Abstract
Cellular senescence is a tumor-suppressive mechanism that permanently arrests cells at risk for malignant transformation. However, accumulating evidence shows that senescent cells can have deleterious effects on the tissue microenvironment. The most significant of these effects is the acquisition of a senescence-associated secretory phenotype (SASP) that turns senescent fibroblasts into proinflammatory cells that have the ability to promote tumor progression.
Keywords: aging, cancer, inflammation, proliferation, invasion
INTRODUCTION
The tissue microenvironment is defined by the phenotypes of the cells in the immediate area and by the physical and chemical properties of the soluble and insoluble factors surrounding cells within a given tissue. These properties include temperature and oxygen tension, as well as various molecules that may be produced locally—for example, growth factors and cytokines. Further, cells within tissues form a dynamic network that contributes to their microenvironment. At the same time, the tissue microenvironment regulates cell behavior. This reciprocal relationship determines tissue function and repair and is also central to a number of pathologies, including cancer.
A permissive microenvironment supports and promotes tumor growth and cancer cell aggressiveness (1–4). Alterations in the cellular and molecular composition of the connective tissues surrounding carcinomas allow tumors to evade detection by the immune system as well as to proliferate inappropriately, invade the surrounding tissue structure, and eventually metastasize. The synergy between an altered microenvironment and the genetic alterations acquired by tumor cells allows these cells to evade preventive mechanisms and become fully malignant. Cellular senescence is now recognized as a potent tumor-suppressive mechanism that arrests the growth of cells at risk for malignant transformation (5–12). However, recent studies show that senescent cells develop altered secretory activities that may induce changes in the tissue microenvironment, relaxing its control over cell behavior and promoting tumorigenesis (13–18).
How can the senescence response be both tumor suppressive and procarcinogenic? It is important to consider that a biological process such as cellular senescence can be both beneficial and deleterious. The idea that processes can have such dual effects is consistent with a major evolutionary theory of aging termed antagonistic pleiotropy (19). The senescence-associated secretory phenotype (SASP) represents one of the darkest sides of the senescence response and is the focus of this review. We particularly emphasize the potential effects of the SASP (20) on cell behavior in the context of tumor progression.
CELLULAR SENESCENCE
Cellular senescence occurs in culture and in vivo as a response to excessive extracellular or intracellular stress. The senescence program locks the cells into a cell-cycle arrest that prevents the spread of damage to the next cell generation and precludes potential malignant transformation (19). Senescent cells have been shown to accumulate over the life span of rodents, nonhuman primates, and humans (21). These cells are found primarily in renewable tissues and in tissues that experience prolonged inflammation.
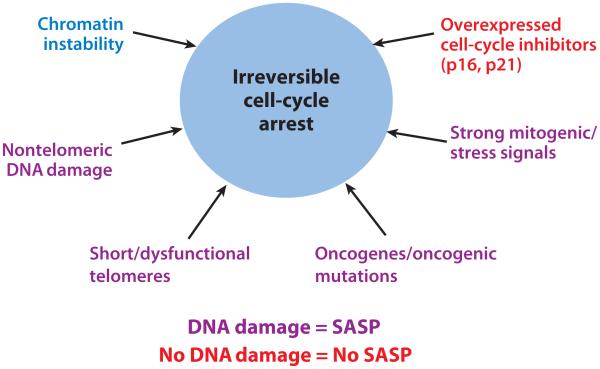
A plethora of stresses can provoke cellular senescence (22, 23). These stresses include telomeric dysfunction (telomere uncapping) resulting from repeated cell division (termed replicative senescence), mitochondrial deterioration, oxidative stress, severe or irreparable DNA damage and chromatin disruption (genotoxic stress), and the expression of certain oncogenes (oncogene-induced senescence) (see Figure 1) (24–31). Stresses that cause cellular senescence can be induced by external or internal chemical and physical insults encountered during the course of the life span, during therapeutic interventions (for example, X-irradiation or chemotherapy), or as a consequence of endogenous processes such as oxidative respiration and mitogenic signals. External mitogenic signals, for example growth-related oncogene alpha (GROα) secretion by tumor cells in close proximity to normal cells (32) or circulating angiotensin II (33, 34), have also been shown to induce cellular senescence. All somatic cells that have the ability to divide can undergo senescence. Regardless of the disparate mechanisms of senescence-inducing stresses, the senescence program is activated once a cell has sensed a critical level of damage or dysfunction. So far, the senescence growth arrest has been shown to depend on the activities of the major tumor-suppressor pathways controlled by p16INK4a and pRB (retinoblastoma protein), as well as by p53. Some of the molecules involved in pathways upstream and downstream of the senescence-associated phenotype have been used as markers to detect senescent cells in culture and in vivo.
Figure 1.
Multiple types of stimuli can provoke cellular senescence and a senescence-associated secretory phenotype (SASP). When irreversible cell-cycle arrest is triggered by severe DNA damage (i.e., dysfunctional telomeres or oncogenic stress), the SASP occurs in senescent cells. However, when a senescent-like phenotype is triggered in cells that overexpress cell-cycle inhibitors such as p16 or p21, cells undergo a growth arrest with many characteristics of senescent cells, but not a SASP.
THE SECRETORY PHENOTYPE OF SENESCENT CELLS
The senescent phenotype is not limited to an arrest of cell proliferation. In fact, a senescent cell is a potentially persisting cell that is metabolically active and has undergone widespread changes in protein expression and secretion, ultimately developing the SASP. This phenotype has also been termed the senescence-messaging secretome (35). We recently provided a large-scale characterization of the SASP, using antibody arrays to quantitatively measure factors secreted by human fibroblasts and epithelial cells (18), as well as mouse fibroblasts (J.P. Coppé & J. Campisi, unpublished data). The potential existence of the SASP was already suggested by large-scale comparative gene (mRNA) expression studies performed on fibroblasts from different-aged donors and different tissues of origin (36–46). Among the cells that have been shown to senesce and secrete biologically active molecules are liver stellate cells (47), endothelial cells (36, 48–51), and epithelial cells of the retinal pigment, mammary gland, colon, lung, pancreas, and prostate (8, 18, 36, 41, 52–56).
Senescence-associated changes in gene expression are specific and mostly conserved within individual cell types. Most differences between the molecular signatures of presenescent and senescent cells entail cell-cycle- and metabolism-related genes, as well as genes encoding the secretory proteins that constitute the SASP. The SASP includes several families of soluble and insoluble factors (see Table 1). These factors can affect surrounding cells by activating various cell-surface receptors and corresponding signal transduction pathways that may lead to multiple pathologies, including cancer. SASP factors can be globally divided into the following major categories: soluble signaling factors (interleukins, chemokines, and growth factors), secreted proteases, and secreted insoluble proteins/extracellular matrix (ECM) components. SASP proteases can have three major effects: (a) shedding of membrane-associated proteins, resulting in soluble versions of membrane-bound receptors, (b) cleavage/degradation of signaling molecules, and/or (c) degradation or processing of the ECM. These activities provide potent mechanisms by which senescent cells can modify the tissue microenvironment. In the following sections, we discuss these SASP subsets and some of their known paracrine effects on nearby cells, with an emphasis on their ability to facilitate cancer progression.
Table 1.
