Abstract
Despite their differential cell tropisms, HIV-1 and HCV dramatically influence disease progression in coinfected patients. Macrophages are important target cells of HIV-1. We hypothesized that secreted HCV core protein might modulate HIV-1 replication. We demonstrate that HCV core significantly enhances HIV-1 replication in human macrophages by upregulating TNF-α and IL-6 via TLR2-, JNK-, and MEK1/2-dependent pathways. Furthermore, we show that TNF-α and IL-6 secreted from HCV core-treated macrophages reactivates monocytic U1 cells latently infected with HIV-1. Our studies reveal a previously unrecognized role of HCV core by enhancing HIV-1 infection in macrophages.
Keywords: Hepatitis C virus core protein, HIV-1, Macrophages, Toll-like receptor 2, MAPK kinase, IL-6, TNF-α
1. Introduction
Increased progression rates to acquired immunodeficiency syndrome (AIDS) and liver disease have been reported in individuals coinfected with human immunodeficiency virus type 1 (HIV-1) and hepatitis C virus (HCV) [1, 2]. Strikingly, activation of CD4+ T cells, macrophages, and dendritic cells enhances HIV-1 viral replication [3–6]. In HIV-1-infected individuals, higher viral loads and faster progression to AIDS correlate with systemic markers of immune activation [7]. Furthermore, cells latently infected by HIV-1 serve as long-lasting viral reservoirs and hinder the eradication of HIV-1 from infected patients with antiretroviral treatment.
HCV infection causes chronic inflammation of the liver and affects 3% of the human population worldwide [8]. The molecular mechanism(s) underlying uncontrolled, chronic liver inflammation remain poorly understood but may include direct viral effects and immune activation and dysregulation.
Combined immune dysregulation, immune deficiency, and immune activation have been proposed to worsen disease progression in HIV-1/HCV-coinfected patients [9]. The influence of HCV on HIV-1 infection remains poorly understood [10, 11]. HIV-1 infection of macrophages plays a key role in viral pathogenesis, allowing accumulating replication-competent HIV-1, even in patients receiving antiretroviral treatment [12]. Although there is evidence supporting HCV infection of macrophages in vivo [13, 14], the ability of macrophages to support productive HCV infection remains controversial ([15], and recently reviewed in [16]). In addition, HIV has been shown to facilitate HCV infection of native human macrophages in vitro [17]. Macrophage phagocytic uptake of HCV triggers immune activation [18], suggesting their contribution to chronic inflammation in infected individuals. Even though these viruses infect distinct cell types, a potential mechanism of interaction between HIV-1 and HCV is through the action of viral secreted proteins that takes place during viral replication.
HCV core is a secreted viral protein that has been detected in the circulation of HCV-infected patients [19]. Although several HCV proteins exhibit immunomodulatory activity, HCV core is unique in its pleiotropic effects [20]. In addition to its structural role in the encapsidation of the viral RNA, HCV core exhibits multiple regulatory functions, including the induction of tumorigenesis, regulation of viral and cellular gene expression, modulation of apoptosis, and suppression of host immunity [21–24]. The potential contribution of HCV core to HIV pathogenesis remains poorly understood. HCV core has been shown to mediate repression of the HIV-1 protein Tat-mediated transactivation of HIV-1 long terminal repeat (LTR) in a hepatoma cell line [25]. In contrast, Khan et al [26] have shown that HCV core and HIV-1 protein Nef upregulated HIV-1 LTR-driven luciferase expression in a transiently-transfected human monocytic cell line. Strikingly, the potential role of HCV core on HIV-1 infectivity in the context of infection, rather than plasmid-based transcription analysis, remains unknown.
Tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) are key proinflammatory cytokines known to enhance HIV-1 replication [27–30]. Interestingly, stimulation of naïve, uninfected human macrophages with HCV core induces TNF-α and IL-6 production through a Toll-like receptor 2 (TLR2)–mediated pathway [31].
Because HCV core has been shown to both inhibit and enhance HIV-1 transcription [25, 26, 32], and the impact of HCV core on HIV-1 infection in macrophages is not well known, we aimed to determine the effect of HCV core stimulation in HIV-1 infection of THP-1 promonocytic cells, primary monocyte-derived macrophages (MDMs), and in the HIV-1 latently-infected U1 monocytic cell line. We found that HCV core mediates enhancement of HIV-1 infection in macrophages through a TLR2-, JNK-, and MEK1/2-mediated pathway that results in upregulation of TNF-α and IL-6 as final molecular effectors. Our study identifies a novel role of HCV core-TLR2 interaction in augmenting HIV-1 infection in macrophages and reactivation of HIV-1-infected latent reservoirs.
2. Materials and methods
2.1. Ethics statement
De-identified human monocytes from healthy blood donors were obtained from the University of Pennsylvania’s Human Immunology Core (operating under the supervision of the University of Pennsylvania’s Institutional Review Board). We did not have any interaction with human subjects or protected information, and therefore no informed consent was required. All studies were approved and supervised by Drexel University’s Institutional Review Board.
2.2. Cell culture
A promonocytic cell line (THP-1) was differentiated in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (hiFBS) certified for low endotoxin and hemoglobin levels (BenchMark FBS, Gemini Bio-Products, West Sacramento, CA), 100 ng/ml of phorbol 12-myristate 13-acetate (PMA), antibiotics, and L-glutamine for 3 days. Cells rested in media containing no PMA for 3 more days to obtain macrophages (referred to as THP-1 macrophages). Primary MDMs were generated by culturing human monocytes in Dulbecco’s modified Eagle’s Medium (DMEM) supplemented with 10% hiFBS, antibiotics, and 16 U/ml macrophage colony-stimulating factor (eBioscience, San Diego, CA) for 7 days. Human embryonic kidney (HEK) 293T cells were cultured in DMEM supplemented with 10% DFBS and antibiotics. The HIV-1 latently-infected U1 monocytic cell line was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH from Dr. Thomas Folks. These cells were cultured in RPMI 1640 medium containing 10% hiFBS and L-glutamine. All experiments involving U1 cells were performed in a biosafety level 3 laboratory under standard operating procedures. All cells were maintained at 37°C with 5% CO2.
2.3. Single-cycle HIV-1 BaL infection assay
Single-round infectious, luciferase-reporter HIV-1 BAL pseudotyped virions were produced by cotransfecting HEK 293T cells and normalized by p24 as previously described [33]. Infectivity was measured by luciferase activity in cell lysates using a microplate luminometer (GloMax, Promega, Madison, WI).
2.4. Stimulation of macrophages with recombinant HCV core and β-galactosidase proteins
THP-1 macrophages and MDMs were treated with either 5 μg/ml of recombinant HCV core protein genotype 1b fused to β-galactosidase (Virogen, Watertown, MA), or 5 μg/ml of β-Galactosidase (β-Gal) control protein (Virogen). THP-1 and MDMs were first infected with HIV-1 BAL pseudovirus and treated with either β-Gal or HCV core recombinant proteins at 14 hours after infection. HIV-1-infected, HCV core-stimulated macrophages were incubated at 37°C for 48 hours; supernatants were collected for assessing cytokine production and cells were lysed to determine HIV-1 infectivity by luciferase activity (Promega).
2.5. Pharmacological inhibition of MAP kinases
THP-1 macrophages and human primary MDMs were treated with JNK [50 μM SP600125 (Invivogen, San Diego, CA)], p38 [20 μM SB203580 (Invivogen)], and MEK1/2 [50 μM PD98059 and 10 μM U0126 (both from Cell Signaling, Danvers, MA)] kinase inhibitors. To evaluate effects of MAP kinase inhibitors on HCV core-mediated induction of HIV-1 infectivity, macrophages were prestimulated with the inhibitors as above, then infected with HIV-1 BAL for 12 hours (in the presence or absence of these inhibitors) and treated with β-Gal or HCV core proteins, and finally lysed to determine luciferase activity 48 hours after infection. To define the effects of the MAP kinase inhibitors on HCV core–mediated induction of TNF-α and IL-6 in uninfected macrophages, cells were stimulated with MAP kinase inhibitors for 1 hour before exposure to HCV core or β-Gal, and during stimulation for 14 hours. Supernatants were collected and assayed by ELISA to determine cytokine induction.
