Abstract
The low bioavailability of most flavonoids limits their application as anti-carcinogenic agents in humans. A novel approach of treatment with a mixture of bioactive compounds that share molecular anti-carcinogenic targets may enhance the effect on these targets at low concentrations of individual compound, thereby overcoming the limitations of reduced bioavailability. We therefore investigated whether a combination of three natural products arctigenin (Arc), a novel anti-inflammatory lignan from the seeds of Arctium lappa, green tea polyphenol (−)-epigallocatechin gallate (EGCG) and curcumin (Cur) increases the chemopreventive potency of individual compounds. LNCaP prostate cancer and MCF-7 breast cancer cells were treated with 2–4 mg/L (about 5–10μM) Cur, 1μM Arc and 40μM EGCG alone or in combination for 48h. In both cell lines treatment with the mixture of Cur, Arc and EGCG synergistically increased the antiproliferative effect. In LNCaP cells both Arc and EGCG increased the pro-apoptotic effect of Cur. Whereas in MCF-7 cells Arc increased the cell apoptosis of Cur while EGCG enhanced cell cycle arrest of Cur at G0/G1 phase. The strongest effects on cell cycle arrest and apoptosis were achieved by combining all three compounds in both cell lines. The combination treatment significantly increased the ratio of Bax to Bcl-2 proteins, decreased the activation of NFκB, PI3K/Akt and Stat3 pathways and cell migration compared to individual treatment. These results warrant in vivo studies to confirm the efficacy of this novel regimen by combining Arc and EGCG with Cur to enhance chemoprevention in both prostate and breast cancer.
Keywords: Curcumin, green tea polyphenol, arctigenin, prostate cancer, breast cancer, combination
1. Introduction
Prostate and breast cancers are the most frequently diagnosed malignancy in men and women in the United States, and both are the second leading cause of cancer death by gender 1. Both cancers are typically diagnosed at a later age and subject to dietary factors and obesity 2–4, which makes them good candidates for chemoprevention to delay or suppress their development. Increasing evidence from preclinical studies is demonstrating that bioactive products may be a non-toxic alternative in prevention and treatment of chronic diseases including cancer 5, 6. However, translation of these results to clinical studies is limited, mainly due to the low bioavailability of these compounds and their extensive biotransformation in vivo into less active metabolites 5, 6. To overcome these limitations mixtures of bioactive compounds, as traditionally used in Chinese and Indian/Ayurvedic medicine, may be employed. Many bioactive compounds share the same molecular anticarcinogenic targets. Therefore, the sum activity of combination treatment with several bioactive compounds may enhance the effect on their molecular targets at low concentrations of individual compound.
Curcumin (structure shown in Figure 1A) is a hydrophobic polyphenol derived from the root of the plant Curcuma longa (commonly known as turmeric). The commercial curcumin products usually contain curcuminoids including curcumin (77%), demethoxycurcumin (17%), and bisdemethoxycurcumin (3%). The chemopreventive activity of curcumin has been well demonstrated in vitro and in animal models against most cancers such as colorectal, pancreatic, liver, lung, prostate and breast cancer 7. Curcumin targets multiple molecules and signaling pathways involved in carcinogenesis, including the nuclear factor kappa B (NFκB), signal transducer and activator of transcription (Stat) 3 and phosphatidylinositide 3-kinases (PI3K)/Akt pathways, epidermal growth factor (EGF) and its receptor (EGFR), and angiogenesis 7. A number of clinical trials with curcumin have been completed, and the majority supports a cancer preventive potential of curcumin 7, 8. However, the efficacy of curcumin is limited by its poor bioavailability. Biologically effective levels of curcumin may be only achievable in the gastrointestinal tract while not in other parts of the body by oral consumption 9. Less than 2 μM of curcumin was detected in human serum after oral uptake of 8g of curcumin 8, while no curcumin was detectable in liver tumor tissues from patients with hepatic metastases from colorectal cancer after consumption of 3.6g of curcumin for one week prior to surgery 10.
Fig. 1.
Chemical structures of curcumin, green tea polyphenols and arctigenin. A, curcumin; B, green tea polyphenols; C, arctigenin. EC, (−)-epicatechin; EGC, (−)-epigallocatechin; ECG, (−)-epicatechin-3-gallate; EGCG, (−)-epigallocatechin-3-gallate.
A combination treatment of green tea (GT) with curcumin has demonstrated a synergistic effect in inhibition of oral epithelial and breast cancer cell growth 11, 12. GT is produced from the leaves of the plant Camellia sinensis. The major bioactive components of GT are GT polyphenols (GTPs, structures in Figure 1B) including (−)-epigallocatechin, (−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin, and (−)-epicatechin-3-gallate, with EGCG being the major component 13. GT targets multiple signaling pathways in anti-carcinogenesis, and the chemopreventive effect of GT has been demonstrated in several cancers including prostate and breast cancer 5, 6, 14. A previous study showed that the combination of 20–25μM EGCG and 2–3μM curcumin synergistically increased the anti-proliferative effect in cultured breast cancer MDA-MB-231 cells, and the combined effect was further confirmed in vivo in the inhibition of MDA-MB-231 xenograft tumor growth in athymic nude mice 12.
In a screening of 15 commonly studied anti-carcinogenic natural compounds, we found that arctigenin synergistically enhanced the anti-proliferative effect of both curcumin and EGCG in prostate cancer LNCaP cells. Therefore it may be an ideal candidate to further enhance the efficacy of curcumin plus GT combination. Arctigenin (structure in Figure 1C) is a lignan derived mainly from the seeds of the plant Arctium lappa which is widely used in traditional Chinese medicine to treat inflammation related diseases such as cough, cold and swelling of throat 15. In the plant arctigenin is present as glucoside (arctiin) and arctigenin is released during the digestive process 16. Both have been detected in rat plasma after oral administration of arctiin 16. Arctigenin has exhibited multiple activities including the antioxidant, antiproliferative and anti-inflammatory activities 15, 17. The anti-carcinogenic property of arctigenin has been demonstrated in several cancers including pancreatic and colorectal cancer 17, 18. The present study investigated the combined effect of the three chemicals, arctigenin, EGCG and curcumin, in prostate cancer LNCaP and breast cancer MCF-7 cells. This study is expected to provide a novel non-toxic formulation to enhance the chemoprevention in prostate and breast cancer particularly for those high-risk populations.
