Summary
Culture-independent microbiological techniques have revealed a previously unappreciated complexity to the bacterial microbiome of the respiratory tract, forcing reconsideration of the interactions between host, bacteria and the pathogenesis of exacerbations of chronic lung disease. The composition of the lung microbiome is determined by microbial immigration, elimination, and the relative growth rates of its members; all of these change dramatically in chronic lung disease and further during exacerbations. Exacerbations lack key features of bacterial infections, including increased bacterial burden and decreased community diversity. We propose instead that exacerbations are occasions of respiratory dysbiosis: a disordered respiratory microbial ecosystem with negative effects on host biology. Respiratory dysbiosis provokes a dysregulated host immune response, which in turn alters microbial growth conditions in patient airways, further promoting dysbiosis and perpetuating a coupled cycle of inflammation and disordered microbiota. Differences in baseline respiratory microbiota may help explain the “frequent-exacerbator” phenotype observed across multiple disease states, and may provide novel targets for therapeutic intervention.
Introduction
The natural histories of numerous chronic lung diseases are punctuated by exacerbations, characterized by abrupt worsening of respiratory symptoms and pulmonary function. Exacerbations are responsible for much of the morbidity, mortality, and expense of chronic lung diseases1-3, and are associated with acceleration of disease progression4-7. Exacerbations have long been associated with viral exposure and bacterial growth from respiratory cultures, but the precise relationships between resident bacteria, acute infection, and exacerbation pathogenesis have been unsettled and controversial8, 9.
In the past decade, novel culture-independent techniques of microbial identification have revealed a previously unappreciated complexity to the bacterial microbiome of the respiratory tract. The lungs and airways, whether in health or afflicted by chronic or acute lung disease, harbor diverse communities of microbes undetected by conventional culture-based approaches10. This revolution in lung microbiology has called into question long-held beliefs regarding the pathogenesis of exacerbations of chronic lung disease, our understanding of which derives from a half-century of experimentation and observation using culture-based techniques.
What is an Exacerbation?
Exacerbations of chronic lung disease are periods of acute worsening of respiratory symptoms. They arise abruptly, over hours to days, and generally prompt an escalation in medication therapy. Symptoms may include focal respiratory symptoms such as cough, increased sputum production, dyspnea or wheeze, but may include systemic complaints such as fever, fatigue or malaise. Symptom onset often precedes objective worsening in lung function11, though for some patients with impaired perception of dyspnea, symptom onset occurs relatively late in the course of an exacerbation12. Exacerbations are typically followed by recovery to the patient's prior lung function, though in many cases lung function returns to a newly compromised baseline4, 13. Exacerbations are distinct from primary acute lung infections (such as a lobar bacterial pneumonia) and from irreversible progression of underlying lung disease (as with progressively obstructive bronchiolitis obliterans).
Modern Techniques of Lung Microbiome Study
Though a comprehensive discussion of modern techniques of microbial identification would exceed the scope of this Seminar, a familiarity with basic principles of study is key to understanding the recent revelations and persistent limitations of the field. We provide in references several sources for further reading10, 14, 15.
While early lung microbiome studies employed a variety of molecular techniques to characterize microbial communities in respiratory specimens, the most commonly used modern approach employs high-throughput sequencing of amplicons of the 16S rRNA gene, a small and highly conserved locus of the bacterial genome. A single sequencing run of a respiratory specimen yields thousands of short genome sequences that are then aligned, sorted and classified according to established taxonomic databases. This sequencing-based approach describes communities of microbes in tissue specimens, permitting analysis of community features such as diversity and relative membership of specific taxonomic groups. Quantitative PCR of the 16S rRNA gene provides an approximation of bacterial burden that correlates grossly with quantitative culture results16. Sequencing-based approaches thus do not rely upon microbes reproducing in the narrow growth conditions of conventional culture techniques, which fail to identify most human-associated microbes17.
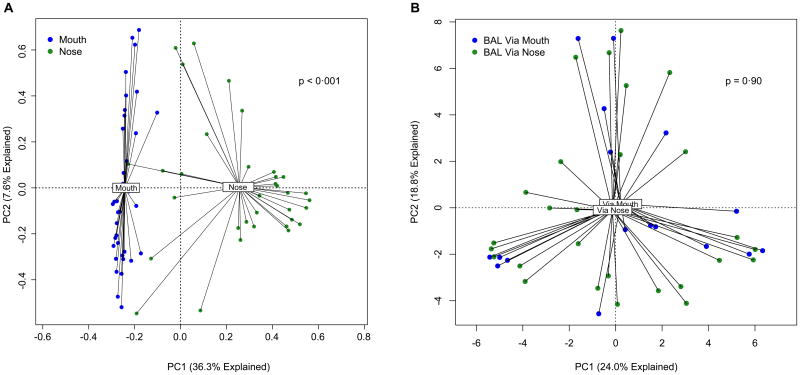
The type of respiratory specimen studied and its route of acquisition is an important consideration in interpreting lung microbiome studies. Many studies in the field have employed bronchoalveolar lavage (BAL) fluid or protected specimen brushings (PSB). Though passage of a bronchoscope through the upper airways introduces a theoretical risk of contamination via pharyngeal microbiota18, the anatomic route of bronchoscope insertion (oral or nasal) has no detectable impact on BAL microbiota despite the markedly divergent microbiota present in these body sites16, 19, 20 (Figure 1), illustrating the minimal influence of upper respiratory tract contamination on microbiota detected via bronchoscopy. Additionally, as detailed below, significant associations have been reported between the microbiota of BAL and PSB specimens and numerous clinically significant parameters: severity of airway obstruction21, airway reactivity22, inhaled medication exposure23, disease prognosis24, clinical response to therapeutic intervention22, 25, and the identity, number and behavior of pulmonary inflammatory cells16, 25, 26. Every study using molecular-based culture-independent techniques that has compared the microbiota of healthy control subjects obtained via bronchoscope with that of subjects with lung disease has found significant differences in bacterial community composition10. These observations provide multifaceted validation of the biologic reality and significance of the lung microbiome, as detected in BAL fluid and PSB specimens.
Figure 1. Lack of influence of upper respiratory tract microbiota on BAL microbiota.
Though the microbial communities of the mouth and nose differ significantly20 (A), route of bronchoscope insertion (via mouth or via nose) has no appreciable effect on BAL microbiota (B). A: Ordination generated from the University of Michigan data collected as part of the NHLBI Lung HIV Microbiome Project37, 138. B: Reproduced from Dickson and colleagues16.
For some lung diseases (cystic fibrosis [CF], bronchiectasis, and chronic obstructive pulmonary disease [COPD]), spontaneously expectorated and induced sputum has been employed for analysis10. While this introduces additional risk of upper airway contamination27, features of microbiota detected in sputum have been significantly associated with patient age28, disease severity29, airway inflammation21, 30, antibiotic exposure31, and response to controlled viral exposure30. Thus any “noise” introduced to sputum specimens by oropharyngeal microbiota does not entirely obscure the meaningful “signal” that correlates consistently with other well-established indices of lung health and disease.
The Microbial Ecology and Topography of the Human Respiratory Tract in Health, Chronic Disease and Exacerbation
A key principle of microbial ecology is the central importance of local environmental conditions in determining the constitution of its resident microbial communities. An oft-cited tenet in the field is that “Everything is everywhere, but the environment selects.”32 The growth of a single species, and its relative abundance in its microbial community, is a function of nutrient availability, temperature, pH, oxygen concentration, and innumerable other environmental factors. Thus any inquiry into the respiratory microbiome must begin both with consideration of the unique local microbial growth conditions of the human respiratory tract, in health and disease, as well as the dynamics of microbial immigration and elimination.
The airways and alveoli are topologically outside of the body and thus continually exposed to the environment. Though the linear distance from nares to alveolus is only half a meter in the average adult, the internal surface area of the lungs is 30 times that of the skin33. Each day, this enormous terrain is exposed to more than 8000 liters of inhaled air, carried to within 0·1 micrometers of one tenth of the body's total blood volume34. The lungs are arguably the human body's largest and most intimate interface with the outside environment33, 35.