The senescence-associated secretory phenotype (SASP). Factors significantly altered between presenescent and senescent states are listed
| SASP factorsa | Secretory profile of senescent cellsb |
Changes in the SASP due to the loss of p53 and/or gain of oncogenic RAS |
|---|---|---|
| Soluble factors | ||
| Interleukins (IL) | ||
| IL-6 | ↑ | ↑ |
| IL-7 | ↑ | ↑ |
| IL-1a, -1b | ↑ | ↑ |
| IL-13 | ↑ | ↑ |
| IL-15 | ↑ | ↑ |
| Chemokines (CXCL, CCL) | ||
| IL-8 | ↑ | ↑ |
| GRO-a,-b,-gc | ↑ | ↑ |
| MCP-2 | ↑ | ↑ |
| MCP-4 | ↑ | × |
| MIP-1a | ↑ | ↑ |
| MIP-3a | ↑ | × |
| HCC-4 | ↑ | × |
| Eotaxin | × | ↑ |
| Eotaxin-3 | ↑ | ↑ |
| TECK | × | ↑ |
| ENA-78 | × | ↑ |
| I-309 | × | ↑ |
| I-TAC | × | ↓ |
| Other inflammatory factors | ||
| GM-CSE | ↑ | ↑ |
| G-CSE | × | ↑ |
| IFN-γ | × | ↑ |
| BLC | × | ↑ |
| MIF | ↑ | ↓ |
| Growth factors and regulators | ||
| Amphiregulin | ↑ | × |
| Epiregulin | ↑ | × |
| Heregulin | ↑ | × |
| EGF | ↑ or × | ↑ |
| bFGF | ↑ | ↑ |
| HGF | ↑ | × |
| KGF (FGF7) | ↑ | ↑ |
| VEGF | ↑ | × |
| Angiogenin | ↑ | × |
| SCF | ↑ | × |
| SDF-1 | ↑ or × | ↑ |
| PIGF | ↑ | × |
| NGF | × | ↓ |
| IGFBP-2, -3, -4, -6, -7 | ↑ | ↑ or × |
| Proteases and regulators | ||
| MMP-1, -3, -10, -12, -13, -14 | ↑ | ↑ or × |
| TIMP-1 | ↓ or × | × |
| TIMP-2 | ↑ | × |
| PAI-1, -2; tPA; uPA | ↑ | × |
| Cathepsin B | ↑ | × |
| Soluble or shed receptors or ligands | ||
| ICAM-1, -3 | ↑ | × |
| OPG | ↑ | ↑ |
| sTNFRI | ↑ | × |
| TRAIL-R3, Fas, sTNFRII | ↑ | × |
| Fas | ↑ | × |
| uPAR | ↑ | ↑ |
| SGP130 | ↑ | ↑ |
| EGF-R | ↑ | × |
| Nonprotein soluble factors | ||
| PGE2 | ↑ | − |
| Nitric oxide | ↑ | − |
| Reactive oxygen species | Altered | − |
| Insoluble factors (ECM) | ||
| Fibronectin | ↑ | − |
| Collagens | Altered | − |
| Laminin | Altered | − |
Factors are arranged by family.
The secretory changes that occur at senescence are indicated by upward arrows (increase), crosses (no change), and downward arrows (decrease). Loss of p53 or gain of oncogenic RAS increases (upward arrows) or decreases (downward arrows) the secretion of several SASP factors.
Abbreviations: bFGF, basic fibroblast growth factor; ECM, extracellular matrix; EGF, endothelial growth factor; GRO, growth-related oncogene; HGF, hepatocyte growth factor; ICAM, intercellular adhesion molecule; IGFBP, insulin-like growth factor\p=n-\binding protein; MCP, membrane cofactor protein; MMP, matrix metalloproteinase; NGF, nerve growth factor; OPG, osteoprotegerin; PAI, plasminogen activator inhibitor; PGE2, prostaglandin E2; PIGF, placental growth factor; SCF, stem cell factor; SDF, stromal cell\p=n-\derived factor; sTNFR, soluble tumor necrosis factor receptor; t-PA, tissue-type plasminogen activator; TIMP, tissue inhibitor of metalloproteinases; TRAIL, tumor necrosis factor\p=n-\related apoptosis-inducing ligand; u-PA, urokinase-type plasminogen activator; uPAR, u-PA receptor; VEGF, vascular endothelial growth factor.
Soluble Signaling Factors as Major Components of the Senescence-Associated Secretory Phenotype
Senescent cells secrete interleukins, inflammatory cytokines, and growth factors that can affect surrounding cells.
IL-6
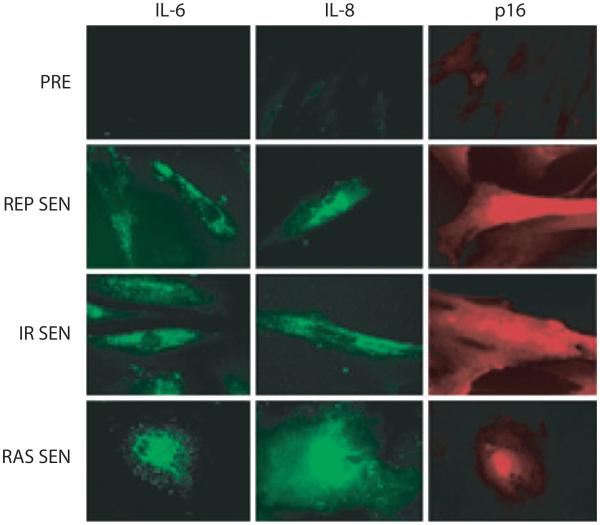
The most prominent cytokine of the SASP is interleukin-6 (IL-6), a pleiotropic proinflammatory cytokine (see Figure 2). IL-6 has been shown to be associated with DNA damage– and oncogenic stress–induced senescence of mouse and human keratinocytes, melanocytes, monocytes, fibroblasts, and epithelial cells (16, 18, 57, 58). Further, IL-6 secretion appears to be directly controlled by persistent DNA-damage signaling (ATM and CHK2), independent of the p53 pathway (59). Through IL-6 expression, senescent cells can directly affect neighboring cells that express the IL-6R (gp80) and gp130 signaling complex at their surface, such as epithelial and endothelial cells of various functions and origins.
Figure 2.
Human fibroblasts, either presenescent (PRE) or senescent (SEN), were immunostained for the inflammatory cytokines interleukin (IL)-6 and IL-8, as well as the senescence marker p16. Cells were made senescent either by replicative exhaustion (REP) or ionizing radiation (IR) or by expression of oncogenic RAS (RAS).
IL-1
Another interleukin signaling pathway demonstrated to be upregulated by senescent cells is that of IL-1 (60, 61). Both IL-1α and -β are overexpressed and secreted by senescent endothelial cells (62), fibroblasts (63, 64), and chemotherapy-induced senescent epithelial cells (53). These cytokines can affect neighboring cells through the cell-surface receptors (IL-1 receptor/Toll-like receptor superfamily), which act primarily to trigger the nuclear factor kappa B and activating protein 1 pathways (65).
Chemokines (CXCL and CCL)
Most senescent cells overexpress IL-8 (CXCL-8) (see Figure 2), along with GROα and GROβ (CXCL-1 and -2; the murine CXCL-1 is named KC) (58, 66, 67). CCL family members that are generally upregulated in senescent cells include MCP-2, -4, and -1 (CCL-8, -13, and -2); HCC-4 (CCL-16); eotaxin-3 (CCL-26); and macrophage inflammatory protein (MIP)-3α and -1α (CCL-20, -3). MCP-3 (CCL-7) is overexpressed by senescent liver stellate cells and by prostate and skin fibroblasts. Fibroblasts induced to senesce by oncogenic RAS secrete high levels of MCP-3 as well as I-309 (CCL-1). In addition, both fibroblasts induced to senesce by RAS and stellate cells induced to senesce by liver damage secrete high levels of another two members of the CXCL family, GCP-2 (CXCL-6) and ENA-78 (CXCL-5). Overexpression of PF-4 (CXCL-4) and SDF-1 (CXCL-12) was observed in senescent prostate fibroblasts (46, 68). Recently, it was shown that cells undergoing oncogene-induced senescence secrete multiple CXCR-2 (IL-8RB)-binding chemokines (15). It was proposed that senescent cells activate a self-amplifying secretory network in which CXCR-2-binding chemokines reinforce growth arrest.
IGF pathway
The insulin-like growth factor (IGF)/IGF receptor network may also contribute to the effect senescent cells exert on their microenvironment. Senescent endothelial, epithelial, and fibroblast cells express high levels of almost all the IGF-binding proteins (IGFBPs), including IGFBP-2, -3, -4, -5, and -6 (18, 69, 70) and their regulators, IGFBP-rP1 and -rP2 [also known as connective tissue growth factor (CTGF)] (44, 71). Recently, activation of the BRAF oncogene in primary fibroblasts was shown to lead to the secretion of IGFBP-7, which acts through autocrine/paracrine pathways to induce senescence and apoptosis in neighboring cells (72).
Other soluble factors
There are additional soluble factors associated with the SASP. For example, inflammatory cytokines such as the colony-stimulating factors (CSFs, including GM-CSF and G-CSF) are secreted at high levels by senescent fibroblasts (18). In addition, osteoprotegerin, a secreted decoy receptor for tumor necrosis factor alpha, is present at high levels in the extracellular milieu of senescent fibroblasts. Other molecules upregulated at senescence include prostaglandin E2 (PGE2) (57, 73) and Cox-2, the enzyme responsible for the production of PGE2 and other prostaglandins.
Extracellular Proteases as an Important Subset of the Senescence-Associated Secretory Phenotype
In addition to secreting soluble signaling cytokines and growth factors, senescent cells also secrete proteases such as matrix metalloproteinases (MMPs).
