Abstract
Lysophosphatidic acid (LPA, 1- or 2-acyl-sn-glycerol 3-phosphate) mediates a plethora of physiological and pathological activities via interactions with a series of high affinity G protein-coupled receptors (GPCR). Both LPA receptor family members and autotaxin (ATX/LysoPLD), the primary LPA-producing enzyme, are aberrantly expressed in many human breast cancers and several other cancer lineages. Using transgenic mice expressing either an LPA receptor or ATX, we recently demonstrated that the ATX-LPA receptor axis plays a causal role in breast tumorigenesis and cancer-related inflammation, further validating the ATX-LPA receptor axis as a rich therapeutic target in cancer.
Keywords: Breast cancer, ATX, LPA, G protein-coupled receptor, inflammation, cytokines, target therapy
Lysophosphatidic acid – a structurally simple lipid with diverse actions
Lysophosphatidic acid (LPA) is a simple lipid with a single fatty acyl chain, a glycerol backbone and a free phosphate group.1 Despite the simplicity of its structure, LPA exerts multifaceted bioactivities including stimulation of proliferation, migration, and survival of many cell types. LPA binds to specific cell-surface G protein-coupled receptors (GPCRs). So far, there are six identified as bona fide LPA receptors, LPA1–6,2–7, and several others which include intracellular receptor PPAR©8, putative LPA receptors (GPR879 and p2Y10). The best-characterized LPA receptors are those of the endothelial differentiation gene (Edg) family including LPA1/Edg2, LPA2/Edg4 and LPA3/Edg7. The other Edg-family proteins, Edg1, 3, 5, 6 and 8, are receptors for a closely-related bioactive lipid, sphingosine 1-phosphate, which by itself, can exert an array of functions both similar and distinct from those of LPA.11
Early evidence suggested that LPA is primarily synthesized in extracellular fluids. We found a marked elevation of lysophosphatidylcholine (LPC) in the plasma of ovarian cancer patients, suggesting that LPA was produced via a lysoPLD activity.12 Umezu-Goto et al. demonstrated for the first time that the ectoenzyme ATX, possessed lysoPLD activity and is the main source of LPA production, resulting in LPA-mediated growth and motility of cancer cells.13 Interestingly, levels of the circulating LPA were decreased ~50% in mice carrying a heterozygous null mutation of Enpp2, the gene encoding ATX.14,15 It is now established that ATX is a key enzyme that produces LPA in cancer.
LPA receptors are ubiquitously and variably expressed in most cell types. Upon binding to its receptors, LPA can elicit divergent signaling pathways through LPA1–3 via at least three subunits of G proteins, Gq/11, Gi/o and G12/13.16 First, LPA induces transient elevation of cytosolic free Ca2+ concentration ([Ca2+]i) through a pathway involving Gq/11, phospholipase C and protein kinase C. Second, LPA receptors couple to Gi/o leading to inhibition of adenylate cyclase, activation of the Ras/mitogen-activated protein kinase (MAPK/ERK) pathway and activation of protein kinase B/Akt pathway. Third, LPA stimulates a family of guanine exchange factor RhoA GEFs and RhoA GTPase through G12/13.17 Together with the appropriate contexts, these pathways display potential to interact with one another, and in turn, integrate to regulate cell proliferation, survival, differentiation and motility through several immediate, early and late-onset genes.
In order to further and unambiguously identify the specific function of each Edg-family LPA receptor, mice with targeted deletion of the genes encoding LPA1–3 were created. Edg2−/− mice showed ~50% neonatal lethality, a defect in suckling behavior in neonatal pups and craniofacial dysmorphism.18 However, Edg2−/−/Edg4−/− mice revealed no additional phenotype to those found in Edg2−/− mice,19 suggesting that LPA2 may serve similar role to the LPA1 during development. Blastocysts of mice with null mutation of Edg7−/− developed delayed implantation and improper spacing due to a decrease in prostaglandin-induced uterine contraction.20 Enpp2−/− mice are embryonic lethal with severe attenuation of blood vessel formation.14,15 Together, these compelling genetic models point to the critical roles of LPA in fetal development. However, the early embryonic lethality in some of the LPA knockout mice hinders evaluating the significance of LPA signaling in late-onset disease models. Nevertheless, mice lacking LPA2 show a decrease in tumor development induced by colitis.21 demonstrating a critical role for LPA receptors and in this case LPA2 for development of adult onset diseases.
ATX-LPA receptor axis in cancer related- inflammation
In breast cancer, epidemiological evidence suggests that inflammation is associated with poor prognosis.22 Release and activation of growth factors and cytokines provide biochemical cues that exert a major influence on tumor cell survival. In addition, inflammatory cells show pro-tumorigenic roles, specifically tumor-associated macrophages and B cells, which are postulated to foster tumor growth and metastasis through release of cytokines and matrix remodeling enzymes.23, 24 The role of LPA and LPA receptor function in airway inflammation has been studied extensively revealing that LPA regulates receptor-mediated pro-inflammatory transcriptional factors including NF-κB, AP1, which regulate cytokine and lipid mediator production and secretion.25–29
Until now, the role of LPA in mammary gland inflammatory diseases had not been defined. We recently demonstrated for the first time that the ATX-LPA receptor axis induces inflammation and tumor formation in the mammary gland by establishing transgenic mouse models expressing human ATX, LPA1, LPA2 or LPA3 under the MMTV-LTR promoter. Individual overexpression of each receptor resulted in a high frequency of chronic mastitis, hyperplasia, mammary intraepithelial neoplasia and invasive and metastatic tumors. Strikingly, inflammation was present in mammary glands whether or not tumors were present in the transgenic mice. Furthermore inflammation was detected earlier than tumorigenesis in the transgenic mice. The high frequency and early onset of chronic mastitis suggests that chronic inflammation could contribute to tumor development in these models.30
We recently demonstrated that over expression of the Edg family of LPA receptors enhances the production of IL-6, IL-8, and VEGF in SKOV-3 and OVCAR-3 cells, while knockdown decreases production.31 This establishes a strong correlation between LPA function and production of pro-inflammatory cytokines, at least in ovarian cancer cells. Multiple mechanisms could contribute to LPA stimulating proinflammatory gene expression. NF-κB and ATF-2 both regulation expression of genes contributing to inflammatory reactions.32–35 LPA enhances NF-κB expression via activation of PKC or AKT pathways and LPA induces ATF-2 production through Rho-CDC42-p38 MAPK pathways36–38 (Figure 1). LPA stimulation also induces the activation of signal transducer and activator of transcription 3 (STAT3) and STAT5.30 Together, these transcription factors coordinate the production of inflammatory cytokines, chemokines and the production of cyclooxygenase 2 (COX2).38–41 Subsequently, cytokines bind to their own receptors and activate STAT family members,42, 43 further contributing to inflammation and to tumor cell activation44, 45 in an autocrine and paracrine feedforward cycle, stabilizing the production of inflammatory factors and the generation of an inflammatory tumor microenvironment. Finally, LPA also transactivates receptor tyrosine kinases (RTKs), such as EGFR,26, 46–49 PDGFRβ50 and c-Met,51, 52 which can contribute to cytokine production.
