, 1500) as the within and diet (ID, ED) and drug (vehicle, 20, 40 mg/kg) as the between-subject factors; D5 BEL data were analyzed using a 2-way ANOVA with diet (ID, ED) and drug (vehicle, 20, 40 mg/kg) as the between-subject factors. As BEL and BCL data from withdrawal day (D5) were collected and analyzed at only 1 time point, a 2-way ANOVA was employed using diet (ID, ED) and drug (vehicle, 20, 40 mg/kg) as the between-subject factors. The data are expressed as mean and SEM. The accepted level of significance for all tests was p < 0.05.
RESULTS
Body Weight and Food Consumption
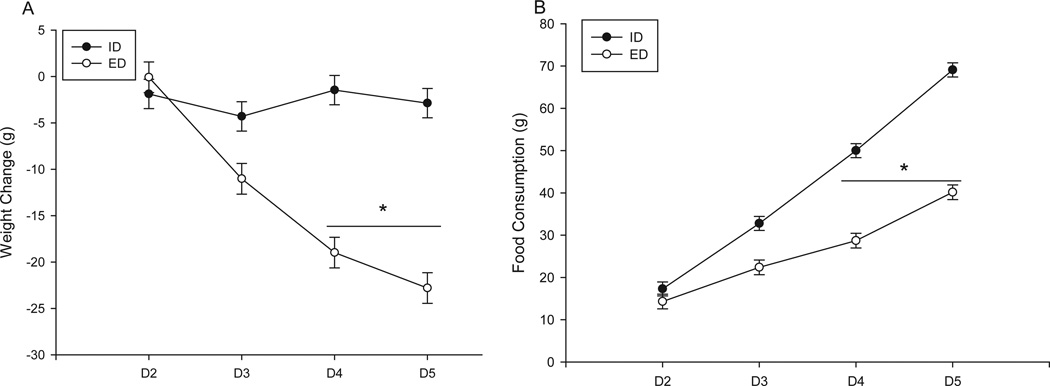
Body weights were measured prior to the 0800-hour treatment time point on each day of the 4-day regimen. Weights did not differ significantly between groups prior to EtOH administration. Analysis of total weight loss/gain during the experiment revealed a significant day by diet interaction, F(3, 144) = 45.7, p < 0.001 (Fig. 1A). Post hoc analysis revealed that on D4 and D5 of the dosing regimen, animals receiving EtOH diet lost significantly more weight than did those receiving the isocaloric diet (total mean weight loss (g): ED = 22.8 ± 1.65; ID = 2.8 ± 1.58). Analysis of food consumption also revealed a significant interaction between day and diet, F(3,144) = 67.4, p < 0.001 (Fig. 1B). Similar to weight loss data, post hoc analysis of food consumption revealed that on D4 and D5, animals receiving EtOH diet consumed significantly less food than did group-matched control fanimals (total mean food consumption (g): ED = 40.2 ± 1.74; ID = 69.1 ± 1.66).
Fig. 1.
Mean weight change and food consumption during a 4-day gavage regimen of EtOH diet (ED) or an isocaloric diet (ID) and receiving either vehicle, 20, or 40 mg/kg of mifepristone (s.c.). On Days 4 (D4) and 5 (D5), animals receiving ED lost significantly more weight (A) and consumed less food (B) than animals receiving ID. (ID+veh n = 8; ED+veh n = 10; ID+20 mg/kg n = 9; ED+20 mg/kg n = 9; ID+40 mg/kg n = 10; ED+40 mg/kg n = 10); *p < 0.05 as compared to EtOH naïve animals (p < 0.05).