MMP family
The MMP family members that are consistently upregulated in human and mouse fibroblasts undergoing replicative or stress-induced senescence are stromelysin-1 and -2 (MMP-3 and -10, respectively) and collagenase-1 (MMP-1) (74–78). In some instances, the MMP-1 and -3 produced by senescent cells (79) can also regulate the activity of the soluble factors present in the SASP. For example, these MMPs can cleave MCP-1, -2, and -4 and IL-8 (80). A variety of other CXCL/CCL family members that constitute the SASP can also be cleaved by MMP-9, -2, or -7. These CXCL and CCL cytokines can originate from neighboring cells, such as leukocytes or tumor cells (81).
Serine proteases and their inhibitors
Another family of proteases involved in carcinogenesis and present in the SASP comprises serine proteases and regulators of the plasminogen activation pathway. Members of this family include urokinase- or tissue-type plasminogen activators (uPA or tPA, respectively), the uPA receptor (uPAR), and inhibitors of these serine proteases (PAI-1 and -2) (82). Indeed, a >50-fold increase in plasminogen activator activity has been reported in senescent endothelial cells and lung and skin fibroblasts (83, 84). PAI-1 is also upregulated in fibroblasts and endothelial cells from aged donors (85–87). Like the CXCR-2 cytokines, PAI-1 also seems to reinforce the senescence growth arrest (88).
Extracellular Insoluble Molecules
Fibronectin is a large multidomain glycoprotein found in connective tissue, on cell surfaces, and in plasma and other body fluids. It interacts with a variety of macromolecules, including cell-surface receptors, components of the cytoskeleton, and other ECM molecules. Through its interactions with cell-surface receptors, primarily integrins, fibronectin can affect cell adhesion, survival, growth, and migration. Fibronectin production is upregulated in premature aging Werner syndrome fibroblasts (89). Moreover, cells undergoing senescence in culture and in vivo (90) increase fibronectin expression.
Nonprotein Secretions
As a result of senescence-induced changes in cellular metabolism, senescent cells may exert influences on tissue microenvironments due to the secretion of molecules other than proteins. These molecules include reactive oxygen species and transported ions. For example, senescent cells have been shown to release nitric oxide and reactive oxygen species due to alterations in inducible nitric oxide synthase, endothelial nitric oxide synthase, and superoxide-dismutase activities (91–95). These reactive molecules are known modulators of cellular phenotype, such as the differentiation of monocytes. In addition, these molecules can enhance cancer cell aggressiveness and can promote aging and age-related degeneration (96, 97).
SPECIFICITY OF THE SENESCENCE-ASSOCIATED SECRETORY PHENOTYPE
Despite the fact that a significant number of factors increase their secretion upon senescence, the SASP is not a general or nonspecific upregulator of secretion. The levels of expression of many secreted factors do not change when cells senesce. Interestingly, among these unchanged secreted molecules are anti-inflammatory soluble factors such as IL-2, -4, -10, -11, and -12 (18). Fractalkine (CX3CL-1), GCP-2, GITR, PDGF-BB, and LIGHT (all essential to leukocyte differentiation or proliferation) also remain unchanged when fibroblasts are induced to senesce by X-irradiation, RAS overexpression, or replicative exhaustion. Intriguingly, no factor was significantly downregulated in different senescent states (18).
Despite a specific, conserved core of upregulated and unchanged secreted molecules, different senescent states appear to display some unique features. Whereas cells induced to senesce by replicative exhaustion, telomere disruption, X-irradiation, or chromatin disruption seem to express closely related SASPs (18, 59), fibroblasts induced to senesce by oncogenic RAS oversecrete more GM-CSF, IL-6, -7, -8, -1β, -13, and GROα than do cells induced to senesce by other means. Moreover, such cells secrete high levels of factors such as ENA-78, I-309, G-CSF, and interferon (IFN)-γ that are not secreted by other senescent cells. By contrast, cells induced to senesce by overexpression of the p16INK4a tumor-suppressor protein do not express a SASP despite other hallmarks of senescence (J.P. Coppé, F. Rodier & J. Campisi, unpublished data). Cells that senesce with dysfunctional p53 develop a SASP that resembles the SASP caused by oncogenic RAS (18). Thus, although a core of SASP factors is a feature of all senescent cells (with the exception of p16INK4a-induced senescence), there are variations in the quantity and quality of the SASP that depend on the cell type and senescence inducer.
Another feature of the SASP is its dynamic development over time (18). In culture, cells develop a full SASP >5 days after senescence induction, and the cells’ growth arrests within 24 h of damage. Not all SASP factors begin to be secreted at the same time. This gradual phenotypic transition is a feature conserved between cell types and senescence inducers. Genetic alterations, such as loss of p53 or gain of oncogenic RAS, lead to a more rapid acquisition of the SASP, suggesting that the SASP is a specific program triggered by genotoxic stress.
Finally, mouse senescent fibroblasts also display a SASP. Under standard cell-culture conditions, which include 20% oxygen, mouse cells undergo an arrest that has been termed senescence but that does not include a SASP. By contrast, under physiological 3% oxygen, the mouse SASP closely resembles the human SASP. These findings suggest that the senescent cell secretome is specific and evolutionarily conserved (J.P. Coppé & J. Campisi, unpublished data).
REGULATORY MECHANISMS OF THE SENESCENCE-ASSOCIATED SECRETORY PHENOTYPE
Intracellular Signaling, Transcription, and Chromatin: Locking in the Senescence-Associated Secretory Phenotype
Overall, the gene (mRNA) expression profiles of senescent cells determined by microarrays resemble the profiles of secreted proteins determined by antibody arrays (18; J.P. Coppé & J. Campisi, unpublished data). This finding suggests that the secretory phenotype of senescent cells is at least partly regulated at the transcriptional level. However, because the changes in gene expression that occur at senescence are so widespread, the transcriptional control may well be at the level of chromatin organization, rather than due to changes in specific transcription factors. In fact, dramatic chromatin alterations are known to occur at senescence (98–101). Further support for the idea that gene expression specific to the senescence program may be partially attributed to larger changes in chromatin conformation is suggested by the physical clustering of genes that constitute the SASP (J.P. Coppé & J. Campisi, unpublished data).
Our data suggest that a large proportion of the SASP of senescent fibroblasts is irreversible once established (18). Indeed, senescent human fibroblasts that express low levels of p16INK4a can revert and resume proliferation upon p53 inactivation (102). These reverted cells, however, retain the SASP (18). This may imply that, once senescence is established, unknown mechanisms—potentially related to chromatin alterations—permanently lock the SASP in an irreversible open chromatin confirmation, analogous to the way the p16INK4a/pRB pathway is proposed to lock growth-promoting genes into a heterochromatic state (103). Another implication of these findings is that the senescence-associated cell-cycle arrest and the SASP can be uncoupled (see Figure 1). Further, the SASP is a more permanent characteristic of senescence than is the growth-arrested state.
Cell-Nonautonomous Tumor Suppressors: The Guardians of the Senescence-Associated Secretory Phenotype
The p16INK4a tumor suppressor is a positive regulator of the pRB tumor-suppressor pathway. High levels of ectopic expression of p16INK4a induce senescence. Induction of endogenous p16INK4a is associated with tumor prevention and the general age-associated decline in stem cell and tissue function (104–109). Although p16INK4a is a very efficient inducer of cell-cycle arrest (including the senescence-associated arrest), p16INK4a does not seem to play a major role in the development of the SASP (J.P. Coppé, F. Rodier & J. Campisi, unpublished data). Cells induced to senesce by ectopic p16INK4a expression secrete significantly lower levels of SASP factors compared to cells induced to senesce by most other senescence inducers. Senescence induced by p16INK4a has potential therapeutic applications. As an example, p16INK4a gene therapy for rheumatoid arthritis was demonstrated to efficiently stop the disease evolution and decrease the inflammatory state (110). In cancer, such an approach could take advantage of both the cell-autonomous properties (inhibition of cell growth) and the cell-nonautonomous properties (senescence arrest without a SASP) of p16INK4a. That is, p16INK4a induction/delivery could actively suppress cell proliferation without triggering the proinflammatory SASP.