Figure 1. Synthetic pathways for LPA and major LPA signaling pathways connecting inflammation and cancer.
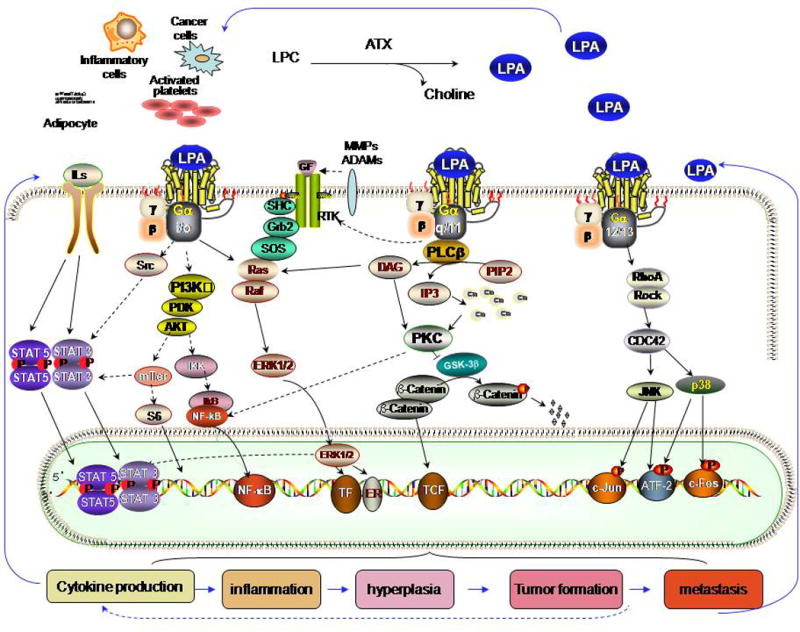
ATX hydrolyzes lysophospholipids, in particular lysophosphatidylcholine (LPC), to produce bioactive LPA. Newly produced LPA acts on its own GPCRs via at least three distinct classes of heterotrimeric G proteins — Gq, Gi and G12/13 activating multiple downstream pathways and evoke its biological effects, including RAS-ERK pathway through Gi and Gq; PI3K-AKT pathway through Gi; PKC –GSK30β – β-catenin pathway through Gq (and /or Gi); Rho-CDC42 pathway through G12/13; Src-Stat pathway through Gi, which induce expression and activation of multiple transcriptors including STAT3, NF-κB, ATF-2, which induce cell proliferation and production of cytokines. The cytokines bind to their receptors inducing Stat 3 and 5 production and activation.
Understanding the mechanism by which the ATX-LPA receptor axis induces inflammation in the mammary gland could enhance our understanding of breast tumorigenesis, and especially inflammatory breast cancer (IBC). IBC is a rapidly progressing and highly aggressive form of cancer characterized by increased angiogenesis, high expression of VEGF, EGF and their receptors, hyperactivation of NF-κB and overexpression of E-cadherin.53 For those patients who have HER2-positive IBC tumors, trastuzumab has been demonstrated activity but unfortunately this group represents only a small portion of the total number affected. The intracellular signaling signature in mammary tumors of transgenic mice over-expressing either ATX or LPA1, LPA2 or LPA3 receptors in mammary glands is remarkably similar to molecular abnormalities in IBC.30 In the LPAR and ATX transgenic mice, we observed mastitis and mammary tumors30 with increased levels of MIP-2 (the murine homolog of IL-8) and VEGF common observations in IBC. Thus, research is warranted into the potential role of LPA and LPA induced inflammation in IBC.
ATX-LPA receptor axis in breast and ovarian cancer
Although significant progress has been made in improving prognosis for patients with breast and ovarian cancer, unfortunately many patients still succumb to these diseases, which necessitate new molecular therapeutics to further improve outcomes. Enhanced ATX expression,54, 55 elevated LPA production,56, 57 decreased lipid phosphate phosphatase expression58 and increased LPA receptor expression59, 60 are found in multiple cancer types, including ovarian and breast cancer. The role of LPA in ovarian cancer has been extensively studied. The most notable finding, which spawned interest in this field, was that LPA is present at high levels in the ascitic fluid of ovarian cancer patients.61 Interestingly, peritoneal-lining mesothelial cells as well as ovarian cancer cells can also produce LPA,56 suggesting that both malignant and benign cells are sources of LPA in the ascites. Consistent with the role of LPA in tumor invasiveness, ovarian cancer cells frequently adhere to the lining cells as well as invade underlying stroma.62, 63 Moreover, LPA2 and LPA3 transcripts were increased between 15% and 49% in benign tumors and early and late stage ovarian carcinomas suggesting that both LPA production and action is aberrant in ovarian cancer.57 Systemic analyses of the LPA transcriptomes in human epithelial ovarian cancer suggested that LPA regulates several genes associated with cell adhesion and migration, which in turn, correlate with a poor prognosis in patients.64
In accord with ovarian cancer, breast cancer cells exhibit aberrant expression of ATX, which promotes tumor aggressiveness.65 Expression of ATX is markedly upregulated by the Jun oncoprotein in embryonic chick fibroblasts.66 In contrast, transcript levels of ATX were decreased by tumor suppressor CST6 in human breast cancer cells.67 A role for LPA receptors has also been suggested in the development and progression of breast cancer.68 LPA2 expression was upregulated in patients with invasive mammary ductal carcinoma,69 whereas the tumor suppressor Nm23-H1 downregulated LPA1 expression and inhibited metastasis in breast cancer cells.70 Inhibition of LPA1 receptor expression suppressed tumor growth and bone metastasis in the xenografts of MDA-BO2 breast cancer cells in mice.71
Thus, it is clear that the aberrant regulations of ATX-LPA signaling are strongly associated with the pathogenesis of both breast and ovarian cancer. We therefore sought to determine whether ATX-LPA signaling by itself is sufficient to induce tumorigenesis. Strikingly, transgenic mice expressing either ATX or each of the three major Edg family LPA receptors under the MMTV-LTR promoter exhibited chronic mastitis, mammary intraepithelial neoplasia together with invasive and metastatic breast cancer.30 In support of a role for LPA and its receptors in tumor pathophysiology, overexpression of each of the Edg family LPA receptors in SKOV-3 cells that endogenously produce LPA enhanced cell proliferation and invasion in vitro as well as formation of ascites and metastasis in vivo.31 Together, our data from animal models of breast and ovarian cancer indicate that LPA production and action and specifically ATX and LPA Edg family receptors are candidates for drug development in breast and ovarian cancer.