2. Materials and Methods
2.1 Cell line and cell culture
The androgen-dependent LNCaP human prostate cancer cells, estrogen receptor positive MCF-7 breast cancer cells, and mouse embryonic fibroblasts NIH-3T3 cells were purchased from American Type Culture Collection (ATCC, Chicago, IL). LNCaP cells were cultured in RPMI 1640 medium, MCF-7 and NIH-3T3 cells in Dulbecco modified Eagle medium (DMEM), supplemented with 10% (v:v) of fetal bovine serum (FBS), 100,000 IU/L of penicillin and 100 mg/L of streptomycin at 37 °C in a 5% CO2 incubator.
2.2 Cell proliferation assay
Both LNCaP and MCF-7 cells were seeded into 96-well plates at a density of 8×103 per well. Curcumin extract in capsules was purchased from the LifeExtension inc. (Ft. Lauderdale, FL). The content of curcuminoids in the capsules is 95%, which was confirmed by high-performance liquid chromatography (HPLC) in our laboratory. An inhibition curve was achieved for individual compound including curcumin, EGCG and arctigenin by incubation of both cell lines with multiple doses of each compound. A dose that led to 10–30% cell growth inhibition by each compound was selected for the combination study. Cells were treated with the following: vehicle control (DMSO), 40μM EGCG (Sigma-Aldrich, St Louis, MO), 1μM arctigenin (Sigma-Aldrich), low dose (LD) curcumin (2mg/L, about 5μM), 2mg/L curcumin + 40μM EGCG, 2mg/L curcumin + 1μM arctigenin, 2mg/L curcumin + 40μM EGCG + 1μM arctigenin for 48h. In addition, a higher dose (HD) of curcumin (4mg/L, about 10μM) was added to determine a dose-response relationship. The cells were treated with 4mg/L curcumin, 4mg/L curcumin + 40μM EGCG, 4mg/L curcumin + 1μM arctigenin, or 4mg/L curcumin + 40μM EGCG + 1μM arctigenin for 48h. Cell proliferation was measured with adenosine triphosphate (ATP) assay using the CellTiter-Glo® Luminescent cell viability assay kit (Promega Corporation, Madison, WI). To minimize the effect of hydrogen peroxide (H2O2) that may be formed by autoxidation and/or dimerization of EGCG and other phytochemicals in cell culture medium 19, 50,000 units/L of catalase was added to the medium prior to EGCG, curcumin and arctigenin for all the experiments. The experiment was repeated twice with five wells for each treatment in each experiment.
In addition, a combination index (CI) was calculated for the mixture of all three chemicals using the CompuSyn software (ComboSyn, Inc., Paramus, NJ) which is based on the widely-accepted Chou-Talalay equation and mass-action law 20. The value of CI less than 1 indicates a synergistic effect of a combination, equal to 1 additive, and greater than 1 antagonistic 20.
2.3 Cell cycle and apoptosis analysis using Cellometer imaging cytometry
LNCaP and MCF-7 cells were cultured in T25 flasks. When 50–60% confluent both cell lines were treated with vehicle control, 40μM EGCG, 1μM arctigenin, 2mg/L curcumin, 2mg/L curcumin + 40μM EGCG, 2mg/L curcumin + 1μM arctigenin, or 2mg/L curcumin + 40μM EGCG + 1μM arctigenin for 48h. Cells were trypsinized and monolayers attaching to the bottom were collected. The procedures for cell cycle and apoptosis analysis using a small cytometry system Cellometer Vision (Nexcelom Bioscience LLC, Lawrence, MA) were described previously 21, 22. Results obtained from this system have been shown to be comparable to that from conventional flow cytometry 21, 22. The procedures were modified slightly in the present study. Briefly, for cell cycle assay cells were centrifuged at 2,000 rpm for 5min and the pellet containing 2×106 cells was resuspended and fixed in 500μl of 100% ice cold methanol for 15 min on ice. The cells were then centrifuged at 2,000 rpm for 8 min, and the pellet was stained in 150μl of propidium iodide (PI) solution (Nexcelom Bioscience LLC) at 37°C for 40min. The staining solution was removed after centrifuge of the cells at 2,000 rpm for 8min and the pellet was resuspended in 150ul phosphate buffered saline (PBS) for imaging cytometry analysis. For apoptosis assay, the cells were centrifuged at 2,000 rpm for 5min and the pellet was resuspended in Annexin V binding buffer and double-stained with Annexin V-FITC (Nexcelom Bioscience LLC) and PI (Nexcelom Bioscience LLC) for 15min at room temperature for Cellometer analysis. A positive control was generated by heating LNCaP or MCF-7 cells in a 45°C water bath for 10min. Non-treated cells were used as negative control. Both controls were processed with the samples. The fluorescence data generated by the Cellometer software were converted into FCS files and analyzed by De Novo FCS Express 4 software (Los Angeles, CA). The experiment was performed in triplicate and repeated twice. The results were also confirmed by flow cytometry at the UCLA Janis V. Giorgi cytometry core facility.