Blood EtOH Levels
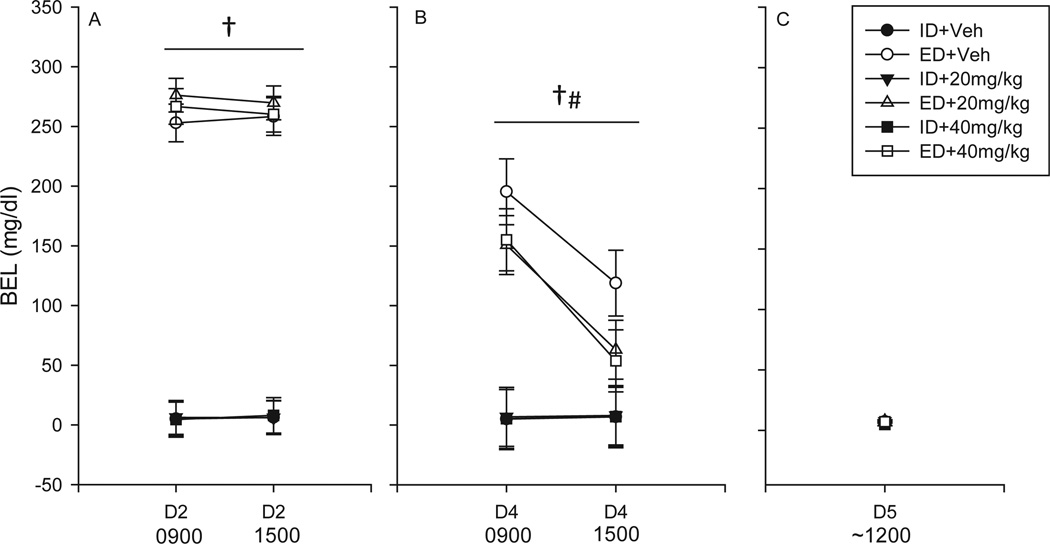
Analysis of BEL data on D2 revealed a main effect of treatment, F(1, 55) = 499.08, (Fig. 2A) such that animals receiving EtOH diet achieved significantly elevated BELs as compared to those receiving isocaloric diet (mean BEL [mg/dl]: ED + veh = 255.6 vs. ID + veh = 5.6; ED + 20 mg/kg = 272.9 vs. ID + 20 mg/kg = 6.1; ED + 40 mg/kg = 263.4 vs. ID + 40 mg/kg = 6.3); no significant differences were found between BEL at 0900 and 1500 on D2. BEL data collected on D4 revealed an interaction of diet and time, F(2, 55) = 96.74, p < 0.001 (Fig. 2B). Post hoc analysis showed that in animals receiving EtOH diet, BELs declined significantly between the 0900 and 1500 blood draws. Additionally, a main effect of diet was found, F(2, 55) = 32.46, p < 0.001 (Fig. 2B), such that animals receiving EtOH diet had significantly higher BELs, as compared to those receiving isocaloric diet (mean BEL [mg/dl]: ID = 6.54 vs. ED = 122.83). BELs did not differ significantly on withdrawal day (D5) (Fig. 2C).
Fig. 2.
Mean blood EtOH levels (BELs) during a 4-day gavage regimen of EtOH diet (ED) or an isocaloric diet (ID) and receiving either vehicle, 20, or 40 mg/kg of mifepristone (s.c.). Animals receiving ED had significantly higher BELs than animals receiving ID on Day 2 (D2) (A) and D4 (B). On D4, EtOH-treated animals had lower BELs at the 1500 time point, as compared with the 0900 time point (B); no significant differences were found between animals receiving EtOH diet on D4. No group differences were observed on D5 (C). (ID+veh n = 8; ED+veh n = 10; ID+20 mg/kg n = 9; ED+20 mg/kg n = 9; ID+40 mg/kg n = 10; ED+40 mg/kg n = 10); †p < 0.05 formain effect of diet; #p < 0.05 for interaction of diet and time.