Beginning with reverse phase protein arrays (RPPA) functional proteomic analysis on mammary cancers isolated from transgenic mouse models30 and combining the data with published reports, a resultant pathway signature mediated by the ATX-LPA receptor axis is illustrated using ScienceSlides 2008 (Figure 1). ATX-LPA receptor axis in cancer signals are mediated by multiple pathways, including at least three distinct G proteins (Gq/11, Gi/o and G12/13) coupled to receptors, inflammatory cytokine pathways, transactivation of receptor tyrosine kinase (RTK) signaling, which, in turn, feeds into multiple transcriptional mediators,72–82 see Figure 1.
Expression of the estrogen receptor (ER) is a key determinant of the pathophysiology of breast cancer.83 ER-positive breast cancers are characterized as luminal subtype associated with a better prognosis and response to hormonal manipulation.72–82 Primary metastatic sites of the ER-positive cancers are lung and bone, and less frequently liver and brain, whereas ER-negative cancers spread mainly to visceral organs.86 The expression of ER was upregulated in a subset of the transgenic mice, providing a novel tumor model for invasive and metastatic ER-positive tumors. Some findings have also suggested that estrogen could regulate the functions of LPA. For example, estrogen has been shown to modulate actions of LPA through LPA1 in C9 hepatic cells.87 Moreover, estrogen was found to induce expression of LPA3 receptors in porcine uterine endometrial explants.88 However, the of ER in LPA-induced breast cancer progression remains to be elucidated.
Targeting the ATX-LPA receptor Axis in Cancer
The mortality rate for breast cancer in the U.S. has been in decline for over a decade and continues to decrease 1.8% every year.89 For patients with this disease, advances in screening, early detection and chemotherapy integrate to contribute to the improved prognosis89 Targeted therapeutics provide best hope for improving outcomes for breast cancer.
For patients with HER-2-positive breast cancer, the advent of trastuzumab (Herceptin™) contributed significantly to improved outcomes for breast cancer patients. It has also provided the ideal model for future breast cancer drug development. In certain breast cancers, the amplified and overactive HER-2 receptor at the cell surface continuously signals cellular growth and survival, which ultimately drives disease development and progression. The combination of an approach to identify patients with amplified HER2 (herceptest™) and an effective therapeutic approach trastuzumab, resulted in benefit to patients with amplified HER2.
Potential of LPA pathway-targeted therapeutics for breast cancer
Previous work by our group and others combined with the new transgenic models30 supports a role for LPA, ATX, LPA receptors and lipid phosphate phosphatases in cancer.31, 69, 79, 90–94 Thus, there are multiple potential therapeutic targets in this pathway that could function as targets for anti-cancer therapeutics. In fact, the therapeutics under development in both academic and biotech centers encompass several different goals: monoclonal antibodies which bind to and inhibit LPA, stabilized analogues of naturally occurring molecules that inhibit ATX and chemical compounds that function as LPA receptor antagonists.95
Applying the current standard of care to breast cancer would necessitate the identification of tumor driven by the LPA pathway, as is already done with hormone receptor and HER2 status at the time of diagnosis. Based on that result, patients with an active LPA pathway would be eligible for therapeutics targeting the LPA production and function.
Targeting LPA receptors
Since most actions mediated by LPA occur through GPCRs, and GPCRs have been fruitful targets, multiple groups have developed inhibitors to LPA receptors.95 Each of the Edg family LPA receptors has functional antagonists, although not all are specific to one receptor, many have dual receptor antagonism because of technical obstacles and similarities in receptor binding. For examples, there are number synthetic compounds (e.g. Ki16425,96 DGPP 8:0,97 PA 8:0,97 VPC1224998) that are antagonists of both LPA1 and LPA3. Since Edg family LPA receptors retain the capability of inducing similar signaling events and functional outcomes, although each of them preferentially link to particular pathways and functional outcomes, a lack of exquisite receptor selectivity may not be a problem.30, 31 Indeed, many current targeting therapeutics are pleiomorphic in activity blocking multiple kinases.
Previous work implicated that LPA1 receptor contributes to metastasis.99 Interestingly, siRNA targeting either LPA1 receptors or a pharmacological blockade of LPA1 receptor activity with the LPA1/LPA3 antagonist Ki16425 inhibited metastasis to bone in vivo.71 In support of this concept, cDNA array analysis demonstrated that the LPA1 receptor plays a major role expression of genes involved in invasion and motility.100 Furthermore, a comprehensive screen using cDNA overexpression in breast cancer cells expressing inducible HER-2 variants demonstrated that the LPA1 receptor cooperates with HER-2 though a hypothesized mechanism of ligand-independent dimerization of LPA1 with HER-2 facilitating cell proliferation, morphogenesis and migration.68 In addition, our studies suggest that the LPA1 receptor may contribute to cancer metastasis.30, 31. Ki16425, DGPP 8:0, PA 8:0, VPC-32183, Darmstoff analogues, tetradecyl-phosphonate, phosphonothioate and fluoromethylene phosphonate ccPA analogues, methylene phosphonate analogues, isoxazole derivative may thus be useful in preventing breast cancer metastasis to bone.