2.4 Western blot analysis of protein biomarkers
When 50–60% confluent in 60 mm Petri dishes, both LNCaP and MCF-7 cells were treated with vehicle control, 40μM EGCG, 1μM arctigenin, 2mg/L curcumin, 2mg/L curcumin + 40μM EGCG, 2mg/L curcumin + 1μM arctigenin, or 2mg/L curcumin + 40μM EGCG + 1μM arctigenin for 48h. The procedure for cell harvest and protein extraction was described before 23. For the Western blot analysis, 50 μg of protein was loaded and separated on a 4–12% Bis-Tris gel (Invitrogen, Carlsbad, CA). Proteins were electrotransferred to nitrocellulose membranes and blocked in Tris-buffered saline with 0.1% Tween 20 and 5% nonfat milk for 1 hour at room temperature. Membranes were incubated with primary anti-human antibodies for the detection of Bax (sc-493), Bcl-2 (sc-509, Santa Cruz Technology, CA), NFκB (3034), p-NFκB (3031), p-NFκB inhibitor protein (IκB)-α (2859), Akt (4685), p-Akt (Ser473) (4058), Stat3 (9132), and p-Stat3 (9131, Cell Signaling Technology, Danvers, MA). GAPDH protein was used as loading control. Protein was visualized and analyzed using a ChemiDoc XRS chemiluminescence detection and imaging system (Bio-Rad Laboratories, Irvine, CA). The experiment was done in duplicate and repeated twice.
2.5 Migration assay
The capacity of these compounds and their combinations to inhibit cell migration was tested in MCF-7 cells using both transwell chamber and scratch assays. The transwell chamber is 24-well plate based with an insert of 8μm pore size polyethylene terephthalate membrane (Corning Life Sciences, Tewksbury, MA). MCF-7 cells were cultured in T25 flasks to 50–60% confluency and treated with vehicle control, 40μM EGCG, 1μM arctigenin, 2mg/L curcumin, 2mg/L curcumin + 40μM EGCG, 2mg/L curcumin + 1μM arctigenin, or 2mg/L curcumin + 40μM EGCG + 1μM arctigenin for 48h. The cells were starved in serum-free medium overnight. After trypsinization 1×105 cells were collected, suspended in 200μl serum-free medium and added on the upper well. NIH-3T3 mouse embryonic fibroblasts were seeded on the bottom well in 500μl of complete DMEM medium one day prior to the adding of MCF-7 cells to produce conditioned media as chemoattractant 24. After 40h incubation, cells were fixed with 5% glutaraldehyde and stained with 0.5% toluidine blue as desribed previously 25. Cells on the upper membrane were wiped off with a cotton swab. Migrated cells on the lower membrane were counted under a microscope at x200 magnification. Three fields for each wells were counted and the experiment was done in triplicate.
2.6 Scratch wound assay
MCF-7 cells were cultured in 24-well plate to 90–100% confluency. Cells were starved in serum-free medium overnight. A line was drawn with a marker pen on the bottom of the plate across the middle of wells. Scratches were made perpendicular to the line using a 200μl pipette tip. Three separate wounds were made for each well. The cells were rinsed with PBS and treated with vehicle control, 40μM EGCG, 1μM arctigenin, 2mg/L curcumin, 2mg/L curcumin + 40μM EGCG, 2mg/L curcumin + 1μM arctigenin, or 2mg/L curcumin + 40μM EGCG + 1μM arctigenin for 48h. Pictures were taken both above and below each of the intersections using a microscope camera at x100 magnification at 0h and 48h (total six pictures for each well at each time point). The gaps were measured and percent wound closure was calculated. The experiment was done in duplicate.
2.7 Statistical analysis
SPSS (Version 20.0, Chicago, IL) was used for statistical analyses. All the data collected from each experiment were used to calculate the mean values and standard deviations (SD). Comparison of means was performed by one-way analysis of variance (ANOVA) with Tukey’s posttest. Differences were considered significant if P<0.05.
3. Results
3.1 Enhanced antiproliferative effect by combination treatment
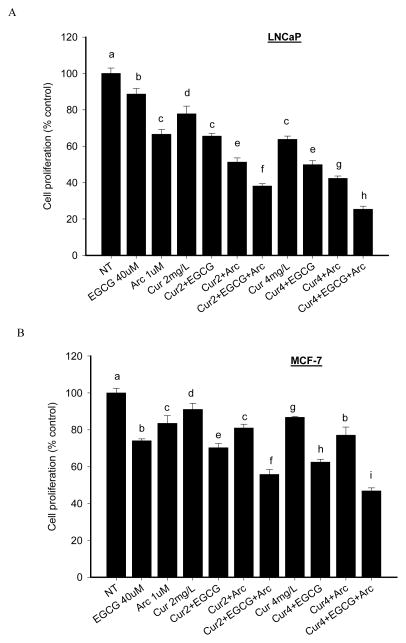
The IC50 values of curcumin, EGCG and arctigenin in LNCaP cells were 12.5μM, 130μM and 13μM, respectively, and 30μM, 100μM and 16μM in MCF-7 cells. The combination treatment increased the antiproliferative effect in both LNCaP cells and MCF-7 cells compared to individual treatment (Figure 2). LNCaP cell growth was inhibited by 11% (EGCG), 29% (arctigenin), 22% (LD curcumin), 34% (LD curcumin+EGCG), 49% (LD curcumin+arctigenin), and 62% (LD curcumin+EGCG+ arctigenin) compared to control. The combined effect was further enhanced by increasing curcumin concentration (HD), and LNCaP cell growth was inhibited by 75% by the combination of all three chemicals (Figure 2). Overall, arctigenin and EGCG increased the antiproliferative effect of curcumin by 40% in both LNCaP and MCF-7 cells. A combination index (CI) of 0.8 was achieved by the combination of LD curcumin + EGCG + arctigenin, and 0.6 by HD curcumin + EGCG + arctigenin combination. Similar pattern on cell proliferation was observed in MCF-7 cells (Figure 2). The CI values are 0.7 and 0.6 by LD and HD curcumin, respectively, in combination with EGCG and arctigenin. The three chemicals in combination did not increase cytotoxicity in normal prostate epithelial PrEC cells or breast MCF-12A cells compared to individual treatment (data not shown).
