Introduction
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is a debilitating chronic condition associated with urinary symptoms including urgency, frequency, nocturia and pelvic pain. Berry and co-authors report the prevalence of IC/BPS at between 3 and 8 million women in the United States [3]. Few patients achieve long-term remission of symptoms despite a range of treatments [1,33]. A substantial body of research has explored bladder pathology and other peripheral tissue abnormalities, however, a large number of IC/BPS patients lack any discernible end organ inflammation, and do not respond to treatment of peripheral tissues (e.g. hydrodistension of bladder) [31]. This suggests that investigation of more systemic factors (e.g., inflammation, inflammatory control) may yield important insights. IC/BPS patients have a high prevalence of comorbid Functional Somatic Syndromes (FSSs), such as irritable bowel syndrome (IBS) and fibromyalgia (FM), which are also characterized by chronic pain in the absence of clearly identifiable peripheral pathology [6,28]. This suggests that altered central pain processing (e.g. hyperalgeisa, allodynia), termed central sensitization [49] could be a contributory factor to the chronic pain seen in IC. Consistent with this notion, a recent study found that IC/BPS patients have reduced pain thresholds and pain tolerance compared to healthy controls [25].
Toll-Like Receptors (TLR), particularly TLR-4, have been identified as critical factors in central pain sensitization. Evidence in animal models of chronic pain suggests that inflammatory signaling secondary to TLR-4 stimulation plays a critical part in the development of hyperalgesia and allodynia [10,14,24]. Preliminary evidence suggests that heightened inflammatory responses to TLR stimulation may be a feature of human pain syndromes as well [19], but these have not been evaluated in IC/BPS. Further, no studies have determined if inflammatory responses to TLR stimulation are associated with the magnitude of painful symptoms. Endogenous control of inflammation, one of the central functions of the Hypothalamic-Pituitary-Adrenal (HPA) axis may also be a critical factor in IC/BPS. We previously found that higher levels of morning salivary cortisol, an endogenous HPA axis glucocorticoid with antiinflammatory properties, are associated with less severe symptoms in IC/BPS patients [23]. TLR-mediated inflammation and HPA axis activity may be interactive, as glucocorticoids have been shown to potentiate inflammatory responses in animal models of pain [21]. The goals of the current study were to identify differences in inflammatory processes, including those mediated by TLRs, and HPA axis function as assessed by diurnal cortisol between IC/BPS patients and healthy controls, and to determine whether these factors were associated with IC/BPS symptoms.
Methods
MAPP Study and Recruitment
The Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) study is a multi-site research effort sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases to better characterize syndrome phenotypes, syndrome etiology, and the natural history of chronic urologic pelvic pain patients [50]. The University of Iowa is a participating institution collecting site-specific data on biomarkers of chronic pelvic pain. Inclusion criteria for IC/BPS included being at least 18 years of age and reporting pain, pressure or discomfort associated with the bladder or pelvic region present the majority of the time during the most recent 3 months. Additionally, IC/BPS participants were negative on urine culture for any uropathogens. Exclusion criteria included a history of urethral stricture, neurological disorder affecting the bladder or bowel, cystitis caused by tuberculosis, radiation therapy or Cytoxan/cyclophosphamide therapy, augmentation cystoplasty or cystectomy, active autoimmune or infectious disorder, history of cancer, major psychiatric illness, or cardiac, pulmonary, renal, or hepatic disease, or pregnancy. Of the 98 female Iowa IC/BPS and HC participants enrolled in the broader trans-MAPP study at the University of Iowa, 86 (88%) agreed to an additional blood draw for isolation of PBMCs, and 72 (73%) agreed and were able to collect salivary cortisol. Study participants were 58 (48 for cortisol analyses) IC/BPS patients and 28 (24 for cortisol analyses) HCs who met study criteria. In addition to meeting exclusion criteria, healthy controls had no urinary, pelvic, or bladder symptoms, and met no criteria for common FSSs IBS, FM, or Chronic Fatigue Syndrome [9,12,48]. All participants provided informed consent and all procedures were cleared by the Institutional Review Board of the participating MAPP institutions.
Demographic and Symptom Information
Participants provided demographic information at the time of eligibility screening. Upon study entry, participants had a blood draw, urine collection, physical examination and completed a battery of trans-MAPP questionnaires relating to pelvic and bladder symptoms which have been previously used to assess pain and symptom severity in urologic conditions. These included the 9-item Genitourinary Pain Index (GUPI)[7], the 19-item Female Sexual Functioning Inventory (FSFI) [34], and the 4-item Interstitial Cystitis Symptom Index (ICSI) [29]. The GUPI includes pain and urinary symptom subscales. The pain subscale contains questions indicating the number of painful areas in the genitourinary region (e.g. the bladder, urethra), the number of activities (i.e. filling, voiding of bladder) that are painful, and two questions relating to the frequency and intensity of pain experience regardless of area or activity. The FSFI contains 3 questions relating specifically to the frequency and intensity of pain during and after intercourse. The ICSI contains questions specific to bladder pain, and to the urgency and frequency of urination and nocturia.
Cortisol
Salivary cortisol was collected in salivettes by participants at 3 time points (upon waking: 4–9am, afternoon: 4–6:30pm, and bedtime: 8pm-12am) for three consecutive days. Samples collected outside this time frame were excluded to maintain homogeneity. Participants were instructed not to eat, exercise or consume caffeine for thirty minutes prior to collecting a sample. Self-report of collection time has been demonstrated to be reliable and salivary cortisol is stable at room temperature [18]. Salivettes were analyzed by chemiluminescence immunoassay (IBL, Hamburg, Germany) at the Technical University of Dresden. The lower detection limit is 0.41 nmol/L and inter-assay and intra-assay coefficients of variance are less than 10%.
