Abstract
Trait anxiety, a stable personality trait associated with increased fear responses to threat, is regarded as a risk factor for the development and maintenance of anxiety disorders. Although the effect of trait anxiety has been examined with regard to explicit threat cues, little is known about the effect of trait anxiety on contextual threat learning. To assess this issue, extreme groups of low and high trait anxiety underwent a contextual fear conditioning protocol using virtual reality. Two virtual office rooms served as the conditioned contexts. One virtual office room (CXT+) was paired with unpredictable electrical stimuli. In the other virtual office room, no electrical stimuli were delivered (CXT−). High-anxious participants tended to show faster acquisition of startle potentiation in the CXT+ versus the CXT− than low-anxious participants. This enhanced contextual fear learning might function as a risk factor for anxiety disorders that are characterized by sustained anxiety.
Keywords: Trait anxiety, Contextual fear conditioning, Startle reflex, Virtual reality
1. Introduction
Identifying signals that indicate threat is vitally important for the survival of an organism. Fear and anxiety are important emotional reactions that help to mobilize appropriate behavioral responses in dangerous situations (Mineka and Öhman, 2002). Fear is a phasic reaction toward an explicit threatening stimulus, whereas anxiety is defined as a diffuse and longer-lasting state related to more unspecific and unpredictable threats (Davis et al., 2010; Marks, 1987). Moreover, fear and anxiety also vary in their neurobiological bases. Phasic responses to a threat cue have been related to the amygdala (Alvarez et al., 2008; Knight et al., 2005; LeDoux, 2000; Phelps, 2006), whereas sustained responses to unpredictable stressors have been found to be additionally mediated by the bed nucleus of the stria terminalis (BNST; Alvarez et al., 2011; Luyten et al., 2011; Zimmerman and Maren, 2011) and the hippocampus (Alvarez et al., 2008; Hasler et al., 2007; Lang et al., 2009; Marschner et al., 2008).
Cue conditioning, where a discrete cue (conditioned stimulus, CS) is predictably paired with an aversive event (unconditioned stimulus, US), is regarded as a model for phasic fear learning (Grillon, 2002a). However, in the case of an unpredictable US, the individual experiences a chronic state of anxiety because of an inability to identify periods of safety (Seligman, 1968; Seligman and Binik, 1977). Studies on contextual fear conditioning frequently combine the presentation of predictable and unpredictable aversive events in one experiment. In these studies, the US is predictably paired with a discrete cue (CS/US are paired) in one condition, whereas in another condition, the US is presented without being associated with a cue (context conditioning; CS/US are unpaired) (Grillon et al., 2004, 2006; Vansteenwegen et al., 2008). In the latter condition, the context becomes associated with the unpredictable US because the context is the best predictor of it. Thus, contextual fear conditioning induces a state of chronic anticipatory anxiety as the occurrence of the US is not time-bound and experienced as unpredictable (Grillon, 2008). Therefore, contextual fear conditioning may be relevant for anxiety disorders characterized by sustained and longer lasting anxiety states (Grillon, 2002a; Lang et al., 2000; Marks, 1987). As a matter of fact, there is evidence that in patients with anxiety disorders, the processing of unpredictable threats is altered. Panic disorder patients, but not healthy controls, have been found to show increased anxiety in a context in which unpredictable aversive events occurred, compared to a safe context where no aversive event appeared (Grillon et al., 2008). Thus, we conclude that increased contextual anxiety elicited by unpredictable aversive events may be an important pathogenic marker for anxiety disorders characterized by diffuse and sustained anxiety states.
Inter-individual differences in trait anxiety are thought to have an impact on the acquisition and extinction of conditioned fear responses and are regarded as a risk factor for anxiety disorders (Chambers et al., 2004; Mineka and Oehlberg, 2008). Trait anxiety refers to the State-Trait-Anxiety Model and indicates relatively firm inter-individual differences in the tendency to rate situations as threatening. Thus, the individual reacts with increased state anxiety (Spielberger et al., 1970). Consequently, high-anxious individuals tend to feel threatened and respond with fear more often than low-anxious subjects. Interestingly, an animal study investigating the influence of a trait-like measure of anxiety on cued fear conditioning (Duvarci et al., 2009) revealed that high-anxious rats were characterized by an impaired discrimination of cues in a conditioning protocol, but displayed high freezing in the context. By contrast, low-anxious rats exhibited enhanced discrimination during cue conditioning, but showed lower contextual freezing. In the face of a predictable threat, high trait anxiety might lead to diminished discrimination between fear and safety cues and might therefore result in increased contextual anxiety. Additionally, the combination of cued and contextual fear conditioning in one paradigm was used in an fMRI study investigating the influence of trait anxiety in humans (Indovina et al., 2011). On the one hand, high trait-anxious participants showed an increased amygdala response to cues that predicted the US, suggesting vulnerability for anxiety disorders characterized by phasic fear. On the other hand, these individuals displayed decreased activity of the ventral prefrontal cortex (vPFC) to cues and contexts, indicating reduced control mechanisms to downregulate both phasic fear and sustained anxiety during acquisition, which may account for inhibitory deficits or the maintenance of anxiety disorders. Supporting this idea and in analogy to clinical samples, a cue (Sehlmeyer et al., 2011) and a contextual fear conditioning study (Barrett and Armony, 2009) employing fMRI have identified resistance to extinction as indexed by an elevated amygdala response in extinction in individuals with high trait anxiety. Other studies have reported heightened amygdala responses to unattended or unconscious fearful faces in high trait-anxious individuals (Dickie and Armony, 2008; Etkin et al., 2004). In sum, high trait anxiety seems to be a vulnerability factor for the development and maintenance of different anxiety disorders as it is associated with an increased amygdala response to fearful stimuli, resistance to extinction, and altered control mechanisms. However, studies that have investigated the influence of trait anxiety on the acquisition of contextual anxiety are lacking.
In humans, contextual fear conditioning can be conducted using virtual reality (VR). With the help of VR, it is possible to change location in spatial contexts while being physically stationary. This increases the controllability of the experiment considerably and makes it possible to register behavioral characteristics of anxiety, like avoidance or approach behavior (Glotzbach et al., 2012; Grillon et al., 2006) and physiological variables simultaneously (Baas et al., 2004, 2008; Mühlberger et al., 2008; Tröger et al., 2012).
To assess the influence of trait anxiety on contextual anxiety, participants scoring high versus low on the trait version of the State-Trait-Anxiety-Inventory (STAI; Spielberger et al., 1970) underwent contextual fear conditioning in a VR paradigm with two contexts and no cues. One context (the anxiety context) was paired with unpredictable slightly painful electric stimuli, whereas the other context (the safety context) was never paired with any aversive stimulus. Based on fear conditioning studies on highly trait-anxious individuals and clinical samples, we first hypothesized that high-anxious individuals would show greater physiological arousal overall (higher baseline SCL and startle magnitudes) compared to low-anxious individuals (Blechert et al., 2007; Glover et al., 2011; Orr et al., 2000). Second, we hypothesized that the high-anxious group would exhibit enhanced context conditioning (greater differences between the CXT+ and CXT− in startle, SCL, and ratings measurements) compared to the low-anxious group (Grillon et al., 2008).