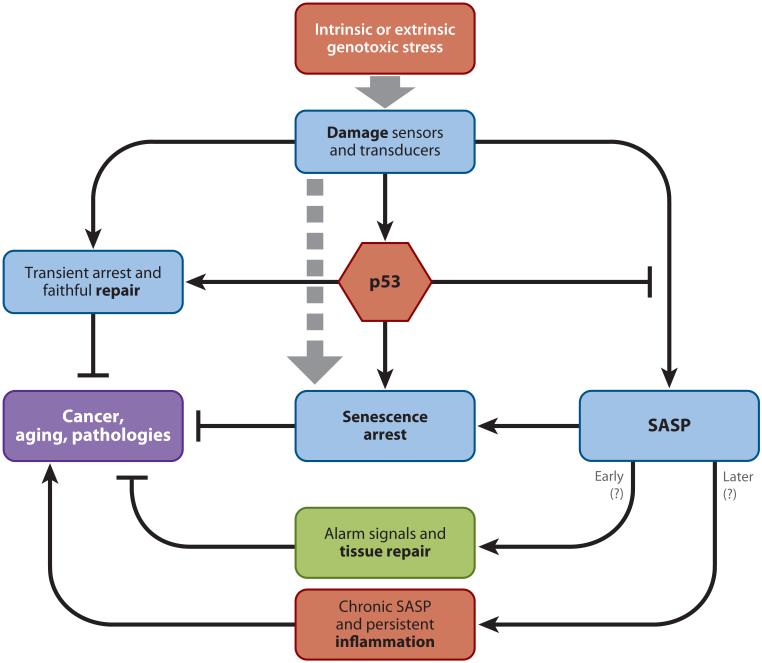
The p53 tumor suppressor can also promote aging (111, 112) and senescence (7, 11, 12) in mice (see Figure 3). Along with mutations in p16INK4a, mutations leading to p53 inactivation occur very frequently in cancer cells; that is, p53 is well known to act as a cell-autonomous tumor suppressor by controlling apoptosis and cell-cycle arrest, both in culture and in vivo. However, p53 mutations have also been found in the stromal vicinity of carcinomas, and this p53-deficient stroma was shown to promote tumorigenesis (32). These data suggest that p53 may have beneficial cell-nonautonomous effects in preventing cancer development (32, 113). Strikingly, we found that p53 actively restrains the SASP, suggesting a potential mechanism by which p53 may suppress tumorigenesis—that is, by restraining the development of a protumorigenic/proinflammatory tissue microenvironment (18). Thus, loss of p53 activity by senescing or damaged fibroblasts greatly enhances the SASP and the stimulatory effects of the SASP on malignant epithelial cells (as discussed further below) (18).
Figure 3.
The DNA damage signaling pathway leads to the activation of the p53 tumor suppressor. Activated p53 triggers cell fate decisions, such as senescence or apoptosis. Depending on the cell context, p53 can suppress cancer through transient cell-cycle arrest and activation of the DNA-repair machinery. Additionally, p53 restrains the senescence-associated secretory phenotype (SASP). Regulation of the SASP by p53 suggests a cell-nonautonomous function of this tumor suppressor. In the short term, the SASP may promote tissue repair. In the long term, it may promote chronic inflammation, which in turn can drive cancer and aging.
EFFECTS ON CELL BEHAVIOR
Factors secreted by senescent cells can promote tumor development in vivo and malignant phenotypes such as proliferation and invasiveness in cell-culture models. These effects have been observed in a number of tissues, including breast (13, 18, 77, 78, 114), skin (115), prostate (18, 116), pancreas (117), and oropharyngial mucosa (14). The effects of the complex SASP are, of course, dependent on the tissue context. Thus, different models show different effects of the SASP. In the following sections, we discuss in greater detail the various behavioral changes cells can undergo when residing in the proximity of senescent cells and how the senescent tissue microenvironment can facilitate tumor initiation and progression.
The Senescence-Associated Secretory Phenotype Promotes Cell Proliferation
One of the most protumorigenic effects of the SASP is to promote the proliferation of epithelial cells.
Breast cancer
In the case of breast epithelial cells, senescent human fibroblasts can stimulate the growth of premalignant and malignant mammary epithelial cells (13, 18, 77). This stimulation may be due in large measure to secretion of GROα, which is a prominent SASP component (J.P. Coppé & J. Campisi, unpublished data). Irradiated stromal cells, which are presumed to be senescent, have been shown to perturb mammary epithelial microenvironment and to fuel inappropriate epithelial cell growth in the mammary gland (114, 118). Furthermore, MMPs secreted by senescent fibroblasts have been shown to be responsible for the higher tumorigenicity of breast epithelial cell xenografts in mice, most likely by allowing mitogenic and chemotactic signals greater access to breast cancer cells (78, 114). In addition to secreted soluble factors, there is evidence that the matrix laid down by senescent cells can also stimulate mammary epithelial cell growth (13).
Prostate cancer
Fibroblasts from the human prostate gland that undergo senescence in culture have been shown to create a local tissue environment that favors prostate epithelial cell hyperproliferation, in part owing to amphiregulin secretion (46). Furthermore, CTGF (or IGFBP-rP2) is upregulated in senescent fibroblasts (44), and this protein was shown to regulate prostate tumor progression in xenografts and to be expressed by the cancer-associated reactive stroma (119). Whereas upregulation of CXCR-4 is observed in most cancer cells, only senescent stromal cells of the prostate display high levels of expression of its ligand SDF-1α (CXCL-12). Thus, SDF-1α secretion by senescent fibroblasts may therefore play a selective role in fueling prostate cancer. It has recently been determined that senescence induced by irradiation in prostate cancer patients is associated with a significantly increased re lease of exosome-like microvesicles (120). This novel secretory phenotype depends on the activation of p53. Finally, the propensity of prostate cancer patients to relapse after chemotherapy may be due to the accumulation of senescent tumor cells with inflammatory characteristics (18).
Other carcinomas
In the skin, unidentified factors secreted by human fibroblasts were shown to be capable of inducing clonal expansion of keratinocytes (121). In addition, senescent endometrial fibroblasts promoted anchorage-independent epithelial cell growth, primarily because of IL-1 oversecretion (64). In the orobucal cavity, tobacco-driven senescence of supportive stromal cells was shown to stimulate the hyperplastic growth of epithelial cells and was associated with the loss of tight junctions and epithelial integrity (14).
Melanoma
Melanocytic nevi (moles) are often composed of senescent melanocytes that are induced owing to oncogenic mutations in BRAF (V600E mutations) (9). Only rare cell variants in nevi can evolve into melanoma. Malignant melanocytes express high levels of the CXCR-2 receptor (122) and can be stimulated to grow by its ligands GROα (123) and IL-8 (124). Given that both GROα and IL-8 form part of the core SASP, the senescent microenvironment may therefore stimulate the proliferation of rare premalignant cells in nevi, thereby leading to the development of melanoma.
Other tumor-associated cells
During angiogenesis, endothelial cells can undergo proliferation, which is stimulated by vascular endothelial growth factor (VEGF), IL-8, I-309, and eotaxin (125–127). The proangiogenic effects of the SASP were shown in vivo in mouse xenografts of breast cancer cells. The blood vessel density was significantly higher when the tumors developed in the presence of senescent but not presenescent fibroblasts (127). RAS-driven tumors are also known to contain significant numbers of senescent cells (8), and these tumors are also highly vascularized (128). Many of the SASP factors can also affect leukocyte proliferation during the course of cancer development. For example, IL-7 directly promotes lymphocyte proliferation in peripheral tissues, and GM-CSF stimulates myeloid suppressor cells, which are known to have important immunosuppressive functions that affect cancer progression (129).
The Senescence-Associated Secretory Phenotype Stimulates Cell Motility (Invasion, Migration, Metastasis)
Senescent cells secrete an array of factors that can create a gradient to promote cell migration and invasion.
Epithelial cells
In pancreatic cancer, hepatocyte growth factor (HGF), and to a lesser degree basic fibroblast growth factor (bFGF), promotes cancer cell invasion in culture and can potentially drive cancer dissemination in vivo (117, 130). In breast cancer, high levels of IL-6 and -8 secreted by senescent fibroblasts are responsible for enhancing the invasiveness of a panel of cancer cell lines in cell-culture models (18). Moreover, the secretion of MMP-2 and -3 by senescent cells can also promote the invasion of multiple epithelial cell types (77, 78, 114, 131, 132). Other proteases, such as uPA and its regulator (PAI-1), are likewise implicated in cancer cell invasion. Senescent stromal cells may promote an epithelial-to-mesenchymal transition (18), which is an important phenotypic switch that enables cancer cells to migrate and invade. Through use of co-cultures of smokeless tobacco extract–exposed fibroblasts and human epidermal keratinocytes, factors secreted by extract-modified fibroblasts increased the invasiveness of partially transformed epithelial cells in conjunction with a loss of E-cadherin, zonula occludens 1, and involucrin expression (14). Thus, senescent cells and the SASP can induce phenotypes in nearby human epithelial cells that are common during cancer progression.