Inflammatory Measures
Blood samples were collected between approximately 11:30am and 12:30pm. PBMCs were separated by Ficoll-paque gradient centrifugation within 30 minutes of blood collection and cultured in RPMI 1640 medium containing 10% fetal bovine serum, 100 U/ml penicillin and 100 ug/ml streptomycin for 3 days at 37°C in a humidified incubator with 5% CO2 and TLR agonists. TLR-2 and 4 agonists were selected on the basis of the role of these receptors in chronic pain in animal models [2,14]. For stimulation of TLR-4, 50 ng/ml of Lipopolysaccharide (LPS) was used; For TLR-2 stimulation, 0.04 ng/ml of Staphylococcus aureus Cowan I (SAC) was used. Conditioned media was then harvested and frozen at −80°C prior to batch ELISA analysis. Each well contained 1×10^6 cells in 24 well plates, with one well per subject for TLR-4 and TLR-2 stimulation. Cytokines were assayed in duplicate by DuoSet ELISAs (R&D Systems) according to instructions included with the kit. Plasma cytokines were assayed with high sensitivity Quantikine ELISAs. Similar approaches in populations with complex presentations of pain have been reported and are able to distinguish patients from healthy controls [5,16,17,19].
Statistical analyses
Statistical analyses were performed using SPSS v. 21. Inflammatory variables (plasma interleukin-6 (IL-6), pro-inflammatory cytokine response to TLR stimulation in PBMCs) were log-10 transformed to normalize their distribution. Composite inflammation scores for stimulated cytokine responses were calculated by summing the z-scores ([individual score- group mean]/ group standard deviation) for the IL-6 and interleukin-1 beta (IL-1β) response in PBMCs following either LPS or SAC stimulation. Both cytokines have been identified as mediators of pain amplification by spinal glia and are released following TLR-2 and 4 stimulation, in part, by transcription of nuclear factor-kappaB (NFκB) [24]. Cortisol values were normalized using natural log transformations. Distributions of transformed variables were examined for confirmation of normality. Salivary cortisol values at each of the collection points were regressed on the time of collection over the three-day period to calculate cortisol slope, a measure of the average hourly decrease in cortisol over the course of the day as described previously [18]. To determine if diurnal patterns of cortisol secretion differed between groups, a repeated measures ANOVA was used with time of cortisol sample (morning, afternoon, nocturnal) and group membership as factors. A significant time by group interaction indicates different patterns of diurnal secretion between groups. Post-hoc comparisons with Sidak adjustment were used to determine which, if any, time-points differed in salivary cortisol concentrations between groups.
BMI was used as a covariate in all analyses of biomarkers due to well-established relationships between adiposity and inflammation [11,47]. While all participants were free of major psychiatric diagnoses, levels of negative affect (i.e. anxious and depressive symptoms) have previously been linked to self-reported pain measures in chronic pain populations so the participant score on the Positive and Negative Affect Schedule (PANAS) negative affect scale was included as a covariate in analyses of symptoms [39,42]. Group differences between IC/BPS and HC in demographic and symptom data were tested by one-way analysis of variance (ANOVA) and chi−squared tests. Within IC/BPS participants, One-way ANOVAS were used to test mean differences in inflammatory variables for the use of tri-cyclic antidepressants, pentosan polysulfate, opioids, SSRI/SNRIs, and NSAIDs and the presence of a comorbid FSS. Differences in inflammatory variables between IC/BPS and HC were tested with General Linear Models controlling for BMI. Relationships between inflammatory variables and symptom scores were assessed using multiple regression controlling for BMI and negative affect.
Because use of tricyclic anti-depressants and the presence of a comorbid FSS were each associated with marginal differences in TLR responses, these variables were also controlled for in additional analyses to determine if the magnitude of the association between inflammatory variables and self-reported painful symptoms remained statistically significant and comparable to reduced models. Similarly, because duration of symptom in years was associated with TLR-4 inflammatory response, models were also tested which controlled for duration of symptoms. To determine which inflammatory measures had the strongest association with painful symptoms, all inflammatory variables (IL-6, cortisol slope, TLR-2 inflammatory score, and TLR-4 inflammatory score) were used simultaneously as predictors of painful symptom scores in models with BMI and negative affect.
To determine if dysregulated HPA activity and heightened TLR-4 inflammatory responses combine to exacerbate symptoms of pain, we divided patients on median splits of cortisol slope (steep, i.e. healthier; flat, i.e. less healthy) and TLR-4 inflammatory response, and compared measures of pain in the resulting 4 groups by One-Way ANOVA. Post-hoc comparisons were conducted with Sidak adjustment.
Results
Participant Characteristics
The mean age of participants was approximately 42 years (range 20–68). IC/BPS patients and healthy controls did not differ on potential confounding variables such as income, education, race, ethnicity, employment status or age (all p>.15). BMI of HCs was significantly elevated compared to that of IC/BPS patients (p=.021). As expected, IC/BPS participants reported more genitourinary symptoms including pain and urinary dysfunction, elevated IC symptoms, and more compromised sexual functioning (all p<.004) when compared to HCs. In IC/BPS patients the average duration of symptoms was 7.7 (SD=7.3) years. See Table 1. IC/BPS participants on tricyclic antidepressants (n=29, 50%) showed marginally lower IL-6 responses following TLR-4 stimulation (p=.056) and lower IL-6 following TLR-2 stimulation (p=.044) but no differences in any other inflammatory measure (p values>.42). IC/BPS participants on opioids (n=11, 19%), pentosan polysulfate (n=29, 50%), SSRI/SNRIs (n=9, 16%), or NSAIDs (n=7, 12%) did not differ on any inflammatory variable from patients not taking those medications (all p>.12). The presence of a comorbid FSS was associated with marginally higher IL-1β following TLR-4 stimulation (p=.07) but no differences in any other inflammatory variable (p values>.27).
Table 1.