BCL and EtOH Withdrawal Behavior
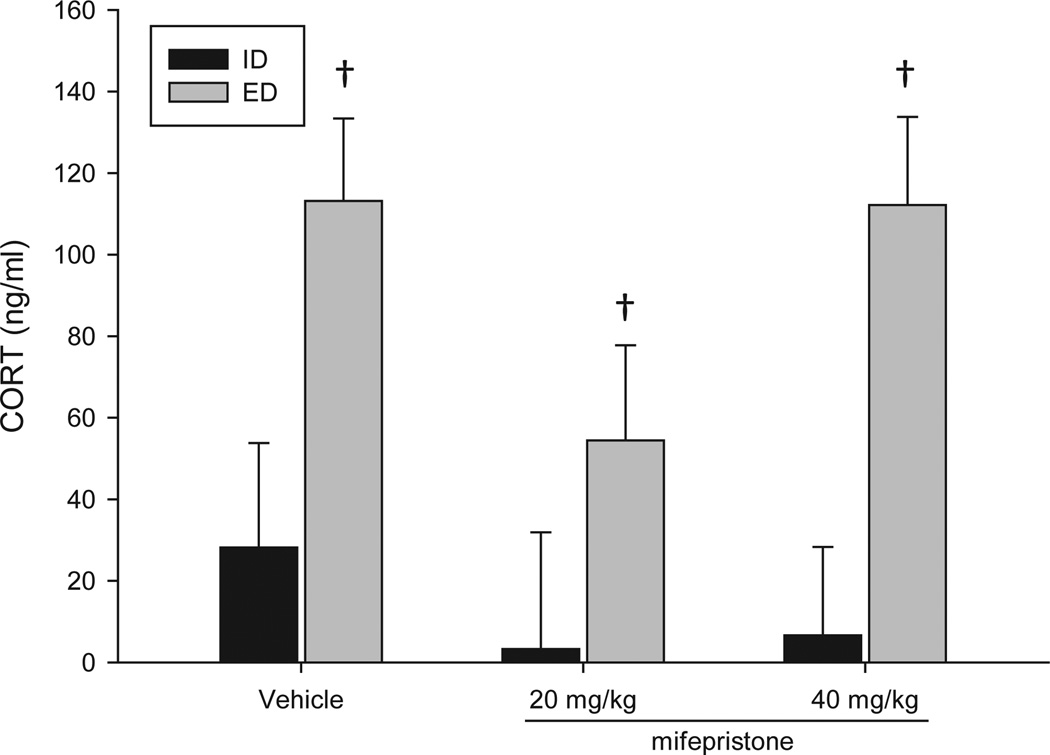
Analysis of BCL data collected on withdrawal day revealed a significant main effect of treatment, F(1, 36) = 17.35, p < 0.05, such that animals treated with EtOH diet had significantly elevated levels of CORT compared to those receiving the isocaloric diet (Fig. 3). BCL in EtOH-treated animals ranged from approximately 60 to 112 ng/ml ~12 hours after the final EtOH administration.
Fig. 3.
Mean blood corticosterone (CORT) levels (BCLs) during withdrawal (day 5) from a 4-day gavage regimen of EtOH diet (ED) or an isocaloric diet (ID) and receiving either vehicle, 20, or 40 mg/kg of mifepristone (s.c.). Animals receiving ED had significantly higher BCLs than animals receiving ID. (ID+veh n = 5; ED+veh n = 4; ID+20 mg/kg n = 4; ED+20 mg/kg n = 6; ID+40 mg/kg n = 5; ED+40 mg/kg n = 6); †, p < 0.05 for main effect of diet.
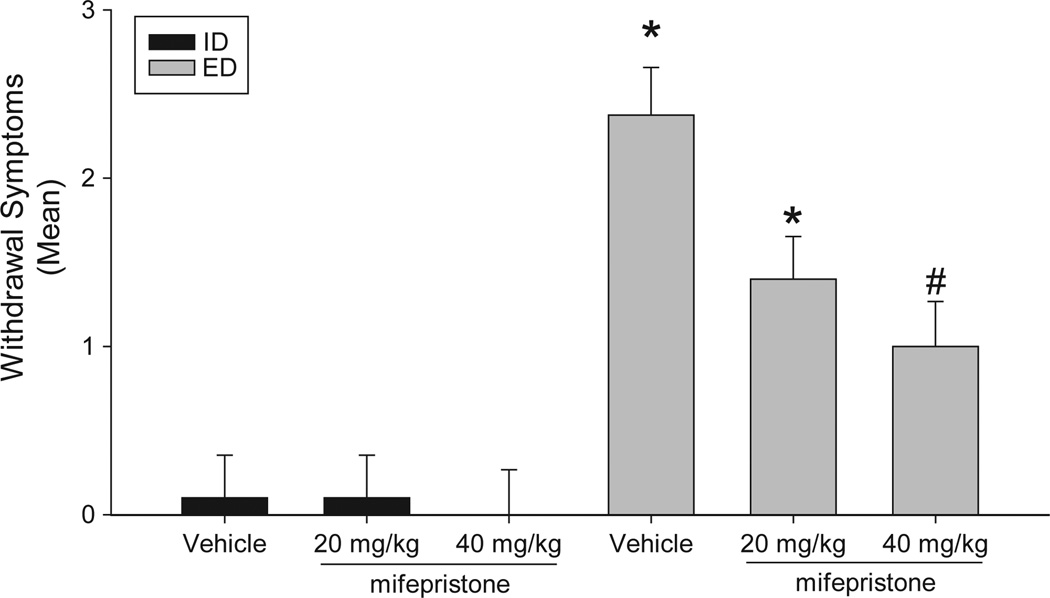
Analysis of behavioral data revealed a significant interaction between diet and drug, F(5, 55) = 3.92, p < 0.05, such that EtOH-treated animals receiving vehicle or 20 mg/kg of mifepristone displayed significantly more signs of EtOH withdrawal than did EtOH-naïve animals receiving the same drug treatment. Importantly, treatment with 40 mg/kg of mifepristone significantly reduced the severity of EtOH withdrawal, in a dose-dependent manner (Fig. 4). The most prominent behavioral signs of EtOH withdrawal were tremor (n = 12), rigidity (n = 10), splayed paws (n = 11), and “wet dog shakes” (n = 10). Administration of mifepristone did not alter behavior of animals treated with an isocaloric diet.