2.6. Neutralization of TLR2, TNF-α, and IL-6
To determine the role of TLR2 in HCV core-mediated stimulation of uninfected macrophages, cells were prestimulated with 10 μg/ml of a TLR2 neutralizing antibody (nAb) (Invivogen) for 1 hour and then treated with 5 μg/ml of HCV core and 5 μg/ml of β-Gal as a negative control, in the continuous presence of the nAb. To determine the role of TLR2 in HCV core-mediated enhancement of HIV-1 infectivity in THP-1 macrophages and primary MDMs, cells were infected with HIV-1 BaL pseudovirus. Fourteen hours after infection, cells were treated with 10 μg/ml of the TLR2 nAb for 1 hour. We next treated the cells with 5 μg/ml of HCV core, and 5 μg/ml of β-Gal as a negative control, in the continuous presence of the nAb. Macrophages were lysed 48 hours after infection and tested for HIV-1 infection.
THP-1 macrophages and primary MDMs were infected with HIV-1 BAL pseudovirus and treated with 5 μg/ml of β-Gal or 5 μg/ml of HCV core, in the presence or absence of 20 μg/ml of an anti-TNF-α nAb (Thermo Fisher Scientific, Rockford, IL) and/or 20 μg/ml of an anti-IL-6 nAb (Thermo Fisher). Supernatants were collected for measuring cytokine production (ELISA) 48 hours after incubation, and cells were lysed to measure HIV-1 BaL infection.
2.7. TNF-α and IL-6 determination by ELISA
Supernatants were analyzed for secretion of proinflammatory cytokines TNF-α and IL-6 using commercial ELISA kits (eBioscience). Limit of detection of TNF-α and IL-6 ELISAs are 0.13 and 0.03 pg/ml, respectively.
2.8. Western blotting assays
Western blot analysis was performed in unstimulated and HCV core-stimulated THP-1 differentiated macrophages. Cells were stimulated with 5.0 μg/ml HCV core or β-Gal protein control for 20 and 60 minutes. Cells were washed with ice-cold PBS and then lysed. Whole-cell lysates (35 μg) were mixed with Laemmli loading buffer containing β-mercaptoethanol and heated at 95°C for 5 minutes. The samples were loaded onto 10% precast polyacrylamide gels (Bio-Rad, Hercules, CA) and electrophoresis was performed at 90 V for 90 minutes. Proteins were transferred onto methanol-activated PVDF membranes (EMD Millipore, Billerica, MA) by wet-transfer system (Bio-Rad) at 100 V for 60 minutes. Membranes were blocked in LI-COR Odyssey blocking buffer (LI-COR, Lincoln, NE) for 1 h and scanned with the LI-COR Odyssey IR imager using appropriate secondary antibodies coupled to IR680 or IR800 dye. All primary antibodies used were purchased from Cell Signalling Technology (Beverly, MA); anti-p-ERK1/2 (1:500), anti-ERK1/2 (1:500), anti-SAPK/JNK (1:1000), anti-p-SAPK/JNK (1:1000), anti-p38 MAPK (1:500), anti-p-p38 MAPK (1:500), and anti-HSP90 (1:5000) as endogenous control.
2.9. HIV-1 latency reactivation and macrophage-conditioned media experiments
U1 cells were directly treated with 5 μg/ml of β-Gal, or 5 μg/ml HCV core, or 100 ng/ml of PMA (as a TLR2-independent positive control for HIV-1 reactivation) for 14 hours. Cells were next centrifuged and supernatants collected. For macrophage-conditioned media (CM) experiments, U1 cells were treated with supernatants from macrophages that were either unstimulated or stimulated with β-Gal or HCV core, either individually or in the presence of neutralizing TNF-α antibody, neutralizing IL-6 antibody, or both. U1 cells were centrifuged and supernatants were collected 14 hours after stimulation with CM. Reactivation of the latent HIV-1 in U1 cells was determined using a commercial HIV-1 p24 ELISA (ABL, Rockville, MD HIV p24 ELISA; limit of detection is 3.1 pg/ml).
2.10. Statistical analysis
Statistical significance of experimental data was determined by unpaired Student’s t-test and P < 0.05 was considered statistically significant.
3. Results
3.1. HCV core enhances HIV-1 infection of THP-1 cells and primary macrophages
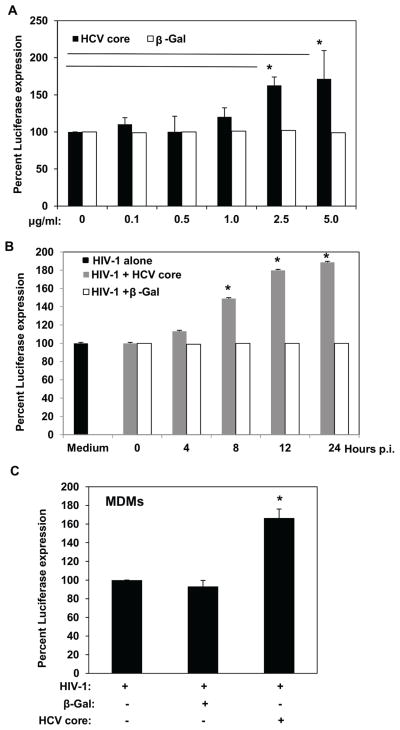
We investigated the potential effects of HCV core in HIV-1 infectivity in THP-1 cells and in primary MDMs. For this purpose, we used a single-round infectious, luciferase-reporter HIV-1 pseudotype that express the envelope of CCR5-using, macrophage-tropic BaL [33, 34]. Using this system, LTR-driven luciferase expression is a measure of HIV-1 infection. We performed a dose response curve to investigate the effects of HCV core on differentiated, HIV-1-infected THP-1 cells. For all our experiments we used an HCV genotype 1b recombinant core protein fused to β-galactosidase simultaneously, or β-Gal protein alone as a negative control. The use of these HCV core and β-Gal recombinant proteins has been previously established and a dose of 5 μg/ml has been determined to exhibit various effects on different cell types [35, 36]. We then investigated the effects of 0.1, 0.5, 1.0, 2.5, and 5.0 μg/ml of HCV core or β-Gal protein. After differentiation, THP-1 cells were infected with HIV-1 BaL pseudovirus for 12 h and treated with HCV core protein or β-Gal protein alone as a negative control; luciferase was measured 48 h after HIV-1 infection. As shown in Figure 1A, HCV core concentrations below 2.5 μg/ml did not affect HIV-1 infectivity. In contrast, stimulation of infected THP-1 cells with 2.5 μg/ml or 5.0 μg/ml HCV core significantly increased LTR-driven luciferase expression (P < 0.001), suggesting that HCV core can enhance HIV-1 replication in infected macrophages. As expected, treatment with β-Gal protein did not affect HIV-1 infectivity (Fig. 1A).
Fig. 1.