Participant Characteristics. IC/BPS=Interstitial Cystitis/Bladder Pain Syndrome. BMI=Body Mass Index.
| IC/BPS n=58 |
Healthy Controls n=28 |
p | |
|---|---|---|---|
| Age Mean(SD) | 41.1 (14.8) | 42.9 (13.1) | .59 |
| BMI Mean(SD) | 27.2 (5.8) | 30.5 (6.9) | .021 |
| Race % (n) | |||
| White | 97 (56) | 100 (28) | .61 |
| Asian | 2 (1) | 0 | |
| Multi Race | 2 (1) | 0 | |
| Ethnicity % (n) | |||
| Non-Hispanic | 100 (58) | 100 (28) | n/a |
| Education % (n) | |||
| High School or GED | 14 (8) | 11 (3) | .93 |
| Some College | 24 (14) | 25 (7) | |
| Graduated College | 33 (19) | 39 (11) | |
| Graduate Degree | 29 (17) | 25 (7) | |
| Employment % (n) | |||
| Employed | 60 (35) | 90 (25) | .15 |
| Unemployed | 12 (7) | 4 (1) | |
| Disabled | 9 (5) | 0 (0) | |
| Retired | 7 (4) | 4 (1) | |
| Full Time Homemaker | 10 (6) | 4 (1) | |
| Annual Income/$ % (n) | |||
| <10,000 | 14 (8) | 7 (2) | .92 |
| <25,000 | 7 (4) | 7 (2) | |
| <50,000 | 21 (12) | 18 (5) | |
| < 100,000 | 36 (21) | 46 (13) | |
| > 100,000 | 17 (10) | 18 (5) | |
| Prefer not to answer | 5 (3) | 4 (1) | |
| Comorbid Conditions % (n) |
|||
| None | 33 (47) | 0 (100) | N/A |
| Irritable Bowel Syndrome | 23 (40) | ||
| Fibromyalgia | 2 (3) | ||
| Chronic Fatigue Syndrome | 8 (14) | ||
Inflammatory Markers and Cortisol in IC/BPS patients and Healthy Controls
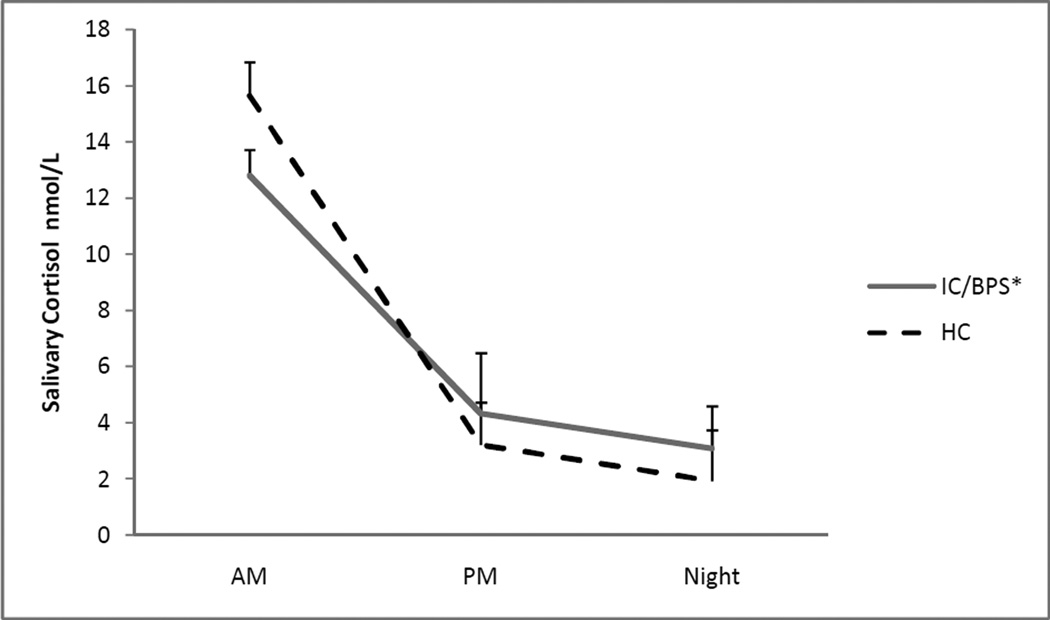
Cortisol slope was significantly flatter (p=.010) in IC/BPS participants compared to HCs, indicating a smaller hourly decrease in salivary cortisol for IC/BPS patients. Additionally, the results of the repeated measures ANOVA revealed a significant time by group interaction (p=.016). Post-hoc comparisons revealed no significant differences in morning cortisol (p=.14) but marginally higher afternoon cortisol (p=.07) and significantly higher nocturnal cortisol (p=.019) in the IC/BPS group, indicating a blunting of the diurnal cortisol rhythm. See Figure 1. As seen in Table 2, IC/BPS participants had significantly elevated plasma IL-6 compared to HC’s (p=0.040), suggesting an elevated level of basal inflammation. When stimulated with TLR-2 receptor agonist SAC, PBMCs isolated from IC/BPS participants demonstrated greater IL-1β responses (p=.040), but no difference in IL-6 responses (p=.10), compared to PBMCs of HC participants. There were no statistically significant differences between IC/BPS and HC participants in the pro-inflammatory cytokine response in LPS stimulated PBMCs (see Table 2.)
Figure 1.
Means and standard deviations of diurnal salivary cortisol concentrations in IC/BPS participants and HCs. Natural log-transformed values are back transformed.
*Significant time x group interaction in repeated measures ANOVA (F2,68 =4.414, p=.016)
Table 2.
Symptoms and Biomarkers in IC/BPS patients and Healthy Controls. Inflammatory variables are adjusted for BMI. GUPI=Genitourinary Pain Index, IC= Interstitial Cystitis, BPS=Bladder Pain Syndrome, FSFI=Female Sexual Functioning Inventory. IL-6=Interleukin-6. IL-1β=Interleukin-1 beta. LPS= Lipopolysaccharide. SAC= Staphylococcus aureus Cowan.