Fig. 4.
Mean number of ethanol (EtOH) withdrawal-related behavioral abnormalities, most prominently observed signs were tremor, tail rigidity, splayed paws, and “wet dog shakes”, observed ~10 hours after the final EtOH administration (at 0000 hours) of a 4-day EtOH gavage regimen. Withdrawal from the regimen produced moderate EtOH withdrawal signs that were significantly attenuated by prior exposure (s.c.) to 40 mg/kg mifepristone. (ID+veh n = 8; ED+veh n = 10; ID+20 mg/kg n = 9; ED+20 mg/kg n = 9; ID+40 mg/kg n = 10; ED+40 mg/kg n = 10); *p < 0.05 as compared with group-matched control; #p < 0.05 vs. EtOH diet+vehicle.
DISCUSSION
Pharmacological manipulation of HPA axis function and extra-hypothalamic CRF systems may represent novel means of treating alcohol dependence, detoxification and/or maintenance of abstinence from drinking. This has become increasingly clear given the large body of evidence implicating extra-hypothalamic CRF systems in EtOH preference. These findings demonstrate a role for CRF-1 receptor antagonists and, possibly CRF-2 receptor agonists, in attenuating EtOH intake in rodents (Lowery et al., 2010; Sabino et al., 2006; Sparta et al., 2008), although it must be noted that findings not consistent with this hypothesis have been reported. For example, Yang and colleagues (2008) reported that the CRF-1 receptor antagonists R121919 and Antalarmin did not alter stress-induced increases in EtOH preference in 129SVEV mice. The CORT synthesis inhibitor metyrapone was reported to reduce EtOH preference in high- and low-preferring mice with chronic administration (O’Callaghan et al., 2005). Prior work examining effects of mifepristone on EtOH-associated behaviors has demonstrated that mifepristone is ineffective at reducing EtOH preference in 129SVEV mice exposed to restraint stress (Yang et al., 2008) or binge-like intake in C57BL/6J mice using a “drinking in the dark” procedure (Lowery et al., 2010). In contrast, others have reported that chronic treatment with mifepristone reduced the increased EtOH intake in mice with low, but not high, preference for EtOH following injection-induced stress (O’Callaghan et al., 2005), and Koenig and Olive (2004) reported that mifepristone reduced EtOH intake in rats under a limited access regimen. Similarly, Vendruscolo and colleagues (2012) reported that chronic mifepristone treatment via s.c. pellet (150 mg/kg for 21 days) was associated with reduced alcohol intake following intermittent alcohol vapor exposure and alcohol intake associated with long-term abstinence from exposure.