HCV core enhances HIV-1 BaL infection of THP-1 macrophages and MDMs. (A) HCV core dose curve experiment. THP-1 differentiated cells were stimulated with 0.1, 0.5, 1.0, 2.5, and 5 μg/ml of HCV core, or β-Gal recombinant protein control at 12 hours after infection with a luc-reporter, macrophage-tropic BaL HIV-1 pseudotype. (B) HCV core time course experiment. THP-1 differentiated cells were stimulated with 5 μg/ml of HCV core, or β-Gal recombinant protein control at the time of infection (time 0) and 4, 8,12, and 24 hours after HIV-1 BaL infection. (C) Primary human macrophages (MDMs) were stimulated with 5 μg/ml of HCV core, or β-Gal recombinant protein control at 12 hours after HIV-1 BaL infection. LTR-driven Luciferase activity was measured 2 days after infection and results from 5 independent experiments (THP-1) and 2 independent MDMs donors are shown. Luciferase activity in cell lysates was measured as relative light units per second. Data are shown as percent luciferase expression relative to HIV-1 infected alone cells (mean ± SD). Values that were significantly different (P < 0.05) from the value of HIV-infection alone group are indicated (*).
To determine the kinetics of HCV core-mediated enhancement of HIV-1 infection in macrophages, we performed time course experiments. We found that THP-1 macrophages treated with HCV core at the time of infection or at 4 hours post-infection showed no significant change in HIV-1 infectivity compared with untreated, or β-Gal-treated, HIV-1-infected cells (Fig. 1B). Interestingly, stimulation of THP-1 macrophages with HCV core at 8, 12 and 24 hours post-infection significantly increased LTR-driven luciferase expression (8 h p.i., P < 0.05; and 12 and 24 h p.i., P < 0.01). These findings suggest that HCV core-mediated enhancement of HIV-1 infection in macrophages occurs when entry and early post-entry events have been completed.
To determine that HCV core increases HIV-1 infection in primary human macrophages, we stimulated MDMs with 5.0 μg/ml of HCV core or β-Gal protein control 12 hours after HIV-1 BaL infection. As shown in Fig. 1C, HCV core induced a significant upregulation of LTR-driven luciferase expression in HIV-1 infected MDMs (Fig. 1C, P < 0.05). Taken together, these results suggested that HCV core stimulation after HIV-1 infection of macrophages enhances HIV-1 replication.
3.2. HCV core enhances HIV-1 infectivity in macrophages via a TLR2- JNK- MEK1/2-dependent pathway
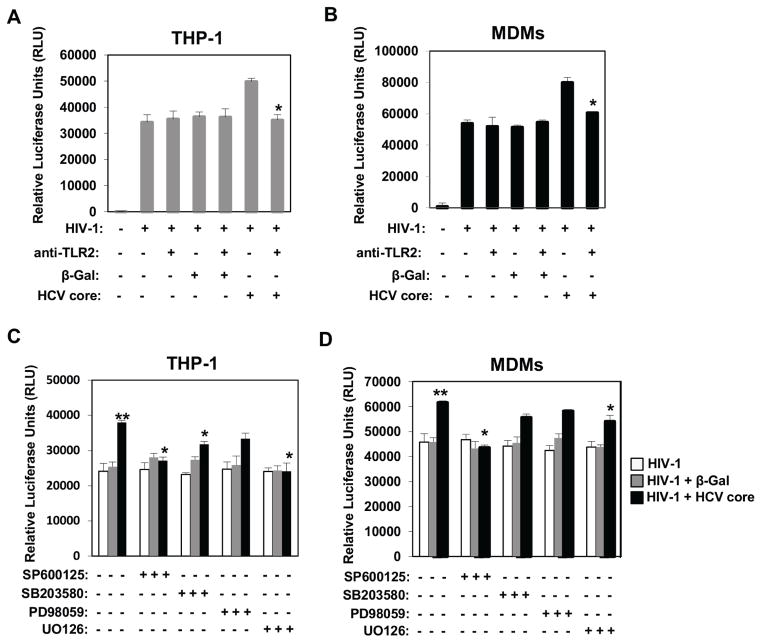
Previous studies have demonstrated that HCV core but not HCV virion can trigger TLR2-mediated pathways [31, 36–38]. To determine the role of TLR2 in HCV core-mediated enhancement of HIV-1 infectivity in macrophages, we used a nAb against TLR2. Twelve hours after HIV-1 BaL infection, THP-1 macrophages and primary MDMs were treated with HCV core, or β-Gal protein, in the presence or the absence of anti-TLR2 nAb. Strikingly, blocking TLR2 abrogated HCV core enhancement of HIV-1 replication (Fig. 2A and B).
Fig. 2.
HCV core enhances HIV-1 infectivity via TLR2. THP-1 macrophages (A) and MDMs (B) were infected with a luc-reporter, macrophage-tropic BaL HIV-1 pseudotype. Cells were unstimulated or stimulated with 5 μg/ml of HCV core or β-Gal recombinant protein control in the presence of a neutralizing human monoclonal anti-TLR2 Ab or isotype control (Invivogen) 12 hours after infection. Luc activity was measured 2 days after infection and results from 3 independent experiments (THP-1) and 2 independent MDMs donors are shown. Luciferase activity in cell lysates was measured as relative light units per second. Results represent means ± SD. To determine the role of MAP kinases in HCV core-mediated enhancement of HIV infection in THP-1 macrophages (C) and MDMs (D), cells were prestimulated with specific inhibitors of JNK (50 μM SP600125), p38 (20 μM SB203580), and MEK1/2 (50 μM PD98059, and 20 μM U0126) for 30 minutes and then infected with HIV-1 BaL pseudotype. Infected cells were left unstimulated or treated with HCV core or β-Gal 12 hours after infection. Luc activity was measured 2 days after infection and results from 3 independent experiments (THP-1) and 2 independent MDM donors are shown. Luciferase activity in cell lysates was measured as relative light units per second. Data are shown as relative luciferase units (RLU) of HIV infectivity (mean ± SD). Values that were significantly different (P < 0.05) from the value of HIV-infection alone group are indicated (*).
MAP kinases play a prominent role as molecular mediators of TLR-induced inflammation, and as critical regulators of transcriptional regulation, and cross-talk of TLRs [39]. Therefore, we investigated the involvement of the c-Jun aminoterminal kinase (JNK), the p38 MAP kinases (p38), and the extracellular signal-regulated protein kinase 1/2 (ERK) in HCV core-mediated enhancement of HIV-1 infectivity in macrophages. None of the MAP kinase inhibitors tested affected HIV-1 infectivity in macrophages (white bars in Fig. 2C and D). In contrast, pharmacological inhibition of JNK (SP600125) and MEK1/2 (U0126) significantly reduced HCV core enhancement of HIV-1 infectivity (P < 0.05) in THP-1 and MDMs (Fig. 2C and D). A significant difference was observed after inhibition of p38 MAPK (SB203580) in THP-1 cells. Although HCV core effect was reduced in MDMs, the difference was not statistically significant (Fig. 2C and D). Since U0126 is a highly selective inhibitor of both MEK1 and 2, we investigated the effects of another MEK1/2 inhibitor (PD98059) that inhibits MEK1 more potently than MEK2. In contrast to the significant effect of U0126, we found that PD98059 only partially reduced HCV core enhancement of HIV-1 infection (Fig. 2C and D). These data suggest that MEK1 and MEK2 have differential contributions to HCV core effect (being more dependent on MEK2 than MEK1), or that both MEK1 and MEK 2 are equally required. Taken together, our data demonstrated that HCV core promotes HIV-1 infectivity in macrophages via a TLR2- JNK- MEK1/2-dependent pathway. Furthermore, our data suggest differential activation/regulation of p38 kinase in THP-1 and MDMs.