| Variable Mean (95% CI) | IC/BPS n=58 |
Healthy Controls n=28 |
p |
|---|---|---|---|
| GUPI score total |
24.7 (22.7,26.8) | 1.4(.5,2.3) | <.001 |
| GUPI score pain | 12.0(10.8,13.1) | .3(−.1,.6) | <.001 |
| GUPI score urinary subscale | 5.6(4.8,6.4) | .7(.3,1.1) | <.001 |
| IC Symptom Index | 10.5(9.3,11.6) | 2.5(1.6,3.5) | <.001 |
| FSFI total | 16.5(13.78,19.1) | 25.3(21.2,29.5) | <.001 |
| FSFI Pain | 6.00(4.5,7.4) | 11.9(9.7,14.1) | <.001 |
| Morning Cortisola nmol/L |
2.55 (2.40, 2.71) | 2.75 (2.54, 2.96) | .14c |
| Afternoon Cortisola nmol/L |
1.48 (1.28, 1.67) | 1.17 (.90, 1.44) | .07c |
| Nocturnal Cortisola nmol/L |
1.13 (.91, 1.36) | .66 (.35, .97) | .019c |
| Cortisol Slopea nmol/L, hourly decrease |
−.10(−.12, −.08) | −.14(−.17, −.12) | .010 |
| IL-6 plasmab pg/mL |
.45(.39,.52) | .34(.25,.43) | .040 |
| IL-β + LPSb pg/mL |
3.34(3.16,3.50) | 3.53(3.27,3.77) | .24 |
| IL-β + SACb pg/mL |
1.22(.93,1.52) | .67(.24,1.10) | .039 |
| IL-6 + LPSb pg/mL |
4.20(4.09,4.31) | 4.13(3.96,4.29) | .48 |
| IL-6 + SACb pg/mL |
1.71(1.35,2.07) | 1.15(.63,1.67) | .09 |
natural log transformed
log 10 transformed
post-hoc comparison by Sidak adjustment
Associations between Inflammation and IC/BPS Symptoms
The TLR-4 inflammation score was associated with multiple measures of non-specific pain intensity and frequency. The composite TLR-4 inflammation score (calculated from the response of IL-6 and IL-1β to LPS) was significantly associated with higher total GUPI scores (p=.005), GUPI pain subscale scores (p=.010), and marginally with GUPI urinary symptom subscale scores (p=.062). On the GUPI pain subscale, the relationship with TLR-4 inflammation score was strongest for two items; pain frequency (p=.001) and intensity (p=.008). The TLR-4 inflammation score was also associated with reduced FSFI sexual functioning (p=.001); the strongest relationships were seen with pain variables in this scale: e.g., pain frequency during intercourse (p=.002), pain frequency after intercourse (p=.002), and sexual pain severity (p=0.003). The TLR-4 inflammation score was marginally associated with higher IC symptom index scores (p=.068). However, there were no significant relationships between the TLR-4 inflammation score and the ICSI urinary urgency, frequency, or nocturia items (p-values > 0.17) or with the pain/burning specific to the bladder item (p=.13). Interestingly, TLR-4 inflammatory response was associated with longer duration of symptoms (years; p=.047) and with flatter cortisol slopes (p=.043). In contrast to these findings, TLR-2 inflammatory response for SAC-stimulated PBMCs, plasma IL-6, and cortisol slope were not significantly associated with any symptom scale (all p>.10). See Table 3 for associations of TLR-4 inflammatory response with scales. See Table 4 for associations of TLR-4 inflammatory response with specific items.
Table 3.
Relationship of TLR-4 inflammatory response with symptom scores (controlling for BMI and negative affect), and inflammatory variables (controlling for BMI) in IC/BPS participants (n=58). GUPI=Genitourinary Pain Index, IC= Interstitial Cystitis, BPS=Bladder Pain Syndrome, FSFI=Female Sexual Functioning Inventory. IL-6=Interleukin-6. LPS= Lipopolysaccharide. SAC= Staphylococcus aureus Cowan. TLR=Toll-Like Receptor.
| TLR-4 (LPS) inflammation score | ||
|---|---|---|
| β | p | |
| GUPI score total | .333 | .005 |
| GUPI score pain | .310 | .010 |
| GUPI score urine | .252 | .062 |
| FSFI total | −.407 | .001 |
| FSFI pain | −.393 | .002 |
| IC symptom Index | .246 | .068 |
| Cortisol slope | .299 | .043 |
| Duration of symptoms (years)a |
.256 | .047 |
| TLR-2 (SAC) inflammation score |
.236 | .080 |
| Plasma IL-6 pg/mL | .037 | .78 |
adjusted for age
Table 4.
Relationships between TLR-4 inflammatory response and scale items controlling for MI and negative affect. Lower FSFI scores reflect worse symptom severity. GUPI=Genitourinary Pain Index, ICSI= Interstitial Cystitis Symptom Index, BPS=Bladder Pain Syndrome, FSFI=Female Sexual Functioning Inventory.
| Item | β | p |
|---|---|---|
| GUPI pain frequency | .401 | .001 |
| GUPI pain intensity | .339 | .008 |
| FSFI pain frequency during intercourse |
−.405 | .002 |
| FSFI pain frequency after intercourse |
−.389 | .002 |
| FSFI pain severity | −.368 | .003 |
| Urinary Urgency (ICSI Q1) | .174 | .18 |
| Urinary Frequency (ICSI Q2) |
.117 | .40 |
| Nocturia (ICSI Q3) | .182 | .18 |
| Burning/Pain in Bladder (ICSI Q4) |
.201 | .13 |
The relationships between TLR-4 inflammation scores and symptom scales were not attenuated or rendered insignificant when analyses adjusted for use of tri-cyclic anti-depressants and presence of comorbid FSSs, nor were either of these associated with differences in painful symptoms (p values >.19). Adjusting for the duration of symptoms in years similarly did not affect the relationship between TLR-4 inflammation and painful symptoms and duration of symptoms was not itself associated with pain (p values > 0.14). The relationship between TLR-4 inflammation score and painful symptoms was similarly unaffected when all other inflammatory variables were included simultaneously as predictors of painful symptoms, and no other inflammatory variable was associated with pain in these models (p values >.16).