The present studies employed a forced intragastric intoxication model to specifically examine the role that GR activation had in the development of physical dependence of EtOH (as reflected in metabolic tolerance and withdrawal), independent of its effects on voluntary intake of EtOH or motivational factors potentially influencing drinking behavior. Findings demonstrate that administration of EtOH in a 4-day fixed variable dosing schedule produced BELs commonly reported in the binge drinking population (Perkins et al., 2001). Further, these BELs were similar to previously published levels, which were shown to produce both metabolic tolerance and marked EtOH withdrawal (Self et al., 2009). In the current studies, EtOH withdrawal-associated behavioral abnormalities were observed in 87% of animals administered EtOH without prior mifepristone pretreatment. Notably, resting tremor and splayed paws were greater than 2-fold more common than other signs of withdrawal. Prior treatment with 40 mg/kg significantly reduced the severity of EtOH withdrawal, with this dose producing a 60% reduction in the number of withdrawal signs. The effects of mifepristone on withdrawal behavior observed in the present study were not associated with significant changes in body weight or, most importantly, BEL during EtOH administration or on the day of withdrawal, although nonsignificant decreases in BELs were observed in mifepristone-treated subjects at some time points. A prior study demonstrated that a single i.p. administration of mifepristone immediately prior to withdrawal from prolonged oral intake of EtOH in outbred TO mice reduced handling-induced convulsions (Jacquot et al., 2008), suggesting an acute effect of GR blockade. However, in the current studies, the final administration of mifepristone preceded withdrawal by approximately 26 hours. As the half-life of mifepristone is estimated to be 1 to 2 hours in the rat (Heikinheimo et al., 1994) and accumulation of drug is not observed with repeated administration of low doses (1 to 50 mg/kg) (Gaillard et al., 1984), the 26-hour interval likely allowed for clearance of the drug. However, this was not specifically examined and the potential for persistence of relevant mifepristone concentrations in circulation following repeated depot injection exists. Data from the current experiment suggest that chronic GR antagonism attenuated the development of EtOH dependence, as reflected in reduced EtOH withdrawal severity. Although it must be noted that mifepristone is also a potent progesterone receptor antagonist. Thus, it will be of importance to extend the current findings with the use of more selective GR antagonist(s).
One hour after administration of EtOH on D2 at 0900 of the regimen, peak BELs were approximately 260 mg/dl, a level that was maintained at the 1500-hour time point. This stasis of BEL likely reflects the large doses of EtOH being delivered during the initial phase of the model (9.4 g/kg in the 24 hours prior to blood draw). The mean EtOH dose administered on D4 at 0800 was lower than that administered at the same time on D2, and produced correspondingly lower peak BELs at the 0900 time points. Although EtOH withdrawal behaviors were not formally assessed at this time, it was noted that some animals displayed withdrawal-like tremor, and seizure was observed in 1 animal at this time. On D5, ~12 hours after the last EtOH administration, BELs of EtOH and control animals did not differ. Prior EtOH exposure resulted in elevated BCLs during the withdrawal period, as compared to those of EtOH-naïve subjects. Additionally, this demonstrates a discord between plasma CORT levels and behavioral signs of EtOH withdrawal, which were markedly attenuated by mifepristone preexposure.
Neuroadaptations resulting from prolonged EtOH exposure are diverse and highly complex, but are likely influenced by EtOH-induced elevations in circulating CORT. The current studies suggest that antagonism of CORT effects in this regard can markedly reduce the behavioral consequences of these neuroadaptations. Expression and/or function of many ionotropic neurotransmitter receptors and voltage-sensitive Ca2+ channels are increased with extended glucocorticoid exposure or exposure to prolonged stress. For example, elevated glucocorticoid levels have been associated with increased abundance of the NR2B subunits of NMDA receptors in the central nervous system (Weiland et al., 1997) and NMDA receptor antagonists clearly ameliorate the severity of EtOH withdrawal–related damage in vitro (Mulholland et al., 2005) and in vivo (Dahchour and De Witte, 2003). Additionally, the synthesis of polyamines, positive allosteric modulators of NR2B subunits, is also increased following CORT elevations via activation of its rate-limiting enzyme ornithine decarboxylase (Cousin et al., 1982; Nsi-Emvo et al., 1996; for review, see Prendergast and Mulholland, 2012). Glucocorticoids increase signaling of L-type Ca2+ channels (Chameau et al., 2007) and pharmacological inhibition of these channels attenuates severity of EtOH withdrawal (Brooks et al., 2008). It is feasible that antagonizing GRs during 4 days of EtOH exposure interferes with these alterations in receptor activity, composition and/or function, and thereby result in decreased neural hyperexcitibility and attenuation of the behavioral signs of withdrawal. In support of this proposition, Wulsin and colleagues (2010) found that mifepristone decreased neural excitation in the CA2 and CA3 regions of the hippocampus following exposure to the forced swim test.
In sum, the present findings demonstrate a significant role for GR activity in promoting EtOH dependence and subsequent EtOH withdrawal that are independent of effects on the motivation to consume EtOH. The present findings and others demonstrating that administration of a single dose of mifepristone prior to withdrawal reduced cognitive impairment in EtOH-dependent mice (Jacquot et al., 2008); that mifepristone reduced voluntary intake of EtOH in rats under a limited access schedule (Koenig and Olive, 2004); and that mifepristone reduced escalation of EtOH intake following intermittent EtOH vapor exposure (Vendruscolo et al., 2012) suggest that GRs may be targets to be exploited pharmacologically in the treatment of alcohol dependence.