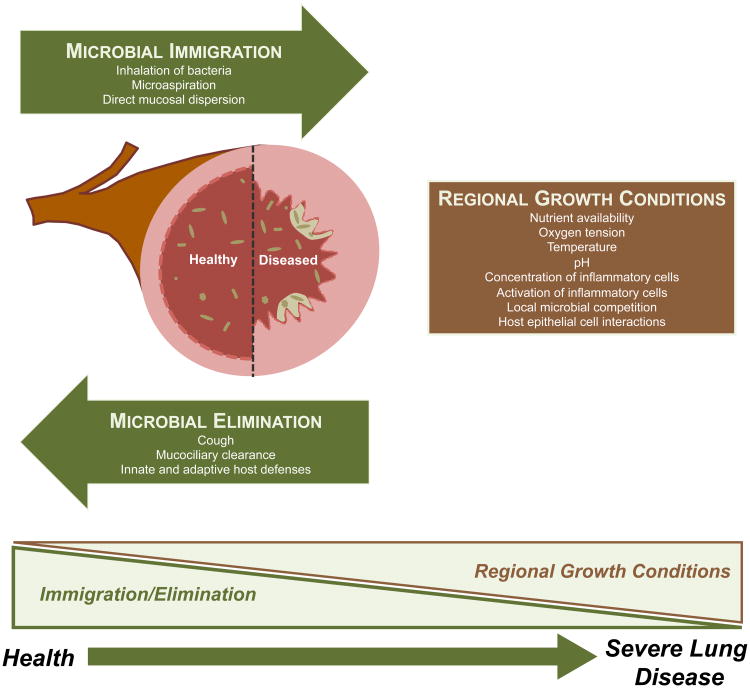
The constitution of the lung microbiome is determined by three factors: microbial immigration, microbial elimination and the relative reproduction rates of its members36 (Figure 2). Any alteration of the lung microbiome detected in disease states must be attributable to some combination of these three factors. The proportion of the lung microbiome in healthy individuals that are resident, reproducing members (subject to regional differences in environmental growth conditions) versus transient microbes (determined solely by rates of immigration and elimination) is a topic of active investigation in the field37. Microbes enter the lungs continually via inhalation of air (which contains 104 - 106 bacterial cells per cubic meter even before reaching the microbe-dense upper airways38), microaspiration (which is ubiquitous among healthy subjects39, 40), and direct dispersion along mucosal surfaces. The high degree of shared membership between the oral and lung microbiome, compared to the air, suggests that microaspiration and direct mucosal dispersion contribute more to microbial immigration than inhalation of bacteria37, 41, 42. Microbes exit the respiratory tract via mucociliary clearance, cough (which is frequent even among healthy subjects43) and the highly active and diverse antimicrobial mechanisms of innate and adaptive immunity. Local microbial growth conditions within the respiratory tract are heterogenous. Within a single lung, dramatic regional variation can be found in oxygen tension, pH, relative blood perfusion, relative alveolar ventilation, temperature, epithelial cell structure, deposition of inhaled particles and in the concentration and behavior of inflammatory cells36, 44-46, all of which have demonstrable effects on microbial growth rates. The distal alveoli are bathed in pulmonary surfactant, which has bacteriostatic activity against some (but not all) bacterial strains, further creating selective pressure on reproducing communities47. Thus the “steady state” of the lung microbiome is one of constant influx, constant efflux, and spatial heterogeneity in local microbial growth conditions.
Figure 2. Determinants of the respiratory microbiome.
The constitution of the respiratory microbiome is determined by three factors: microbial immigration, microbial elimination and the relative reproduction rates of its members. Any alteration detected in disease states must be attributable to some combination of these three factors. In health, community membership is primarily determined by immigration and elimination; in advanced lung disease, membership is primarily determined by regional growth conditions.
Chronic lung disease alters both the topography of the respiratory tract and the dynamics of microbial turnover. Destructive diseases such as emphysema and pulmonary fibrosis dramatically reduce the internal surface area of the lungs by as much as 90%48, 49. Esophageal dysfunction and reflux is extremely common (>70%) among patients with advanced lung disease, increasing the microbial immigration rate and introducing an additional (gastric) microbial source50, 51. Chronic airway diseases such as CF, bronchiectasis and chronic bronchitis are characterized by impaired mucociliary clearance, impairing microbial elimination. The same diseases are associated with increased baseline mucus production, providing both nutrient-rich growth medium as well as pockets of decreased oxygen concentration52 and increased temperature53. The inflammatory cells of the alveolus and airway are both more numerous and more activated in chronic lung disease than in health, even in the absence of cigarette smoking or active exacerbation54, 55. Many therapies for chronic lung disease have profound effects on microbial growth conditions: supplemental oxygen, systemic and inhaled corticosteroids, and systemic and inhaled antibiotics all likely have pleiotropic effects on the influx, efflux and reproduction rates of lung microbiota. As evidenced by the association between disease severity and the identification of persistent bacterial species (“colonizers”) via culture-based testing56-58, as the severity of chronic lung disease worsens, the composition of the respiratory microbiome is determined less by the balance of microbial immigration and elimination (the primary determinant in healthy lungs) and more by regional growth conditions and differential reproduction rates within the respiratory tract (Figure 2).
In the context of a respiratory exacerbation, the topography of the respiratory tract changes further. Hyperventilation accelerates the influx of air-borne microbes and markedly lowers airway temperature59. Increased cough accelerates microbial efflux, and the numbers and activation of inflammatory cells increase60. Byproducts of the host inflammatory response such as inflammatory cytokines61-63, catecholamines64, increased temperature53, 65, glucose and free ATP65 are known growth factors for select bacterial species, creating selectively favorable growth conditions. Bronchoconstriction alters regional oxygen concentration and pH. Acute mucus production and vascular permeability increase local nutrient supply; airway mucus also introduces further gradients of local anoxia and hyperthermia, which selectively favor the growth of specific lung pathogens52, 53, 66, 67.
The Microbiome and Exacerbations of Chronic Lung Diseases
Chronic Obstructive Pulmonary Disease
Exacerbations of COPD are associated with high mortality, accelerated loss of lung function and much of the health care costs of the disease68. Susceptibility to frequent exacerbations both increases with disease severity and also represents an independent clinical phenotype of the disease that is stable over years69. Exacerbations are associated with systemic inflammation70, airway inflammation60 and worsened obstruction due to airway edema, increased sputum production and bronchoconstriction71.
A relationship between COPD exacerbations and respiratory viral infection is inarguable. In case-control and serially-sampled cohort studies, respiratory viruses have been identified in respiratory specimens of 39% - 56% of COPD patients at exacerbation compared to 6% - 19% at clinical baseline.60, 72, 73. When COPD subjects are administered a controlled rhinovirus exposure, they develop features of COPD exacerbations (cough, sputum production, airflow limitation and inflammation) significantly more than do non-COPD controls subjects74.
In contrast, the relationship between bacteria and COPD exacerbations has been unclear and controversial for decades. While potentially pathogenic bacteria are cultured from respiratory specimens in 51% - 70% of patients during exacerbations, the same organisms are grown from 25 - 48% of specimens acquired during clinically stable baseline periods75-77. The culture-determined density of bacteria in sputum is not significantly increased during exacerbation78. One hypothesis has attributed COPD exacerbations to the introduction of new pathogenic bacterial strains to the airways. In a longitudinal study of cultured sputum in outpatients with COPD, new strains were more frequently isolated during exacerbation compared to clinical stability (29% vs. 13%); however, over 70% of exacerbations were not associated with detection of a newly identified strain77. Thus, within the limitations of culture-based identification and a heterogeneous clinical syndrome, the causal relationship between bacteria and COPD exacerbations remains undetermined8. Accordingly, the benefit of antibiotic therapy in the treatment of COPD exacerbations remains controversial; recent metaanalyses suggest that antibiotics reduce the frequency of treatment failure in hospitalized patients with severe exacerbations, but are of unclear benefit for outpatients with mild or moderate disease79. Recently, a large trial demonstrated reduced frequency of exacerbations in patients receiving chronic azithromycin80, sparking renewed interest in the role of bacteria and host inflammation in exacerbation pathogenesis.
Recent culture-independent studies have revealed an unappreciated complexity to the microbiota of COPD exacerbations. Beyond the occasional bacterial species identified in roughly half of specimens via culture, the airways of patients with exacerbations in fact harbor dozens or hundreds of phylogenetically distinct bacterial taxonomic groups. In an early study, Huang et al. observed that even in subjects both receiving antibiotics and with positive P. aeruginosa growth from respiratory cultures, endotracheal aspirate specimens contained hundreds of distinct bacterial taxa representing an average of 140 distinct bacterial families. Subsequent studies have found comparably diverse microbial communities in spontaneously expectorated81 and induced 30 sputum specimens. In all reports, the bacteria identified by culture are detected via concurrent culture-independent techniques, but the converse is not the case: prominent culture-independent community members are often not cultured.