Fig. 2.
Enhanced antiproliferative effect by combination of arctigenin and EGCG with curcumin in both LNCaP and MCF-7 cells. LNCaP (A) and MCF-7 cells (B) were treated with the indicated concentrations of curcumin, arctigenin and EGCG alone or in combination for 48h. Cell proliferation was measured by ATP assay. Data are presented as mean ± SD. NT: non-treatment, DMSO control; Cur: curcumin; Arc: arctigenin. Columns with different letters represent significant difference between treatments (P<0.05).
3.2 Effect on apoptosis and cell cycle distribution
The individual treatment with curcumin, EGCG or arctigenin significantly increased cell apoptosis in LNCaP cells compared to control, and the effect was further enhanced by the combination of either EGCG or arctigenin with curcumin with the strongest effect by combining all three compounds (Table 1). Similarly, all the three compounds induced cell apoptosis in MCF-7 cells when used alone. However, only arctigenin enhanced the pro-apoptotic effect of curcumin (Table 2). EGCG significantly increased the effect of curcumin on cell cycle arrest at G0/G1 phase in MCF-7 cells, and the effect was further enhanced by the addition of arctigenin (Table 2).
Table 1.
Cell cycle distribution and apoptosis in LNCaP cells
| Cell cycle distribution (%)
|
|||||
|---|---|---|---|---|---|
| Treatment | Pre-G0 | G0/G1 | S | G2/M | Apoptosis |
| DMSO | 0.2 ± 0.1 | 73.1 ± 1.6 | 11.3 ± 0.2 | 14.8 ± 1.7 | 3.1 ± 0.4a |
| EGCG 40μM | 0.1 ± 0.1 | 75.0 ± 1.0 | 11.3 ± 0.4 | 13.4 ± 0.5 | 6.9 ± 1.3b |
| Arctigenin 1μM | 0.2 ± 0.3 | 75.7 ± 1.6 | 11.1 ± 0.8 | 12.8 ± 2.0 | 7.1 ± 1.2b |
| Curcumin 2mg/L | 0.2 ± 0.3 | 73.7 ± 1.4 | 11.2 ± 1.3 | 14.4 ± 0.6 | 6.2 ± 1.3b |
| Cur+E | 0.2 ± 0.2 | 72.5 ± 0.3 | 12.7 ± 0.3 | 14.2 ± 0.3 | 9.0 ± 0.3c |
| Cur+A | 0.2 ± 0.2 | 74.5 ± 1.0 | 11.0 ± 2.5 | 13.5 ± 1.5 | 8.7 ± 0.4c |
| Cur+E+A | 0.1 ± 0.1 | 73.9 ± 1.5 | 12.9 ± 0.9 | 13.1 ± 1.6 | 14.3 ± 0.6e |
LNCaP cells were cultured in T25 flasks until 50–60% confluent. Cells were treated with indicated treatments for 48h. Cells were trypsinized and monolayers attaching to the bottom were collected for cell cycle and apoptosis analysis using a small cytometry system Cellometer Vision. Cells were stained with propidium iodide (PI) for cell cycle assay, double-stained with Annexin V-FITC and PI for apoptosis assay. Data are presented in mean ± SD. Values with different superscripts in each of the columns are significantly different (P<0.05).
Table 2.
Cell cycle distribution and apoptosis in MCF-7 cells
| Cell cycle distribution (%)
|
|||||
|---|---|---|---|---|---|
| Treatment | Pre-G0 | G0/G1 | S | G2/M | Apoptosis |
| DMSO | 0.8 ± 0.2 | 65.8 ± 1.7a | 12.1 ± 1.1a | 19.9 ± 0.7a | 5.1 ± 0.8a |
| EGCG 40μM | 0.5 ± 0.1 | 69.7 ± 2.4ab | 10.0 ± 1.1a | 19.1 ± 2.0a | 7.7 ± 0.3b |
| Arctigenin 1μM | 1.9 ± 0.3 | 66.9 ± 2.4a | 10.5 ± 0.8a | 20.2 ± 1.9a | 8.3 ± 0.6b |
| Curcumin 2mg/L | 0.7 ± 0.0 | 68.3 ± 2.1ab | 12.3 ± 1.0a | 18.4 ± 1.4a | 8.4 ± 0.7b |
| Cur+E | 0.6 ± 0.2 | 71.4 ± 1.3b | 10.5 ± 1.0a | 16.4 ± 0.7b | 8.0 ± 1.5b |
| Cur+A | 1.6 ± 0.5 | 65.6 ± 5.1a | 10.8 ± 0.7a | 21.6 ± 4.3a | 14.2 ± 1.3c |
| Cur+E+A | 0.5 ± 0.2 | 78.8 ± 0.8c | 8.3 ± 0.4b | 12.2 ± 0.4c | 15.2 ± 1.5c |
MCF-7 cells were cultured in T25 flasks until 50–60% confluent. Cells were treated with indicated treatments for 48h. Cells were trypsinized and monolayers attaching to the bottom were collected for cell cycle and apoptosis analysis using a small cytometry system Cellometer Vision. Cells were stained with propidium iodide (PI) for cell cycle assay, double-stained with Annexin V-FITC and PI for apoptosis assay. Data are presented in mean ± SD. Values with different superscripts in each of the columns are significantly different (P<0.05).