The LPA2 receptor is preferentially and often exclusively expressed in more than half of bowel cancers. In addition, the expression of the LPA2 receptor is correlated with a higher rate of invasion and metastasis93 and is significantly overexpressed in patients with invasive ductal carcinoma, particularly in postmenopausal women.69 Multiple cancer cells have been found to expresses abundant LPA2 receptor levels, including colon, ovary, breast, and thyroid. This receptor may regulate LPA-induced stimulation of the chemokine growth regulated oncogene (GROα) which contributes to angiogenesis and tumorigenesis of ovarian cancer cells.101 Expression of the human LPA2 receptor in the breast of multiply pregnant mice was sufficient to result in the development of mammary carcinomas with a high incidence and short tumor-free period compared to other LPA receptors,30 suggesting that the LPA2 receptor may be a particularly attractive target in breast cancer. A number of compounds including Tetradecyl-phosphonate, Darmstoff analogues, FDP, FMP, methylene phosphonate analogues and Compound 35102–105 have been developed as antagonists of LPA2 receptor.
So far, more antagonists exist for the LPA3 receptor than other LPA receptors because of its unique and advantageous basic residue near the phosphate headgroup (e.g. Ki16425, DGPP 8:0, PA 8:0, VPC-32183, VPC-12249, FAP-12, Darmstoff analogues, tetradecyl- phosphonate, (2R)-TPA 8:0, SDP, FR, FDP, FMP).102–105 Our demonstration that transgenic expression of the LPA3 receptor is sufficient to result in mammary carcinomas with a high frequency of tumor metastasis, combined with other studies suggests that LPA3 may be a particularly attractive target in breast and ovarian cancer.30,31
Targeting ATX and LPA
An alternative strategy to LPA receptor antagonism is to inhibit the production of LPA or to immunoneutralize LPA. Inhibiting LPA directly could have all of the effects described above as well as blocking yet unknown or uncharacterized LPA receptors such as LPA4–8 and bypassing potential partial agonist effects against receptor subsets. Thus, directly targeting LPA and/or targeting the enzyme responsible for the bulk of LPA production are logical approaches. Development of monoclonal antibodies targeting LPA has commenced with Lpathomab™95 and monocolonal antibodies that bind to S1P have been successfully developed and tested in small animal models, including Asonep (Sphingomab®)106. Indeed, Asonep is progressing through phase I clinical trials.
LPA is produced through at least two pathways: 1) in blood, mainly from lysophospholipids by ATX; 2) by inflammatory cells, activated platelets, adipocytes, mesothelial cells and some cancer cells, mainly from phosphatidic acid by phospholipases. ATX plays a critical role in both pathways of LPA production.107 Cell line and our recent transgenic studies identified potential roles for ATX in cancer progression, tumor cell invasion and metastasis including promotion of tumor angiogenesis108. LPA and S1P are natural ATX inhibitors providing an approach for the development of ATX inhibitors. These include synthetic analogues of the naturally-occurring ATX-inhibitor cyclic phosphatidic acid (carba analogues of cyclic phosphatidic acid or ccPA),109, 110 L-histidine,111 VPC8a202,112, 113 Darmstoff analogues,114 Thiophosphoric acid O-octadec-9-enyl ester115 and small molecular inhibitors identified using high-throughput screening.116 Additional ATX inhibiting compounds are under development by multiple groups world-wide, attesting to the importance of this enzyme as a therapeutic target in tumorigenesis.
Research in progress and perspective
Aberrant expression of LPA receptors and ATX enhance ER expression in mammary glands and induced ER positive mammary cancer compatible with functional ER signaling.1 Studies are underway to verify the mechanism by which the ATX-LPA receptors axis enhances ER expression. Both in vitro and in vivo studies suggest that the ATX-LPA receptors axis promote tumor metastasis, but further studies are needed to determine the mechanism. Indeed, the late and variable onset of tumors suggest that other mutational events in the transgenic mice cooperate with LPA signaling to induce tumorigenesis. Alternatively, since LPA is a potent survival factor, LPA signaling may allow cells to survive oncogenic success leading to tumor development. Studies have demonstrated that augmented LPA signaling contributes to cancer related inflammation and breast cancer initiation and progression, indicating that LPA producing enzymes and LPA receptors can be targets for treatment or chemoprevention of inflammation and cancer. Development of compounds targeting LPA production and action has been initiated and hopefully will soon enter clinical trials determining whether the ATX-LPA axis is indeed a useful target in cancer.
Acknowledgments
The work was supported by DOD Breast Cancer Research Program DAMD17-03-1-0409 (to S.L.), by National Institute of Health Grants CA82716, CA64602 and CA099031 (to G.B.M.)