2. Method
2.1. Participants
A large screening of N = 1031 students was conducted to identify high- and low-anxious participants. Students were recruited in lectures or on a university campus to complete the Trait version of the STAI (Spielberger et al., 1970; German version: Laux et al., 1981). The STAI Trait is a 20-item questionnaire measuring the tendency to rate situations as threatening. Based on the distribution of the Trait sum score in this screening sample, participants in the upper 20% percentile were chosen for the high-anxious group, whereas participants in the lower 20% percentile built the low-anxious group. Only 445 of the screened students (42.9%) agreed to participate in further experimental studies. Of these subjects, there were 83 in the low- and 91 in the high-anxious group. We found no significant difference in willingness to participate between these two groups, χ2 = 0.96, P = .327, phi = −.05. Students in these two groups were contacted by phone and invited to take part in the experiment. Participants were excluded if they admitted by self-report to consuming illegal drugs, taking a centrally active medication, having a current psychiatric, neurological, or other severe illness, studying psychology, or participating in a similar study. Finally, 59 subjects participated in the study and were tested via a double-blind procedure for trait-anxiety group membership. Five further participants had to be excluded for several reasons: startle nonresponding (n = 2, see Section 2.4), regular drug consumption (n = 1), technical problems (n = 1), or noncompliance (n = 1). To assure group membership of high versus low anxiety, participants had to complete the STAI-Trait questionnaire at the beginning of the experiment once again. We calculated a median split of this score and excluded participants who did not remain in the high or low anxiety group compared to the screening (n = 5). The final sample consisted of 49 participants, with 24 participants in the low- and 25 in the high-anxiety group.
Before the experiment, participants completed the German versions of the Anxiety-Sensitivity-Index (ASI; Reiss et al., 1986; German version: Alpers and Pauli, 2001), the Behavioral Inhibition System and Behavioral Approach System (BIS-BAS; Carver and White, 1994; German version: Strobel et al., 2001), both scales of the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988; German version: Krohne et al., 1996), and the STAI-State (a 20-item questionnaire conceptualizing fear as a state variable). The ASI measures the tendency to respond fearfully to one’s own bodily sensations on a 16-item scale, whereas the BIS-BAS measures sensitivity to punishment (BIS) on a 7-item scale and sensitivity to reward (BAS) on a 13-item scale. BIS depicts an aversive motivational system, which correlates with avoidance behavior and negative feelings such as fear and anxiety, whereas BAS is regarded as an appetitive motivational system, which drives goal-directed behavior and positive affect (Gray, 1990). The PANAS can be used to determine current positive and negative mood on a 10-item scale each. After the experiment, participants were required to fill out the Igroup Presence Questionnaire (IPQ; original version in German: Schubert et al., 2001), which measures the experience of feeling presence in a virtual reality environment retrospectively on a 14-item scale. As Table 1 shows, the two groups did not differ in age, handedness, gender, and IPQ. But there were significant group differences on the STAI Trait, STAI-State, BIS, BAS, both PANAS subscales (PA and NA) and the ASI.
Table 1.
Demographic and psychometric data for low and high anxiety groups: frequencies, means, standard deviations, and results of χ2 or t tests. Significant group differences are displayed in bold. STAI, State-Trait-Anxiety-Inventory; BIS, Behavior Inhibition Scale; BAS, Behavior Avoidance Scale; PA, positive affect; NA, negative affect; ASI, Anxiety Sensitivity Index; IPQ, Igroup Questionnaire.
| Low anxiety group (n = 24)
|
High anxiety group (n = 25)
|
χ2, t | p | |||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | |||||
| Gender | 15 female | 16 female | 0.012 | .913 | ||||
| Handedness | 23 right | 24 right | 0.001 | .976 | ||||
| Age in years | 22.33 | 2.62 | 21.32 | 1.60 | 1.644 | .107 | ||
| STAI-Trait | 28.00 | 3.13 | 50.68 | 5.09 | 18.690 | <.001 | ||
| STAI-State | 35.17 | 9.00 | 44.68 | 6.31 | 4.299 | <.001 | ||
| BIS | 2.42 | 0.50 | 3.38 | 0.48 | 6.873 | <.001 | ||
| BAS | 3.15 | 0.29 | 2.94 | 0.27 | 2.555 | .014 | ||
| PA | 32.83 | 5.72 | 26.96 | 5.68 | 3.607 | .001 | ||
| NA | 12.08 | 2.26 | 15.48 | 5.36 | 2.867 | .006 | ||
| ASI | 13.17 | 5.80 | 21.24 | 7.14 | 4.335 | <.001 | ||
| IPQ | −1.17 | 14.65 | −3.56 | 13.16 | 0.602 | .550 | ||
All participants gave their written informed consent and were paid 16€ for their participation. The study was approved by the Ethics committee of the Medical Faculty of the Julius-Maximilians-University of Würzburg.
2.2. Stimuli and apparatus
Contextual stimuli
The virtual reality environment was created with the Source Engine from the Valve Corporation (Bellevue, USA), which is also used for the computer game Half-Life 2. The VR environment consisted of two office rooms that were arranged opposite each other and separated by a corridor. Participants were situated in the middle of the corridor in such a way that they could see only one room. This was the starting point for all passages through the rooms. The two office rooms served as the different contexts. They differed in layout, window style, carpet color, view (big city vs. small village), and arrangement of furniture (Fig. 1). The VR environment, instructions, and ratings were presented with a Z800 3D Visor head-mounted display (HMD; eMagin, Hopewell Junction, NY, USA) with a resolution of 600 × 800 pixels. The head position was monitored by an electromagnetic tracking device (Patriot, Polhemus Corp., Colchester, VT, USA) in order to adapt the field of view to head movements and to assess head orientation. The experimental procedure was controlled by the software Cyber Session (version 5.3.38), developed in the Department of Psychology I, University of Würzburg.
Fig. 1.

Screenshots of the two office rooms and the connecting corridor (intertrial interval, ITI). During acquisition one office room was paired with mildly painful electrical stimuli (anxiety context, CXT+), whereas the other office room was never paired with electrical stimuli (safety context, CXT−).