To investigate a potential direct role of HCV core on MAP kinases stimulation in macrophages, we stimulated differentiated THP-1 cells with 5.0 g/ml of HCV core and β–Gal as a negative control. Because we aimed to elucidate direct effects of HCV core stimulation on MAP kinases, we stimulated cells using short times of incubation (20 and 60 minutes). Twenty minutes was selected because there is previous data in the literature demonstrating that HCV core activates ERK1/2 phosphorylation in human monocytes at this short time after stimulation [31]. As shown in supplementary Figure 1A, we observed a two-fold induction of ERK1/2 phosphorylation after stimulation with 5.0 μg/ml HCV core for 20 minutes. Pharmacological inhibition of ERK1/2 (PD98059, UO126) in HCV core-stimulated cells reduced ERK1/2 phosphorylation to levels below those observed in unstimulated cells, whereas SP600125 (a JNK inhibitor) exhibited only a slight reduction on HCV core-mediated ERK1/2 phosphorylation (supplementary Fig. 1A). Interestingly, we observed a stimulatory effect of SB203580 (p38 kinase inhibitor) on ERK1/2 phosphorylation that has been previously noted in other cell types (supplementary Fig. 1A) [40]. It should be noted that a dominant negative mutant of p38 also increased ERK1/2 phosphorylation in response to UVA [41]. Therefore, it is unlikely that the effects on ERK1/2 are due to non-selectivity of SB203580 p38 inhibitor. Indeed, a crosstalk between ERK1/2 and p38 kinases has been previously demonstrated in various cell types, and usually these kinases have opposing effects. Interestingly, p38 exhibits a negative regulation on the ERK pathway in response to redox and mitogen stimulation with PMA in various cell types [40, 42]. In addition, HCV core stimulation activated p38 kinase phosphorylation, but it did not affect JNK after 20 minutes of HCV core stimulation. Similar results were obtained 60 minutes after stimulation (data not shown). β–Gal protein did not affect kinase activation (data not shown). Taken together, these data demonstrated that HCV core stimulation directly activates pathways that mediate rapid phosphorylation of ERK1/2 and p38 kinases shortly after stimulation of macrophages.
3.3. Role of TNF-α and IL-6 in HCV core enhancement of HIV-1 infectivity in macrophages
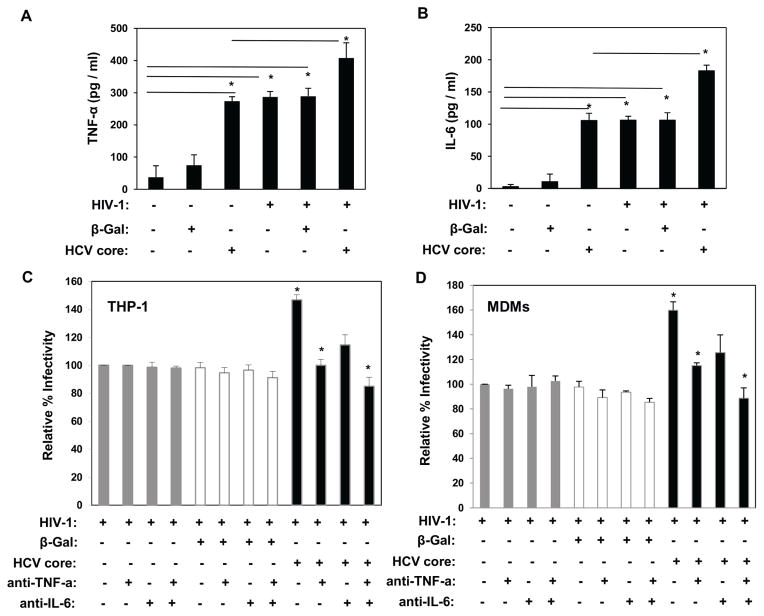
The proinflammatory cytokines IL-6 and TNF-α upregulate production of HIV-1 in acutely as well as in chronically infected cells of monocytic lineage [43]. Furthermore, extracellular HCV core induces secretion of proinflammatory cytokines, including TNF-α and IL-6 in antigen-presenting cells [35, 44, 45]. Thus, we investigated the role of TNF-α and IL-6 in HCV core-mediated upregulation of HIV-1 infectivity in macrophages. As shown in Fig. 3A and B, stimulation with HCV core induced TNF-α and IL-6 production in uninfected macrophages. While HIV-1 BaL infection or HCV core stimulation alone resulted in secretion of these cytokines, treatment of macrophages with HCV core after HIV-1 BaL infection resulted in significant overproduction of both TNF-α and IL-6. To determine whether TNF-α and/or IL-6 contribute to HCV core-mediated enhancement of HIV-1, we stimulated HIV-1 BaL-infected THP-1 macrophages and MDMs with HCV core in the presence or absence of nAbs against TNF-α and IL-6. Blockade of TNF-α suppressed HCV core-mediated enhancement of HIV-1 infectivity whereas blocking IL-6 partially prevented this effect (Fig. 3C and D). Simultaneous blockade of TNF-α and IL-6 significantly abrogated HCV core-mediated enhancement of HIV-1 infectivity. These findings demonstrate that TNF-α and to a lesser extent IL-6, mediated HCV core enhancement of HIV-1 infectivity in macrophages.
Fig. 3.
HCV core-induced TNF-α and IL-6 mediate the enhancement of HIV-1 infection in THP-1 macrophages and MDMs. (A) TNF-α and (B) IL-6 cytokine induction in naïve, HIV-BaL-infected, and/or stimulated with HCV core, and β-galactosidase protein control was measured by ELISA. (C) THP-1 macrophages and (D) MDMs were infected with HIV-BaL for 12 hours. Cells were left unstimulated or treated with HCV core, or β-galactosidase protein control, in the presence or absence of specific human monoclonal nAbs anti-TNF-α and/or anti-IL-6. HIV infectivity was determined by luciferase activity in cell lysates 48 hours after infection. Data are shown as relative fold induction of HIV infectivity (mean ± SD). Results from 3 independent experiments (THP-1) and 2 independent MDM donors are shown. *Significantly different (P < 0.05) from the HIV-infection alone group.
3.4. Role of MAP kinases in HCV core induction of TNF-α and IL-6 in macrophages
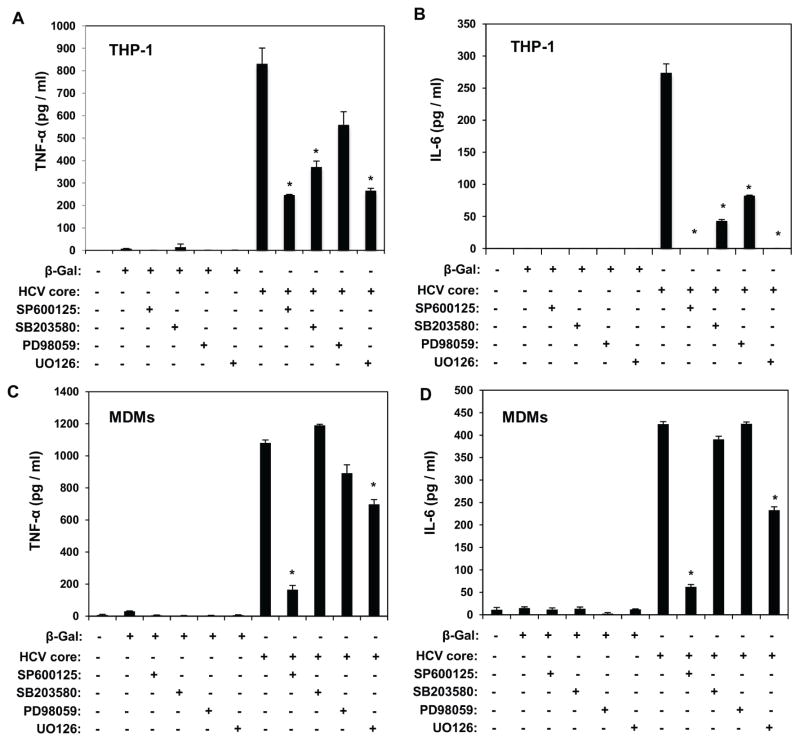
Our data demonstrated the involvement of JNK and MEK1/2 kinases in the TLR2-mediated, HCV core enhancement of HIV-1 infectivity in THP-1 macrophages and MDMs (Fig. 2C and D). Thus we investigated whether MAP kinases play a role in HCV core–mediated induction of TNF-α and IL-6 in THP-1 macrophages and primary MDMs. Production of TNF-α by HCV core was significantly reduced by SP600125, SB203580, and U0126, whereas the effect of PD98059 did not reach statistical significance (Fig. 4A). These findings confirmed the differential contribution of JNK, MEK1/2, and p38 MAP kinases in HCV core-mediated TNF-α production in uninfected macrophages. Importantly, these findings correlated with inhibition of HIV-1 BaL infectivity in HCV core-stimulated THP-1 macrophages (Fig. 2C). In addition, although SP600125, SB203580, U0126, and PD98059 inhibitors significantly affected HCV core-mediated IL-6 production, only SP600125 and U0126 suppressed IL-6, suggesting a prominent role of JNK and MEK1/2 kinases in HCV core-mediated IL-6 induction in THP-1 macrophages (Fig. 4B).