Abbreviations
- LPA
lysophosphatidic acid
- ATX
autotaxin
- GPCR
G protein-coupled receptors
- Edg
endothelial differentiation gene
- LPC
lysophosphatidylcholine
- MAPK
mitogen-activated protein kinase
- PKB/AKT
protein kinase B
- STAT
signal transducer and activator of transcription
- ATF-2
activating transcription factor 2
- COX2
cyclooxygenase 2
- RTK
receptor tyrosine kinase
- IBC
inflammatory breast cancer
- NF- κB
nuclear factor kappa B
- IL
Interleukin
- EGFR
epidermal growth factor receptor
- PDGF
platelet-derived growth factor
- MIP-2
macrophage-inflammatory protein-2
- VEGF
vascular endothelial growth factor
- RPPA
reverse phase protein arrays
- ER
estrogen receptor
- GRO
growth regulated oncogene
References
- 1.Tokumura A. A family of phospholipid autacoids: occurrence, metabolism and bioactions. Prog Lipid Res. 1995;34:151–84. doi: 10.1016/0163-7827(95)00001-g. [DOI] [PubMed] [Google Scholar]
- 2.Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. The Journal of cell biology. 1996;135:1071–83. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. The Journal of biological chemistry. 1998;273:7906–10. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- 4.Bandoh K, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K, et al. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. The Journal of biological chemistry. 1999;274:27776–85. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- 5.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. The Journal of biological chemistry. 2003;278:25600–6. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 6.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. The Journal of biological chemistry. 2006;281:23589–97. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 7.Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y, et al. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. The Journal of biological chemistry. 2009;284:17731–41. doi: 10.1074/jbc.M808506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARγ agonist. Proc Natl Acad Sci USA. 2003;100:131–6. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2007;363:861–6. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 10.Murakami M, Shiraishi A, Tabata K, Fujita N. Identification of the orphan GPCR, P2Y10 receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2008;371:707–12. doi: 10.1016/j.bbrc.2008.04.145. [DOI] [PubMed] [Google Scholar]
- 11.Sabbadini RA. Targeting sphingosine-1-phosphate for cancer therapy. Br J Cancer. 2006;95:1131–5. doi: 10.1038/sj.bjc.6603400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okita M, Gaudette DC, Mills GB, Holub BJ. Elevated levels and altered fatty acid composition of plasma lysophosphatidylcholine (lysoPC) in ovarian cancer patients. Int J Cancer. 1997;71:31–4. doi: 10.1002/(sici)1097-0215(19970328)71:1<31::aid-ijc7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. The Journal of cell biology. 2002;158:227–33. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Molecular and cellular biology. 2006;26:5015–22. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. The Journal of biological chemistry. 2006;281:25822–30. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 16.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nature reviews. 2003;3:582–91. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XF, Schaefer AW, Burnette DT, Schoonderwoert VT, Forscher P. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron. 2003;40:931–44. doi: 10.1016/s0896-6273(03)00754-2. [DOI] [PubMed] [Google Scholar]
- 18.Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci USA. 2000;97:13384–9. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, et al. Characterization of lpa2 (Edg4) and lpa1/lpa2 (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa2. Molecular and cellular biology. 2002;22:6921–9. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–8. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin S, Wang D, Iyer S, Ghaleb AM, Shim H, Yang VW, et al. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology. 2009;136:1711–20. doi: 10.1053/j.gastro.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hojilla CV, Wood GA, Khokha R. Inflammation and breast cancer: metalloproteinases as common effectors of inflammation and extracellular matrix breakdown in breast cancer. Breast Cancer Res. 2008;10:205. doi: 10.1186/bcr1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–23. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature reviews. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 25.Cummings R, Zhao Y, Jacoby D, Spannhake EW, Ohba M, Garcia JG, et al. Protein kinase Cδ mediates lysophosphatidic acid-induced NF-κB activation and interleukin-8 secretion in human bronchial epithelial cells. The Journal of biological chemistry. 2004;279:41085–94. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, He D, Saatian B, Watkins T, Spannhake EW, Pyne NJ, et al. Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cδ, Lyn kinase, and matrix metalloproteinases. The Journal of biological chemistry. 2006;281:19501–11. doi: 10.1074/jbc.M511224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saatian B, Zhao Y, He D, Georas SN, Watkins T, Spannhake EW, et al. Transcriptional regulation of lysophosphatidic acid-induced interleukin-8 expression and secretion by p38 MAPK and JNK in human bronchial epithelial cells. Biochem J. 2006;393:657–68. doi: 10.1042/BJ20050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He D, Natarajan V, Stern R, Gorshkova IA, Solway J, Spannhake EW, et al. Lysophosphatidic acid-induced transactivation of epidermal growth factor receptor regulates cyclo-oxygenase-2 expression and prostaglandin E2 release via C/EBPβ in human bronchial epithelial cells. Biochem J. 2008;412:153–62. doi: 10.1042/BJ20071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barekzi E, Roman J, Hise K, Georas S, Steinke JW. Lysophosphatidic acid stimulates inflammatory cascade in airway epithelial cells. Prostaglandins Leukot Essent Fatty Acids. 2006;74:357–63. doi: 10.1016/j.plefa.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang W, et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion and metastases. Cancer Cell. 2009;15:539–50. doi: 10.1016/j.ccr.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J, et al. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst. 2008;100:1630–42. doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang YC, Kim KM, Lee KS, Namkoong S, Lee SJ, Han JA, et al. P38 MAPK: critical molecule in thrombin-induced NF-kappa B-dependent leukocyte recruitment. Am J Physiol Heart Circ Physiol. 2002;284:1095–103. doi: 10.1152/ajpheart.00016.2002. [DOI] [PubMed] [Google Scholar]