Unconditioned stimulus
A constant current stimulator (Digitimer DS7A, Digitimer Ltd., Welwyn Garden City, UK) was used to generate an electric stimulus which was triggered by a frequency of 50 Hz and a duration of 200 ms by the software Cyber Session. The electric stimulus was applied by a surface electrode placed on the dominant forearm. The intensity of the current was individually adjusted to each participant’s pain threshold as done previously (Andreatta et al., 2010) and increased by 30% to avoid habituation. The two groups did not differ in current intensity (high anxiety: M = 1.71 mA, SD = 1.12 mA; low anxiety: M = 1.65 mA, SD = 1.17 mA), t(47) = 0.16, p = .874, or in pain rating (high anxiety: M =5.30, SD = 1.29; low anxiety: M =5.17, SD = 2.20; on a scale with anchors at 0 = no feeling at all, 4 = just noticeable pain, and 10 = very strong pain), t(47) = 0.26, p = .797.
Recording of physiological data
Startle probes of 50 ms, 98 dB (A) white noise were presented for physiological measures through Sennheiser HD 215 headphones. Startle reflex was measured by electromyographic activity (EMG) from the M. orbicularis oculi with two Ag-AgCl electrodes filled with electrolytes, which were placed centrally under and next to the lateral canthus of the left eye. Ground and reference electrodes were adhered to the mastoids. Impedances were kept below 10 kΩ. The EMG signal was filtered online with a 50 Hz notch filter and sampled at 1000 Hz. Skin conductance level (SCL) was measured on the thenar of the nondominant hand by two Ag-AgCl electrodes. Physiological data were recorded by Vision Recorder software (Brain Products Inc., Munich, Germany).
2.3. Procedure and design
The experiment consisted of five phases: startle habituation, pre-acquisition, Acquisition 1, Acquisition 2, and extinction. During the startle habituation phase, three startle probes were delivered every 18–20 s to habituate initial startle reactivity. Afterwards participants were required to rate the valence of the startle probes on a scale from 0 (very negative) to 100 (very positive). The two anxiety groups did not differ in their rating, t(47) = 0.20, p = .839 (high anxiety: M =21.60, SD = 12.64; low anxiety: M =22.50, SD = 17.94). During the pre-acquisition phase, participants explored the corridor for 1 min and each of the two office rooms for 2 min. In this phase, participants could actively move themselves in the VR environment by using a joystick. Three startle tones were presented in each office and two in the corridor but no US was administered.
There were two successive acquisition phases with each phase consisting of three runs each lasting about 210 s. In each run, participants entered each context once. For example, participants started in the corridor and went first through one office room (ca. 85 s), then through the corridor (intertrial interval: ITI, ca. 35 s) into the other office room (ca. 85 s) and back into the corridor (one run). After each run, the display turned black before a new run was started. SCL was recorded in each context (CXT+, CXT−, ITI), that is between entrance and exit. In each run, there were two to three startle tones in each office room and one to two startle tones in the corridor at intervals of 10–34s. Participants were passively moved through the VR environment (i.e., they could not influence the route they took through the office rooms and corridor). The paths leading through the corridor and office rooms were prerecorded and played back. However, participants were always able to adapt their line of sight in the VR environment by head movements. Participants received one to three mildly painful electric stimuli in one office room (anxiety context, CXT+) but never in the second office room (safety context, CXT−). A total of 12 electric stimuli were presented across both acquisition phases at different locations in the anxiety context, preventing the participants from associating specific cues within this context with shock administration. The corridor served as a control context and as an ITI between the CXT+ and CXT− in one run. There was only one extinction phase. During extinction, no US was delivered. The office rooms were randomly assigned to the two conditions (CXT+ vs. CXT−) and counterbalanced across participants and groups. The sequence of offices was pseudo-randomized and also counterbalanced across participants and groups. Ratings for valence, arousal, and anxiety regarding the three contexts (CXT+, CXT−, ITI) were obtained after the different phases of the experiment (pre-acquisition, Acquisition 1, Acquisition 2, extinction). The anchors of the rating scales were 0 (very negative/no arousal at all/no anxiety at all) and 100 (very positive/very high arousal/very high anxiety). In addition, participants were asked about the contingency between contexts and US after Acquisition 2 and after extinction on a scale from 0 (no expectancy of any US at all) to 100 (US definitely expected). Awareness of the CXT+−US contingency was assessed with an open question (“In which room did you receive electrical stimuli?”) after Acquisition 2, and participants had to describe the room. If participants described only the anxiety context, they were labeled as “aware”; if they described the correct room but also an additional one (CXT− and/or ITI) they were labeled as “uncertain”, and if they described only the wrong room (CXT− or ITI) they were labeled as “unaware”. In total, there were n = 34 aware, n = 14 uncertain and n = 1 unaware (high-anxiety group) participants. Uncertain participants were not significantly over-represented in one trait anxiety group compared to the other trait anxiety group (high anxiety: n = 6, 25%; low anxiety: n = 8, 33%), χ2 (1, N = 48) = 0.40, p = .525, phi = .09. Unaware or uncertain participants were not excluded from any analysis.
2.4. Data analysis
Startle response
Eyeblink EMG data were processed with Vision Analyzer software (Brain Products Inc., Munich, Germany). The signal of orbital electrodes was filtered offline with a 500 Hz High Cut-Off and a 30 Hz Low Cut-Off Filter. The signal was rectified, smoothed (50 ms moving window average), and baseline corrected (50 ms). The peak magnitude was identified within a time window from 20 to 200 ms after the probe onset. Artifact rejection was made by hand excluding responses with baseline shifts above or below 5 μV and pre-blinks 50 ms before probe onsets higher than 5 μV. Magnitudes smaller than 5 μV were coded as zero. Responders versus nonresponders were defined on the basis of sufficient valid responses, meaning artifact free and higher than 5 μV. If there were fewer than two valid responses per stimulus category (CXT+, CXT, ITI) in a given phase (Acquisition 1, Acquisition 2, extinction), the participant was excluded from further analysis (n = 2). Magnitudes in the acquisition and extinction phases were standardized into T-scores for each participant. First, raw magnitudes were z-standardized using the individual mean and standard deviation across all acquisition and extinction trials, z(x) = (x − M)/SD. Second, the T-score calculation was applied, T(x) = 50 + (z× 10). For pre-acquisition data, we did not apply any normalization (log) or standardization (T-scores), but analyzed raw startle magnitude and raw SCL to assess between-subject differences before conditioning.
Skin conductance level
SCL data were filtered with a 1 Hz High Cut-Off Filter. The mean tonic SCL was determined for each context presentation (excluding epochs from US presentation to 10s after US presentation to avoid an increased SCL due to US presentation). SCLs during acquisition and extinction were log-transformed (log 10[SCL+1]) to normalize the distribution.