Fig. 4.
TNF-α and IL-6 cytokine induction mediated by HCV core is dependent on MAP kinases. THP-1 macrophages (A and B) and MDMs (C and D) were stimulated with HCV core or β-galactosidase protein control in the presence or absence of 50 μM SP600125 (JNK inhibitor), 20 μM SB203580 (p38 inhibitor), 50 μM PD98059 (MEK1/2 inhibitor), and 10 μM U0126 (MEK1/2 inhibitor). TNF-α (A and C) and IL-6 (B and D) induction was determined by ELISA (R&D Systems, Minneapolis, MN) 14 hours after stimulation. Results from 3 independent experiments (THP-1) and 2 independent MDM donors are shown. Data are means ± SD. *Significantly different (P < 0.05) from HCV core–stimulated group.
Our data show that in primary MDMs only SP600125 and U0126 inhibitors significantly reduced HCV core-mediated enhancement of HIV-1 infectivity (Fig. 2D). Interestingly, SP600125 and U0126 significantly inhibited both TNF-α and IL-6 production in HCV core-stimulated MDMs, suggesting that JNK and MEK1/2 kinases play a key role in HCV core induction of both cytokines in primary MDMs. In contrast, no significant changes were observed with SB203580 and PD98059 inhibitors, in parallel with results demonstrating a lack of effect of these inhibitors in HCV core enhancement of HIV-1 infectivity in MDMs (Fig. 2D).
Overall, these findings suggest that JNK and MEK1/2 kinases contribute to TNF-α and IL-6 production in HCV core-stimulated THP-1 macrophages and primary MDMs. In contrast, pharmacological inhibition of p38 MAP kinase with SB203580 significantly reduced HCV core induction of TNF-α and IL-6 in THP-1 macrophages, with no effects in primary MDMs. Importantly, these findings correlate with the effects of these MAP kinase inhibitors in HCV core enhancement of HIV-1 infectivity (Fig. 2C and D).
3.5. HCV core -induced TNF-α and IL-6 reactivate HIV-1 latently infected U1 cells
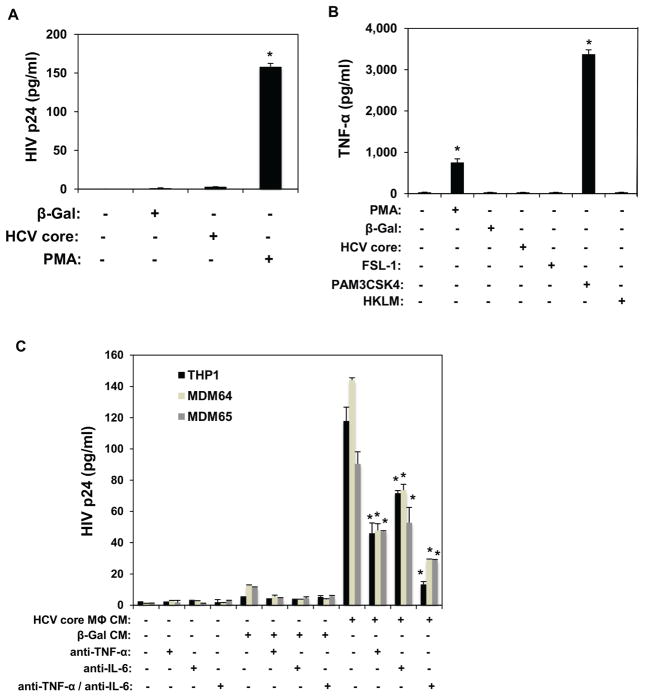
U1 is a cell line derived from U937 promonocytic cells latently infected with HIV-1 that has been established as a model for HIV-1 latency [46]. Although provirus expression in U1 cells is poor (at least in part due to a defective Tat function [47, 48]), HIV-1 provirus expression is highly inducible following treatments with various cytokines or phorbol esters [46, 49]. Therefore, we next investigated the effects of HCV core protein on HIV-1 reactivation in U1 cells. Unexpectedly, stimulation of U1 cells with HCV core did not affect HIV-1 latency (Fig. 5A), similarly to β-Gal negative control. In contrast, PMA induced robust upregulation of HIV-1 p24 (Fig. 5A), demonstrating that U1 cells are responsive to phorbol esters, as expected. Although HCV core stimulation of U1 cells did not directly reactivate HIV-1, we next examined the hypothesis that TNF-α and IL-6 induced in HCV core-stimulated macrophages might reactivate HIV-1 in U1 cells. To test this hypothesis we first stimulated U1 cells with conditioned media (CM) of THP-1 macrophages and primary MDMs that have been stimulated with HCV core or the β-Gal control. Stimulation of U1 cells with CM of HCV core-treated THP-1 macrophages and MDMs induced HIV-1 reactivation similarly to PMA treatment alone (Fig. 5A and C). Strikingly, neutralization of TNF-α and/or IL-6 reduced HIV p24 induction in U1 cells stimulated with CM of HCV core-treated macrophages. The contribution of TNF-α to HIV-1 reactivation in CM-stimulated U1 cells was more profound than that of IL-6. Taken together, these findings suggested that although HCV core lacks the ability to directly reactivate HIV-1 in latently-infected U1 cells, CM of HCV core-stimulated macrophages induced HIV-1 reactivation in U1 cells through TNF-α and IL-6.
Fig. 5.
HCV core and conditioned media (CM) of HCV core-stimulated THP-1 macrophages and MDMs differentially affect reactivation of HIV-1 latent infection in U1 cells. (A) HCV core does not induce p24 production in U1 cells. U1 cells were stimulated with HCV core, β-galactosidase (negative control), or PMA (positive control) for 12 hours. Supernatants of stimulated U1 cells were assayed for p24 secretion by ELISA 24 hours after stimulation. (B) U1 cells show differential TNF-α production in response to TLR2/6 and TLR2/1 agonists. U1 cells were stimulated with PMA (positive control), HCV core, β-galactosidase protein, TLR2/6 agonist FSL-1, TLR2/1 agonist PAM3CSK4, and TLR2/2 agonist HKLM for 12 hours. Supernatants of stimulated U1 cells were assayed for p24 production by ELISA 24 hours after stimulation. (C) Stimulation of U1 cells with CM of HCV core-stimulated THP-1 macrophages and MDMs induced significant p24 production through the induction of the proinflammatory cytokines TNF-α and IL-6. U1 cells were stimulated with supernatants of HCV core–stimulated THP-1 macrophages and MDMs collected 14 hours after stimulation (CMs). Supernatants from U1 cells stimulated with CMs were assayed for p24 production by ELISA 24 hours after stimulation. Results from 3 independent experiments (THP-1 and U1) and CMs of 2 independent MDM donors are shown. Data are mean ± SD; *significantly different (P < 0.05) from control.