Exacerbations of COPD are associated with changes both in respiratory microbiota and associated airway inflammation. Using paired sputum specimens from COPD subjects at baseline and during exacerbations, Millares et al. found that exacerbations were associated with selective increase in relative abundance of taxa classically associated with exacerbations (e.g. Haemophilus, Pseudomonas, Moraxella) despite inconsistent detection via culture81. Huang et al., using paired sputum specimens of COPD subjects at baseline and during exacerbation, similarly found a shift in community composition towards the Proteobacteria phylum82. Molyneaux et al. employed a human model of virus-induced COPD exacerbations to systematically compare sputum microbiota in subjects at baseline and during exacerbation as well as with healthy controls30. Compared to “baseline” sputum and that of post-viral-exposure control subjects, sputum acquired from “exacerbation” COPD subjects contained a significant shift in community composition towards Proteobacteria. None of these three studies observed a decrease in community diversity, as would be expected in the context of acute infection16. Importantly, Molyneaux et. al. found that key taxonomic groups that spiked in abundance during exacerbation exposure were detected in the same subjects both at baseline before viral exposure as well as after clinical resolution of the exacerbation.
Asthma
Exacerbations are responsible for much of the mortality, morbidity, and expense of asthma2, 83. Precipitants include allergens, air pollution, and exercise, though, as with COPD, some patients exhibit a “frequent-exacerbator” phenotype independent of other risk factors84. Asthma exacerbations are even more strongly associated with viral infections than those of COPD: respiratory viruses (most commonly rhinovirus) are detectable in respiratory specimens of more than 75% of patients with asthma exacerbations85, and controlled rhinovirus instillation provokes the airway hyperresponsiveness observed in allergic asthma86. Bacteria have not traditionally been implicated in asthma exacerbations, as they are infrequently cultured from patient sputum, and early antibiotic trials showed no clinical benefit87. Yet recent serology- and PCR-based approaches have revealed a surprising prevalence of C. pneumoniae and M. pneumoniae infections among patients with acute exacerbations9.
Though to date no culture-independent studies have included respiratory specimens acquired from asthmatic subjects in the context of an exacerbation, multiple studies have demonstrated that the airways in asthma harbor a microbiome distinct from that of healthy subjects, suggesting an association between respiratory microbiota, airway inflammation, and susceptibility to exacerbations. In four separate studies analyzing respiratory microbiota (using BAL25, 88, bronchial brushings22, 88, and induced sputum89), the microbiota detected in asthmatic airways at baseline has been consistently and significantly distinct from that of control subjects. All four studies demonstrated significantly increased community abundance of Proteobacteria, the bacterial phylum that contains common gram-negative respiratory pathogens such as Haemophilus spp., Pseudomonas spp., and Klebsiella spp.. Goleva et. al. observed an increased concentration of LPS in Proteobacteria-rich specimens25, providing in vivo validation of this difference in community membership.
These studies have demonstrated provocative associations between the respiratory microbiome and the clinical, physiological, and therapeutic aspects of asthma. Huang et al. demonstrated that airway hyperresponsiveness (assessed by methacholine challenge) was positively associated both with community diversity and community composition22 (Figure 3A). This association was driven by the increased community abundance of the Proteobacteria phylum among patients with hyperresponsive airways. The authors also demonstrated that clinical improvement to clarithromycin was significantly predicted by differences in patients' baseline microbiota (Figure 3B). Separately, Goleva et al. observed that differences in baseline microbiota were associated with patients' clinical response to systemic corticosteroids25 (Figure 3C). In vitro co-culture of BAL-acquired macrophages with Haemophilus parainfluenzae (which they observed only in corticosteroid-resistant subjects) blunted response of airway macrophages to corticosteroid effects, demonstrating the biologic plausibility of a relationship between airway microbiota and the hyperresponsiveness that is accentuated during exacerbations.
Figure 3. Associations between respiratory microbiota and clinical features of asthma.

Among asthmatics, respiratory microbial diversity is positively associated both with bronchial hyperresponsiveness (A) and whether bronchial reactivity improved with clarithromycin therapy (B). Bacterial community composition is significantly different among asthmatics whose airway obstruction improves with systemic corticosteroids (C). A, B: Reproduced from Huang and colleagues22. C: Reproduced from Goleva and colleagues25.
Cystic Fibrosis
Exacerbations in CF are associated with much of the mortality and expense of the diagnosis, as well as acceleration of lung function decline3, 6. With increasing age, nearly all CF patients grow select respiratory pathogens from sputum (most commonly Staphylococcus aureus, Pseudomonas aeruginosa and Haemophilus influenzae)57, both during exacerbations and during clinical stability. Exacerbations have thus classically been considered infectious events and treated primarily with antibiotics, though data supporting this is surprisingly sparse90-92. Provocatively, two large trials have found no detectable association between patients' clinical response to antibiotic therapy during exacerbations and the in vitro susceptibility of their cultured organism to their administered antibiotic93, 94 (Figure 4A); further, the clinical course of patients receiving antibiotics during exacerbations is unrelated to concurrent change in culture-determined sputum bacterial burden95.
Figure 4. Cystic fibrosis exacerbations lack key features of bacterial infections.

A: CF patients' clinical response to antibiotic therapy is not correlated with the in vitro susceptibility of organisms cultured from sputum at the onset of exacerbation. B: Bacterial density in sputum does not increase prior to or at the onset of exacerbation. C: Sputum bacterial community diversity is not lower during exacerbation compared to clinical baseline. A: Reproduced from Hurley and colleagues93. B: Reproduced from Stressmann and colleagues102. C: Reproduced from Carmody and colleagues101.
In the past decade, use of culture-independent techniques have revolutionized our understanding of the CF microbiome at baseline and during exacerbation. Early studies revealed a far greater microbial diversity in sputum than was appreciated via culture96, with viability of many microbes verified both via amplification of transcribed RNA97 and advanced culture techniques98. Dozens of studies have since verified that the sputum of CF patients contains diverse bacterial communities, replete both with classically-recognized pathogens and previously unrecognized microbes99. Several studies have shown an overall decrease in community diversity with age and disease severity10, 28, 29; this loss of diversity is more strongly associated with cumulative antibiotic exposure than disease severity29, 31.
Culture-independent analysis has upended long-held assumptions about the bacterial pathogenesis of CF exacerbations. Multiple studies have analyzed paired specimens acquired from patients during periods of stability and subsequent exacerbation29, 100-102. All have been remarkably consistent in their key findings: CF exacerbations are not associated with increased bacterial density and are not associated with decreased community diversity (Figures 4B, 4C). These results differ starkly from those found in patients with bacterial pneumonia, for which, as expected, bacterial burden is high, community diversity is low, and the intensity of the host inflammatory response correlates tightly with bacterial burden16, 103. These findings, considered with the lack of association between culture-identified pathogens and clinical response summarized above, strongly challenge the conventional understanding of CF exacerbations as acute infections of the airways.
These same studies have asked whether bacterial community membership changes at the time of exacerbation, and no consistent pattern of microbial change has emerged31, 97, 98. Carmody et al. found that compared to baseline, respiratory microbiota at the time of exacerbation contain a consistent and significant increase in abundance of Gemella sp., a gram-positive anaerobe associated with the mouth and upper GI tract29, 100, 101. It is unknown how baseline differences in microbiota influence the frequency or character of CF exacerbations; Carmody et. al. observed that dissimilarity between baseline and exacerbation microbiota was significantly associated both with high baseline diversity and baseline community domination by Pseudomonas sp.101.
Non-CF Bronchiectasis
Patients with bronchiectasis not attributable to CF comprise a heterogenous group with a variety of predisposing conditions, though the plurality of cases are idiopathic. Compared to CF, little is known about the pathophysiology and treatment of the chronic inflammation and episodic exacerbations that characterize its clinical course. New evidence suggests that chronic macrolide therapy decreases the frequency of exacerbations in this population104, though as with CF and COPD, it is unknown whether this is due to antimicrobial effects.