Secondary Analyses
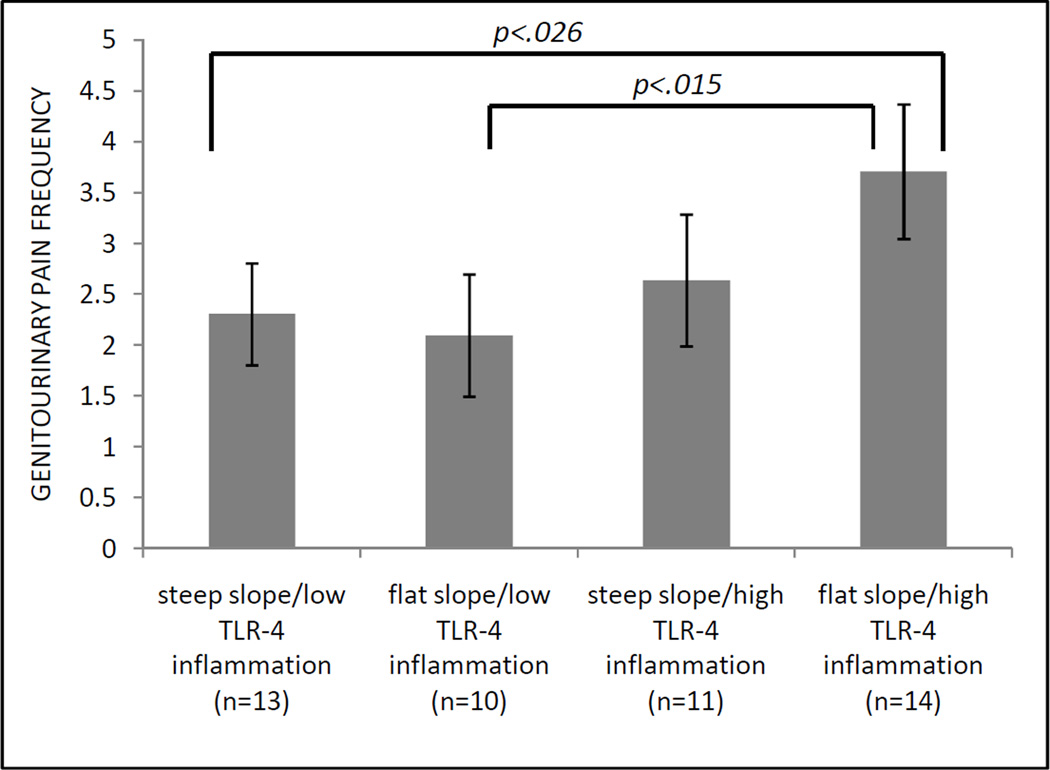
Consistent with the hypothesis that HPA dysregulation and TLR-4 inflammation may act synergistically on measures of pain, we found that, amongst the four groups, the most severe pain scores on the GUPI pain subscale, GUPI pain frequency and severity items, and FSFI pain subscale were all reported in the group with flatter cortisol slopes and high TLR-4 inflammatory responses (Table 5). Despite relatively small groups, post-hoc comparisons demonstrated that differences between the flat cortisol slope/high TLR-4 inflammation group and steep cortisol slope/low TLR-4 inflammation group were statistically significant for the GUPI pain frequency and FSFI pain scores (both p<.027). See Figure 2.
Table 5.
Means and standard deviations of selected scales and items by patient groups divided on median splits of cortisol slope and TLR-4 inflammatory response. Steeper cortisol slopes indicate more normal diurnal cortisol profiles. GUPI=Genitourinary Pain Index. FSFI=Female Sexual Functioning Inventory. TLR=Toll-Like Receptor.
| Group | GUPI pain scale | GUPI pain frequency** |
GUPI pain severity |
FSFI pain scale ** + |
| steep slope/low TLR-4 inflammation (n=13) |
11.055 (5.29) | 2.31 (1.03)* | 4.38 (2.63) | 10.54 (4.86)* |
| flat slope/low TLR-4 inflammation (n=10) |
10.50 (4.35) | 2.1 (1.2)* | 4.00 (1.89) | 4.4 (5.03) |
| steep slope/high TLR-4 inflammation (n=11) |
11.42 (4.45) | 2.64 (1.29) | 4.82 (1.89) | 4.36 (4.18) |
| flat slope/high TLR-4 inflammation (n=14) |
13.81 (3.32) | 3.71 (1.33) | 5.86 (1.46) | 3.00 (4.22) |
omnimbus test, p<.05
post-hoc comparison vs. flat slope/high TLR-4 inflammation group, p<.05
lower scores indicate greater pain
Figure 2.
Means and standard deviations of GUPI pain frequency item for IC/BPS participants grouped by median split of cortisol slope and TLR-4 inflammation response. GUPI=Genitourinary Pain Index. TLR=Toll-Like Receptor.
Discussion
One of the major findings of this study is that TLR-4 stimulated inflammatory cytokine responses are robustly associated with measures of painful symptoms in women with IC/BPS. These findings were not altered when controlling for multiple other measures of inflammation and potential confounding variables. The symptoms most strongly associated with TLR-4 stimulated inflammatory responses were non-specific pain severity and pain frequency. Further, IC/BPS is marked by inflammatory dysregulation, indicated by flattened diurnal cortisol rhythmicity, elevated plasma IL-6 and an exaggerated IL-1β response to TLR-2 stimulation in PBMCs. TLR-2 stimulated IL1-β responses in PBMCs were recently shown to be capable of differentiating a population of chronic pain patients from HCs [19]. This study extends that finding to a chronic pelvic pain population. More generally, these findings are in agreement with the results of other studies demonstrating that inflammation from TLR stimulated PBMCs are able to differentiate patients with Inflammatory Bowel Disease, recent onset rheumatoid arthritis, and persistent fatigue from healthy controls [5,16,17]. Importantly, none of the aforementioned studies determined if the magnitude of the response to TLR stimulation was associated with the magnitude of reported painful symptoms, or examined HPA activity in conjunction with TLR responses. Ours is the first study we know of to report these associations between pain and TLR inflammatory response in a clinical population; importantly, this sample was screened extensively for medical comorbidities and conditions which could simultaneously affect both inflammation and pain (e.g. recurrent bacterial infection) and many potential confounding variables were controlled. For instance, while the use of tri-cyclic anti-depressants was associated with lower TLR inflammatory responses, its inclusion as a covariate had no effect on the relationship between TLR-4 mediated inflammation and pain. In models controlling for all other measures of inflammation, the relationships between TLR-4 inflammatory responses and pain scales were not attenuated; this provides strong evidence that TLR-4 mediated inflammation specifically plays a critical role in IC/BPS pain symptoms. These findings may be related to recent work demonstrating that experimental induced endotoxemia via LPS administration reduces pain thresholds in healthy subjects [8].
The present findings indicate that HPA dysregulation is a feature of IC/BPS. Compromised GC diurnal variation has been associated with a reduced ability to respond to acute stressors with rapid GC release [37]. This may be of particular importance in IC/BPS, where symptom flares are often preceded by acute psychosocial stress, and these findings suggest one potential process by which this may occur [35]. Thus, HPA dysregulation may limit the ability of IC/BPS sufferers to control pain-promoting inflammation; the association of flattened cortisol slope with greater TLR-4 inflammatory cytokine responses suggests that this is true. Early life traumatic events, such as sexual trauma, are associated with HPA axis dysregulation, and there is evidence that chronic pain patients, including pelvic pain patients, report more early life trauma [30].