ACKNOWLEDGMENT
The authors acknowledge the support of AA013388.
REFERENCES
- Addolorato G, Abenavoli L, Leggio L, Gasbarrini G. How many cravings? Pharmacological aspects of craving treatment in alcohol addiction: a review. Neuropsychobiology. 2005;51:59–66. doi: 10.1159/000084161. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Iranmanesh A, Veldhuis J, Fisher L. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alele PE, Devaud LL. Sex differences in steroid modulation of ethanol withdrawal in male and female rats. J Pharmacol Exp Ther. 2007;320:427–436. doi: 10.1124/jpet.106.107896. [DOI] [PubMed] [Google Scholar]
- von Bardeleben U, Heuser I, Holsboer F. Human CRH stimulation response during acute withdrawal and after medium-term abstention from alcohol abuse. Psychoneuroendocrinology. 1989;14:441–449. doi: 10.1016/0306-4530(89)90043-7. [DOI] [PubMed] [Google Scholar]
- Besemer F, Pereira AM, Smit JW. Alcohol-induced Cushing syndrome. Hypercortisolism caused by alcohol abuse. Neth J Med. 2011;69:318–323. [PubMed] [Google Scholar]
- Brooks SP, Croft AP, Norman G, Shaw SG, Little HJ. Nimodipine prior to alcohol withdrawal prevents memory deficits during the abstinence phase. Neuroscience. 2008;157:376–384. doi: 10.1016/j.neuroscience.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Chameau P, Qin Y, Spijker S, Smit AB, Joels M. Glucocorticoids specifically enhance L-type calcium current amplitude and affect calcium channel subunit expression in the mouse hippocampus. J Neurophysiol. 2007;97:5–14. doi: 10.1152/jn.00821.2006. [DOI] [PubMed] [Google Scholar]
- Clarke TK, Treutlein J, Zimmermann US, Kiefer F, Skowronek MH, Rietschel M, Mann K, Schumann G. HPA-axis activity in alcoholism: examples for a gene-environment interaction. Addict Biol. 2008;13:1–14. doi: 10.1111/j.1369-1600.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Lando D, Moguilewsky M. Ornithine decarboxylase induction by glucocorticoids in brain and liver of adrenalectomized rats. J Neurochem. 1982;38:1296–1304. doi: 10.1111/j.1471-4159.1982.tb07904.x. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effects of acamprosate on excitatory amino acids during multiple ethanol withdrawal periods. Alcohol Clin Exp Res. 2003;27:465–470. doi: 10.1097/01.ALC.0000056617.68874.18. [DOI] [PubMed] [Google Scholar]
- Ellis FW. Effect of ethanol on plasma corticosterone levels. J Pharmacol Exp Ther. 1966;153:121–127. [PubMed] [Google Scholar]
- Esel E, Sofuoglu S, Aslan SS, Kula M, Yabanoglu I, Turan MT. Plasma levels of beta-endorphin, adrenocorticotropic hormone and cortisol during early and late alcohol withdrawal. Alcohol Alcohol. 2001;36:572–576. doi: 10.1093/alcalc/36.6.572. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz KM, Bergquist K, Anderson G, Kreek MJ, Sinha R. Sex-specific dissociations in autonomic and HPA responses to stress and cues in alcohol-dependent patients with cocaine abuse. Alcohol Alcohol. 2009;44:575–585. doi: 10.1093/alcalc/agp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard RC, Riondel A, Muller AF, Herrmann W, Baulieu EE. RU 486: a steroid with antiglucocorticosteroid activity that only disinhibits the human pituitary-adrenal system at a specific time of day. Proc Natl Acad Sci USA. 1984;81:3879–3882. doi: 10.1073/pnas.81.12.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O, Personen U, Huupponen R, Koulu M, Lähteenmäki P. Hepatic metabolism and distribution of mifepristone and its metabolites in rats. Hum Reprod. 1994;9:40–46. doi: 10.1093/humrep/9.suppl_1.40. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Ferdinand RF, Ormel J, Verhulst FC. Hypothalamicpituitary-adrenal axis activity and early onset of cannabis use. Addiction. 2006;101:1581–1588. doi: 10.1111/j.1360-0443.2006.01570.x. [DOI] [PubMed] [Google Scholar]