Several recent studies have employed culture-independent analysis to study the microbiome of non-CF bronchiectasis. A single study by Tunney et al. has directly compared paired sputum specimens of patients with non-CF bronchiectasis at stability and during exacerbation21. They observed no difference in community diversity at the time of exacerbation, and by quantitative culture analysis they observed no increase in aerobic, anaerobic or total bacterial growth. Communities both at baseline and exacerbation were largely dominated by Proteobacteria (e.g. Haemophilus sp. and Pseudomonas sp.). Rogers et al. observed that the composition of sputum microbiota at clinical baseline was predictive of subsequent exacerbation frequency; importantly, this association was not detected when limited to culture-based microbiologic results105. A study comparing explanted lung tissue found comparable microbiota in CF and non-CF bronchiectasis lungs106, but it is unknown how generalizable findings of CF studies are to non-CF bronchiectasis patients.
Idiopathic Pulmonary Fibrosis
While the natural history of idiopathic pulmonary fibrosis (IPF) has traditionally been characterized as one of slowly progressive decline in lung function, the clinical phenomenon of acute exacerbations of IPF (AE-IPF) has been increasingly recognized as a major cause of mortality in the disease7. Exacerbations arise over the course of weeks and are characterized by progressive hypoxemia, lung infiltrates, the histological presence of diffuse alveolar damage and the clinical exclusion of infection or heart failure. Mortality is high and no therapies are of demonstrated benefit. Many patients with AE-IPF exhibit clinical features of infection (fever, cough, BAL neutrophilia)107, 108, but no infectious etiology has been identified.
Several recent observations have provoked interest in a potential role of the bacterial lung microbiome in the progression of IPF and its exacerbations. Richter et al. observed a surprising frequency of culture-positivity in the BAL fluid of patients with IPF at clinical baseline109, and subsequent culture-independent reports have confirmed that IPF lungs harbor a distinct microbiome from that of healthy lungs24, 110. Han et al. recently observed a positive association between the presence of specific microbial community members (Staphylococcus sp. and Streptococcus sp.) in BAL and disease progression24, and a randomized controlled trial of co-trimoxazole found reduced mortality among IPF patients receiving antibiotic therapy111.
Key Lessons and Directions for Study
Exacerbations are not acute bacterial infections of the airways but rather instances of dysregulated host immune response coupled with respiratory dysbiosis
Despite the unambiguous presence of airway inflammation in respiratory exacerbations, a startling and consistent finding across disease states has been the conspicuous lack of evidence that exacerbations represent acute bacterial infections of the airways. Of the eight culture-independent studies across three disease states that have compared patients' respiratory specimens acquired at baseline and during exacerbation, all have found no change in bacterial density or community diversity during exacerbation21, 29, 30, 81, 82, 100-102. This differs starkly from studies of bacterial pneumonia, in which (as expected) increased inflammation is strongly associated with increases in bacterial burden and decreases in community diversity36, 103. The airway inflammation common to all respiratory exacerbations is surprisingly dissociated from microbial load and community domination by one or few pathogenic species.
Exacerbations differ from acute bacterial infections in numerous important respects (Table 1). Acute bacterial infections respond rapidly and consistently to antibiotics, while data showing benefit of antibiotics in treating respiratory exacerbations ranges from subtle to inconsistent to absent79, 87, 92. In vitro susceptibility testing is of clear utility in predicting response to therapy in bacterial lung infections112, but bears no correlation with response to therapy in CF exacerbations93, 94. While bacterial lung infections are the most common etiology of severe sepsis113, respiratory exacerbations rarely (if ever) directly provoke severe sepsis and shock.
Table 1. Comparison of respiratory exacerbations with acute bacterial infections.
| Acute bacterial respiratory infections | Respiratory exacerbations | |
|---|---|---|
| Bacterial density (compared to baseline) | High | Normal |
| Bacterial community diversity (compared to baseline) | Low | Normal |
| Culture growth | Usually | Occasionally |
| Airway inflammation | High Proportionate to microbial burden |
High Disproportionate to microbial burden |
| Sepsis | Common | Never/rare |
| Clinical benefit of antibiotics | Rapid, unambiguous | Inconsistent, subtle |
| Relevance of in vitro susceptibility | Critical to clinical response | No relation with clinical response |
These key differences between exacerbations and bacterial infections do not imply that bacteria are uninvolved in exacerbation pathogenesis. Rather, we propose that exacerbations are occasions of respiratory dysbiosis: disorder and dysregulation of the microbial ecosystem of the respiratory tract, coupled with a dysregulated host immune response, resulting in negative effects on host biology. Paired specimen studies have observed significant changes in the constitution of respiratory bacterial communities during exacerbations, often with phylum-level shifts away from Bacteroidetes (the most abundant phylum reported in the lungs of healthy subjects10, 16, 88) and towards members of Proteobacteria30, 81, 82 and other disease-associated taxonomic groups101. The increase in frequency of novel strain isolation in sputum observed in COPD exacerbations compared to baseline (from 13% to 29%)77 is consistent with the phenomenon of respiratory dysbiosis. Altered environmental conditions provoke a disturbance in bacterial community composition, both increasing relative abundance of previously undetectable species as well as increasing the community's susceptibility to invasion via new strains.
A rich analogy may be drawn between exacerbations of chronic lung disease and exacerbations of inflammatory bowel disease. Both are acute clinical worsening of chronic inflammatory conditions of mucosa-lined luminal organs, associated with profound dysbiosis and dysregulated host inflammatory response114. Both are characterized by an erosion of the homeostatic balance between resident microbiota and host immunity115. Neither is an instance of “acute infection” in any traditional sense, in which one or few pathogenic species overtake a body site and directly mediate tissue injury. In both cases, the benefit of antibiotics, if present, is not via eradication of a targeted pathogen but instead via selective manipulation of microbial community composition or indirect immunomodulatory effects (potentially explaining the discordance between culture sensitivities and clinical response observed in CF93, 94). Both are either prevented or treated by macrolides, which have both antimicrobial and immunomodulatory effects80, 104, 116, 117.
We propose in Figure 5 a model of the cycle of host inflammation and respiratory dysbiosis that typifies a respiratory exacerbation. An inflammatory trigger (e.g. viral infection, allergic exposure, etc.) initiates a cascade of host inflammatory responses that acutely and profoundly alter the microbial growth conditions of the airways. Permeability of the airway wall and mucus production introduce nutrient-dense substrate for bacterial growth52, 66. Free catecholamines and inflammatory cytokines (e.g. TNF-α, IL-1, IL-6, IL-8) directly promote growth of select bacterial species (e.g. P. aeruginosa, S. aureus, S. pneumoniae, B. cepacia complex) via interkingdom signaling61-65. Inflammatory cells are recruited and activated, killing and clearing bacteria with highly variable effectiveness118, creating a gradient of negative selective pressure across species. Airway mucus creates local pockets of increased temperature and decreased oxygen tension, selectively favoring growth of prominent disease-associated microbes52, 53. Within this model, these diverse effects on microbial growth conditions result in disorder and dysregulation of the dynamic homeostasis of the airway microbiome, i.e. respiratory dysbiosis. Newly-prominent community members, some with altered expression of virulence traits and some with enhanced immunogenicity compared to previously prominent members, provoke further airway inflammation via pathogen-associated molecular pattern (PAMP) – pattern recognition receptor (PRR) interactions119, including differential activation of toll-like receptors120, 121. This inflammation further alters growth conditions within the airway, resulting in a self-amplifying feedback loop. Tissue injury arises via a combination of direct injury from newly prominent community members, alteration in microbial behavior, and indirect effects of the dysregulated inflammatory response122. Homeostasis is attained only after the feedback loop of dysbiosis and inflammation is severed. While the initial dysbiosis observed in exacerbations may be secondary to a primary inflammatory trigger, the causal relationship between inflammation and dysbiosis is likely bidirectional and self-perpetuating, providing a plausible explanation for why the airway inflammation of exacerbations persists long after direct exposure to the inflammatory precipitant13, 30, 68.
Figure 5. The dysbiosis-inflammation cycle.
An inflammatory trigger initiates airway inflammation, which alters environmental growth conditions of airway microbiota via positive and negative selective pressures. Disordered growth conditions result in a disordered microbiome, which provokes further airway inflammation via pathogen-associated molecular pattern (PAMP) – pattern recognition receptor (PRR) interactions, microbial metabolite signaling to leukocytes and epithelial cells, and other pathways. This results in a self-amplifying cycle of airway inflammation and respiratory dysbiosis.