Statistical analysis
Prior to statistical analysis, physiological data were averaged across the three runs in each phase (Acquisition 1, Acquisition 2, extinction). Pre-acquisition, acquisition, and extinction data were analyzed separately, as we were specifically interested in the time course of fear acquisition and extinction. Statistical analyses of pre-acquisition and extinction data were conducted with a 3 × 2 multivariate analysis of variance (MANOVA) for repeated measures with context (CXT+, CXT−, ITI) as a within-subjects factor and group (high-anxious vs. low-anxious) as a between-subjects factor. To analyze the acquisition data, we added a within-factor phase (Acquisition1, Acquisition 2) resulting in a 3 × 2 × 2 MANOVA. To clarify significant main effects or interactions, t tests were calculated. In all analyses, the alpha level was set at p ≤ .05. Because of a violation of sphericity, multivariate procedures were used. Effect sizes were calculated using partial eta squared .
3. Results
3.1. Pre-acquisition
The MANOVA on the pre-acquisition raw startle magnitudes revealed no significant differences between contexts at the beginning of the experiment (all ps > .5), but returned a significant main effect of group, F(1, 47) = 5.31, p = .026, , due to a larger mean raw startle magnitude in high-anxious (M = 67.25, SD = 31.80) compared to low-anxious (M = 47.07, SD = 25.44) participants across all contexts. Further exploratory analyses of pre-conditioning differences between contexts or groups also were negative: high-anxious (all ps > .20) as well as low-anxious participants (all ps > .90) did not show higher startle magnitudes in the CXT+ compared to the CXT−, and groups did not differ in ITI startle magnitudes prior to conditioning. Regarding the pre-acquisition raw SCL, we also found no significant differences between groups or contexts (all ps > .10). Analyses of valence ratings yielded a significant main effect of context, F(2, 46) = 32.66, p < .001, , which however was due to more negative ratings for the ITI period (M = 43.78, SD = 15.26) compared to the CXT+ (M = 62.45, SD = 13.19), t(48) = 8.09, p < .001, or the CXT− (M = 59.59, SD = 17.41), t(48) = 4.61, p < .001, with no difference between the CXT+ and CXT−, t(48) = 0.93, p = .356. There were no significant main effects of context or group or a significant Context × Group interaction for pre-acquisition arousal and anxiety ratings (all ps > .10).
3.2. Acquisition
Fig. 2 and Table 2 display the means (SDs) for all dependent measures separately for each anxiety group.
Fig. 2.
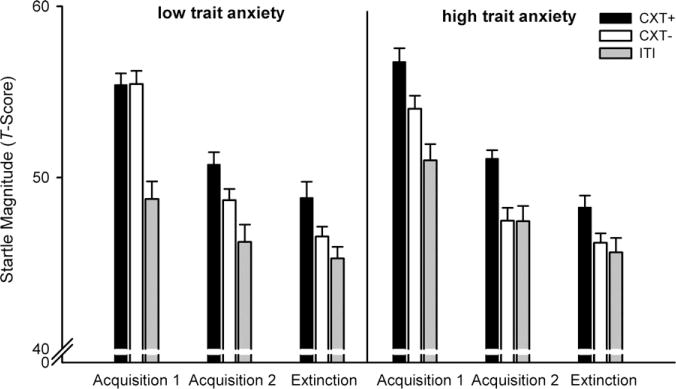
Fear potentiated startle magnitude (T-scores) during Acquisition 1, Acquisition 2 and Extinction in low- (left) and high-anxious participants (right). Error bars represent standard errors of the mean (SEM). CXT+, anxiety context (paired with US); CXT−, safety context (no US); ITI, intertrial interval.
Table 2.
Means (SDs) for dependent variables in low and high anxiety groups. CXT+, anxiety context (paired with US during acquisition); CXT−, safety context (no US); ITI, intertrial interval. Valence, arousal, anxiety, and US-expectancy ratings were assessed on a scale from 0 (very negative/no arousal at all/no anxiety at all/no expectancy at all) to 100 (very positive/very high arousal/very high anxiety/definitely expected).
| Low anxiety group (n = 24)
|
High anxiety group (n = 25)
|
|||||
|---|---|---|---|---|---|---|
| CXT+ | CXT− | ITI | CXT+ | CXT− | ITI | |
| SCL (log) | ||||||
| Acquisition 1 | 0.609 (0.156) | 0.606 (0.152) | 0.609 (0.153) | 0.607 (0.178) | 0.599 (0.174) | 0.600 (0.177) |
| Acquisition 2 | 0.579 (0.179) | 0.568 (0.177) | 0.573 (0.176) | 0.570(0.179) | 0.562 (0.186) | 0.568 (0.181) |
| Extinction | 0.552 (0.186) | 0.547 (0.185) | 0.548 (0.185) | 0.558 (0.182) | 0.560 (0.180) | 0.558 (0.181) |
| Valence | ||||||
| Acquisition 1 | 52.29 (16.02) | 55.63 (14.32) | 48.96 (15.88) | 44.00(18.65) | 59.32 (15.05) | 46.40 (16.04) |
| Acquisition 2 | 47.92 (14.52) | 53.67 (17.46) | 49.17 (13.49) | 41.60(20.60) | 59.20 (22.90) | 44.40 (16.09) |
| Extinction | 50.83 (12.83) | 53.54(15.21) | 44.17 (16.13) | 50.80(16.50) | 60.12 (19.74) | 45.20 (14.18) |
| Arousal | ||||||
| Acquisition 1 | 30.21 (25.13) | 23.54(25.56) | 14.79 (22.53) | 38.40(27.49) | 23.80 (23.15) | 19.20 (21.39) |
| Acquisition 2 | 31.25 (25.21) | 24.79(25.17) | 19.37 (21.48) | 32.60(32.95) | 19.20 (23.26) | 18.00(23.63) |
| Extinction | 25.21 (24.65) | 19.79(21.79) | 13.75 (21.63) | 23.00(23.09) | 14.20 (16.43) | 12.20 (16.46) |
| Anxiety | ||||||
| Acquisition 1 | 17.71 (22.26) | 14.17 (21.70) | 9.37 (13.70) | 27.00(25.66) | 12.12 (14.34) | 12.00(15.81) |
| Acquisition 2 | 18.33 (23.02) | 12.71 (17.51) | 10.00 (14.14) | 26.60(30.37) | 13.20 (18.81) | 14.00(18.93) |
| Extinction | 15.00 (19.78) | 12.92(16.81) | 11.04 (16.15) | 19.00(21.36) | 11.28 (17.11) | 11.68 (18.81) |
| US-expectancy | ||||||
| Acquisition 2 | 80.21 (25.47) | 27.38 (33.20) | 20.62 (32.18) | 82.36 (18.51) | 29.96 (30.18) | 26.80 (27.04) |
| Extinction | 57.50 (32.74) | 30.63 (28.07) | 17.29 (23.82) | 51.20(29.77) | 27.40 (30.18) | 17.20 (23.37) |
Startle response
The MANOVA revealed significant main effects of phase, F(1, 47) = 86.58, p < .001, , and context, F(2, 46) = 36.61, p < .001, . There were significant Context × Group, F(2, 46) = 3.43, p = .041, , and Phase × Context, F(2, 46) = 3.87, p = .028, , interactions. The Context × Phase × Group interaction did not reach significance, F(2, 46) < 1. The Group × Context interaction was due to the fact that high-anxious participants demonstrated a clear discrimination between anxiety and safety contexts, whereas low-anxious participants did not (Fig. 2). Indeed, follow-up t tests revealed that startle was not larger during the CXT+ compared to the CXT− in low-anxious participants, t(23) = 1.20, p = .243. In addition, their startle was larger during the CXT+ and CXT− compared to the ITI, t(23) = 6.45, p < .001, and t(23) = 5.27, p < .001, respectively. By contrast, high-anxious participants showed significantly higher startle response in the CXT+ compared to both the CXT−, t(24) = 4.47, p < .001, and the ITI, t(24) = 5.76, p < .001, but there was only a trend for a significant difference between the CXT− and the ITI, t(24) = 1.79, p = .086. For the Phase × Context interaction, follow-up t tests indicated that in Acquisition 1, startle responses were higher in the CXT+ compared to the ITI, t(48) = 8.05, p < .001, and the CXT− compared to the ITI, t(48) = 5.24, p < .001. The difference between the CXT+ and CXT− reached only the trend level, t(48) = 1.75, p = .087. In Acquisition 2, startle responses in the CXT+ were significantly higher both compared to the CXT−, t(48) = 3.99, p < .001, and the ITI, t(48) = 5.02, p < .001. The difference between the CXT− and the ITI was not significant, t(48) = 1.34, p = .186.