3.3 Modulation on protein markers of apoptosis and cell proliferation
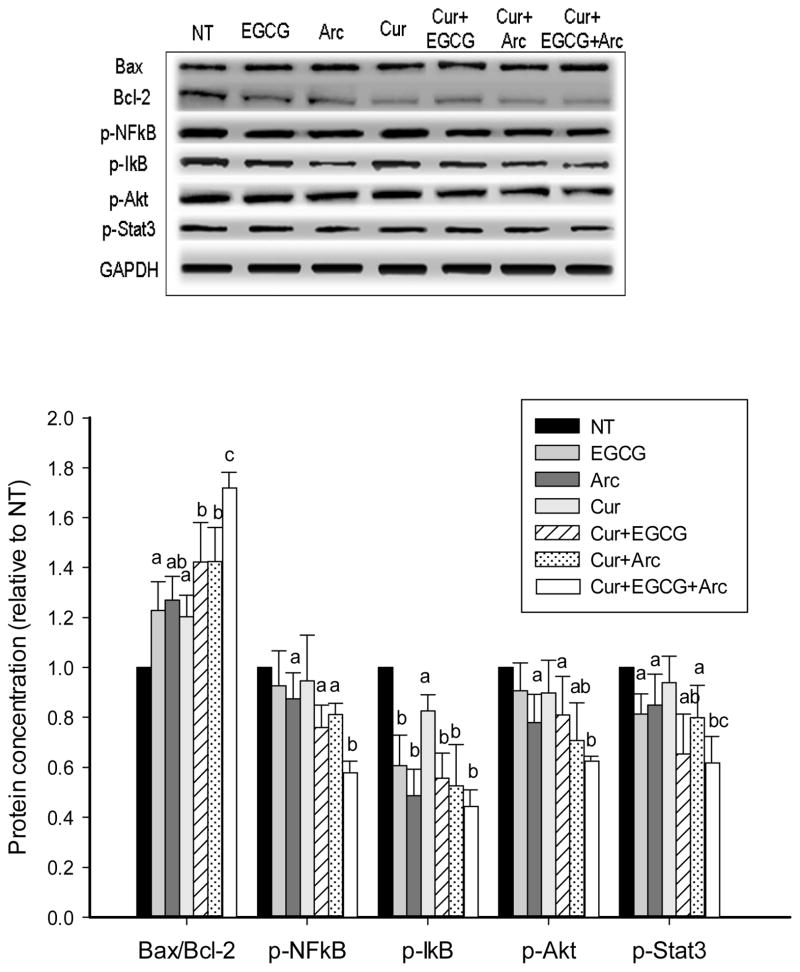
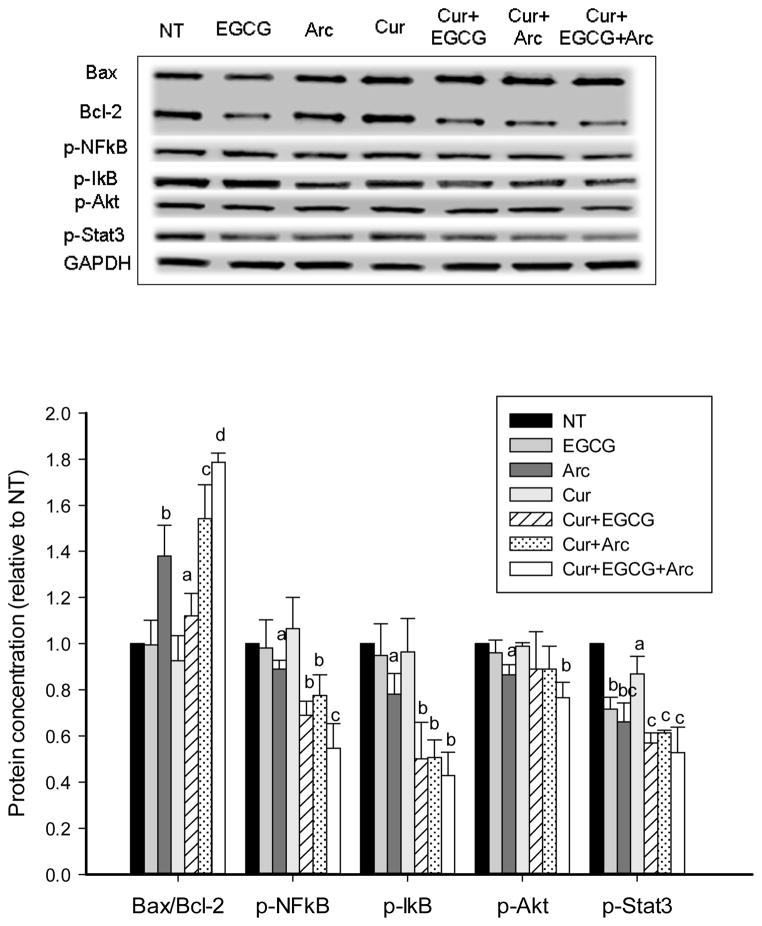
We examined the effect of these compounds particularly their combinations on several important signaling pathways involved in cell apoptosis and proliferation. An increased ratio of Bax to Bcl-2 proteins was observed by individual treatment of curcumin, EGCG and arctigenin in LNCaP cells (Figure 3), and by arctigenin in MCF-7 cells (Figure 4). The ratio was significantly increased by combination treatment and the highest ratio was achieved with the mixture of all three compounds in both LNCaP and MCF-7 cells (Figures 3 & 4). The phosphorylation of NFκB was increasingly inhibited by the combination treatment along with decreased p-IκB levels in both cell lines (Figures 3 & 4). In addition, the three chemicals in combination significantly decreased the phosphorylation of Akt and Stat3 compared to individual treatment (Figures 3 & 4).
Fig. 3.
Increased modulation of protein expression and phosphorylation in LNCaP cells by combination treatment. LNCaP cells were treated with the indicated concentrations of curcumin, arctigenin and EGCG alone or in combination for 48h. The protein expression and phosphorylation were evaluated by Western blot. Data are presented as mean ± SD. NT: non-treatment, DMSO control; Cur: curcumin; Arc: arctigenin. a,b,c - compared to control, P<0.05; columns with different letters represent significant difference between treatments (P<0.05).