- 33.Petro TM. ERK-MAP-kinases differentially regulate expression of IL-23 p19 compared with p40 and IFN-beta in Theiler’s virus-infected RAW264. 7 cells. Immunol Lett. 2005;97:47–53. doi: 10.1016/j.imlet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Bhat NR, Feinstein DL, Shen O, Bhat AN. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. J Biol Chem. 2002;277:29584–92. doi: 10.1074/jbc.M204994200. [DOI] [PubMed] [Google Scholar]
- 35.Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, et al. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J Biol Chem. 2007;282:27229–38. doi: 10.1074/jbc.M703250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estrella VC, Eder AM, Liu S, Pustilnik TB, Tabassam FH, Claret FX, et al. Lysophosphatidic acid induction of urokinase plasminogen activator secretion requires activation of the p38MAPK pathway. Int J Oncol. 2007;31:441–49. [PubMed] [Google Scholar]
- 37.Hao F, Tan M, Xu X, Han J, Miller DD, Tigyi G, et al. Lysophosphatidic acid induces prostate cancer PC3 cell migration via activation of LPA (1), p42 and p38alpha. Biochim Biophys Acta. 2007;1771:883–92. doi: 10.1016/j.bbalip.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalari S, Zhao Y, Spannhake EW, Berdyshev EV, Natarajan V. Role of acylglycerol kinase in LPA-induced IL-8 secretion and transactivation of epidermal growth factor-receptor in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L328–36. doi: 10.1152/ajplung.90431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenkol L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007;19:251–60. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Panne D, Maniatis T, Harrison SC. An atomic model of the interferon-beta enhanceosome. Cells. 2007;129:1111–23. doi: 10.1016/j.cell.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, et al. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157–68. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 43.Wegenka UM, Lutticken C, Buschmann J, Yuan J, Lottspeich F, Müller-Esterl W, et al. The interleukin-6-activated acute-phase response factor is antigenically and functionally related to members of the signal transducer and activator of transcription (STAT) family. Mol Cell Biol. 1994;14:3186–96. doi: 10.1128/mcb.14.5.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Du H, Qin Y, Roberts J, Cummings OW, Yan C. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res. 2007;67:8494–503. doi: 10.1158/0008-5472.CAN-07-0647. [DOI] [PubMed] [Google Scholar]
- 45.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 46.Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997;16:7032–44. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mori K, Kitayama J, Shida D, Yamashita H, Watanabe T, Nagawa H. Lysophosphatidic acid-induced effects in human colon carcinoma DLD1 cells are partially dependent on transactivation of epidermal growth factor receptor. J Surg Res. 2006;132:56–61. doi: 10.1016/j.jss.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 48.Shah BH, Baukal AJ, Shah FB, Catt KJ. Mechanisms of extracellularly regulated kinases 1/2 activation in adrenal glomerulosa cells by lysophosphatidic acid and epidermal growth factor. Mol Endocrinol. 2005;19:2535–48. doi: 10.1210/me.2005-0082. [DOI] [PubMed] [Google Scholar]
- 49.Umata T, Hirata M, Takahashi T, Ryu F, Shida S, Takahashi Y, et al. A dual signaling cascade that regulates the ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. The Journal of biological chemistry. 2001;276:30475–82. doi: 10.1074/jbc.M103673200. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Cummings R, Zhao Y, Kazlauskas A, Sham JK, Morris A, et al. Involvement of phospholipase D2 in lysophosphatidate-induced transactivation of platelet-derived growth factor receptor-beta in human bronchial epithelial cells. The Journal of biological chemistry. 2003;278:39931–40. doi: 10.1074/jbc.M302896200. [DOI] [PubMed] [Google Scholar]
- 51.Rosen EM, Nigam SK, Goldberg ID. Scatter factor and the c-met receptor: a paradigm for mesenchymal/epithelial interaction. The Journal of cell biology. 1994;127:1783–7. doi: 10.1083/jcb.127.6.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin JS, Bottaro DP, Aaronson SA. Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochim Biophys Acta. 1993;1155:357–71. doi: 10.1016/0304-419x(93)90015-5. [DOI] [PubMed] [Google Scholar]
- 53.Yamauchi H, Cristofanilli M, Nakamura S, Hortobagyi GN, Ueno NT. Molecular targets for treatment of inflammatory breast cancer. Nat Rev Clin Oncol. 2009;6:387–94. doi: 10.1038/nrclinonc.2009.73. [DOI] [PubMed] [Google Scholar]
- 54.Kishi Y, Okudaira S, Tanaka M, Hama K, Shida D, Kitayama J, et al. Autotaxin is overexpressed in glioblastoma multiforme and contributes to cell motility of glioblastoma by converting lysophosphatidylcholine to lysophosphatidic acid. The Journal of biological chemistry. 2006;281:17492–500. doi: 10.1074/jbc.M601803200. [DOI] [PubMed] [Google Scholar]
- 55.Masuda A, Nakamura K, Izutsu K, Igarashi K, Ohkawa R, Jona M, et al. Serum autotaxin measurement in haematological makignancies: a promising marker for follicular lymphoma. Br J Haematol. 2008;143:60–70. doi: 10.1111/j.1365-2141.2008.07325.x. [DOI] [PubMed] [Google Scholar]
- 56.Ren J, Xiao YJ, Singh LS, Zhao X, Zhao Z, Feng L, et al. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 2006;66:3006–14. doi: 10.1158/0008-5472.CAN-05-1292. [DOI] [PubMed] [Google Scholar]
- 57.Sengupta S, Xiao YJ, Xu Y. A novel laminin-induced LPA autocrine loop in the migration of ovarian cancer cells. FASEB J. 2003;17:1570–2. doi: 10.1096/fj.02-1145fje. [DOI] [PubMed] [Google Scholar]
- 58.Tanyi JL, Hasegawa Y, Lapushin R, Morris AJ, Wolf JK, Berchuck A, et al. Role of decreased levels of lipid phosphate phosphatase-1 in accumulation of lysophosphatidic acid in ovarian cancer. Clin Cancer Res. 2003;9:3534–45. [PubMed] [Google Scholar]