A distinct but related observation in the field is that the virulence and immunogenicity of a single bacterial species within the respiratory tract is not static, but rather contingent on numerous host- and microbe-derived environmental factors. A rich recent literature has demonstrated that the behavior of a given bacterial strain (as assessed either by gene expression or in vitro or in vivo virulence and immunogenicity) is strongly influenced by the same environmental factors that differentially alter bacterial growth rates: temperature53, 65, concentration of host-derived cytokines and catecholamines63, 64 and presence of free glucose65. Further, the behavior and virulence of a single strain is dramatically influenced by the composition of the bacterial community sharing its ecologic niche123, mediated by direct and indirect microbe-microbe interactions124, 125. Changes in the metabolism and virulence of microbes (even independent of community membership) can modulate inflammatory cascades, as has been proposed for the gastrointestinal tract and modeled for P. aeruginosa in Drosophilia123, 126, 127. Thus the environmental conditions of the inflamed airways can alter the virulence of individual community members both directly (via the virulence-promoting factors listed above) as well as indirectly (via the alteration of community composition and microbe-microbe interactions observed in respiratory dysbiosis). This recent insight will be critical for subsequent study of bacterial lung disease: the pathogenicity of a single species cannot be understood outside of its ecological milieu.
The observation that “respiratory exacerbations” do not occur in individuals without chronic lung disease is self-evident but telling. The response of healthy airways to an inflammatory exposure (e.g. viral infection) is measured, proportionate to the insult, and self-limited. Any disruption in baseline lung microbiota is relatively muted and short-lived30. The key phenomena of exacerbations -- sustained respiratory dysbiosis coupled with a prolonged immune response disproportionate to microbial burden -- arise only when dysfunction already exists at baseline on both sides of the microbiome-immune interface. Even when clinically stable (outside of exacerbation states), patients with chronic lung disease, when compared to healthy controls, have alterations both in respiratory microbiota10, 128 and in the constitution and activation of airway inflammatory cells54, 55, 129 (Table 2). The composition of respiratory microbiota, determined primarily by the balance of immigration and elimination in health, is increasingly determined by regional growth conditions with increasing severity of lung disease (Figure 2); in an exacerbation, the contribution of regional growth conditions abruptly becomes all-important. We propose that the coupled and self-perpetuating dysregulation of dysbiosis and airway inflammation of exacerbations is an abrupt, emergent phenomenon arising from the chronically disordered but compensated, fragile homeostasis present in stable chronic lung disease.
Table 2. Comparison of respiratory health with stable and exacerbated chronic lung disease.
| Health | Chronic Lung Disease (Stable) | Chronic Lung Disease (Exacerbation) | |
|---|---|---|---|
| Primary ecological determinant of community composition | Immigration/elimination | Mild: Immigration/elimination Severe: Regional growth conditions |
Regional growth conditions |
| Respiratory dysbiosis | Absent | Moderate, varies with disease severity | Severe |
| Airway inflammation | Absent/mild Proportionate to microbial burden | Increased Proportionate to microbial burden | Increased Disproportionate to microbial burden |
An important consequence of understanding the airways as an ecosystem is recognition of the limitations of conventional analytic approaches in modeling its behavior. While the conventional model of airway infection implies a linear system of pathogenesis (a suitably large bacterial inoculum enters the airways and overwhelms host defenses with unrestrained growth and resultant inflammation), within our modern model, exacerbations instead emerge abruptly from the complex adaptive system of the respiratory ecosystem36. Ecosystems are “prototypical complex adaptive systems”130, defined by the presence of diverse entities, interacting with each other within a common space, exhibiting interdependent actions and possessing the capacity to adapt to changes in conditions131, 132. Within linear systems, small changes in conditions result in proportionately small changes in outcomes, whereas in complex adaptive systems outcomes can be abruptly and disproportionately altered by small differences in conditions. Unlike linear systems, complex adaptive systems defy reductionist modeling and are instead better modeled via computational techniques (e.g. agent-based modeling)133, 134. Such techniques may prove essential in understanding how the phenomena of respiratory exacerbations emerge from the homeostasis of clinical stability36.
The respiratory microbiome may partly explain the “frequent-exacerbator” phenotype of chronic lung diseases
In multiple chronic lung diseases, a subgroup of patients experience exacerbations more frequently than predicted by established risk factors69, 84. This “frequent-exacerbator” phenotype is consistent across years, implying a persistent biologic predisposition to exacerbations that is unexplained by our current understanding of their pathogenesis. The revelations of the culture-independent era have prompted a novel hypothesis: differences in baseline respiratory microbiota may explain the differences observed in exacerbation frequency among otherwise clinically equivalent patients.
Several preliminary observations lend plausibility to this hypothesis. Among patients with non-CF bronchiectasis, Rogers et al. observed that differences in baseline respiratory microbiota were strongly and significantly associated with the number of exacerbations experienced in the subsequent year105; importantly, no association was observed between culture-based microbiology results and exacerbation frequency. Among patients with asthma, as discussed above, several associations have been reported between baseline respiratory microbiota and both airway hyperresponsiveness and clinical response to therapy22, 25. Molyneaux et al. demonstrated in COPD patients that after rhinovirus exposure, alterations in the respiratory microbiome were both heterogenous among patients and persistent at six weeks30. Chronic azithromycin therapy has been shown to decrease the frequency of exacerbations in COPD, CF and non-CF bronchiectasis80, 104, 116, though it is unknown whether this benefit is attributable to its unquestionable effects on respiratory microbiota135. Two randomized controlled trials have observed a benefit of enteric probiotics in decreasing the frequency of cystic fibrosis exacerbations136, 137; given the anatomic and ecologic continuity of the aerodigestive tract, it is plausible that this benefit was mediated in part via alteration of respiratory microbiota. Given the plausibility of this hypothesis and the high morbidity and expense associated with exacerbations of chronic lung disease, an immediate goal of further study should be to determine both whether the respiratory microbiome can be used to predict exacerbations, and to determine if its therapeutic manipulation can decrease exacerbation frequency128.
Culture-based approaches are inadequate to completely understand the interactions of the host, microbiome and disease pathogenesis
Every study of respiratory exacerbations that has employed both culture-dependent and culture-independent results has found marked divergence in their results; while culture-independent techniques faithfully confirm the presence of culture-identified organisms, culture-based approaches grossly understate the complexity, richness, and dynamism of respiratory microbiota. Importantly, culture-independent approaches have identified significant associations between respiratory microbial community features and airway hyperresponsiveness22, disease prognosis24, susceptibility to exacerbations105, and response to a controlled respiratory viral exposure30; each association would have been unappreciated by culture-based approaches. Given the intimate relationship between the respiratory microbiome and the host, and given the plausible role of the microbiome as mediator of acute and chronic inflammation, changes to the respiratory microbiome should be considered as a secondary outcome of all clinical trials targeting a reduction in exacerbation frequency.
Conclusions
Use of culture-independent techniques of microbial identification has upended our understanding of the role of bacteria in exacerbations of chronic lung disease. Exacerbations are not bacterial infections of the airways, but instead occasions of respiratory dysbiosis: disorder and dysregulation of the microbial ecosystem that provoke a dysregulated host inflammatory response, which in turn promotes further dysbiosis. Future studies should investigate which features of the respiratory microbiome are associated with subsequent exacerbations and determine whether therapeutic manipulation of the microbiome can decrease exacerbation frequency.
Search Strategy and Selection Criteria
We searched Medline with no date nor language restrictions. Initial search phrases were “exacerbation[All Fields] AND (“microbiota”[MeSH Terms] OR “microbiota”[All Fields] OR “microbiome”[All Fields])” and “DISEASE[All Fields] AND exacerbation[All Fields] AND (“microbiology”[Subheading] OR “microbiology”[All Fields] OR “bacteria”[All Fields] OR “bacteria”[MeSH Terms])” where DISEASE represents “cystic fibrosis,” “COPD,” “bronchiectasis,” “asthma,” or “pulmonary fibrosis.”
Acknowledgments
Funding: Funding provided by NIH grants T32HL00774921 (RPD), U01HL098961 (GBH), R01HL114447 (GBH, FJM), the Nesbitt Family Charitable Foundation (GBH), the Nesbitt Program For Cystic Fibrosis Research (GBH) and the University of Michigan. Support provided by the Host Microbiome Initiative of the University of Michigan.