Endothelial cells
In cell-culture models, endothelial cells are induced to migrate by factors secreted by senescent fibroblasts (127). This is in part due to VEGF secretion and chemokine gradients set up by senescent cells (133). Neoangiogenesis, which is dependent on endothelial cell motility and invasion, is enhanced in xenograft models containing senescent fibroblasts (127). Further, it is known that IL-1, a SASP component, activates the endothelium and consequently increases the adhesive potential of cancer cells to vessel walls (134). Thus, senescent cells may promote extravasation of cancer cells to secondary metastatic sites. However, the effects of senescent cells on angiogenesis may depend on cell type. For example, senescent keratinocytes oversecrete maspin, which displays paracrine antiangiogenic activity and acts as a dominant inhibitor of endothelial cell migration (135).
Leukocytes
Senescent fibroblasts may promote leukocyte recruitment because they chronically release chemokines (136). In p53-deficient RAS-driven tumors induced to senesce through reestablishment of p53 function (12), innate immune cells were shown to migrate into the vicinity of the senescent tumor area. CSF-1, CXCL-1, or MCP-1 and ICAM-1 transcripts were found to be higher in these senescent tumor masses, and they may be responsible for the immune response. For example, neutrophils express CXCR-1, -2, and -4 to sense their microenvironment and to invade tissues; eosinophils use the broad-spectrum receptor CCR-3 to fulfill their function; monocytes use CCR-1, -2, and -5, CXCR-4, and CX3CR1 to extravasate and enter peripheral sites, where they differentiate; natural killer cells express CCR-2 and -5, CXCR-4, CX3CR1, and XCR1; and immature myeloid dendritic cells display CCR-1, -2, -5, and -6 and CXCR-4, which facilitate their transport, migration, and function (136–139).
The Senescence-Associated Secretory Phenotype Regulates Cell Differentiation
The factors secreted by senescent cells can alter the differentiation status of neighboring cells.
Epithelial cells
Senescent human and mouse fibroblasts disrupt the differentiation of mammary epithelial cells and inhibit the expression of differentiation markers (77, 114). This activity is due in large measure to the secretion of MMP-3 by the senescent cells. Furthermore, weakly tumorigenic pancreatic (117) and mammary (18) epithelial cells undergo morphologic changes in culture that resemble an epithelial-to-mesenchymal transition in the presence of a senescent conditioned medium. The effect on mammary epithelial cells is attributable to IL-6 and -8 (18), as well as to HGF, uPAR, and MMPs (77), all of which can disrupt epithelial cell clusters and stimulate dedifferentiation in culture and in vivo (140–142).
Endothelial cells
Strikingly, no angiostatic factors have been reported among SASP constituents (e.g., IFN-γ, TSP-1, MIG, PF4, IP-10, IL-4 and -12, and endostatin). This contrasts with the largely proangiogenic profile of the SASP (which includes IL-8, MCP-1 and -2, GROs, PGE2, VEGF, EGF, CSFs, u-/tPA, MMPs, fibronectin, and laminin) (143). Furthermore, there may be an amplifying activation loop because senescent stromal cells secrete MCPs, CSFs, MIPs, GROs, and CXCLs, which in turn recruit inflammatory and immune cells that also secrete proangiogenic factors (VEGFs, IL-8, and MMPs). Thus, senescent cells are poised to support the differentiation of a new vasculature around and within a progressing tumor.
The Senescence-Associated Secretory Phenotype Affects Leukocyte Infiltration and Tumor Immunology
No anti-inflammatory factors (e.g., IFN-α, IFN-γ, IL-3, and IL-5) are significantly secreted by senescent fibroblasts, and some of these factors are even downregulated upon senescence (e.g., IL-2 and -12). Nonetheless, some reports show that massive amounts of either MCP-1 or IL-8, which are prominent components of the SASP, lead to tumor destruction (144, 145). Senescent fibroblasts may influence the macrophage balance in the tumor environment. Molecules that are implicated in the recruitment and differentiation of circulating monocytes to tumor sites also happen to be overexpressed by senescent fibroblasts (146). These molecules can lead to an inadequate immune response within the close proximity of senescent cells. Senescent fibroblasts may affect lymphocytic populations infiltrating the tumor. Specific T cell populations associated with tumor progression (i.e., Th2 and regulatory T cells) respond to inflammatory cytokines that are commonly present in the fibroblast SASP. Other cells of the specific and innate immune system, such as natural killer cells, neutrophils, eosinophils, dendritic cells, and B cells, are also subject to regulation by cytokines that are produced by senescent fibroblasts.
CONCLUSIONS AND FUTURE DIRECTIONS
Most insoluble components of the ECM are enzymatic targets of secreted proteases. Therefore, the senescence-associated changes in proteolytic activities could affect the physical properties of the tissue structure around cells. In particular, the accumulation of senescent cells could lessen the supportive role of the ECM, globally diminishing tissue tension and elasticity. In addition, the relaxed tissue structure and higher levels of MMPs may help tumor cells migrate and invade through the ECM, thus enabling metastasis. The panel of proteases secreted by senescent cells extensively overlaps with those found in malignant tumors. Further, senescence-induced alterations in the secretion of interleukins, chemokines, growth factors, proteases, and associated processing activities tend to establish the SASP as protumorigenic.
Overall, senescence is a molecular program with a unique phenotypic outcome. How its extracellular molecular signature is activated and maintained and the extent to which it influences the tissue milieu in healthy tissues, aged tissues, and diseased tissues are some of the many questions that remain unanswered. However, even with our currently limited knowledge of the SASP and its potential effects on carcinogenesis, promising new strategies for cancer therapies are possible. For example, restoring the activity of tumor-suppressor proteins is an attractive, potentially powerful therapeutic approach. Taking into account our present understanding of the cell-nonautonomous effects of tumor-suppressor genes such as p53, small chemicals that can pharmacologically restore their normal function would help reestablish the proper tissue and cell signals, thereby stimulating cancer regression (147–150). Such approaches could stimulate cancer elimination for two reasons: First, they would limit inflammation and thus possibly allow proper tissue repair; second, they would directly promote the immune-mediated clearance of cells that drive cancer progression.