It is now well established that pro-inflammatory TLR-4 activation of spinal cord glial cells is a key factor in the development and maintenance of chronic pain [24,10,14]. It has been hypothesized that PBMC cytokine responsiveness to TLRs may mirror the cytokine responsiveness to TLRs in the glial cells of the spinal cord, and a recent study in a sciatic constriction animal model of pain demonstrated concordance between TLR-2 and TLR-4 stimulated PBMCs and inflammation assayed in the supernatant of lumbar spinal cultures [19,20]. Therefore, it is possible that the difference in TLR-2 inflammation between IC/BPS and HCs reflects underlying processes in the spinal cord. That TLR mediated inflammation in PBMCs would distinguish pain populations as different in presentation as IC/BPS patients and the pain group used in Kwok, et al. (primarily chronic back, shoulder and leg pain, and osteoarthritis) is noteworthy [19]. Inflammation in PBMCs may therefore serve as a useful biomarker of persistent pain in clinical settings and may help phenotype pain patients. In animal models, TLR-4 stimulation specifically in spinal glial cells has been shown to lead to amplified ascending pain signaling via, in part, the release of pro-inflammatory cytokines This TLR-4-stimulated glial activation is thought to underlie the initiation of chronic pain and its extension beyond the original site [24]. However, recent experiments in preclinical models of pain call into question the role of TLR-4 in female pain. These found that TLR-4 stimulation in the spinal cord only produced a heightened pain response in male mice, an effect possibly mediated by testosterone [40], and that TLR-4 knockout did not reliably attenuate the pain response in female mice [41]. As administration of TLR-4 agonists to the brain and hindpaw still produced heightened pain in both female and male mice, this sex difference may be limited to direct stimulation of the spinal cord [40]. Clearly, further mechanistic investigation with an animal model of IC/BPS would be required to determine relevant sex differences in TLR activity for IC/BPS.
That TLR-4 mediated inflammation was a robust predictor of painful symptoms in female IC/BPS patients is interesting, as interactions between TLR-4 mediated inflammation and sex hormones have been posited as a one potential mechanism in the greater prevalence of pain conditions in women [27]. Glial cells are known to express estrogen receptors [27], and chronic estrogen stimulation in vivo enhances pro-inflammatory gene expression in microglia following LPS stimulation [4]. Further, IC/BPS symptoms are known to differ according to phases of the menstrual cycle [32]. Therefore, exploring the interaction between TLR mediated inflammation and sex hormones/phases of the menstrual cycles may reveal insight into the painful symptoms experienced by female IC/BPS patients.
Importantly, TLR-4 responsiveness was associated most strongly with pain frequency and intensity, rather than specific urologic symptoms (i.e. pain on filling of the bladder) or anatomical regions (i.e. the urethra). This suggests that peripheral tissue inflammation or damage is not solely responsible for the initiation of painful signaling, and is consistent with the hypothesis that some pain in IC/BPS may be mediated by central pain amplification. The marginal difference in IL-1β response to TLR-4 stimulation between IC patients with pelvic pain only and comorbid FSSs is intriguing and requires further investigation. That TLR-4 stimulated inflammatory cytokine responses are associated with longer symptom duration and flattened cortisol slopes raises the possibility that that TLR-4-mediated pain may be progressive. Other studies have demonstrated that longer symptom duration is associated with a greater number of problematic symptom domains in IC/BPS [26]. These findings suggest possible parallel physiologic processes. Glucocorticoid signaling is known to mediate sensitization of microglial proinflammatory responses, a pathway that may additionally provoke stress-induced symptom flares in IC/BPS [21]. Analyses demonstrating that the most severe pain was experienced by those with flat cortisol slopes and high TLR-4 inflammatory responses provides some evidence that inflammatory responses and poor endogenous inflammatory control may contribute to pain exacerbation synergistically, though longitudinal analyses are required to clarify this relationship. Compromised inflammatory control may permit sensitization of TLRs in immune cells and the spinal cord, and repeated inflammatory insults from reactive immune cells may disrupt HPA activity; more likely, the relationship is bi-directional.
These novel findings offer compelling evidence that the inflammatory response to TLR-4 stimulation is associated with pain in IC/BPS patients, and suggest that PBMC responsiveness to TLR-2 stimulation holds promise as a biomarker for IC/BPS pain. Importantly, these findings also suggest that TLR-4 may be a therapeutic target in IC/BPS. Animal research has demonstrated that TLR4 antagonism can reverse neuropathic pain in a sciatic nerve constriction model [14]. Further, recent work has demonstrated that TLR-4 antagonism may increase the effectiveness of opioids while reducing tolerance and dependence [13].
Limitations
These analyses were cross-sectional and thus do not provide information about causality or about the longitudinal course of IC/BPS symptoms, nor can temporal precedence for any measure of inflammatory dysregulation be established. We used self-report measures of pain; future work should incorporate quantitative sensory pain tests. We used a single concentration of LPS; thus it is possible that examination of dose-response kinetics with higher concentrations may have revealed that TLR4 cytokine response can differentiate between IC/BPS and HCs as reported by Kwok et al. in other chronic pain populations [19].
Future Directions
Understanding the longitudinal course of TLR-4 mediated pain is a critical endeavor. In particular, it would be important to determine if TLR-4 mediated pain is an early or late feature of IC/BPS pain, and if it is only an important mechanism in a subset of patients. Animal models need to be developed to allow for greater mechanistic investigation of TLR-mediated pain in IC/BPS, and to confirm if PBMC cytokine responsiveness is reflective of glial cytokine responsiveness in the spinal cord. Additionally, the role of TLR-2 mediated inflammation, which differentiates IC/BPS patients from HCs, but does not predict painful symptoms, needs to be elucidated. Characterizing TLR-2 and TLR-4 receptor expression in IC/BPS patients may provide important insights, as might investigation of cytokine and TLR polymorphisms. Finally TLR-4 antagonists may provide an important avenue of future treatment for those suffering from IC/BPS.
Inflammatory dysregulation marks Interstitial Cystitis/Bladder Pain Syndrome. Inflammatory responses in Peripheral Blood Mononuclear Cells following Toll-Like Receptor 4 stimulation predict the magnitude of painful symptoms.