Footnotes
Declaration of Interests: Dr. Dickson and Professor Huffnagle report no conflicting interests. Professor Martinez has served on advisory boards relating to COPD-related topics for Almirall, AstraZeneca, Forest Laboratories, GlaxoSmithKline, MedImmune, Merck, Novartis Pharmaceuticals, Pearl Therapeutics, United BioSource. He has served on data safety monitoring boards of Novartis, Sanofi. He has consulted for Actelion, Bayer, Boehringer Ingelheim, BoomComm, Comgenex, F Hoffmann-La Roche, FB Communications, Forest Laboratories, HLS, Merck/Schering-Plough, Nycomed, Pfizer, Quark, Sanofi, Talecris Biotherapeutics. He has served on speaker's bureaus for Altana/Nycomed, American Lung Association, AstraZeneca, Boehringer Ingelheim, CME Incite, ePocrates, France Foundation, GlaxoSmithKline, Med-Ed, Merck/Schering–Plough, National Asssociation for Continuing Education, Pfizer, Potomac, Vox Medica, WebMD. His institution has received funds from Boehringer Ingelheim for a clinical trial. He has received royalties from Associates in Medical Marketing and Castle Connolly. He has developed educational materials for the France Foundation, HIT Global, ePocrates. He has served on steering committees for clinical trials supported by Actelion, Centocor, Forest Laboratories, GlaxoSmithKline, MPex, Takeda.
References
- 1.Strassels SA, Smith DH, Sullivan SD, Mahajan PS. The costs of treating COPD in the United States. Chest. 2001;119(2):344–52. doi: 10.1378/chest.119.2.344. [DOI] [PubMed] [Google Scholar]
- 2.Smith DH, Malone DC, Lawson KA, Okamoto LJ, Battista C, Saunders WB. A national estimate of the economic costs of asthma. Am J Respir Crit Care Med. 1997;156(3 Pt 1):787–93. doi: 10.1164/ajrccm.156.3.9611072. [DOI] [PubMed] [Google Scholar]
- 3.Lieu TA, Ray GT, Farmer G, Shay GF. The cost of medical care for patients with cystic fibrosis in a health maintenance organization. Pediatrics. 1999;103(6):e72. doi: 10.1542/peds.103.6.e72. [DOI] [PubMed] [Google Scholar]
- 4.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–13. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–52. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153(4):345–52. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyzy R, Huang S, Myers J, Flaherty K, Martinez F. Acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2007;132(5):1652–8. doi: 10.1378/chest.07-0299. [DOI] [PubMed] [Google Scholar]
- 8.Hirschmann JV. Do bacteria cause exacerbations of COPD? Chest. 2000;118(1):193–203. doi: 10.1378/chest.118.1.193. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland ER, Martin RJ. Asthma and atypical bacterial infection. Chest. 2007;132(6):1962–6. doi: 10.1378/chest.06-2415. [DOI] [PubMed] [Google Scholar]
- 10.Dickson RP, Erb-Downward JR, Huffnagle GB. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med. 2013;7(3):245–57. doi: 10.1586/ers.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan-Yeung M, Chang JH, Manfreda J, Ferguson A, Becker A. Changes in peak flow, symptom score, and the use of medications during acute exacerbations of asthma. Am J Respir Crit Care Med. 1996;154(4 Pt 1):889–93. doi: 10.1164/ajrccm.154.4.8887581. [DOI] [PubMed] [Google Scholar]
- 12.Magadle R, Berar-Yanay N, Weiner P. The risk of hospitalization and near-fatal and fatal asthma in relation to the perception of dyspnea. Chest. 2002;121(2):329–33. doi: 10.1378/chest.121.2.329. [DOI] [PubMed] [Google Scholar]
- 13.Sanders DB, Hoffman LR, Emerson J, et al. Return of FEV1 after pulmonary exacerbation in children with cystic fibrosis. Pediatr Pulmonol. 2010;45(2):127–34. doi: 10.1002/ppul.21117. [DOI] [PubMed] [Google Scholar]
- 14.Hamady M, Knight R. Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res. 2009;19(7):1141–52. doi: 10.1101/gr.085464.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan XC, Huttenhower C. Chapter 12: Human microbiome analysis. PLoS Comput Biol. 2012;8(12):e1002808. doi: 10.1371/journal.pcbi.1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickson RP, Erb Downward JR, Freeman CM, et al. Changes in the lung microbiome following lung transplantation include the emergence of two distinct pseudomonas species with distinct clinical associations. PLoS One. 2014;9(5):e97214. doi: 10.1371/journal.pone.0097214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suau A, Bonnet R, Sutren M, et al. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65(11):4799–807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184(8):957–63. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassis CM, Erb Downward JR, Venkataraman A, et al. Bronchoalveolar lavage, oral rinse, nasal swab and gastric aspirates each identify distinct but related microbial communities in healthy subjects. preparation [Google Scholar]
- 20.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers GB, van der Gast CJ, Cuthbertson L, et al. Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax. 2013;68(8):731–7. doi: 10.1136/thoraxjnl-2012-203105. [DOI] [PubMed] [Google Scholar]
- 22.Huang YJ, Nelson CE, Brodie EL, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127(2):372–81 e1-3. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One. 2012;7(10):e47305. doi: 10.1371/journal.pone.0047305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han MK, Zhou Y, Murray S, et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med. 2014 doi: 10.1016/S2213-2600(14)70069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goleva E, Jackson LP, Harris JK, et al. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med. 2013;188(10):1193–201. doi: 10.1164/rccm.201304-0775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segal LN, Alekseyenko AV, Clemente JC, et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome. 2013;1(1):19. doi: 10.1186/2049-2618-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goddard AF, Staudinger BJ, Dowd SE, et al. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc Natl Acad Sci U S A. 2012;109(34):13769–74. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox MJ, Allgaier M, Taylor B, et al. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS One. 2010;5(6):e11044. doi: 10.1371/journal.pone.0011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J, Schloss PD, Kalikin LM, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci U S A. 2012;109(15):5809–14. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molyneaux PL, Mallia P, Cox MJ, et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(10):1224–31. doi: 10.1164/rccm.201302-0341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, Murray S, Lipuma JJ. Modeling the impact of antibiotic exposure on human microbiota. Sci Rep. 2014;4:4345. doi: 10.1038/srep04345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baas Becking LGM. Geobiologie, of Inleiding Tot de Milieukunde: Met Literatuurlijst en Ind. Van Stockum; 1934. [Google Scholar]
- 33.Hasleton PS. The internal surface area of the adult human lung. J Anat. 1972;112(Pt 3):391–400. [PMC free article] [PubMed] [Google Scholar]
- 34.Dock DS, Kraus WL, Mc GL, Hyland JW, Haynes FW, Dexter L. The pulmonary blood volume in man. J Clin Invest. 1961;40:317–28. doi: 10.1172/JCI104259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helander HF, Fandriks L. Surface area of the digestive tract - revisited. Scand J Gastroenterol. 2014;49(6):681–9. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- 36.Dickson RP, Erb-Downward JR, Huffnagle GB. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir Med. 2014;2(3):238–46. doi: 10.1016/S2213-2600(14)70028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris A, Beck JM, Schloss PD, et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187(10):1067–75. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lighthart B. Mini-review of the concentration variations found in the alfresco atmospheric bacterial populations. Aerobiologia. 2000;16(1):7–16. [Google Scholar]
- 39.Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111(5):1266–72. doi: 10.1378/chest.111.5.1266. [DOI] [PubMed] [Google Scholar]
- 40.Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64(4):564–8. doi: 10.1016/0002-9343(78)90574-0. [DOI] [PubMed] [Google Scholar]
- 41.Bowers RM, Sullivan AP, Costello EK, Collett JL, Jr, Knight R, Fierer N. Sources of bacteria in outdoor air across cities in the midwestern United States. Appl Environ Microbiol. 2011;77(18):6350–6. doi: 10.1128/AEM.05498-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertolini V, Gandolfi I, Ambrosini R, et al. Temporal variability and effect of environmental variables on airborne bacterial communities in an urban area of Northern Italy. Appl Microbiol Biotechnol. 2013;97(14):6561–70. doi: 10.1007/s00253-012-4450-0. [DOI] [PubMed] [Google Scholar]
- 43.Munyard P, Bush A. How much coughing is normal? Arch Dis Child. 1996;74(6):531–4. doi: 10.1136/adc.74.6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.West JB. Regional differences in the lung. Chest. 1978;74(4):426–37. doi: 10.1378/chest.74.4.426. [DOI] [PubMed] [Google Scholar]
- 45.Ingenito EP, Solway J, McFadden ER, Jr, et al. Indirect assessment of mucosal surface temperatures in the airways: theory and tests. J Appl Physiol. 1987;63(5):2075–83. doi: 10.1152/jappl.1987.63.5.2075. [DOI] [PubMed] [Google Scholar]
- 46.Hatch TF. Distribution and deposition of inhaled particles in respiratory tract. Bacteriol Rev. 1961;25:237–40. doi: 10.1128/br.25.3.237-240.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu H, Kuzmenko A, Wan S, et al. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003;111(10):1589–602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coxson HO, Hogg JC, Mayo JR, et al. Quantification of idiopathic pulmonary fibrosis using computed tomography and histology. Am J Respir Crit Care Med. 1997;155(5):1649–56. doi: 10.1164/ajrccm.155.5.9154871. [DOI] [PubMed] [Google Scholar]
- 49.Coxson HO, Rogers RM, Whittall KP, et al. A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med. 1999;159(3):851–6. doi: 10.1164/ajrccm.159.3.9805067. [DOI] [PubMed] [Google Scholar]
- 50.D'Ovidio F, Singer LG, Hadjiliadis D, et al. Prevalence of gastroesophageal reflux in end-stage lung disease candidates for lung transplant. Ann Thorac Surg. 2005;80(4):1254–60. doi: 10.1016/j.athoracsur.2005.03.106. [DOI] [PubMed] [Google Scholar]
- 51.Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27(1):136–42. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 52.Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109(3):317–25. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt A, Belaaouaj A, Bissinger R, et al. Neutrophil elastase-mediated increase in airway temperature during inflammation. J Cyst Fibros. 2014 doi: 10.1016/j.jcf.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150(2):448–54. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 55.Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14(5):1015–22. doi: 10.1183/09031936.99.14510159. [DOI] [PubMed] [Google Scholar]
- 56.Zalacain R, Sobradillo V, Amilibia J, et al. Predisposing factors to bacterial colonization in chronic obstructive pulmonary disease. Eur Respir J. 1999;13(2):343–8. doi: 10.1034/j.1399-3003.1999.13b21.x. [DOI] [PubMed] [Google Scholar]
- 57.Cystic Fibrosis Foundation. [accessed April 25 2014];Patient Registry Report. 2012 http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf.