Footnotes
DISCLOSURE STATEMENT
A.K. is CEO and CSO of StemLifeLine, Inc., a biotech company that uses stem cells for drug screening and therapy. The other authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–67. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vakkila J, Lotze MT. Inflammation and necrosis promote tumor growth. Nat. Rev. Immunol. 2004;4:641–48. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 3.Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell. 2005;7:513–20. doi: 10.1016/j.ccr.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 4.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 5.Narita M, Lowe SW. Senescence comes of age. Nat. Med. 2005;11:920–22. doi: 10.1038/nm0905-920. [DOI] [PubMed] [Google Scholar]
- 6.Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–65. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Trotman LC, Shaffer D, Lin H, Dotan ZA, et al. Critical role of p53 dependent cellular senescence in suppression of Pten deficient tumourigenesis. Nature. 2005;436:725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collado M, Gil J, Efeyan A, Guerra C, Schumacher AJ, et al. Tumor biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 9.Michaloglou C, Vredeveld LCW, Soengas MS, Denoyelle C, Kuilman T, et al. BRAFE600-associated senescence-like cell cycle arrest of human nevi. Nature. 2005;436:720–24. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 10.Courtois-Cox S, Genther Williams SM, Reczek EE, Johnson BW, McGillicuddy LT, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–72. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, et al. Restoration of p53 function leads to tumor regression in vivo. Nature. 2007;445:661–65. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 12.Xue W, Zender L, Miething C, Dickins RA, Hernando E, et al. Senescence and tumor clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krtolica A, Parrinello S, Lockett S, Desprez P, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl. Acad. Sci. USA. 2001;98:12072–77. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppé JP, Boysen M, Sun CH, Wong BJ, Kang MK, et al. A role for fibroblasts in mediating the effects of tobacco-induced epithelial cell growth and invasion. Mol. Cancer Res. 2008;6:1085–98. doi: 10.1158/1541-7786.MCR-08-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Kuilman T, Michaloglou C, Vredeveld LCW, Douma S, van Doorn R, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Green MR. Senescence: not just for tumor suppression. Cell. 2008;134:562–64. doi: 10.1016/j.cell.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Coppé JP, Patil CK, Rodier F, Sun Y, Munoz DP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell. Biol. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 20.Young AR, Narita M. SASP reflects senescence. EMBO Rep. 2009;10:228–30. doi: 10.1038/embor.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimri GP, Lee X, Basile G, Acosta M, Scott G, et al. A novel biomarker identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA. 1995;92:9363–67. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben-Porath I, Weinberg RA. When cells get stressed: an integrative view of cellular senescence. J. Clin. Investig. 2004;113:8–13. doi: 10.1172/JCI200420663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005;37:961–76. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt CA. Senescence, apoptosis and therapy—cutting the lifelines of cancer. Nat. Rev. Cancer. 2003;3:286–95. doi: 10.1038/nrc1044. [DOI] [PubMed] [Google Scholar]
- 25.Campisi J. Senescent cells, tumor suppression and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Martin GM. Genetic modulation of senescent phenotypes in Homo sapiens. Cell. 2005;120:523–32. doi: 10.1016/j.cell.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 27.Chien KR, Karsenty G. Longevity and lineages: toward the integrative biology of degenerative diseases in heart, muscle, and bone. Cell. 2005;120:533–44. doi: 10.1016/j.cell.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Braig M, Schmitt CA. Oncogene-induced senescence: putting the brakes on tumor development. Cancer Res. 2006;66:2881–84. doi: 10.1158/0008-5472.CAN-05-4006. [DOI] [PubMed] [Google Scholar]
- 31.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat. Rev. Cancer. 2006;6:472–76. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 32.Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–11. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: Is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–62. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 34.Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–60. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 35.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 36.Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr. Biol. 1999;9:939–45. doi: 10.1016/s0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- 37.Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science. 2000;287:2486–92. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- 38.Park WY, Hwang CI, Kang MJ, Seo JY, Chung JH, et al. Gene profile of replicative senescence is different from progeria or elderly donor. Biochem. Biophys. Res. Commun. 2001;282:934–39. doi: 10.1006/bbrc.2001.4632. [DOI] [PubMed] [Google Scholar]
- 39.Kyng KJ, May A, Brosh RM, Jr, Cheng WH, Chen C, et al. The transcriptional response after oxidative stress is defective in Cockayne syndrome group B cells. Oncogene. 2003;22:1135–49. doi: 10.1038/sj.onc.1206187. [DOI] [PubMed] [Google Scholar]
- 40.Kulaeva OI, Draghici S, Tang L, Kraniak JM, Land SJ, Tainsky MA. Epigenetic silencing of multiple interferon pathway genes after cellular immortalization. Oncogene. 2003;22:4118–27. doi: 10.1038/sj.onc.1206594. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Pan KH, Cohen SN. Senescence-specific gene expression fingerprints reveal cell-type-dependent physical clustering of up-regulated chromosomal loci. Proc. Natl. Acad. Sci. USA. 2003;100:3251–56. doi: 10.1073/pnas.2627983100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Csoka AB, English SB, Simkevich CP, Ginzinger DG, Butte AJ, et al. Genome-scale expression profiling of Hutchinson-Gilford progeria syndrome reveals widespread transcriptional misregulation leading to mesodermal/mesenchymal defects and accelerated atherosclerosis. Aging Cell. 2004;3:235–43. doi: 10.1111/j.1474-9728.2004.00105.x. [DOI] [PubMed] [Google Scholar]
- 43.Yoon IK, Kim HK, Kim YK, Song IH, Kim W, et al. Exploration of replicative senescence-associated genes in human dermal fibroblasts by cDNA microarray technology. Exp. Gerontol. 2004;39:1369–78. doi: 10.1016/j.exger.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Kim KH, Park GT, Lim YB, Rue SW, Jung JC, et al. Expression of connective tissue growth factor, a biomarker in senescence of human diploid fibroblasts, is up-regulated by a transforming growth factor-β-mediated signaling pathway. Biochem. Biophys. Res. Commun. 2004;318:819–25. doi: 10.1016/j.bbrc.2004.04.108. [DOI] [PubMed] [Google Scholar]
- 45.Kyng KJ, May A, Stevnsner T, Becker KG, Kolvra S, Bohr VA. Gene expression responses to DNA damage are altered in human aging and in Werner syndrome. Oncogene. 2005;24:5026–42. doi: 10.1038/sj.onc.1208692. [DOI] [PubMed] [Google Scholar]
- 46.Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- 47.Schnabl B, Purbeck CA, Choi YH, Hagedorn CH, Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–64. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, Brodsky SV, Goligorsky DM, Hampel DJ, Li H, et al. Glycated collagen I induces premature senescence-like phenotypic changes in endothelial cells. Circ. Res. 2002;90:1290–98. doi: 10.1161/01.res.0000022161.42655.98. [DOI] [PubMed] [Google Scholar]
- 49.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17:1183–85. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 50.Kamino H, Hiratsuka M, Toda T, Nishigaki R, Osaki M, et al. Searching for genes involved in arteriosclerosis: proteomic analysis of cultured human umbilical vein endothelial cells undergoing replicative senescence. Cell Struct. Funct. 2003;28:495–503. doi: 10.1247/csf.28.495. [DOI] [PubMed] [Google Scholar]
- 51.Eman MR, Regan-Klapisz E, Pinkse MW, Koop IM, Haverkamp J, et al. Protein expression dynamics during replicative senescence of endothelial cells studied by 2-D difference in gel electrophoresis. Electrophoresis. 2006;27:1669–82. doi: 10.1002/elps.200500746. [DOI] [PubMed] [Google Scholar]
- 52.Schwarze SR, DePrimo SE, Grabert LM, Fu VX, Brooks JD, Jarrard DF. Novel pathways associated with bypassing cellular senescence in human prostate epithelial cells. J. Biol. Chem. 2002;277:14877–83. doi: 10.1074/jbc.M200373200. [DOI] [PubMed] [Google Scholar]
- 53.Chang BD, Swift ME, Shen M, Fang J, Broude EV, Roninson IB. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc. Natl Acad. Sci. USA. 2002;99:389–94. doi: 10.1073/pnas.012602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Untergasser G, Koch HB, Menssen A, Hermeking H. Characterization of epithelial senescence by serial analysis of gene expression: identification of genes potentially involved in prostate cancer. Cancer Res. 2002;62:6255–62. [PubMed] [Google Scholar]