Finally, in order to understand the inability of HCV core to directly reactivate HIV-1 in U1 cells, we determined TNF-α induction in U1 cells stimulated with HCV core, β-Gal, and various TLR2 agonists [FSL-1, PAM3CSK4, and heat-killed Listeria monocytogenes (HKLM)], or PMA (as a positive control of TLR2-independent activation). Ligand recognition and signaling through TLR2 occurs via heterodimerization with TLR1 or TLR6. In the absence of TLR1 and TLR6, TLR2 homodimerization has been proposed, but it remains to be fully demonstrated [50]. Interestingly, stimulation with FSL-1 (TLR2/TLR6) and HKLM (proposed TLR2/TLR2) agonists did not induce TNF-α production in U1 cells. In contrast, both PMA and the TLR2/TLR1 agonist PAM3CSK4 induced robust upregulation of TNF-α in U1 cells (Fig. 5B). These findings demonstrate that TLR2 heterodimerization with TLR1 is functional in U1 cells. In contrast, stimulation of TLR2 heterodimerization with TLR6, or TLR2/TLR2 homodimerization with FSL-1 and HKLM, respectively, failed to activate U1 cells. Taken together, these data suggest that the absence of functional TLR2/TLR6 heterodimerization or TLR2/TLR2 homodimerization may account for the inability of HCV core to stimulate U1 cells.
4. Discussion
HCV-associated liver disease is accelerated in HIV-1-infected individuals, and several mechanisms have been proposed [11, 51]. The impact of HCV on HIV-1 is even less understood, although the potential contribution of HCV core to HIV pathogenesis is emerging. Because of their differential tropism, it is tempting to speculate that HCV core, the only known HCV protein that is secreted into the blood of infected individuals, may impact HIV-1 infectivity of macrophages.
Here we investigated the impact of HCV core protein on acute and latently HIV-infected macrophages. Our findings demonstrate that HCV core stimulation of HIV-1 acutely infected THP-1 macrophages and primary MDMs induced enhancement of HIV-1 infectivity through a mechanism that is dependent on TLR2, JNK, and MEK1/2 kinases. In addition, we have identified TNF-α and, to a lesser extent, IL-6 as final molecular effectors in this pathway. Furthermore, using the promonocytic U1 cell line as a model system for HIV-1 latency, we have shown that conditioned media from THP-1 macrophages and primary MDMs stimulated with HCV core significantly upregulated HIV-1 production in U1 cells through a TNF-α and IL-6–dependent mechanism. Intriguingly, HCV core direct stimulation of U1 cells failed to directly reactivate latent HIV-1 infection, at least in part because of the lack of functional TLR2/TLR6 heterodimerization or TLR2/TLR2 homodimerization in U1 cells.
It is well established that systemic markers of immune activation are associated with higher HIV-1 viral loads and faster progression to AIDS [52]. The effects of various TLR agonists and microbial components of frequent opportunistic infections such as Mycobacterium spp., Neisseria gonorrhoeae, and others, in acute and latently infected HIV-1 cell cultures, and in a few in vivo models, have been previously reported [53–56]. In particular, TLR2 has been implicated in enhancing susceptibility to HIV infection of Langerhans cells and naïve and memory CD4+ T cells [57–62], reactivation of latently infected central memory CD4+ T cells [63], as well as reducing susceptibility of macrophages to HIV-1 infection [64]. Interestingly, we have found that HCV core-induced enhancement of HIV-1 infectivity in THP-1 macrophages and primary MDMs is dependent on TLR2 (Fig. 2A and B). Our findings are in agreement with previous data demonstrating that HCV core triggers TLR2-mediated pathways in macrophages [31]. Using pharmacological inhibitors of JNK, p38, and MEK1/2 MAP kinases, we identified a key role of JNK and MEK1/2 kinases in HCV core enhancement of HIV-1 infection in THP-1 and primary MDMs (Fig. 2C and D). Importantly, treatment with kinase inhibitors alone did not affect HIV-1 infection. We have investigated the effects of HCV core in differentiated THP-1 cells in the absence of HIV-1 infection. Although we have not determined the kinetics of activation of MAP kinases in response to HCV core stimulation, our data demonstrates that HCV core stimulation induces rapid phosphorylation of ERK1/2 and p38 kinases. However we did not observed phosphorylation of JNK at 20 or 60 minutes after stimulation suggesting that HCV core may mediate JNK phosphorylation at later times after stimulation, or that its phosphorylation is mediated indirectly by the production of pro-inflammatory cytokines, likely TNF-α that is known to induce transient and sustained activation of JNK [65]. It is important to note that HIV-1 infection alone may affect MAP kinases activation, and that synergistic, additive or antagonistic mechanisms may regulate the precise role of ERK1/2, p38, and JNK kinases in HIV-1 -infected macrophages that are stimulated with HCV core. Further investigations will define their specific roles in HCV core-mediated enhancement of HIV-1 infectivity in macrophages.
Proinflammatory cytokines such as TNF-α and IL-6 can enhance HIV-1 infection and replication [43]. We found that HCV core stimulation induced both cytokines in uninfected macrophages (Fig. 3A and B). Furthermore, THP-1 macrophages and primary MDMs treated with HCV core after HIV-1 infection produced significantly higher TNF-α and IL-6 compared with HIV-1-infected, unstimulated cells. Using nAbs anti-TNF-α, and anti-IL-6, we demonstrate that HCV core’s pro-HIV-1 effect is mediated by TNF-α and, to a lesser extent, IL-6 induction.
Stimulation of macrophages by TLR ligands leads to the activation of MAP kinases and the subsequent induction of cytokine gene expression. Our data demonstrate that HCV core-mediated induction of TNF-α and IL-6 in THP-1 macrophages and in primary MDMs is significantly reduced in the presence of SP600125, and U0126 (Fig. 4C and D). Taken together with the effects of these inhibitors on HCV core enhancement of HIV-1 infection (Fig. 2C and D), our data suggest that JNK and MEK1/2 play key roles in HCV core-induced upregulation of HIV-1 infection through the induction of TNF-α and IL-6 in macrophages. Inhibition of p38 kinase with SB203580 significantly reduced TNF-α and IL-6 in THP-1 macrophages, but not in MDMs (Fig. 4). These data correlate with the lack of inhibitory effect of SB203580 on HCV core enhancement of HIV-1 infection in MDMs (Fig. 2D) compared with the reduction in THP-1 macrophages (Fig. 2C).
To investigate the potential effects of HCV core stimulation on HIV-1 latently infected monocytic cells, we used the U1 cell line. Surprisingly, HCV core failed to induce upregulation of latent HIV-1 in U1 cells (Fig. 5A). However, conditioned media (CM) of HCV core-stimulated THP-1 macrophages and primary MDMs induced profound HIV-1 reactivation through the induction of TNF-α and IL-6 (Fig. 5B). Although our goal was not to investigate potential effects of TLR agonists on HIV-1 infection, we provide evidence that the latently HIV-1-infected promonocytic U1 cell line is responsive to a TLR2/1 agonist (PAM3CSK4), but fails to be stimulated by a TLR2/6 agonist (FSL-1). These data are in agreement with recent findings demonstrating that PAM3CSK4, but not FSL-1 agonist, reactivates HIV-1 latent infection in U1 cells and in latently infected central memory T cells and resting CD4+ T cells from aviremic patients [63]. Because PAM3CSK4 reactivated latent HIV-1 in the absence of T cell activation or proliferation, these authors suggested that further investigation of the TLR1/2 pathway may lead to the identification of agonists that like PAM3CSK4 have the potential to be explored as antilatency drugs. Our findings demonstrate that TLR2 heterodimerization with TLR1 is functional in U1 cells. In contrast, activation of TLR2 heterodimerization with TLR6 (FSL-1) or TLR2/TLR2 homodimerization (HKLM) failed to activate U1 cells. It has been previously shown that TNF-α and IL-6 induced after stimulation with HCV core in primary MDMs are dependent on TLR2 heterodimerization with both TLR1 and TLR6 [45]. Taken together, these data suggest that the absence of functional TLR2/TLR6 heterodimerization may account for the inability of HCV core to stimulate U1 cells.