SCL
The MANOVA revealed a significant main effect of context, F(2, 46) = 6.07, p = .005, , due to (a) higher SCL in the CXT+ (M = 0.591, SD = 0.169) compared to the CXT− (M = 0.583, SD = 0.169), t(48) = 3.50, p = .001, and the ITI (M = 0.587, SD = 0.169), t(48) = 2.17, p = .035, and (b) higher SCL in the ITI compared to the CXT−, t(48) = 2.68, p = .010. The main effect of phase was also significant, F(1, 47) = 25.30, p < .001, , reflecting the habitation of SCL from Acquisition 1 (M = 0.605, SD = 0.164) to Acquisition 2 (M = 0.570, SD = 0.177). None of the interactions with group reached significance (all ps > 1).
Valence
There was only a significant main effect of context, F(2, 46) = 7.97, p = .001, ; the CXT+ (M = 46.38, SD = 16.57) and the ITI (M = 47.19, SD = 13.77) were both rated as more negative than the CXT− (M = 57.00, SD = 16.01), t(48) = 3.50, p = .001, and t(48) = 3.38, p = .001.
Arousal
There was only a significant main effect of context, F(2, 46) = 29.07, p < .001, , with significantly higher arousal in the CXT+ (M = 33.16, SD = 25.47) compared to both the CXT− (M = 22.81, SD = 21.76), t(48) = 4.61, p < .001, and the ITI (M = 17.86, SD = 19.30), t(48) = 7.73, p < .001, and higher arousal in the CXT− compared to the ITI, t(48) = 2.44, p = .019.
Anxiety
The main effect of context was significant, F(2, 46) = 9.78, p < .001, ; the CXT+ (M = 22.50, SD = 24.53) elicited higher anxiety levels than the CXT− (M = 13.04, SD = 16.53), t(48) = 3.82, p < .001, and the ITI (M = 11.38, SD = 13.36), t(48) = 4.48, p < .001. There was also a marginally significant Context × Group interaction, F(2, 46) = 2.77, p = .073, . Descriptive data (see Fig. 3) showed a pattern similar to the startle magnitudes, with higher reported anxiety in the CXT+ compared to the CXT− in the high-anxious group and no difference between the CXT+ and CXT− in the low-anxious group. To confirm this observation, we conducted exploratory t tests comparing the CXT+ and CXT− separately for each anxiety group and indeed found higher anxiety in the CXT+ compared to the CXT− in the high-anxious group, t(24) = 3.73, p = .001, but not in the low-anxious group, t(23) = 1.57, p = .129.
Fig. 3.
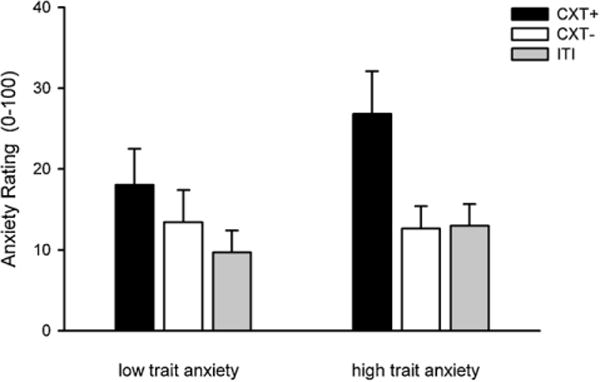
Anxiety ratings from 0 (no anxiety) to 100 (very strong anxiety) across Acquisitions 1 and 2 in low- (left) and high-anxious participants (right). Error bars represent standard errors of the mean (SEM). CXT+, anxiety context (paired with US); CXT−, safety context (no US); ITI, intertrial interval.
Contingency ratings
There was only a significant main effect of context, F(2, 46) = 51.98, p< .001, ; contingency rating for the CXT+ (M = 81.31, SD = 21.99) was higher compared to both the CXT− (M =28.69, SD = 31.39), t(48) = 8.42, p < .001, and the ITI (M = 23.78, SD = 29.52), t(48) = 10.39, p < .001, but there was no difference between the CXT− and the ITI, t(48) = 1.25, p = .216.
3.3. Extinction1
Fig. 2 and Table 2 display the results for all dependent measures separately for both anxiety groups.
Startle response
The MANOVA revealed a significant main effect of context, F(2, 46) = 8.73, p = .001, , with higher startle responses in the CXT+ (M = 48.54, SD = 4.00) compared to the CXT− (M = 46.37, SD = 2.77), t(48) = 3.37, p = .001, and to the ITI (M = 45.46, SD = 3.76), t(48) = 4.11, p < .001. The Context × Group interaction was not significant, F(2, 46) < 1. This suggests that both groups showed an increased startle response in the CXT+ compared to the CXT−.
SCL
There were no significant main effects or interactions in the extinction phase (all Fs < 1), suggesting no differences between contexts or groups during extinction.
Valence
The MANOVA revealed only a significant main effect of context, F(2, 46) = 9.21, p < .001, , indicating that the CXT+ (M = 50.82, SD = 14.66) was rated as more negative than the CXT− (M = 56.90, SD = 17.80), t(48) = 2.66, p = .012; and the ITI (M = 44.69, SD = 15.01) was rated as more negative than both the CXT+, t(48) = 2.77, p = .008, and the CXT−, t(48) = 4.36, p < .001.