Fig. 4.
Increased modulation of protein expression and phosphorylation in MCF-7 cells by combination treatment. MCF-7 cells were treated with the indicated concentrations of curcumin, arctigenin and EGCG alone or in combination for 48h. The protein expression and phosphorylation were evaluated by Western blot. Data are presented as mean ± SD. NT: non-treatment, DMSO control; Cur: curcumin; Arc: arctigenin. a,b,c - compared to control, P<0.05; columns with different letters represent significant difference between treatments (P<0.05).
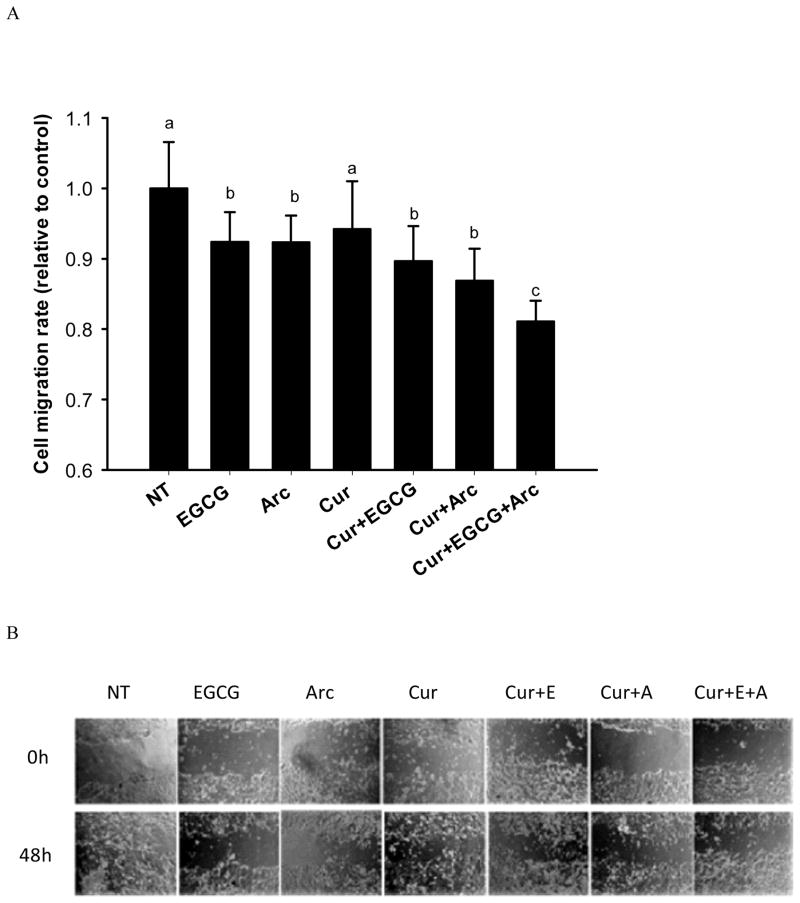
3.4 Inhibition of cell migration
In the transwell chamber assay, both EGCG and arctigenin alone or in combination with curcumin significantly decreased the number of migrated MCF-7 cells compared to control (Figure 5A). The effect was further enhanced by combining all three compounds, leading to a 20% inhibition of cell migration compared to control (Figure 5A). The same pattern was observed in the scratch assay. All the treatments significantly inhibited the closure of wounds compared to control, and the combination of the three chemicals demonstrated a trend to increase the inhibitory effect (Figure 5B). At 48h, the wound was closed by 71% (control), 40% (40μM EGCG), 42% (1μM arctigenin), 40% (2mg/L curcumin), 41% (2mg/L curcumin + 40μM EGCG), 36% (2mg/L curcumin + 1μM arctigenin), and 28% (2mg/L curcumin + 40μM EGCG + 1μM arctigenin).
Fig. 5.
Enhanced inhibition of cell migration in MCF-7 cells by combination treatment. MCF-7 cells were treated with the indicated concentrations of curcumin, arctigenin and EGCG alone or in combination for 48h. The cells were starved in serum-free medium overnight. Then the cells were seeded on the upper membrane of transwell chamber and incubated for 40h. NIH-3T3 conditioned medium was used as chemoattractant. Cells on the lower membrane of chambers were counted. For the scratch assay, wounds were made when cells were 90–100% confluent and after an overnight starvation. The cells were treated with indicated concentrations of curcumin, arctigenin and EGCG alone or in combination for 48h. The closure of the wounds were imaged and measured at 0h and 48h. Data are presented as mean ± SD. NT: non-treatment, DMSO control; Cur: curcumin; Arc: arctigenin. Columns with different letters represent significant difference between treatments (P<0.05).
4. Discussion
The major finding of this study is that although all three phytochemicals have similar molecular targets and mode of action, a mixture of curcumin, EGCG and arctigenin synergistically enhanced the anti-proliferative effect in both prostate and breast cancer cells. In the combination treatment low concentrations were effective, which when used alone induced minor changes in proliferation. The enhanced anti-proliferative effect by combination of Arc with curcumin and EGCG was associated with increased apoptosis and cell cycle arrest, through an enhanced modulation of multiple important signaling pathways involved in carcinogenesis including the PI3K/Akt and NFκB pathways. Natural products are a major source for development of non-toxic chemopreventive agents. However, the low bioavailability of these compounds hinders their application in humans, and effective doses as demonstrated in vitro can barely be achieved in the human body by oral consumption 26. A combination of these compounds may enhance their anti-cancer efficacy in a less-toxic manner, and several promising combinations have been demonstrated in vitro and in animal models 27–29. The combination of GT with curcumin has been shown to synergistically enhance the inhibition of breast cancer MDA-MB-231 cell growth both in vitro and in vivo 12. A similar effect was also observed in non-small cell lung cancer cells under GT plus curcumin treatment 30. The present study provides a novel regimen to further enhance the combined effect of GT and curcumin by the combination with arctigenin. In light of relatively high IC50s of curcumin as we observed in other cell lines (androgen-independent prostate cancer PC-3 cells and HER-2 overexpressing breast cancer SKBR3 and BT474 cells, with IC50 values from 40μM to 60 μM), combination treatment with GT and arctigenin may also be beneficial as therapy or adjuvant therapy for various subtypes of prostate and breast cancer.