Overall, these studies identify a novel role of HCV core in augmenting acute, and latent HIV-1 infection in macrophages, with implications towards a better understanding of the inter-influential role of HIV-1 and HCV in coinfected patients.
Supplementary Material
Effects of HCV core stimulation on MAP kinases phosphorylation in differentiated THP-1 cells. Differentiated THP-1 cells were left untreated, or stimulated with 5.0 μg/ml of HCV core or β-Gal protein for 20 and 60 minutes in the presence or absence of MAP kinases inhibitors 50 μM PD98059 and 10 μM UO126 (ERK1/2), 20 μM SB203580 (p38), and 50 μM SP600125 (JNK). Cell were pre-stimulated with inhibitors for 1 hour before HCV core stimulation. Cell lysates were prepared and immunoblot analysis was performed as described in Methods. Panels A and B show data after 20 minutes of stimulation with HCV core. (A) HCV core stimulates ERK1/2 phosphorylation and this effect is inhibited by its pharmacological inhibition (PD98059 and OU126) and augmented by SB203580, suggesting that p38 is a negative regulator of HCV core-mediated early ERK1/2 phosphorylation. JNK phosphorylation inhibition only slightly reduced ERK1/2 phosphorylation. (B) Effects of HCV core stimulation on p38 and JNK phosphorylation in THP-1 macrophages. Results are representative of two independent experiments with 4 replicates per experiment.
Highlights.
HCV core enhances HIV-1 in macrophages via a TLR2, MAP kinase, TNF-α/IL-6 pathway.
HCV core-induced TNF-α/IL-6 reactivate HIV-1 in latently-infected monocytic cells.
HCV core does not activate latently-infected U1 cells.
U1 cells lack functional TLR2/TLR6 or TLR2/TLR2 dimerization.
HCV core’s pro-HIV-1 effects in macrophages may be harmful in coinfected patients.
Acknowledgments
This work was supported in part by Public Health Service Grants AI088423 and DK089314 to S.N.M., and NS065727, and AI098549 to J.M.G., from the National Institute of Allergy and Infectious Disease (AI), and the National Institute of Diabetes and Digestive and Kidney Diseases, and National Institute of Neurological Disorders and Stroke (NS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Diana Winters (Drexel University College of Medicine Academic Publishing Services) is acknowledged for manuscript editing.
Abbreviations
- APCs
antigen-presenting cells
- ERK
Extracellular signal-regulated protein kinase 1/2
- HKLM
Heat Killed Listeria monocytogenes
- IL-6
Interleukin 6
- JNK
c-Jun amino-terminal kinase
- MDMs
primary monocyte derived macrophages
- p38
p38 MAP kinase
- PD98059
2′-Amino-3′-methoxyflavone
- PMA
Phorbol myristate acetate
- SB203580
4-(4′-Fluorophenyl)-2-(4′-methylsulfinylphenyl)-5- (4′-pyridyl)-imidazole
- SP600125
c-Jun N-terminal kinase inhibitor
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor alpha
- TRAFs
TNF receptor-associated factors
- U0126
1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanchez-Quijano A, Andreu J, Gavilan F, Luque F, Abad MA, Soto B, Munoz J, Aznar JM, Leal M, Lissen E. Influence of human immunodeficiency virus type 1 infection on the natural course of chronic parenterally acquired hepatitis C. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 1995;14:949–53. doi: 10.1007/BF01691375. [DOI] [PubMed] [Google Scholar]
- 2.Taylor LE, Swan T, Mayer KH. HIV coinfection with hepatitis C virus: evolving epidemiology and treatment paradigms. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;55(Suppl 1):S33–42. doi: 10.1093/cid/cis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbein G, Varin A. The macrophage in HIV-1 infection: from activation to deactivation? Retrovirology. 2010;7:33. doi: 10.1186/1742-4690-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JH, Janas AM, Olson WJ, Wu L. functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. Journal of virology. 2007;81:8933–43. doi: 10.1128/JVI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundquist CA, Tobiume M, Zhou J, Unutmaz D, Aiken C. Nef-mediated downregulation of CD4 enhances human immunodeficiency virus type 1 replication in primary T lymphocytes. Journal of virology. 2002;76:4625–33. doi: 10.1128/JVI.76.9.4625-4633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tardif MR, Tremblay MJ. Tetraspanin CD81 provides a costimulatory signal resulting in increased human immunodeficiency virus type 1 gene expression in primary CD4+ T lymphocytes through NF-kappaB, NFAT, and AP-1 transduction pathways. Journal of virology. 2005;79:4316–28. doi: 10.1128/JVI.79.7.4316-4328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paiardini M, Muller-Trutwin M. HIV-associated chronic immune activation. Immunological reviews. 2013;254:78–101. doi: 10.1111/imr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology (Baltimore, Md) 2013;57:1333–42. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, Klein M. HIV/hepatitis C virus coinfection management: changing guidelines and changing paradigms. HIV medicine. 2014 doi: 10.1111/hiv.12161. [DOI] [PubMed] [Google Scholar]
- 10.Klenerman P, Kim A. HCV-HIV coinfection: simple messages from a complex disease. PLoS medicine. 2007;4:e240. doi: 10.1371/journal.pmed.0040240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Operskalski EA, Kovacs A. HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Current HIV/AIDS reports. 2011;8:12–22. doi: 10.1007/s11904-010-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan J, Sattentau QJ. The HIV-1-containing macrophage compartment: a perfect cellular niche? Trends in microbiology. 2013;21:405–12. doi: 10.1016/j.tim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Laskus T, Radkowski M, Piasek A, Nowicki M, Horban A, Cianciara J, Rakela J. Hepatitis C virus in lymphoid cells of patients coinfected with human immunodeficiency virus type 1: evidence of active replication in monocytes/macrophages and lymphocytes. The Journal of infectious diseases. 2000;181:442–8. doi: 10.1086/315283. [DOI] [PubMed] [Google Scholar]
- 14.Radkowski M, Bednarska A, Horban A, Stanczak J, Wilkinson J, Adair DM, Nowicki M, Rakela J, Laskus T. Infection of primary human macrophages with hepatitis C virus in vitro: induction of tumour necrosis factor-alpha and interleukin 8. The Journal of general virology. 2004;85:47–59. doi: 10.1099/vir.0.19491-0. [DOI] [PubMed] [Google Scholar]
- 15.Marukian S, Jones CT, Andrus L, Evans MJ, Ritola KD, Charles ED, Rice CM, Dustin LB. Cell culture-produced hepatitis C virus does not infect peripheral blood mononuclear cells. Hepatology (Baltimore, Md) 2008;48:1843–50. doi: 10.1002/hep.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revie D, Salahuddin SZ. Role of macrophages and monocytes in hepatitis C virus infections. World journal of gastroenterology: WJG. 2014;20:2777–84. doi: 10.3748/wjg.v20.i11.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laskus T, Radkowski M, Jablonska J, Kibler K, Wilkinson J, Adair D, Rakela J. Human immunodeficiency virus facilitates infection/replication of hepatitis C virus in native human macrophages. Blood. 2004;103:3854–9. doi: 10.1182/blood-2003-08-2923. [DOI] [PubMed] [Google Scholar]
- 18.Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH, Gale M., Jr IL-1beta production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoSpathogens. 2013;9:e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannini C, Brechot C. Hepatitis C virus biology. Cell death and differentiation. 2003;10(Suppl 1):S27–38. doi: 10.1038/sj.cdd.4401121. [DOI] [PubMed] [Google Scholar]