Arousal
There was only a significant main effect of context, F(2, 46) = 9.16, p < .001, , indicating higher arousal for the CXT+ (M = 24.08, SD = 23.64) compared to both the CXT− (M = 16.94, SD = 19.52), t(48) = 3.31, p = .002, and the ITI (M = 12.96, SD = 18.98), t(48) = 4.32, p < .001, and higher arousal for the CXT− compared to the ITI, t(48) = 2.86, p = .006.
Anxiety
There was only a significant main effect of context, F(2, 46) = 4.06, p = .024, , indicating higher anxiety for the CXT+(M = 17.04, SD = 20.49) compared to both the CXT− (M = 12.08, SD = 16.80), t(48) = 2.34, p = .023, and the ITI (M = 11.37, SD = 17.38), t(48) = 2.84, p = .007.
Contingency ratings
There was only a significant main effect of context, F(2, 46) = 27.40, p < .001, , indicating higher contingency ratings for the CXT+ (M =54.29, SD = 31.09) compared to both the CXT− (M = 28.98, SD = 28.90), t(48) = 5.05, p < .001, and the ITI (M = 17.24, SD = 23.34), t(48) = 7.50, p < .001, and a higher contingency ratings for the CXT− compared to the ITI, t(48) = 2.69, p = .010.
4. Discussion
In this study, we investigated the influence of trait anxiety on contextual fear conditioning using a virtual reality paradigm. We were able to show, as in previous studies (Baas et al., 2004; Grillon et al., 2006; Tröger et al., 2012), that physiological and explicit anxiety responses increased in an anxiety context (CXT+) where unpredictable USs were administered, compared to a safety context (CXT−) where no US occurred.
Importantly, we found some evidence that high trait anxiety results in enhanced acquisition of context-specific anxiety reactions. During acquisition, high-anxious participants showed differential conditioning effects in startle magnitude and a similar trend for anxiety ratings. By contrast, low-anxious participants showed no differential conditioning effect during acquisition. Additionally, exhibited higher startle magnitudes in the CXT+ versus the CXT− only during the extinction phase as the main effect of context in absence of any group effect revealed. These data suggest that high-anxious participants show faster contextual fear learning, whereas in low-anxious participants, the acquisition of contextual anxiety seems to be slower as they exhibit fear-potentiated startle only during extinction. To demonstrate whether the low-anxiety group indeed showed slower acquisition of contextual fear during the conditioning phases, we analyzed startle data for Acquisitions 1 and 2 separately for the low-anxiety group.2 This detailed analysis on the time course of acquisition revealed that low-anxious participants displayed a slower fear acquisition as they tended to discriminate between the CXT+ and CXT− only during Acquisition 2. We did not find any differences between the anxiety groups in SCL data, consistent with a study that also failed to show differences in SCR between PTSD patients and controls, suggesting that SCL or SCR may not be sensitive enough to differentiate between anxiety groups (Glover et al., 2011) or that they are too sensitive to conditioning to capture a between group difference in speed of learning.
Our results are in line with a study that has shown that panic disorder patients exhibited increased startle magnitudes in a context in which unpredictable shocks were administered, compared to a safe context, whereas healthy controls failed to show this differentiation (Grillon et al., 2008). Increased sensitivity to unpredictable threats seems to play an important role in some anxiety disorders, especially those that are characterized by sustained anxiety but not phasic fear (Davis et al., 2010). Our results suggest that high trait anxiety enhances conditionability to contexts that signal unpredictable threats, which might be regarded as a risk factor or endophenotype for anxiety disorders characterized by sustained anxiety states.
Differential contextual fear learning might be mediated by an increased arousal level or overall heightened sensitivity to threatening contexts in high-anxious individuals or anxiety disorder patients (Blechert et al., 2007; Glover et al., 2011; Orr et al., 2000). Supportively, we found that high-anxious participants displayed higher raw value startle magnitudes in pre-acquisition compared to low-anxious participants, suggesting higher physiological responding in high-trait individuals before conditioning, which might contribute to increased contextual conditioning as measured in CXT+ versus CXT− differences in startle magnitude (Grillon and Baas, 2002). Additionally, enhanced attentional processes might facilitate fear conditioning to a cue or context (Baas et al., 2008; Grillon, 2002a) in a way that the threat signals might be identified more quickly in participants with higher trait anxiety (Massar et al., 2011). As a first hint, a recent study with PTSD patients showed that an attentional bias toward a threat in a dot probe task was positively correlated with fear-potentiated startle in a cued fear conditioning paradigm (Fani et al., 2012). However, further research is needed to disentangle the associations between attentional processes, contextual fear conditioning, and trait anxiety.
At first glance, our results seem to contradict the findings by Lissek et al. (2009), who reported a failure to discriminate between danger and safety cues or rather slower fear acquisition among panic disorder patients; this failure was due to an elevated fear response to the safety cue. The authors interpreted their findings within the framework of stimulus generalization. Panic patients would tend to generalize their fear responses to similar cues or situations. It is important to emphasize that their study employed a cued fear conditioning paradigm, where the US was predictably paired with a discrete cue, in contrast to our study, which used a contextual fear conditioning protocol. Conclusively, cue and contextual fear conditioning as indices for phasic fear or sustained anxiety may vary not only in their neurological bases (Davis et al., 2010; LeDoux, 2000; Phelps, 2006) and characteristics of fear or anxiety responses (Grillon, 2002a; Marks, 1987), but also in differential learning rates. As a suggestion, in the case of a predictable threat, pathologic or high trait anxiety may result in impaired discrimination between cues that signal predictable threat or safety due to inhibitory deficits (Lissek et al., 2005). By contrast, in the face of an unpredictable threat, pathologic anxiety or, as in this study, high trait anxiety, allows for faster discrimination between contexts that signal unpredictable threats compared to safe contexts.