- 58.Evans SA, Turner SM, Bosch BJ, Hardy CC, Woodhead MA. Lung function in bronchiectasis: the influence of Pseudomonas aeruginosa. Eur Respir J. 1996;9(8):1601–4. doi: 10.1183/09031936.96.09081601. [DOI] [PubMed] [Google Scholar]
- 59.McFadden ER, Jr, Pichurko BM. Intraairway thermal profiles during exercise and hyperventilation in normal man. J Clin Invest. 1985;76(3):1007–10. doi: 10.1172/JCI112052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–21. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 61.Kanangat S, Meduri GU, Tolley EA, et al. Effects of cytokines and endotoxin on the intracellular growth of bacteria. Infect Immun. 1999;67(6):2834–40. doi: 10.1128/iai.67.6.2834-2840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaza SK, McClean S, Callaghan M. IL-8 released from human lung epithelial cells induced by cystic fibrosis pathogens Burkholderia cepacia complex affects the growth and intracellular survival of bacteria. Int J Med Microbiol. 2011;301(1):26–33. doi: 10.1016/j.ijmm.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Porat R, Clark BD, Wolff SM, Dinarello CA. Enhancement of growth of virulent strains of Escherichia coli by interleukin-1. Science. 1991;254(5030):430–2. doi: 10.1126/science.1833820. [DOI] [PubMed] [Google Scholar]
- 64.Freestone PP, Hirst RA, Sandrini SM, et al. Pseudomonas aeruginosa-catecholamine inotrope interactions: a contributory factor in the development of ventilator-associated pneumonia? Chest. 2012;142(5):1200–10. doi: 10.1378/chest.11-2614. [DOI] [PubMed] [Google Scholar]
- 65.Marks LR, Davidson BA, Knight PR, Hakansson AP. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. mBio. 2013;4(4) doi: 10.1128/mBio.00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van de Graaf EA, Out TA, Roos CM, Jansen HM. Respiratory membrane permeability and bronchial hyperreactivity in patients with stable asthma. Effects of therapy with inhaled steroids Am Rev Respir Dis. 1991;143(2):362–8. doi: 10.1164/ajrccm/143.2.362. [DOI] [PubMed] [Google Scholar]
- 67.Sass AM, Schmerk C, Agnoli K, et al. The unexpected discovery of a novel low-oxygen-activated locus for the anoxic persistence of Burkholderia cenocepacia. The ISME journal. 2013;7(8):1568–81. doi: 10.1038/ismej.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–96. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 70.Perera WR, Hurst JR, Wilkinson TM, et al. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J. 2007;29(3):527–34. doi: 10.1183/09031936.00092506. [DOI] [PubMed] [Google Scholar]
- 71.O'Donnell DE, Parker CM. COPD exacerbations. 3: Pathophysiology. Thorax. 2006;61(4):354–61. doi: 10.1136/thx.2005.041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–23. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 73.Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mallia P, Message SD, Gielen V, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183(6):734–42. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilkinson TM, Hurst JR, Perera WR, Wilks M, Donaldson GC, Wedzicha JA. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest. 2006;129(2):317–24. doi: 10.1378/chest.129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Monso E, Ruiz J, Rosell A, et al. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med. 1995;152(4 Pt 1):1316–20. doi: 10.1164/ajrccm.152.4.7551388. [DOI] [PubMed] [Google Scholar]
- 77.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–71. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 78.Sethi S, Sethi R, Eschberger K, et al. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(4):356–61. doi: 10.1164/rccm.200703-417OC. [DOI] [PubMed] [Google Scholar]
- 79.Vollenweider DJ, Jarrett H, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD010257. doi: 10.1002/14651858.CD010257. [DOI] [PubMed] [Google Scholar]
- 80.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–98. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Millares L, Ferrari R, Gallego M, et al. Bronchial microbiome of severe COPD patients colonised by Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2014 doi: 10.1007/s10096-013-2044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang YJ, Sethi S, Murphy T, Nariya S, Boushey HA, Lynch SV. Airway Microbiome Dynamics in Exacerbations of Chronic Obstructive Pulmonary Disease. J Clin Microbiol. 2014 doi: 10.1128/JCM.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krishnan V, Diette GB, Rand CS, et al. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med. 2006;174(6):633–8. doi: 10.1164/rccm.200601-007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009;39(2):193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376(9743):826–34. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lemanske RF, Jr, Dick EC, Swenson CA, Vrtis RF, Busse WW. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989;83(1):1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Graham V, Lasserson T, Rowe BH. Antibiotics for acute asthma. Cochrane Database Syst Rev. 2001;(3):CD002741. doi: 10.1002/14651858.CD002741. [DOI] [PubMed] [Google Scholar]
- 88.Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131(2):346–52. doi: 10.1016/j.jaci.2012.11.013. e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wientzen R, Prestidge CB, Kramer RI, McCracken GH, Nelson JD. Acute pulmonary exacerbations in cystic fibrosis. A double-blind trial of tobramycin and placebo therapy. Am J Dis Child. 1980;134(12):1134–8. doi: 10.1001/archpedi.1980.02130240018007. [DOI] [PubMed] [Google Scholar]
- 91.Hyatt AC, Chipps BE, Kumor KM, Mellits ED, Lietman PS, Rosenstein BJ. A double-blind controlled trial of anti-Pseudomonas chemotherapy of acute respiratory exacerbations in patients with cystic fibrosis. J Pediatr. 1981;99(2):307–14. doi: 10.1016/s0022-3476(81)80486-6. [DOI] [PubMed] [Google Scholar]
- 92.Simon RH. Cystic fibrosis: Antibiotic therapy for lung disease. In: Post TW, editor. UpToDate. 2014. [accessed April 24 2014]. [Google Scholar]
- 93.Hurley MN, Ariff AH, Bertenshaw C, Bhatt J, Smyth AR. Results of antibiotic susceptibility testing do not influence clinical outcome in children with cystic fibrosis. J Cyst Fibros. 2012;11(4):288–92. doi: 10.1016/j.jcf.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith AL, Fiel SB, Mayer-Hamblett N, Ramsey B, Burns JL. Susceptibility testing of Pseudomonas aeruginosa isolates and clinical response to parenteral antibiotic administration: lack of association in cystic fibrosis. Chest. 2003;123(5):1495–502. doi: 10.1378/chest.123.5.1495. [DOI] [PubMed] [Google Scholar]
- 95.Gold R, Overmeyer A, Knie B, Fleming PC, Levison H. Controlled trial of ceftazidime vs. ticarcillin and tobramycin in the treatment of acute respiratory exacerbations in patients with cystic fibrosis. Pediatr Infect Dis. 1985;4(2):172–7. doi: 10.1097/00006454-198503000-00012. [DOI] [PubMed] [Google Scholar]