The enhanced anti-proliferative effect was associated with increased apoptosis and/or cell cycle arrest by the combination treatment. EGCG and arctigenin in combination with curcumin produced the strongest activity in stimulation of apoptosis in both LNCaP and MCF-7 cells, as demonstrated by both cytometry analysis and protein expression of Bax/Bcl-2. Interestingly, EGCG and arctigenin enhanced the effect of curcumin by different means in MCF-7 cells. EGCG increased cell cycle arrest when in combination with curcumin, while arctigenin enhanced the pro-apoptotic effect of curcumin. These interactions among individual compounds build a basis for the success of a combination treatment. Although no changes on cell cycle distribution were observed in LNCaP cells under the combination treatment, there is a possibility of increased cell cycle duration and cell doubling time as a result 31.
The combination treatment increased the modulation of multiple signaling pathways involved in cell proliferation, survival and apoptosis compared to individual compound. The activation and upregulation of PI3K/Akt and NFκB pathways are commonly found in different cancers including prostate and breast cancer, and both pathways have been proved to be effective targets in cancer prevention and treatment 32–34. Extracellular stimuli such as tumor necrosis factor (TNF)-α, can stimulate the phosphorylation and dissociation of NFκB inhibitor protein IκB. Once released, NFκB is activated by phosphorylation and migrates to the nucleus to initiate the transcription of many genes promoting cell growth and suppressing apoptosis 32, 33. The PI3K/Akt pathway is another important pathway involved in carcinogenesis 34. Once Akt is activated by phosphorylation it regulates multiple target genes leading to increased cell proliferation and survival 35. In addition, PI3K/Akt pathway may cross-activate the NFκB pathway 34. Among the three chemicals in this study, arctigenin demonstrated the strongest ability to inhibit the activation of both PI3K/Akt and NFκB pathways in both LNCaP and MCF-7 cells. The inhibitory effect of arctigenin on these two pathways was also reported by other investigators, which was found associated with reduced production of nitric oxide synthase and TNF-α 18, 36, 37. An increased inhibition on both pathways was achieved by the combination of arctigenin with curcumin and EGCG, which may be due to a sum of the activities on these molecular targets by the three chemicals. In addition, an inhibitory effect on the signal transducer and activator of transcription (Stat) pathway was observed with all three chemicals, which supports findings from other investigators 38–40. The activities of the transcription factor Stat, particularly the members Stat3 and Stat5, have been shown to be required to sustain a transformed phenotype, and they represent suitable targets for cancer therapy 41. Stat3 is phosphorylated in response to cytokines and growth factors, and enters the nucleus to mediate the expression of various genes in regulation of cell growth, survival and motility 41. A recent study showed that the inhibition of Stat3 protein expression and activation by EGCG significantly inhibited cell motility, migration and invasion, and increased apoptosis in human pancreatic cancer cells 38. The increased inhibition of Stat3 pathway by the mixture of the three chemicals in our study may in part explain the increased inhibition of cell migration in MCF-7 cells.
5. Conclusions
In summary, the combination of three bioactive phytochemicals, curcumin, EGCG and arctigenin, synergistically enhanced the anti-proliferative effect in both prostate and breast cancer cells. The combined effect was associated with increased modulations on several important signaling pathways involved in carcinogenesis. These results warrant future animal studies and clinical trials to confirm the combined effect of this novel regimen in vivo.
Supplementary Material
Acknowledgments
Grant support: This work was supported by the National Institutes of Health (NIH, NCI, NIMHD, NCATS) Grants: U54 CA143931-01, U54MD007598, UL1TR000124 (J.V. Vadgama); and NIH/National Center for Advancing Translational Sciences (NCATS) UCLA CTSI Grant KL2TR000122 (P. Wang).