- 59.Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–64. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 60.Murph MM, Nguyen GH, Radhakrishna H, Mills GB. Sharpening the edges of understanding the structure/function of the LPA1 receptor: expression in cancer and mechanisms of regulation. Biochim Biophys Acta. 2008;1781:547–57. doi: 10.1016/j.bbalip.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mills GB, May C, Hill M, Campbell S, Shaw P, Marks A. Ascitic fluid from human ovarian cancer patients contains growth factors necessary for intraperitoneal growth of human ovarian adenocarcinoma cells. J Clin Invest. 1990;86:851–5. doi: 10.1172/JCI114784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nature reviews. 2009;9:415–28. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang E, Ngalame Y, Panelli MC, Nguyen-Jackson H, Deavers M, Mueller P, et al. Peritoneal and subperitoneal stroma may facilitate regional spread of ovarian cancer. Clin Cancer Res. 2005;11:113–22. [PubMed] [Google Scholar]
- 64.Murph MM, Liu W, Yu S, Lu Y, Hall H, Hennessy BT, et al. Lysophosphatidic acid-induced transcriptional profile represents serous epithelial ovarian carcinoma and worsened prognosis. PLoS One. 2009;4:e5583. doi: 10.1371/journal.pone.0005583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang SY, Lee J, Park CG, Kim S, Hong S, Chung HC, et al. Expression of autotaxin (NPP-2) is closely linked to invasiveness of breast cancer cells. Clin Exp Metastasis. 2002;19:603–8. doi: 10.1023/a:1020950420196. [DOI] [PubMed] [Google Scholar]
- 66.Black EJ, Clair T, Delrow J, Neiman P, Gillespie DA. Microarray analysis identifies autotaxin, a tumour cell motility and angiogenic factor with lysophospholipase D activity, as a specific target of cell transformation by v-Jun. Oncogene. 2004;23:2357–66. doi: 10.1038/sj.onc.1207377. [DOI] [PubMed] [Google Scholar]
- 67.Song J, Jie C, Polk P, Shridhar R, Clair T, Zhang J, et al. The candidate tumor suppressor CST6 alters the gene expression profile of human breast carcinoma cells: down-regulation of the potent mitogenic, motogenic, and angiogenic factor autotaxin. Biochem Biophys Res Commun. 2006;340:175–82. doi: 10.1016/j.bbrc.2005.11.171. [DOI] [PubMed] [Google Scholar]
- 68.Witt AE, Hines LM, Collins NL, Hu Y, Gunawardane RN, Moreira D, et al. Functional proteomics approach to investigate the biological activities of cDNAs implicated in breast cancer. J Proteome Res. 2006;5:599–610. doi: 10.1021/pr050395r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitayama J, Shida D, Sako A, Ishikawa M, Hama K, Aoki J, et al. Overexpression of lysophosphatidic acid receptor-2 in human invasive ductal carcinoma. Breast Cancer Res. 2004;6:R640–R6. doi: 10.1186/bcr935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horak CE, Mendoza A, Vega-Valle E, Albaugh M, Graff-Cherry C, McDermott WG, et al. Nm23-H1 suppresses metastasis by inhibiting expression of the lysophosphatidic acid receptor EDG2. Cancer Res. 2007;67:11751–9. doi: 10.1158/0008-5472.CAN-07-3175. [DOI] [PubMed] [Google Scholar]
- 71.Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clezardin P, Peyruchaud O. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci USA. 2006;103:9643–8. doi: 10.1073/pnas.0600979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- 73.Jalink K, Eichholtz T, Postma FR, van Corven EJ, Moolenaar WH. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 1993;4:247–55. [PubMed] [Google Scholar]
- 74.Hordijk PL, Verlaan I, van Corven EJ, Moolenaar WH. Protein tyrosine phosphorylation induced by lysophosphatidic acid in Rat-1 fibroblasts. Evidence that phosphorylation of map kinase is mediated by the Gi-p21ras pathway. The Journal of biological chemistry. 1994;269:645–51. [PubMed] [Google Scholar]
- 75.Kumagai N, Morii N, Fujisawa K, Nemoto Y, Narumiya S. ADP-ribosylation of rho p21 inhibits lysophosphatidic acid-induced protein tyrosine phosphorylation and phosphatidylinositol 3-kinase activation in cultured Swiss 3T3 cells. The Journal of biological chemistry. 1993;268:24535–8. [PubMed] [Google Scholar]
- 76.van Corven EJ, Hordijk PL, Medema RH, Bos JL, Moolenaar WH. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc Natl Acad Sci USA. 1993;90:1257–61. doi: 10.1073/pnas.90.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Dijk MC, Postma F, Hilkmann H, Jalink K, van Blitterswijk WJ, Moolenaar WH. Exogenous phospholipase D generates lysophosphatidic acid and activates Ras, Rho and Ca2+ signaling pathways. Curr Biol. 1998;8:386–92. doi: 10.1016/s0960-9822(98)70157-5. [DOI] [PubMed] [Google Scholar]
- 78.Kranenburg O, Poland M, van Horck FP, Drechsel D, Hall A, Moolenaar WH. Activation of RhoA by lysophosphatidic acid and Gα12/13 subunits in neuronal cells: induction of neurite retraction. Mol Biol Cell. 1999;10:1851–7. doi: 10.1091/mbc.10.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang X, Yu S, LaPushin R, Lu Y, Furui T, Penn LZ, et al. Lysophosphatidic acid prevents apoptosis in fibroblasts via Gi-protein-mediated activation of mitogen-activated protein kinase. Biochem J. 2000;352(Pt 1):135–43. [PMC free article] [PubMed] [Google Scholar]
- 80.Fang X, Gaudette D, Furui T, Mao M, Estrella V, Eder A, et al. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann NY Acad Sci. 2000;905:188–208. doi: 10.1111/j.1749-6632.2000.tb06550.x. [DOI] [PubMed] [Google Scholar]
- 81.Kranenburg O, Moolenaar WH. Ras-MAP kinase signaling by lysophosphatidic acid and other G protein-coupled receptor agonists. Oncogene. 2001;20:1540–6. doi: 10.1038/sj.onc.1204187. [DOI] [PubMed] [Google Scholar]
- 82.Van Leeuwen FN, Olivo C, Grivell S, Giepmans BN, Collard JG, Moolenaar WH. Rac activation by lysophosphatidic acid LPA1 receptors through the guanine nucleotide exchange factor Tiam1. The Journal of biological chemistry. 2003;278:400–6. doi: 10.1074/jbc.M210151200. [DOI] [PubMed] [Google Scholar]
- 83.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 84.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–52. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 87.Gonzalez-Arenas A, Avendano-Vazquez SE, Cabrera-Wrooman A, Tapia-Carrillo D, Larrea F, Garcia-Becerra R, et al. Regulation of LPA receptor function by estrogens. Biochim Biophys Acta. 2008;1783:253–62. doi: 10.1016/j.bbamcr.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 88.Seo H, Kim M, Choi Y, Lee CK, Ka H. Analysis of lysophosphatidic acid (LPA) receptor and LPA-induced endometrial prostaglandin-endoperoxide synthase 2 expression in the porcine uterus. Endocrinology. 2008;149:6166–75. doi: 10.1210/en.2008-0354. [DOI] [PubMed] [Google Scholar]
- 89.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 90.Nakamoto T, Yasuda K, Yasuhara M, Yoshimura T, Kinoshita T, Nakajima T, et al. Expression of the endothelial cell differentiation gene 7 (EDG-7), a lysophosphatidic acid receptor, in ovarian tumor. J Obstet Gynaecol Res. 2005;31:344–51. doi: 10.1111/j.1447-0756.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 91.Shida D, Watanabe T, Aoki J, Hama K, Kitayama J, Sonoda H, et al. Aberrant expression of lysophosphatidic acid (LPA) receptors in human colorectal cancer. Lab Invest. 2004;84:1352–62. doi: 10.1038/labinvest.3700146. [DOI] [PubMed] [Google Scholar]