- 96.Rogers GB, Hart CA, Mason JR, Hughes M, Walshaw MJ, Bruce KD. Bacterial diversity in cases of lung infection in cystic fibrosis patients: 16S ribosomal DNA (rDNA) length heterogeneity PCR and 16S rDNA terminal restriction fragment length polymorphism profiling. J Clin Microbiol. 2003;41(8):3548–58. doi: 10.1128/JCM.41.8.3548-3558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rogers GB, Carroll MP, Serisier DJ, et al. Bacterial activity in cystic fibrosis lung infections. Respir Res. 2005;6:49. doi: 10.1186/1465-9921-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sibley CD, Parkins MD, Rabin HR, Duan K, Norgaard JC, Surette MG. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc Natl Acad Sci U S A. 2008;105(39):15070–5. doi: 10.1073/pnas.0804326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lynch SV, Bruce KD. The cystic fibrosis airway microbiome. Cold Spring Harb Perspect Med. 2013;3(3):a009738. doi: 10.1101/cshperspect.a009738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Price KE, Hampton TH, Gifford AH, et al. Unique microbial communities persist in individual cystic fibrosis patients throughout a clinical exacerbation. Microbiome. 2013;1(1):27. doi: 10.1186/2049-2618-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carmody LA, Zhao J, Schloss PD, et al. Changes in cystic fibrosis airway microbiota at pulmonary exacerbation. Ann Am Thorac Soc. 2013;10(3):179–87. doi: 10.1513/AnnalsATS.201211-107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stressmann FA, Rogers GB, Marsh P, et al. Does bacterial density in cystic fibrosis sputum increase prior to pulmonary exacerbation? J Cyst Fibros. 2011;10(5):357–65. doi: 10.1016/j.jcf.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 103.Iwai S, Huang D, Fong S, et al. The lung microbiome of ugandan hiv-infected pneumonia patients is compositionally and functionally distinct from that of san franciscan patients. PLoS One. 2014;9(4):e95726. doi: 10.1371/journal.pone.0095726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):660–7. doi: 10.1016/S0140-6736(12)60953-2. [DOI] [PubMed] [Google Scholar]
- 105.Rogers GB, Zain NM, Bruce KD, et al. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann Am Thorac Soc. 2014 doi: 10.1513/AnnalsATS.201310-335OC. [DOI] [PubMed] [Google Scholar]
- 106.Maughan H, Cunningham KS, Wang PW, et al. Pulmonary bacterial communities in surgically resected noncystic fibrosis bronchiectasis lungs are similar to those in cystic fibrosis. Pulm Med. 2012;2012:746358. doi: 10.1155/2012/746358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest. 1993;103(6):1808–12. doi: 10.1378/chest.103.6.1808. [DOI] [PubMed] [Google Scholar]
- 108.Ambrosini V, Cancellieri A, Chilosi M, et al. Acute exacerbation of idiopathic pulmonary fibrosis: report of a series. Eur Respir J. 2003;22(5):821–6. doi: 10.1183/09031936.03.00022703. [DOI] [PubMed] [Google Scholar]
- 109.Richter AG, Stockley RA, Harper L, Thickett DR. Pulmonary infection in Wegener granulomatosis and idiopathic pulmonary fibrosis. Thorax. 2009;64(8):692–7. doi: 10.1136/thx.2008.110445. [DOI] [PubMed] [Google Scholar]
- 110.Russell A, Cox M, Moffatt M, Cookson W, Maher T, Molyneaux P. The respiratory microbiome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:A5174. [Google Scholar]
- 111.Shulgina L, Cahn AP, Chilvers ER, et al. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax. 2013;68(2):155–62. doi: 10.1136/thoraxjnl-2012-202403. [DOI] [PubMed] [Google Scholar]
- 112.Alvarez-Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Med. 1996;22(5):387–94. doi: 10.1007/BF01712153. [DOI] [PubMed] [Google Scholar]
- 113.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(21):2063. doi: 10.1056/NEJMc1312359. [DOI] [PubMed] [Google Scholar]
- 114.Walker AW, Sanderson JD, Churcher C, et al. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11:7. doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380(9853):1606–19. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 116.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(13):1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 117.Fellermann K, Ludwig D, Stahl M, David-Walek T, Stange EF. Steroid-unresponsive acute attacks of inflammatory bowel disease: immunomodulation by tacrolimus (FK506) Am J Gastroenterol. 1998;93(10):1860–6. doi: 10.1111/j.1572-0241.1998.539_g.x. [DOI] [PubMed] [Google Scholar]
- 118.Finlay BB, McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124(4):767–82. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 119.Hansel TT, Barnes PJ. New drugs for exacerbations of chronic obstructive pulmonary disease. Lancet. 2009;374(9691):744–55. doi: 10.1016/S0140-6736(09)61342-8. [DOI] [PubMed] [Google Scholar]
- 120.Hoogerwerf JJ, de Vos AF, Bresser P, et al. Lung inflammation induced by lipoteichoic acid or lipopolysaccharide in humans. Am J Respir Crit Care Med. 2008;178(1):34–41. doi: 10.1164/rccm.200708-1261OC. [DOI] [PubMed] [Google Scholar]
- 121.Muir A, Soong G, Sokol S, et al. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30(6):777–83. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- 122.Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1(1):17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sibley CD, Duan K, Fischer C, et al. Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 2008;4(10):e1000184. doi: 10.1371/journal.ppat.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Short FL, Murdoch SL, Ryan RP. Polybacterial human disease: the ills of social networking. Trends Microbiol. 2014 doi: 10.1016/j.tim.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Twomey KB, O'Connell OJ, McCarthy Y, et al. Bacterial cis-2-unsaturated fatty acids found in the cystic fibrosis airway modulate virulence and persistence of Pseudomonas aeruginosa. The ISME journal. 2012;6(5):939–50. doi: 10.1038/ismej.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bloom SM, Bijanki VN, Nava GM, et al. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell host & microbe. 2011;9(5):390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McNulty NP, Yatsunenko T, Hsiao A, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med. 2011;3(106):106ra. doi: 10.1126/scitranslmed.3002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gollwitzer ES, Marsland BJ. Microbiota abnormalities in inflammatory airway diseases - Potential for therapy. Pharmacol Ther. 2014;141(1):32–9. doi: 10.1016/j.pharmthera.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 129.Kelly C, Ward C, Stenton CS, Bird G, Hendrick DJ, Walters EH. Number and activity of inflammatory cells in bronchoalveolar lavage fluid in asthma and their relation to airway responsiveness. Thorax. 1988;43(9):684–92. doi: 10.1136/thx.43.9.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Levin SA. Ecosystems and the biosphere as complex adaptive systems. Ecosystems. 1998;1(5):431–6. [Google Scholar]
- 131.Holland JH. Complex adaptive systems. Daedalus. 1992;121(1):17–30. [Google Scholar]
- 132.Page SE. Diversity and complexity. Princeton University Press; 2010. [Google Scholar]
- 133.An G, Mi Q, Dutta-Moscato J, Vodovotz Y. Agent - based models in translational systems biology. Wiley Interdiscip Rev Syst Biol Med. 2009;1(2):159–71. doi: 10.1002/wsbm.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Holland JH. Studying complex adaptive systems. Journal of Systems Science and Complexity. 2006;19(1):1–8. [Google Scholar]
- 135.Slater M, Rivett DW, Williams L, et al. The impact of azithromycin therapy on the airway microbiota in asthma. Thorax. 2013 doi: 10.1136/thoraxjnl-2013-204517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bruzzese E, Raia V, Spagnuolo MI, et al. Effect of Lactobacillus GG supplementation on pulmonary exacerbations in patients with cystic fibrosis: a pilot study. Clin Nutr. 2007;26(3):322–8. doi: 10.1016/j.clnu.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 137.Weiss B, Bujanover Y, Yahav Y, Vilozni D, Fireman E, Efrati O. Probiotic supplementation affects pulmonary exacerbations in patients with cystic fibrosis: a pilot study. Pediatr Pulmonol. 2010;45(6):536–40. doi: 10.1002/ppul.21138. [DOI] [PubMed] [Google Scholar]
- 138.Lozupone C, Cota-Gomez A, Palmer BE, et al. Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am J Respir Crit Care Med. 2013;187(10):1110–7. doi: 10.1164/rccm.201211-2145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]