Abbreviations
- Arc
arctigenin
- ATP
adenosine triphosphate
- Cur
curcumin
- EC
(−)-epicatechin
- ECG
(−)-epicatechin-3-gallate
- EGC
(−)-epigallocatechin
- EGCG
(−)-epigallocatechin-3-gallate
- EGF
epidermal growth factor
- GT
green tea
- GTPs
green tea polyphenols
- HD
high dose
- HPLC
high-performance liquid chromatography
- IκB
NFκB inhibitor protein
- LD
low dose
- NFκB
nuclear factor kappa B
- PI3K
phosphatidylinositide 3-kinases
- Stat
signal transducer and activator of transcription
References
- 1.American Cancer Society. Cancer facts & figures 2014. American Cancer Society; Atlanta, GA: 2014. [Google Scholar]
- 2.Griffiths K, Prezioso D, Turkes A, Denis LJ. Recent Results Cancer Res. 2007;175:33–63. doi: 10.1007/978-3-540-40901-4_4. [DOI] [PubMed] [Google Scholar]
- 3.Patterson RE, Rock CL, Kerr J, Natarajan L, Marshall SJ, Pakiz B, Cadmus-Bertram LA. Journal of the Academy of Nutrition and Dietetics. 2013;113:288–296. doi: 10.1016/j.jand.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkissyan M, Wu Y, Vadgama JV. Cancer. 2011;117:3814–3823. doi: 10.1002/cncr.25956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henning SM, Wang P, Heber D. Molecular nutrition & food research. 2011;55:905–920. doi: 10.1002/mnfr.201000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang CS, Wang X. Nutrition and cancer. 2010;62:931–937. doi: 10.1080/01635581.2010.509536. [DOI] [PubMed] [Google Scholar]
- 7.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Cancer letters. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Anticancer research. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 9.Shureiqi I, Baron JA. Cancer Prev Res (Phila) 2011;4:296–298. doi: 10.1158/1940-6207.CAPR-11-0060. [DOI] [PubMed] [Google Scholar]
- 10.Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ, Berry DP. British journal of cancer. 2004;90:1011–1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khafif A, Schantz SP, Chou TC, Edelstein D, Sacks PG. Carcinogenesis. 1998;19:419–424. doi: 10.1093/carcin/19.3.419. [DOI] [PubMed] [Google Scholar]
- 12.Somers-Edgar TJ, Scandlyn MJ, Stuart EC, Le Nedelec MJ, Valentine SP, Rosengren RJ. International journal of cancer Journal international du cancer. 2008;122:1966–1971. doi: 10.1002/ijc.23328. [DOI] [PubMed] [Google Scholar]
- 13.Lambert JD, Yang CS. Mutat Res. 2003;523–524:201–208. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- 14.Yang CS, Wang X, Lu G, Picinich SC. Nature reviews Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao F, Wang L, Liu K. Journal of ethnopharmacology. 2009;122:457–462. doi: 10.1016/j.jep.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 16.He F, Dou DQ, Sun Y, Zhu L, Xiao HB, Kang TG. Planta medica. 2012;78:800–806. doi: 10.1055/s-0031-1298433. [DOI] [PubMed] [Google Scholar]
- 17.Hausott B, Greger H, Marian B. Journal of cancer research and clinical oncology. 2003;129:569–576. doi: 10.1007/s00432-003-0461-7. [DOI] [PubMed] [Google Scholar]
- 18.Awale S, Lu J, Kalauni SK, Kurashima Y, Tezuka Y, Kadota S, Esumi H. Cancer research. 2006;66:1751–1757. doi: 10.1158/0008-5472.CAN-05-3143. [DOI] [PubMed] [Google Scholar]
- 19.Yang GY, Liao J, Li C, Chung J, Yurkow EJ, Ho CT, Yang CS. Carcinogenesis. 2000;21:2035–2039. doi: 10.1093/carcin/21.11.2035. [DOI] [PubMed] [Google Scholar]
- 20.Chou TC. Cancer research. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 21.Chan L, Zhong X, Qiu J, Li P, Lin B. Cytometry A. 2011;79:507–517. doi: 10.1002/cyto.a.21071. [DOI] [PubMed] [Google Scholar]
- 22.Chan LL, Lai N, Wang E, Smith T, Yang X, Lin B. Apoptosis: an international journal on programmed cell death. 2011;16:1295–1303. doi: 10.1007/s10495-011-0651-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang P, Aronson WJ, Huang M, Zhang Y, Lee RP, Heber D, Henning SM. Cancer Prev Res (Phila Pa) 2010;3:985–993. doi: 10.1158/1940-6207.CAPR-09-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamath L, Meydani A, Foss F, Kuliopulos A. Cancer research. 2001;61:5933–5940. [PubMed] [Google Scholar]
- 25.Lochter A, Srebrow A, Sympson CJ, Terracio N, Werb Z, Bissell MJ. The Journal of biological chemistry. 1997;272:5007–5015. doi: 10.1074/jbc.272.8.5007. [DOI] [PubMed] [Google Scholar]
- 26.Yang CS, Sang S, Lambert JD, Lee MJ. Mol Nutr Food Res. 2008;52(Suppl 1):S139–151. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 27.Liu RH. The Journal of nutrition. 2004;134:3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Heber D, Henning SM. Nutrition and cancer. 2012;64:580–587. doi: 10.1080/01635581.2012.661514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang P, Vadgama JV, Said JW, Magyar CE, Doan N, Heber D, Henning SM. The Journal of nutritional biochemistry. 2014;25:73–80. doi: 10.1016/j.jnutbio.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou DH, Wang X, Yang M, Shi X, Huang W, Feng Q. International journal of molecular sciences. 2013;14:12023–12036. doi: 10.3390/ijms140612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moiseeva EP, Almeida GM, Jones GD, Manson MM. Molecular cancer therapeutics. 2007;6:3071–3079. doi: 10.1158/1535-7163.MCT-07-0117. [DOI] [PubMed] [Google Scholar]
- 32.Baldwin AS., Jr J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H, Ouyang W, Huang C. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 34.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. Cancer treatment reviews. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Morgan TM, Koreckij TD, Corey E. Curr Cancer Drug Targets. 2009;9:237–249. doi: 10.2174/156800909787580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho MK, Park JW, Jang YP, Kim YC, Kim SG. International immunopharmacology. 2002;2:105–116. doi: 10.1016/s1567-5769(01)00153-9. [DOI] [PubMed] [Google Scholar]
- 37.Cho MK, Jang YP, Kim YC, Kim SG. International immunopharmacology. 2004;4:1419–1429. doi: 10.1016/j.intimp.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Tang SN, Fu J, Shankar S, Srivastava RK. PloS one. 2012;7:e31067. doi: 10.1371/journal.pone.0031067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glienke W, Maute L, Wicht J, Bergmann L. Cancer investigation. 2010;28:166–171. doi: 10.3109/07357900903287006. [DOI] [PubMed] [Google Scholar]
- 40.Yao X, Zhu F, Zhao Z, Liu C, Luo L, Yin Z. Journal of cellular biochemistry. 2011;112:2837–2849. doi: 10.1002/jcb.23198. [DOI] [PubMed] [Google Scholar]
- 41.Klampfer L. Current cancer drug targets. 2006;6:107–121. doi: 10.2174/156800906776056491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.