Contextual fear conditioning can be established, if predictive cues signaling threat occurrence are lacking or are not explicitly detected (Baas, 2013; Grillon, 2002b). Furthermore, introducing a predictive cue after conditioning to the context results in reduced contextual anxiety (Fonteyne et al., 2009, 2010) and this has been found to correlate with trait anxiety, showing that low-anxious individuals are better at adaptively reducing their contextual anxiety (Baas, 2013). Therefore, one possible mechanism to explain the faster context conditioning in high trait-anxious participants might be that high-anxious compared to low-anxious individuals give up more quickly when searching for specific predictors of threat. As a consequence, high-anxious individuals might associate the context with the threat more quickly than low-anxious individuals. Nevertheless, in our study, one might argue that displaying increased contextual anxiety was the only adaptive response, as we presented only a context without any predictive cue in contrast to other contextual fear conditioning studies that presented predictive cues and measured anxiety responses to the context in which the predictive cue was presented (Baas, 2013; Duvarci et al., 2009). High contextual anxiety in the latter paradigm is of course maladaptive because the threat was predictable. If high-anxious individuals have deficits in searching for and/or identifying specific predictors of threat, this would be an advantage in our context-only paradigm because there are no specific predictors to identify. Another explanation for why low-anxious participants showed less contextual anxiety might be that they are better in identifying safe periods within the anxiety context. Fonteyne et al. (2009, 2010) showed that participants were even able to predict shocks that were presented between cues as indexed by online US-expectancy ratings. However, their finding might be due to the fact that shocks in their experiment appeared at fixed time points throughout a trial. By contrast, shocks in our experiment appeared at different time points in every trial; thus, it is very unlikely that participants were able to identify safe periods, and they therefore very likely perceived shocks to be unpredictable. Nevertheless, we only can speculate on this topic because unfortunately we did not measure online-expectancy ratings during the conditioning trials. Further studies should include online-expectancy ratings to prove unpredictability of the shocks on a trial-by-trial basis and throughout the context presentation. Alternatively, the low-anxious individuals might have been characterized by increased risk taking, sensation seeking, or reduced sensitivity to punishment (lower BIS) and therefore exhibited deficits in identifying threats and safety. This seems plausible because the low-anxious participants were selected based on their trait anxiety levels from the lowest 20% of the screening sample. Additionally, the trait anxiety groups also differed according to other anxiety-related questionnaires such as the ASI, BIS-BAS, STAI State, and PANAS, which are correlated and are all measures of traits or states of anxiety. Therefore, group differences may have been driven by not only trait anxiety in contextual fear conditioning but also related constructs. To further examine possible confounding personality variables (e.g., sensation seeking), future studies might examine alternative control samples (e.g., with “normal” trait anxiety scores lying between 40% and 60% of the screening sample).
Several studies comparing anxiety patients with healthy controls (e.g., Blechert et al., 2007; Wessa and Flor, 2007) as well as correlative studies assessing trait anxiety and conditioning (Barrett and Armony, 2009; Sehlmeyer et al., 2011) have observed a resistance to extinction in cue conditioning paradigms in the high-anxious group, meaning that extinction was slowed or enhanced amygdala activation was found during extinction in these subjects. By contrast, the present study found no effect of anxiety level on extinction in context conditioning, and as a matter of fact, no extinction effects for most dependent variables were found, except for SCL Importantly, the present study examined context conditioning, whereas all previous studies examined cue conditioning; thus, it might be premature to expect comparable results for the two learning paradigms. Additionally, the present study included three extinction trials only (half of the acquisition trials); perhaps additional trials would have been necessary to detect a resistance to extinction in context conditioning. Therefore, future studies using longer extinction phases are needed to come to more definitive conclusions about the effects of trait anxiety on contextual extinction.
In sum, we demonstrated that high trait anxiety results in faster discrimination of situations of anxiety and safety in the face of an unpredictable threat. Our results provide further support for a clear distinction between phasic fear and contextual anxiety, which are differentially influenced by personality traits. Nevertheless, we may only speculate about the differences in cue versus context conditioning depending on trait anxiety because we investigated only context conditioning in our paradigm. Future studies might implement predictable and unpredictable threat conditions to further disentangle the differential role of trait anxiety in these conditions. Furthermore, VR used in our study proved to be a valid examination tool for contextual fear conditioning and for research on anxiety disorders. As contextual fear conditioning may be a more appropriate paradigm for investigating anxiety disorders (Davis et al., 2010), it would be meaningful to deepen the neural underpinnings involved in this paradigm as well as to apply it in a clinical sample. In particular, further studies using the context conditioning paradigm are important because of its relevance for the development of anxiety disorders and the lack of comparability of results from studies using cue conditioning paradigms.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG): Collaborative Research Center “Fear, Anxiety, Anxiety Disorders” SFB-TRR 58 project B1 to Paul Pauli and Andreas Mühlberger and a grant from the University of Würzburg to Andreas Mühlberger for a research visit to the National Institute of Mental Health. Christian Grillon is supported by the Intramural Research Program of the National Institutes of Mental Health.
Footnotes
Conflicts of interest
Paul Pauli is stakeholder of a commercial company that sells virtual environment research systems. Andreas Mühlberger is stakeholder and executive officers of a commercial company that sells virtual environment research systems. No further potential competing financial interests exist.
As awareness could have had an effect on extinction of contextual fear conditioning, we firstly excluded the only unaware participant from the analysis, but results remained the same. Secondly, we added awareness (unaware/uncertain vs. aware) as a between factor to the overall ANOVA. There were neither significant main effects of awareness nor any significant interactions of Context × Awareness for all dependent variables, all ps > .1, except for contingency ratings (Context × Awareness: F(2, 44) = 8.20, p = .001, . As expected, only aware participants reported higher contingency ratings for CXT+ compared to CXT−, t(33) = 5.27, p < .001, but unaware participants did not, t(14)=1.26, p = .229.
In Acquisition 1, low-anxious participants showed higher startle responses in the CXT+, t(23) = 6.41, p < .001, and in the CXT−, t(23) = 5.58, p < .001, both compared to the ITI, but no differentiation between the CXT+ and CXT−, t(23) = 0.04, p = .971. In Acquisition 2, low-anxious participants showed only a discrimination between the CXT+ and CXT−, t(23) = 1.99, p = .059, and between the CXT− and the ITI, t(23) = 1.96, p = .063, at trend level, but they showed a significant difference between the CXT+ and the ITI, t(23)=3.82, p = .001.