- Jacquot C, Croft AP, Prendergast MA, Mulholland P, Shaw SG, Little HJ. Effects of the glucocorticoid antagonist, mifepristone, on the consequences of withdrawal from long term alcohol consumption. Alcohol Clin Exp Res. 2008;32:2107–2116. doi: 10.1111/j.1530-0277.2008.00799.x. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Koenig HN, Olive MF. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology. 2004;29:999–1003. doi: 10.1016/j.psyneuen.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Self RL, Harris BR, Little HJ, Littleton JM, Prendergast MA. Corticosterone increases damage and cytosolic calcium accumulation associated with ethanol withdrawal in rat hippocampal slice cultures. Alcohol Clin Exp Res. 2005;29:871–881. doi: 10.1097/01.alc.0000163509.27577.da. [DOI] [PubMed] [Google Scholar]
- Nsi-Emvo E, Chaton B, Foltzer-Jourdainne C, Gosse F, Raul F. Premature expression of sucrase-isomaltase triggered by corticoid-dependent changes in polyamine metabolism. Am J Physiol. 1996;270:G54–G59. doi: 10.1152/ajpgi.1996.270.1.G54. [DOI] [PubMed] [Google Scholar]
- O’Callaghan MJ, Croft AP, Jacquot C, Little HJ. The hypothalamopituitary-adrenal axis and alcohol preference. Brain Res Bull. 2005;68:171–178. doi: 10.1016/j.brainresbull.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Rivier C. Effect of three different modes of alcohol administration on the activity of the rat hypothalamic-pituitary-adrenal axis. Alcohol Clin Exp Res. 1997;21:467–476. doi: 10.1111/j.1530-0277.1997.tb03792.x. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Perkins HW, Linkenbach J, Dejong W. Estimated blood alcohol levels reached by “binge” and “nonbinge” drinkers: a survey of young adults in Montana. Psychol Addict Behav. 2001;15:317–320. [PubMed] [Google Scholar]
- Prendergast MA, Mulholland PJ. Glucocorticoid and polyamine interactions in the plasticity of glutamatergic synapses that contribute to ethanol-associated dependence and neuronal injury. Addict Biol. 2012;17:209–223. doi: 10.1111/j.1369-1600.2011.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–1849. [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher-Flowers D, Adinoff B, Ravitz B, Bone GH, Martin PR, Nutt D, Linnoila M. Circadian rhythms of cortisol during alcohol withdrawal. Adv Alcohol Subst Abuse. 1988;7:37–41. doi: 10.1300/J251v07n03_06. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamicpituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- Roberts AJ, Crabbe JC, Keith LD. Genetic differences in hypothalamic-pituitary-adrenal axis responsiveness to acute ethanol and acute ethanol withdrawal. Brain Res. 1992;579:296–302. doi: 10.1016/0006-8993(92)90064-g. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Chagnon YC, Chagnon M, Perusse L, Bouchard C, Bjorntorp P. A polymorphism of the 5’-flanking region of the glucocorticoid receptor gene locus is associated with basal cortisol secretion in men. Metabolism. 2000;49:1197–1199. doi: 10.1053/meta.2000.7712. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology. 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Self RL, Smith KJ, Butler TR, Pauly JR, Prendergast MA. Intra-cornu ammonis 1 administration of the human immunodeficiency virus-1 protein trans-activator of transcription exacerbates the ethanol withdrawal syndrome in rodents and activates N-methyl-D-aspartate glutamate receptors to produce persisting spatial learning deficits. Neuroscience. 2009;163:868–876. doi: 10.1016/j.neuroscience.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LJ, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland NG, Orchinik M, Tanapat P. Chronic corticosterone treatment induces parallel changes in N-methyl-D-aspartate receptor subunit messenger RNA levels and antagonist binding sites in the hippocampus. Neuroscience. 1997;78:653–662. doi: 10.1016/s0306-4522(96)00619-7. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Wulsin AC, Herman JP, Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35:1100–1112. doi: 10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang S, Rice KC, Munro CA, Wand GS. Restraint stress and ethanol consumption in two mouse strains. Alcohol Clin Exp Res. 2008;32:840–852. doi: 10.1111/j.1530-0277.2008.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]