- 55.Zhang H, Herbert BS, Pan KH, Shay JW, Cohen SN. Disparate effects of telomere attrition on gene expression during replicative senescence of human mammary epithelial cells cultured under different conditions. Oncogene. 2004;19:6193–98. doi: 10.1038/sj.onc.1207834. [DOI] [PubMed] [Google Scholar]
- 56.Schwarze SR, Fu VX, Desotelle JA, Kenowski ML, Jarrard DF. The identification of senescence-specific genes during the induction of senescence in prostate cancer cells. Neoplasia. 2005;7:816–23. doi: 10.1593/neo.05250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu SY, Chang KW, Liu CJ, Tseng YH, Lu HH, et al. Ripe areca nut extract induces G1 phase arrests and senescence-associated phenotypes in normal human oral keratinocyte. Carcinogenesis. 2006;27:1273–84. doi: 10.1093/carcin/bgi357. [DOI] [PubMed] [Google Scholar]
- 58.Sarkar D, Lebedeva IV, Emdad L, Kang DC, Baldwin AS, Jr, Fisher PB. Human polynucleotide phosphorylase (hPNPaseold-35): a potential link between aging and inflammation. Cancer Res. 2004;64:7473–78. doi: 10.1158/0008-5472.CAN-04-1772. [DOI] [PubMed] [Google Scholar]
- 59.Rodier F, Coppé JP, Patil CK, Hoeijmakers WAM, Munoz D, et al. Persistent DNA damage signaling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009;11:973–79. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garfinkel S, Brown S, Wessendorf JH, Maciag T. Post-transcriptional regulation of interleukin 1α in various strains of young and senescent human umbilical vein endothelial cells. Proc. Natl. Acad. Sci. USA. 1994;91:1559–63. doi: 10.1073/pnas.91.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McLachlan JA, Serkin CD, Morrey-Clark KM, Bakouche O. Immunological functions of aged human monocytes. Pathobiology. 1995;63:148–59. doi: 10.1159/000163946. [DOI] [PubMed] [Google Scholar]
- 62.Maier JAM, Voulalas P, Roeder D, Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1α antisense oligomer. Science. 1990;249:1570–74. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- 63.Kumar S, Millis AJ, Baglioni C. Expression of interleukin 1–inducible genes and production of interleukin 1 by aging human fibroblasts. Proc. Natl. Acad. Sci. USA. 1992;89:4683–87. doi: 10.1073/pnas.89.10.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palmieri D, Watson JM, Rinehart CA. Age-related expression of PEDF/EPC-1 in human endometrial stromal fibroblasts: implications for interactive senescence. Exp. Cell Res. 1999;247:142–47. doi: 10.1006/excr.1998.4341. [DOI] [PubMed] [Google Scholar]
- 65.Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 2001;22:328–36. doi: 10.1016/s1471-4906(01)01941-x. [DOI] [PubMed] [Google Scholar]
- 66.Castro P, Giri D, Lamb D, Ittmann M. Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate. 2003;55:30–38. doi: 10.1002/pros.10204. [DOI] [PubMed] [Google Scholar]
- 67.Bode-Boger SM, Scalera F, Martens-Lobenhoffer J. Asymmetric dimethylarginine (ADMA) accelerates cell senescence. Vasc. Med. 2005;10(Suppl. 1):S65–71. doi: 10.1177/1358836X0501000110. [DOI] [PubMed] [Google Scholar]
- 68.Begley L, Monteleon C, Shah RB, Macdonald JW, Macoska JA. CXCL12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell. 2005;4:291–98. doi: 10.1111/j.1474-9726.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 69.Wang S, Moerman EJ, Jones RA, Thweatt R, Goldstein S. Characterization of IGFBP-3, PAI-1 and SPARC mRNA expression in senescent fibroblasts. Mech. Ageing Dev. 1996;92:121–32. doi: 10.1016/s0047-6374(96)01814-3. [DOI] [PubMed] [Google Scholar]
- 70.Grillari J, Hohenwarter O, Grabherr RM, Katinger H. Subtractive hybridization of mRNA from early passage and senescent endothelial cells. Exp. Gerontol. 2000;35:187–97. doi: 10.1016/s0531-5565(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 71.Lopez-Bermejo A, Buckway CK, Devi GR, Hwa V, Plymate SR, et al. Characterization of insulin-like growth factor-binding protein-related proteins (IGFBP-rPs) 1, 2, and 3 in human prostate epithelial cells: potential roles for IGFBP-rP1 and 2 in senescence of the prostatic epithelium. Endocrinology. 2000;141:4072–80. doi: 10.1210/endo.141.11.7783. [DOI] [PubMed] [Google Scholar]
- 72.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–74. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang NN, Wang DJ, Heppel LA. Stimulation of aged human lung fibroblasts by extracellular ATP via suppression of arachidonate metabolism. J. Biol. Chem. 1993;268:10789–95. [PubMed] [Google Scholar]
- 74.West MD, Pereira-Smith OM, Smith JR. Replicative senescence of human skin fibroblasts correlates with a loss of regulation and overexpression of collagenase activity. Exp. Cell Res. 1989;184:138–47. doi: 10.1016/0014-4827(89)90372-8. [DOI] [PubMed] [Google Scholar]
- 75.Millis AJT, Hoyle M, McCue HM, Martini H. Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in diploid human fibroblasts. Exp. Cell Res. 1992;201:373–79. doi: 10.1016/0014-4827(92)90286-h. [DOI] [PubMed] [Google Scholar]
- 76.Zeng G, Millis AJ. Differential regulation of collagenase and stromelysin mRNA in late passage cultures of human fibroblasts. Exp. Cell Res. 1996;222:150–56. doi: 10.1006/excr.1996.0019. [DOI] [PubMed] [Google Scholar]
- 77.Parrinello S, Coppé JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J. Cell Sci. 2005;118:485–96. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–26. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 79.Hornebeck W, Maquart FX. Proteolyzed matrix as a template for the regulation of tumor progression. Biomed. Pharmacother. 2003;57:223–30. doi: 10.1016/s0753-3322(03)00049-0. [DOI] [PubMed] [Google Scholar]
- 80.McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–67. [PubMed] [Google Scholar]
- 81.Van Den Steen PE, Wuyts A, Husson SJ, Proost P, Van Damme J, Opdenakker G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur. J. Biochem. 2003;270:3739–49. doi: 10.1046/j.1432-1033.2003.03760.x. [DOI] [PubMed] [Google Scholar]
- 82.Blasi F, Carmeliet P. uPAR: a versatile signaling orchestrator. Nat. Rev. Mol. Cell Biol. 2002;3:932–43. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 83.Comi P, Chiaramonte R, Maier JA. Senescence-dependent regulation of type 1 plasminogen activator inhibitor in human vascular endothelial cells. Exp. Cell Res. 1995;219:304–8. doi: 10.1006/excr.1995.1232. [DOI] [PubMed] [Google Scholar]
- 84.West MD, Shay JW, Wright WE, Linskens MH. Altered expression of plasminogen activator and plasminogen activator inhibitor during cellular senescence. Exp. Gerontol. 1996;31:175–93. doi: 10.1016/0531-5565(95)02013-6. [DOI] [PubMed] [Google Scholar]
- 85.Mu XC, Higgins PJ. Differential growth state–dependent regulation of plasminogen activator inhibitor type-1 expression in senescent IMR-90 human diploid fibroblasts. J. Cell Physiol. 1995;165:647–57. doi: 10.1002/jcp.1041650324. [DOI] [PubMed] [Google Scholar]
- 86.Mu XC, Staiano-Coico L, Higgins PJ. Increased transcription and modified growth state–dependent expression of the plasminogen activator inhibitor type-1 gene characterize the senescent phenotype in human diploid fibroblasts. J. Cell Physiol. 1998;174:90–98. doi: 10.1002/(SICI)1097-4652(199801)174:1<90::AID-JCP10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 87.Martens JW, Sieuwerts AM, Vries JB, Bosma PT, Swiggers SJ, et al. Aging of stromal-derived human breast fibroblasts might contribute to breast cancer progression. Thromb. Haemost. 2003;89:393–404. [PubMed] [Google Scholar]
- 88.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 2006;8:877–84. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rasoamanantena P, Thweatt R, Labat-Robert J, Goldstein S. Altered regulation of fibronectin gene expression in Werner syndrome fibroblasts. Exp. Cell Res. 1994;213:121–27. doi: 10.1006/excr.1994.1181. [DOI] [PubMed] [Google Scholar]
- 90.Kumazaki T, Kobayashi M, Mitsui Y. Enhanced expression of fibronectin during in vivo cellular aging of human vascular endothelial cells and skin fibroblasts. Exp. Cell Res. 1993;205:396–402. doi: 10.1006/excr.1993.1103. [DOI] [PubMed] [Google Scholar]
- 91.Sato I, Morita I, Kaji K, Ikeda M, Nagao M, Murota S. Reduction of nitric oxide producing activity associated with in vitro aging in cultured human umbilical vein endothelial cell. Biochem. Biophys. Res. Commun. 1993;195:1070–76. doi: 10.1006/bbrc.1993.2153. [DOI] [PubMed] [Google Scholar]
- 92.Lee AC, Fenster BE, Ito H, Takeda K, Bae NS, et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 1999;274:7936–40. doi: 10.1074/jbc.274.12.7936. [DOI] [PubMed] [Google Scholar]
- 93.Van Der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, et al. Enhanced peroxynitrite formation is associated with vascular aging. J. Exp. Med. 2000;192:1731–44. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Macip S, Igarashi M, Fang L, Chen A, Pan ZQ, et al. Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J. 2002;21:2180–88. doi: 10.1093/emboj/21.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xin MG, Zhang J, Block ER, Patel JM. Senescence-enhanced oxidative stress is associated with deficiency of mitochondrial cytochrome c oxidase in vascular endothelial cells. Mech. Ageing Dev. 2003;124:911–19. doi: 10.1016/s0047-6374(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 96.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 97.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–74. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 98.Funayama R, Ishikawa F. Cellular senescence and chromatin structure. Chromosoma. 2007;116:431–40. doi: 10.1007/s00412-007-0115-7. [DOI] [PubMed] [Google Scholar]