- 92.Umezu-Goto M, Tanyi J, Lahad J, Liu S, Yu S, Lapushin R, et al. Lysophosphatidic acid production and action: validated targets in cancer? J Cell Biochem. 2004;92:1115–40. doi: 10.1002/jcb.20113. [DOI] [PubMed] [Google Scholar]
- 93.Yamashita H, Kitayama J, Shida D, Ishikawa M, Hama K, Aoki J, et al. Differential expression of lysophosphatidic acid receptor-2 in intestinal and diffuse type gastric cancer. J Surg Oncol. 2006;93:30–5. doi: 10.1002/jso.20397. [DOI] [PubMed] [Google Scholar]
- 94.Yun CC, Sun H, Wang D, Rusovici R, Castleberry A, Hall RA, et al. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol Cell Physiol. 2005;289:C2–C11. doi: 10.1152/ajpcell.00610.2004. [DOI] [PubMed] [Google Scholar]
- 95.Murph M, Mills GB. Targeting the lipids LPA and S1P and their signalling pathways to inhibit tumour progression. Expert Rev Mol Med. 2007;9:1–18. doi: 10.1017/S1462399407000476. [DOI] [PubMed] [Google Scholar]
- 96.Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, et al. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- 97.Fischer DJ, Nusser N, Virag T, Yokoyama K, Wang D, Baker DL, et al. Short-chain phosphatidates are subtype-selective antagonists of lysophosphatidic acid receptors. Mol Pharmacol. 2001;60:776–84. [PubMed] [Google Scholar]
- 98.Heise CE, Santos WL, Schreihofer AM, Heasley BH, Mukhin YV, Macdonald TL, et al. Activity of 2-substituted lysophosphatidic acid (LPA) analogs at LPA receptors: discovery of a LPA1/LPA3 receptor antagonist. Mol Pharmacol. 2001;60:1173–80. doi: 10.1124/mol.60.6.1173. [DOI] [PubMed] [Google Scholar]
- 99.Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clezardin P, Peyruchaud O. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci U S A. 2006;103:9643–9648. doi: 10.1073/pnas.0600979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stadler CR, Knyazev P, Bange J, Ullrich A. FGFR4 GLY388 isotype suppresses motility of MDA-MB-231 breast cancer cells by EDG-2 gene repression. Cell Signal. 2006;18:783–94. doi: 10.1016/j.cellsig.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 101.Lee Z, Swaby RF, Liang Y, Yu S, Liu S, Lu KH, et al. Lysophosphatidic acid is a major regulator of growth-regulated oncogene alpha in ovarian cancer. Cancer Res. 2006;66:2740–8. doi: 10.1158/0008-5472.CAN-05-2947. [DOI] [PubMed] [Google Scholar]
- 102.Jiang G, Xu Y, Fujiwara Y, Tsukahara T, Tsukahara R, Gajewiak J, et al. α-Substituted phosphonate analogues of lysophosphatidic acid (LPA) selectively inhibit production and action of LPA. ChemMedChem. 2007;2:679–90. doi: 10.1002/cmdc.200600280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnson EN, Shi X, Cassaday J, Ferrer M, Strulovici B, Kunapuli P. A 1,536-well [35S]GTPgammaS scintillation proximity binding assay for ultra-high-throughput screening of an orphan Gαi-coupled GPCR. Assay Drug Dev Technol. 2008;6:327–37. doi: 10.1089/adt.2007.113. [DOI] [PubMed] [Google Scholar]
- 104.Marx MH, Wiley RA, Satchell DG, Maguire MH. Darmstoff analogues. 3. Actions of choline esters of acetal phosphatidic acids on visceral smooth muscle. J Med Chem. 1989;32:1319–22. doi: 10.1021/jm00126a029. [DOI] [PubMed] [Google Scholar]
- 105.Palomaki VA, Laitinen JT. The basic secretagogue compound 48/80 activates G proteins indirectly via stimulation of phospholipase D-lysophosphatidic acid receptor axis and 5-HT1A receptors in rat brain sections. Br J Pharmacol. 2006;147:596–606. doi: 10.1038/sj.bjp.0706671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–38. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 107.Aoki J, Inoue A, Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta. 2008;1781:513–8. doi: 10.1016/j.bbalip.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 108.Federico L, Pamuklar Z, Smyth SS, Morris AJ. Therapeutic potential of autotaxin/lysophospholipase D inhibitors. Curr Drug Targets. 2008;9:698–708. doi: 10.2174/138945008785132439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu Y, Jiang G, Tsukahara R, Fujiwara Y, Tigyi G, Prestwich GD. Phosphonothioate and fluoromethylene phosphonate analogues of cyclic phosphatidic acid: Novel antagonists of lysophosphatidic acid receptors. J Med Chem. 2006;49:5309–15. doi: 10.1021/jm060351+. [DOI] [PubMed] [Google Scholar]
- 110.Baker DL, Fujiwara Y, Pigg KR, Tsukahara R, Kobayashi S, Murofushi H, et al. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. The Journal of biological chemistry. 2006;281:22786–93. doi: 10.1074/jbc.M512486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Clair T, Koh E, Ptaszynska M, Bandle RW, Liotta LA, Schiffmann E, et al. L-histidine inhibits production of lysophosphatidic acid by the tumor-associated cytokine, autotaxin. Lipids Health Dis. 2005;4:5. doi: 10.1186/1476-511X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cui P, McCalmont WF, Tomsig JL, Lynch KR, Macdonald TL. α- and β-substituted phosphonate analogs of LPA as autotaxin inhibitors. Bioorg Med Chem. 2008;16:2212–25. doi: 10.1016/j.bmc.2007.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cui P, Tomsig JL, McCalmont WF, Lee S, Becker CJ, Lynch KR, et al. Synthesis and biological evaluation of phosphonate derivatives as autotaxin (ATX) inhibitors. Bioorg Med Chem Lett. 2007;17:1634–40. doi: 10.1016/j.bmcl.2006.12.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gududuru V, Zeng K, Tsukahara R, Makarova N, Fujiwara Y, Pigg KR, et al. Identification of Darmstoff analogs as selective agonists and antagonists of lysophosphatidic acid receptors. Bioorg Med Chem Lett. 2006;16:451–6. doi: 10.1016/j.bmcl.2005.08.096. [DOI] [PubMed] [Google Scholar]
- 115.Durgam GG, Virag T, Walker MD, Tsukahara R, Yasuda S, Liliom K, et al. Synthesis, structure-activity relationships, and biological evaluation of fatty alcohol phosphates as lysophosphatidic acid receptor ligands, activators of PPARgamma, and inhibitors of autotaxin. J Med Chem. 2005;48:4919–30. doi: 10.1021/jm049609r. [DOI] [PubMed] [Google Scholar]
- 116.Saunders LP, Ouellette A, Bandle R, Chang WC, Zhou H, Misra RN, et al. Identification of small-molecule inhibitors of autotaxin that inhibit melanoma cell migration and invasion. Mol Cancer Ther. 2008;7:3352–62. doi: 10.1158/1535-7163.MCT-08-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]