Toll-Like Receptors (TLR) are known to play a role in chronic pain from animal models and limited research in humans, but their role in Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is unknown. Similarly, alterations of the Hypothalamic Pituitary Adrenal axis have been reported in some pain conditions. Our objectives were to identify inflammatory processes which might distinguish IC/BPS sufferers from healthy controls (HC) and to examine their associations with IC/BPS symptoms. Female participants (58 IC/BPS and 28 HC) completed pain and urinary symptom questionnaires and collected saliva for cortisol as part of the Multidisciplinary Approach to Pelvic Pain study. Inflammatory cytokines were assayed in plasma, and in TLR2 and TLR4- stimulated peripheral blood mononuclear cells. Controlling for BMI and negative affect, between group differences were analyzed by General Linear Models and relationships between symptoms and inflammatory variables were analyzed by regression. Compared to HCs, IC/BPS patients had higher levels of plasma interleukin-6 (p=.040), greater interleukin-1β responsive to TLR2 stimulation (p=.040) and flatter diurnal cortisol slopes (p=.010), indicating inflammatory dysregulation. In IC/BPS, inflammation following TLR4 stimulation was associated with multiple symptoms, including genitourinary pain (p=.010), sexual pain (p=.002), and marginally with urinary symptoms (p=.068). Genitourinary pain severity (p=.008), frequency (p=.001) and pain with intercourse (p=.002) were strongly associated with TLR4 inflammatory response. TLR4 appears to play a central role in painful symptoms of IC/BPS sufferers, which may be linked to poor endogenous inflammatory control. These findings may help identify new mechanisms in IC/BPS and lead to new therapeutic approaches.
Acknowledgements
The authors gratefully acknowledge the assistance of Mary Eno in carrying out this project.
Funding:
This research was funded by grant UO1DK082344 from the National Institute of Diabetes Digestive and Kidney Diseases and by the Institute for Clinical and Translational Science at the University of Iowa, grant 2 UL1 TR000442-06.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Anger JT, Zabihi N, Clemens JQ, Payne CK, Saigal CS, Rodriguez LV. Treatment choice, duration, and cost in patients with interstitial cystitis and painful bladder syndrome. Int Urogynecol J. 2011;22:395–400. doi: 10.1007/s00192-010-1252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastos LF, Godin AM, Zhang Y, Jarussophon S, Ferreira BC, Machado RR, Maier SF, Konishi Y, de Freitas RP, Fiebich BL, Watkins LR, Coelho MM, Moraes MF. A minocycline derivative reduces nerve injury-induced allodynia, LPS-induced prostaglandin E2 microglial production and signaling via toll-like receptors 2 and 4. Neurosci Lett. 2013;543:157–162. doi: 10.1016/j.neulet.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lelu K, Krust A, Pipy B, Bayard F, Arnal JF, Guery JC, Gourdy P. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185:1169–1176. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- 5.Chao CC, Janoff EN, Hu SX, Thomas K, Gallagher M, Tsang M, Peterson PK. Altered cytokine release in peripheral blood mononuclear cell cultures from patients with the chronic fatigue syndrome. Cytokine. 1991;3:292–298. doi: 10.1016/1043-4666(91)90497-2. [DOI] [PubMed] [Google Scholar]
- 6.Clauw DJ, Schmidt M, Radulovic D, Singer A, Katz P, Bresette J. The relationship between fibromyalgia and interstitial cystitis. J Psychiatr Res. 1997;31:125–131. doi: 10.1016/s0022-3956(96)00051-9. [DOI] [PubMed] [Google Scholar]
- 7.Clemens JQ, Calhoun EA, Litwin MS, McNaughton-Collins M, Kusek JW, Crowley EM, Landis JR Urologic Pelvic Pain Collaborative Research Network. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009;74:983–987. doi: 10.1016/j.urology.2009.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Goeij M, van Eijk LT, Vanelderen P, Wilder-Smith OH, Vissers KC, van der Hoeven JG, Kox M, Scheffer GJ, Pickkers P. Systemic inflammation decreases pain threshold in humans in vivo. PLoS One. 2013;8:e84159. doi: 10.1371/journal.pone.0084159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–241. [PubMed] [Google Scholar]
- 10.Ellis A, Wieseler J, Favret J, Johnson KW, Rice KC, Maier SF, Falci S, Watkins LR. Systemic Administration of Propentofylline, Ibudilast, and (+)-Naltrexone Each Reverses Mechanical Allodynia in a Novel Rat Model of Central Neuropathic Pain. J Pain. 2014:ii. doi: 10.1016/j.jpain.2013.12.007. S1526–5900(14)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Festa A, D'Agostino R, Jr, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, Haffner SM. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord. 2001;25:1407–1415. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. Scientific World Journal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarcho MR, Slavich GM, Tylova-Stein H, Wolkowitz OM, Burke HM. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biol Psychol. 2013;93:150–158. doi: 10.1016/j.biopsycho.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovarik JJ, Tillinger W, Hofer J, Hölzl MA, Heinzl H, Saemann MD, Zlabinger GJ. Impaired anti-inflammatory efficacy of n-butyrate in patients with IBD. Eur J Clin Invest. 2011;41:291–298. doi: 10.1111/j.1365-2362.2010.02407.x. [DOI] [PubMed] [Google Scholar]
- 17.Kowalski ML, Wolska A, Grzegorczyk J, Hilt J, Jarzebska M, Drobniewski M, Synder M, Kurowski M. Increased responsiveness to toll-like receptor 4 stimulation in peripheral blood mononuclear cells from patients with recent onset rheumatoid arthritis. Mediators Inflamm. 2008;2008:132732. doi: 10.1155/2008/132732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraemer HC, Giese-Davis J, Yutsis M, O'Hara R, Neri E, Gallagher-Thompson D, Taylor CB, Spiegel D. Design decisions to optimize reliability of daytime cortisol slopes in an older population. Am J Geriatr Psychiatry. 2006;14:325–333. doi: 10.1097/01.JGP.0000201816.26786.5b. [DOI] [PubMed] [Google Scholar]