- 99.Mehta IS, Figgitt M, Clements CS, Kill IR, Bridger JM. Alterations to nuclear architecture and genome behavior in senescent cells. Ann. N.Y. Acad. Sci. 2007;1100:250–63. doi: 10.1196/annals.1395.027. [DOI] [PubMed] [Google Scholar]
- 100.Narita M. Cellular senescence and chromatin organisation. Br. J. Cancer. 2007;96:686–91. doi: 10.1038/sj.bjc.6603636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adams PD. Remodeling chromatin for senescence. Aging Cell. 2007;6:425–27. doi: 10.1111/j.1474-9726.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 102.Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–22. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Narita M, Nunez S, Heard E, Narita M, Lin AW, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–16. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 104.Itahana K, Zou Y, Itahana Y, Martinez JL, Beausejour CM, et al. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol. Cell. Biol. 2003;23:389–401. doi: 10.1128/MCB.23.1.389-401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol. Cell. 2004;14:501–13. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 106.Benanti JA, Galloway DA. Normal human fibroblasts are resistant to RAS-induced senescence. Mol. Cell. Biol. 2004;24:2842–52. doi: 10.1128/MCB.24.7.2842-2852.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–57. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 108.Janzen V, Forkert R, Fleming H, Saito Y, Waring MT, et al. Stem cell aging modified by the cyclin-dependent kinase inhibitor, p16INK4a. Nature. 2006;443:421–26. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 109.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, et al. Increasing Ink4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–52. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taniguchi K, Kohsaka H, Inoue N, Terada Y, Ito H, et al. Induction of the p16INK4a senescence gene as a new therapeutic strategy for the treatment of rheumatoid arthritis. Nat. Med. 1999;5:760–67. doi: 10.1038/10480. [DOI] [PubMed] [Google Scholar]
- 111.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, et al. p53 mutant mice that display early aging-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 112.Maier B, Gluba W, Bernier B, Turner T, Mohammad K, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–19. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kiaris H, Chatzistamou I, Trimis G, Frangou-Plemmenou M, Pafiti-Kondi A, Kalofoutis A. Evidence for nonautonomous effect of p53 tumor suppressor in carcinogenesis. Cancer Res. 2005;65:1627–30. doi: 10.1158/0008-5472.CAN-04-3791. [DOI] [PubMed] [Google Scholar]
- 114.Tsai KK, Chuang EY, Little JB, Yuan ZM. Cellular mechanisms for low-dose ionizing radiation-induced perturbation of the breast tissue microenvironment. Cancer Res. 2005;65:6734–44. doi: 10.1158/0008-5472.CAN-05-0703. [DOI] [PubMed] [Google Scholar]
- 115.Sun P, Yoshizuka N, New L, Moser BA, Li Y, et al. PRAK is essential for ras-induced senescence and tumor suppression. Cell. 2007;128:295–308. doi: 10.1016/j.cell.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 116.Choi J, Shendrik I, Peacocke M, Peehl D, Buttyan R, et al. Expression of senescence-associated β-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology. 2000;56:160–66. doi: 10.1016/s0090-4295(00)00538-0. [DOI] [PubMed] [Google Scholar]
- 117.Ohuchida K, Mizumoto K, Murakami M, Qian LW, Sato N, et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004;64:3215–22. doi: 10.1158/0008-5472.can-03-2464. [DOI] [PubMed] [Google Scholar]
- 118.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–60. [PubMed] [Google Scholar]
- 119.Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65:8887–95. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]
- 120.Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68:7864–71. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dilley TK, Bowden GT, Chen QM. Novel mechanisms of sublethal oxidant toxicity: induction of premature senescence in human fibroblasts confers tumor promoter activity. Exp. Cell Res. 2003;290:38–48. doi: 10.1016/s0014-4827(03)00308-2. [DOI] [PubMed] [Google Scholar]
- 122.Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J. Leukoc. Biol. 2002;72:9–18. [PMC free article] [PubMed] [Google Scholar]
- 123.Balentien E, Mufson BE, Shattuck RL, Derynck R, Richmond A. Effects of MGSA/GROα on melanocyte transformation. Oncogene. 1991;6:1115–24. [PubMed] [Google Scholar]
- 124.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J. Immunol. 1993;151:2667–75. [PubMed] [Google Scholar]
- 125.Bernardini G, Spinetti G, Ribatti D, Camarda G, Morbidelli L, et al. I-309 binds to and activates endothelial cell functions and acts as an angiogenic molecule in vivo. Blood. 2000;96:4039–45. [PubMed] [Google Scholar]
- 126.Salcedo R, Young HA, Ponce ML, Ward JM, Kleinman HK, et al. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J. Immunol. 2001;166:7571–78. doi: 10.4049/jimmunol.166.12.7571. [DOI] [PubMed] [Google Scholar]
- 127.Coppé JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 2006;281:29568–74. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 128.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–58. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 129.Frey AB. Myeloid suppressor cells regulate the adaptive immune response to cancer. J. Clin. Investig. 2006;116:2587–90. doi: 10.1172/JCI29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 131.Camphausen K, Moses MA, Beecken WD, Khan MK, Folkman J, O’Reilly MS. Radiation therapy to a primary tumor accelerates metastatic growth in mice. Cancer Res. 2001;61:2207–11. [PubMed] [Google Scholar]
- 132.Qian LW, Mizumoto K, Urashima T, Nagai E, Maehara N, et al. Radiation-induced increase in invasive potential of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor, CGS27023. Clin. Cancer Res. 2002;8:1223–27. [PubMed] [Google Scholar]
- 133.Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumor angiogenesis. Eur. J. Cancer. 2006;42:768–78. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 134.Orr FW, Wang HH. Tumor cell interactions with the microvasculature: a rate-limiting step in metastasis. Surg. Oncol. Clin. N. Am. 2001;10:357–81. [PubMed] [Google Scholar]
- 135.Nickoloff BJ, Lingen MW, Chang BD, Shen M, Swift M, et al. Tumor suppressor maspin is up-regulated during keratinocyte senescence, exerting a paracrine antiangiogenic activity. Cancer Res. 2004;64:2956–61. doi: 10.1158/0008-5472.can-03-2388. [DOI] [PubMed] [Google Scholar]
- 136.Mantovani A. Chemokines in neoplastic progression. Semin. Cancer Biol. 2004;14:147–48. doi: 10.1016/j.semcancer.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 137.Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat. Rev. Immunol. 2002;2:175–84. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 138.Balkwill F. Cancer and the chemokine network. Nat. Rev. Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 139.Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin. Cancer Biol. 2006;16:38–52. doi: 10.1016/j.semcancer.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 140.Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor–induced adherens junction disassembly. Mol. Biol. Cell. 1998;9:2185–200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Paumelle R, Tulasne D, Kherrouche Z, Plaza S, Leroy C, et al. Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway. Oncogene. 2002;21:2309–19. doi: 10.1038/sj.onc.1205297. [DOI] [PubMed] [Google Scholar]
- 142.Thiery JP. Epithelial-mesenchymal transitions in tumor progression. Nat. Rev. Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 143.Tonini T, Rossi F, Claudio PP. Molecular basis of angiogenesis and cancer. Oncogene. 2003;22:6549–56. doi: 10.1038/sj.onc.1206816. [DOI] [PubMed] [Google Scholar]
- 144.Nesbit M, Schaider H, Miller TH, Herlyn M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J. Immunol. 2001;166:6483–90. doi: 10.4049/jimmunol.166.11.6483. [DOI] [PubMed] [Google Scholar]
- 145.Schaider H, Oka M, Bogenrieder T, Nesbit M, Satyamoorthy K, et al. Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. Int. J. Cancer. 2003;103:335–43. doi: 10.1002/ijc.10775. [DOI] [PubMed] [Google Scholar]
- 146.Sica A, Schioppa T, Mantovani A, Allavena P. Tumor-associated macrophages are a distinct M2 polarised population promoting tumor progression: potential targets of anticancer therapy. Eur. J. Cancer. 2006;42:717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 147.Selivanova G, Iotsova V, Okan I, Fritsche M, Strom M, et al. Restoration of the growth suppression function of mutant p53 by a synthetic peptide derived from the p53 C-terminal domain. Nat. Med. 1997;3:632–38. doi: 10.1038/nm0697-632. [DOI] [PubMed] [Google Scholar]
- 148.Foster BA, Coffey HA, Morin MJ, Rastinejad F. Pharmacological rescue of mutant p53 conformation and function. Science. 1999;286:2507–10. doi: 10.1126/science.286.5449.2507. [DOI] [PubMed] [Google Scholar]
- 149.Chene P. Inhibiting the p53-MDM2 interaction: an important target for cancer therapy. Nat. Rev. Cancer. 2003;3:102–9. doi: 10.1038/nrc991. [DOI] [PubMed] [Google Scholar]
- 150.Selivanova G, Wiman KG. Reactivation of mutant p53: molecular mechanisms and therapeutic potential. Oncogene. 2007;26:2243–54. doi: 10.1038/sj.onc.1210295. [DOI] [PubMed] [Google Scholar]