References
- Alpers G, Pauli P. Angstsensitivitäts-Index. Julius-Maximilians Universität; Würzburg: 2001. [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. The Journal of Neuroscience. 2008;28:6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreatta M, Mühlberger A, Yarali A, Gerber B, Pauli P. A rift between implicit and explicit conditioned valence after pain-relief learning in humans. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2411–3241. doi: 10.1098/rspb.2010.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas JM, Nugent M, Lissek S, Pine DS, Grillon C. Fear conditioning in virtual reality contexts: a new tool for the study of anxiety. Biological Psychiatry. 2004;55:1056–1060. doi: 10.1016/j.biopsych.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Baas JMP. Individual differences in predicting aversive events and modulating contextual anxiety in a context and cue conditioning paradigm. Biological Psychology. 2013;92:17–25. doi: 10.1016/j.biopsycho.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Baas JMP, Ooijen L, Goudriaan A, Kenemans JL. Failure to condition to a cue is associated with sustained contextual fear. Acta Psychologica. 2008;127:581–592. doi: 10.1016/j.actpsy.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Barrett J, Armony JL. Influence of trait anxiety on brain activity during the acquisition and extinction of aversive conditioning. Psychological Medicine. 2009;39:255–65. doi: 10.1017/S0033291708003516. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behaviour Research and Therapy. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Chambers JA, Power KG, Durham RC. The relationship between trait vulnerability and anxiety and depressive diagnoses at long-term follow-up of Generalized Anxiety Disorder. Journal of Anxiety Disorders. 2004;18:587–607. doi: 10.1016/j.janxdis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie EW, Armony JL. Amygdala responses to unattended fearful faces: interaction between sex and trait anxiety. Psychiatry Research: Neuroimaging. 2008;162:51–57. doi: 10.1016/j.pscychresns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Paré D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. The Journal of Neuroscience. 2009;29:10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, Kamkwalala A, Jovanovic T. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychological Medicine. 2012;42:533–543. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteyne R, Vervliet B, Hermans D, Baeyens F, Vansteenwegen D. Reducing chronic anxiety by making the threatening event predictable: an experimental approach. Behaviour Research and Therapy. 2009;47:830–839. doi: 10.1016/j.brat.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Fonteyne R, Vervliet B, Hermans D, Baeyens F, Vansteenwegen D. Exposure to the context and removing the unpredictability of the US: two methods to reduce contextual anxiety compared. Biological Psychology. 2010;85:361–369. doi: 10.1016/j.biopsycho.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Glotzbach E, Ewald H, Andreatta M, Pauli P, Mühlberger A. Contextual fear conditioning effects predict subsequent avoidance behavior. Cognition & Emotion. 2012;26:1256–1272. doi: 10.1080/02699931.2012.656581. [DOI] [PubMed] [Google Scholar]
- Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, Ressler KJ, Jovanovic T. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depression and Anxiety. 2011;28:1058–1066. doi: 10.1002/da.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA. Brain systems that mediate both emotion and cognition. Cognition & Emotion. 1990;4:269–288. [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context and neurobiology. Biological Psychiatry. 2002a;56:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biological Psychiatry. 2002b;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology. 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Cornwell B, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biological Psychiatry. 2006;60:752–759. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP. Comments on the use of the startle reflex in psychopharmacological challenges: impact of baseline startle on measurement of fear-potentiated startle. Psychopharmacology. 2002;164:236–238. doi: 10.1007/s00213-002-1164-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioural Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. The American Journal of Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. The Journal of Neuroscience. 2007;27:6313–6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Núñez-Elizalde AO, Dunn BD, Bishop SJ. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69:563–571. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. NeuroImage. 2005;26:1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Krohne HW, Egloff B, Kohmann CW, Tausch A. Untersuchungen mit einer deutschen Version der Positive and Negative Affect Schedule (PANAS) Diagnostica. 1996;42:139–156. [Google Scholar]
- Lang PJ, Davis M, Öhman A. Fear and anxiety: animal models and human cognitive psychophysiology. Journal of Affective Disorders. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lang S, Kroll A, Lipinski SJ, Wessa M, Ridder S, Christmann C, Schad LR, Flor H. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. European Journal of Neuroscience. 2009;29:823–832. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux L, Glanzmann P, Schaffner P, Spielberger CD. Das State-Trait Angstinventar. Beltz Test; Weinheim: 1981. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behavior Research and Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, McDowell DJ, Dvir S, Bradford DE, Geraci M, Pine DS, Grillon C. Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behaviour Research and Therapy. 2009;47:111–118. doi: 10.1016/j.brat.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, van Kuyck K, Vansteenwegen D, Nuttin B. Electrolytic lesions of the bed nucleus of the stria terminalis disrupt freezing and startle potentiation in a conditioned context. Behavioural Brain Research. 2011;222:357–362. doi: 10.1016/j.bbr.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Marks IM. Fear, Phobias, and Rituals. Oxford University Press; New York: 1987. [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Büchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. The Journal of Neuroscience. 2008;28:9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massar SAA, Mol NM, Kenemans JL, Baas JMP. Attentional bias in high-and low-anxious individuals: evidence for threat-induced effects on engagement and disengagement. Cognition & Emotion. 2011;25:805–817. doi: 10.1080/02699931.2010.515065. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychologica. 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Mineka S, Öhman A. Phobias and preparedness: the selective, automatic, and encapsulated nature of fear. Biological Psychiatry. 2002;52:927–937. doi: 10.1016/s0006-3223(02)01669-4. [DOI] [PubMed] [Google Scholar]
- Mühlberger A, Wieser MJ, Pauli P. Darkness-enhanced startle responses in ecologically valid environments: a virtual tunnel driving experiment. Biological Psychology. 2008;77:47–52. doi: 10.1016/j.biopsycho.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109:290–298. [PubMed] [Google Scholar]
- Phelps AE. Emotion and cognition: insight from studies of the amygdala. Annual Review Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Schubert T, Friedmann F, Regenbrecht H. The experience of presence: factor analytic insights. Teleoperators and Virtual Environments. 2001;10:266–281. [Google Scholar]
- Sehlmeyer C, Dannlowski U, Schöning S, Kugel H, Pyka M, Pfleiderer B, Zwitser-lood P, Schiffbauer H, Heindel W, Arolt V, Konrad C. Neural correlates of trait anxiety in fear extinction. Psychological Medicine. 2011;41:789–798. doi: 10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- Seligman ME. Chronic fear produced by unpredictable electric shock. Journal of Comparative and Physiological Psychology. 1968;66:402–411. doi: 10.1037/h0026355. [DOI] [PubMed] [Google Scholar]
- Seligman MEP, Binik YM. The safety signal hypothesis. In: Davis H, Hurwitz HMB, editors. Operant-Pavlovian Interactions. Lawrence Erlbaum Associates; Hillsdale, NJ: 1977. pp. 165–187. [Google Scholar]
- Spielberger CD, Gorsuch SL, Edward LR. STAI Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press; Palo Alto, CA: 1970. [Google Scholar]
- Strobel A, Beauducel A, Debener S, Brocke B. Eine deutschsprachige Version des BIS/BAS-Fragebogens von Carver und White. Zeitschrift für Differentielle und Diagnostische Psychologie. 2001;22:216–227. [Google Scholar]
- Tröger C, Ewald H, Glotzbach E, Pauli P, Mühlberger A. Does pre-exposure inhibit fear context conditioning? A Virtual Reality Study. Journal of Neural Transmission. 2012;119:709–719. doi: 10.1007/s00702-011-0757-8. [DOI] [PubMed] [Google Scholar]
- Vansteenwegen D, Iberico C, Vervliet B, Marescau V, Hermans D. Contextual fear induced by unpredictability in a human fear conditioning preparation is related to the chronic expectation of a threatening US. Biological Psychology. 2008;77:39–46. doi: 10.1016/j.biopsycho.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. American Journal of Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiology of Learning and Memory. 2011;95:199–205. doi: 10.1016/j.nlm.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