- 19.Kwok YH, Hutchinson MR, Gentgall MG, Rolan PE. Increased responsiveness of peripheral blood mononuclear cells to in vitro TLR 2, 4 and 7 ligand in chronic pain patients. PLoS One. 2012;7:e44232. doi: 10.1371/journal.pone.0044232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwok YH, Tuke J, Nicotra LL, Grace PM, Rolan PE, Hutchinson MR. TLR 2 and 4 responsiveness from isolated peripheral blood mononuclear cells from rats and humans as potential chronic pain biomarkers. PLoS One. 2013;8:e77799. doi: 10.1371/journal.pone.0077799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loram LC, Taylor FR, Strand KA, Frank MG, Sholar P, Harrison JA, Maier SF, Watkins LR. Prior exposure to glucocorticoids potentiates lipopolysaccharide induced mechanical allodynia and spinal neuroinflammation. Brain Behav Immun. 2011;25:1408–1415. doi: 10.1016/j.bbi.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundh D, Hedelin H, Jonsson K, Gifford M, Larsson D. Assessing chronic pelvic pain syndrome patients: blood plasma factors and cortisol saliva. Scand J Urol. 2013;47:521–528. doi: 10.3109/21681805.2013.769460. [DOI] [PubMed] [Google Scholar]
- 23.Lutgendorf SK, Kreder KJ, Rothrock NE, Hoffman A, Kirschbaum C, Sternberg EM, Zimmerman MB, Ratliff TL. Diurnal cortisol variations and symptoms in patients with interstitial cystitis. J Urol. 2002;167:1338–1343. [PubMed] [Google Scholar]
- 24.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ness TJ, Lloyd LK2, Fillingim RB. An endogenous pain control system is altered in subjects with interstitial cystitis. J Urol. 2014;191:364–370. doi: 10.1016/j.juro.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickel JC, Shoskes D, Irvine-Bird K. Clinical phenotyping of women with interstitial cystitis/painful bladder syndrome: a key to classification and potentially improved management. J Urol. 2009;182:155–160. doi: 10.1016/j.juro.2009.02.122. [DOI] [PubMed] [Google Scholar]
- 27.Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2012;234:316–329. doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novi JM, Jeronis S, Srinivas S, Srinivasan R, Morgan MA, Arya LA. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: a case-control study. J Urol. 2005;174:937–940. doi: 10.1097/01.ju.0000169258.31345.5d. [DOI] [PubMed] [Google Scholar]
- 29.O'Leary MP, Sant GR, Fowler FJ, Jr, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49:58–63. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 30.Paras ML, Murad MH, Chen LP, Goranson EN, Sattler AL, Colbenson KM, Elamin MB, Seime RJ, Prokop LJ, Zirakzadeh A. Sexual abuse and lifetime diagnosis of somatic disorders: a systematic review and meta-analysis. JAMA. 2009;302:550–561. doi: 10.1001/jama.2009.1091. [DOI] [PubMed] [Google Scholar]
- 31.Potts JM, Payne CK. Urologic chronic pelvic pain. Pain. 2012;153:755–758. doi: 10.1016/j.pain.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Powell-Boone T, Ness TJ, Cannon R, Lloyd LK, Weigent DA, Fillingim RB. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832–1836. doi: 10.1097/01.ju.0000176747.40242.3d. [DOI] [PubMed] [Google Scholar]
- 33.Propert KJ, Schaeffer AJ, Brensinger CM, Kusek JW, Nyberg LM, Landis JR. A prospective study of interstitial cystitis: results of longitudinal followup of the interstitial cystitis data base cohort. J Urol. 2000;63:1434–1439. doi: 10.1016/s0022-5347(05)67637-9. [DOI] [PubMed] [Google Scholar]
- 34.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D'Agostino R., Jr The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 35.Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff T, Zimmerman B. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology. 2001;57:422–427. doi: 10.1016/s0090-4295(00)00988-2. [DOI] [PubMed] [Google Scholar]
- 36.Sarabdjitsingh RA, Isenia S, Polman A, Mijalkovic J, Lachize S, Datson N, de Kloet ER, Meijer OC. Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology. 2012;151:1177–1186. doi: 10.1210/en.2009-1119. [DOI] [PubMed] [Google Scholar]
- 37.Scheff JD, Calvano SE, Lowry SF, Androulakis IP. Transcriptional implications of ultradian glucocorticoid secretion in homeostasis and in the acute stress response. Physiol Genomics. 2012;44:121–129. doi: 10.1152/physiolgenomics.00128.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith BW, Zautra AJ. The effects of anxiety and depression on weekly pain in women with arthritis. Pain. 2011;138:354–361. doi: 10.1016/j.pain.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stokes JA, Cheung J, Eddinger K, Corr M, Yaksh TL. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J Neuroinflammation. 2013;10:148. doi: 10.1186/1742-2094-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang NK, Salkovskis PM, Hodges A, Wright KJ, Hanna M, Hester J. Effects of mood on pain responses and pain tolerance: an experimental study in chronic back pain patients. Pain. 2008;138:392–401. doi: 10.1016/j.pain.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 43.Tanriverdi F, Karaca Z, Unluhizarci K, Kelestimur F. The hypothalamo-pituitary-adrenal axis in chronic fatigue syndrome and fibromyalgia syndrome. Stress. 2007;10:13–25. doi: 10.1080/10253890601130823. [DOI] [PubMed] [Google Scholar]
- 44.Teichman JM, Moldwin R. The role of the bladder surface in interstitial cystitis/painful bladder syndrome. Can J Urol. 2007;14:3599–3607. [PubMed] [Google Scholar]
- 45.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 46.Wingenfeld K, Heim C, Schmidt I, Wagner D, Meinlschmidt G, Hellhammer DH. HPA axis reactivity and lymphocyte glucocorticoid sensitivity in fibromyalgia syndrome and chronic pelvic pain. Psychosom Med. 2008;70:65–72. doi: 10.1097/PSY.0b013e31815ff3ce. [DOI] [PubMed] [Google Scholar]
- 47.Wirtz PH, Ehlert U, Emini L, Suter T. Higher body mass index (BMI) is associated with reduced glucocorticoid inhibition of inflammatory cytokine production following acute psychosocial stress in men. Psychoneuroendocrinology. 2008;33:1102–1110. doi: 10.1016/j.psyneuen.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 49.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. http://www.mappnetwork.org/