- 20.Ray RB, Ray R. Hepatitis C virus core protein: intriguing properties and functional relevance. FEMS microbiology letters. 2001;202:149–56. doi: 10.1111/j.1574-6968.2001.tb10796.x. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Ponce C, Dominguez-Villar M, Aguado E, Garcia-Cozar F. CD4+ primary T cells expressing HCV-core protein upregulate Foxp3 and IL-10, suppressing CD4 and CD8 T cells. PloS one. 2014;9:e85191. doi: 10.1371/journal.pone.0085191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moradpour D, Penin F. Hepatitis C virus proteins: from structure to function. Current topics in microbiology and immunology. 2013;369:113–42. doi: 10.1007/978-3-642-27340-7_5. [DOI] [PubMed] [Google Scholar]
- 23.Imran M, Waheed Y, Manzoor S, Bilal M, Ashraf W, Ali M, Ashraf M. Interaction of Hepatitis C virus proteins with pattern recognition receptors. Virology journal. 2012;9:126. doi: 10.1186/1743-422X-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jahan S, Ashfaq UA, Khaliq S, Samreen B, Afzal N. Dual behavior of HCV Core gene in regulation of apoptosis is important in progression of HCC. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2012;12:236–9. doi: 10.1016/j.meegid.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Srinivas RV, Ray RB, Meyer K, Ray R. Hepatitis C virus core protein inhibits human immunodeficiency virus type 1 replication. Virus research. 1996;45:87–92. doi: 10.1016/s0168-1702(96)01361-5. [DOI] [PubMed] [Google Scholar]
- 26.Khan KA, Abbas W, Varin A, Kumar A, Di Martino V, Dichamp I, Herbein G. HIV-1 Nef interacts with HCV Core, recruits TRAF2, TRAF5 and TRAF6, and stimulates HIV-1 replication in macrophages. Journal of innate immunity. 2013;5:639–56. doi: 10.1159/000350517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito M, Baba M, Sato A, Hirabayashi K, Tanabe F, Shigeta S, De Clercq E. Tumor necrosis factor enhances replication of human immunodeficiency virus (HIV) in vitro. Biochemical and biophysical research communications. 1989;158:307–12. doi: 10.1016/s0006-291x(89)80213-x. [DOI] [PubMed] [Google Scholar]
- 28.Mellors JW, Griffith BP, Ortiz MA, Landry ML, Ryan JL. Tumor necrosis factor-alpha/cachectin enhances human immunodeficiency virus type 1 replication in primary macrophages. The Journal of infectious diseases. 1991;163:78–82. doi: 10.1093/infdis/163.1.78. [DOI] [PubMed] [Google Scholar]
- 29.Walsh DG, Horvath CJ, Hansen-Moosa A, MacKey JJ, Sehgal PK, Daniel MD, Desrosiers RC, Ringler DJ. Cytokine influence on simian immunodeficiency virus replication within primary macrophages. TNF-alpha, but not GMCSF, enhances viral replication on a per-cell basis. The American journal of pathology. 1991;139:877–87. [PMC free article] [PubMed] [Google Scholar]
- 30.Dahiya S, Liu Y, Nonnemacher MR, Dampier W, Wigdahl B. CCAAT enhancer binding protein and nuclear factor of activated T cells regulate HIV-1 LTR via a novel conserved downstream site in cells of the monocyte-macrophage lineage. PloS one. 2014;9:e88116. doi: 10.1371/journal.pone.0088116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, Szabo G. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513–24. doi: 10.1053/j.gastro.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta S, Powell E, Kong L, Blackard JT. Effects of HCV on basal and tat-induced HIV LTR activation. PloS one. 2013;8:e64956. doi: 10.1371/journal.pone.0064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swaminathan G, Rossi F, Sierra LJ, Gupta A, Navas-Martin S, Martin-Garcia J. A role for microRNA-155 modulation in the anti-HIV-1 effects of Toll-like receptor 3 stimulation in macrophages. PLoS pathogens. 2012;8:e1002937. doi: 10.1371/journal.ppat.1002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–9. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 35.Vivithanaporn P, Maingat F, Lin LT, Na H, Richardson CD, Agrawal B, Cohen EA, Jhamandas JH, Power C. Hepatitis C virus core protein induces neuroimmune activation and potentiates Human Immunodeficiency Virus-1 neurotoxicity. PloS one. 2010;5:e12856. doi: 10.1371/journal.pone.0012856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung H, Watanabe T, Kudo M, Chiba T. Hepatitis C virus core protein induces homotolerance and cross-tolerance to Toll-like receptor ligands by activation of Toll-like receptor 2. The Journal of infectious diseases. 2010;202:853–61. doi: 10.1086/655812. [DOI] [PubMed] [Google Scholar]
- 37.Duesberg U, von dem Bussche A, Kirschning C, Miyake K, Sauerbruch T, Spengler U. Cell activation by synthetic lipopeptides of the hepatitis C virus (HCV)--core protein is mediated by toll like receptors (TLRs) 2 and 4. Immunology letters. 2002;84:89–95. doi: 10.1016/s0165-2478(02)00178-5. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann M, Zeisel MB, Jilg N, Paranhos-Baccala G, Stoll-Keller F, Wakita T, Hafkemeyer P, Blum HE, Barth H, Henneke P, Baumert TF. Toll-like receptor 2 senses hepatitis C virus core protein but not infectious viral particles. Journal of innate immunity. 2009;1:446–54. doi: 10.1159/000226136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peroval MY, Boyd AC, Young JR, Smith AL. A critical role for MAPK signalling pathways in the transcriptional regulation of toll like receptors. PloS one. 2013;8:e51243. doi: 10.1371/journal.pone.0051243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Q, Hofmann PA. Protein phosphatase 2A-mediated cross-talk between p38 MAPK and ERK in apoptosis of cardiac myocytes. American journal of physiology Heart and circulatory physiology. 2004;286:H2204–12. doi: 10.1152/ajpheart.01050.2003. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Zhong S, Dong Z, Chen N, Bode AM, Ma W, Dong Z. UVA induces Ser381 phosphorylation of p90RSK/MAPKAP-K1 via ERK and JNK pathways. The Journal of biological chemistry. 2001;276:14572–80. doi: 10.1074/jbc.M004615200. [DOI] [PubMed] [Google Scholar]
- 42.Boivin B, Allen BG. p38 MAP kinase attenuates phorbol ester-induced ERK MAP kinase activation in adult cardiac ventricular myocytes. Current Topics in Biochemical Research. 2012;14:57–63. [Google Scholar]
- 43.Poli G, Bressler P, Kinter A, Duh E, Timmer WC, Rabson A, Justement JS, Stanley S, Fauci AS. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. The Journal of experimental medicine. 1990;172:151–8. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tacke RS, Tosello-Trampont A, Nguyen V, Mullins DW, Hahn YS. Extracellular hepatitis C virus core protein activates STAT3 in human monocytes/macrophages/dendritic cells via an IL-6 autocrine pathway. The Journal of biological chemistry. 2011;286:10847–55. doi: 10.1074/jbc.M110.217653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. Journal of leukocyte biology. 2007;82:479–87. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 46.Folks TM, Justement J, Kinter A, Dinarello CA, Fauci AS. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238:800–2. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 47.Emiliani S, Fischle W, Ott M, Van Lint C, Amelia CA, Verdin E. Mutations in the tat gene are responsible for human immunodeficiency virus type 1 postintegration latency in the U1 cell line. Journal of virology. 1998;72:1666–70. doi: 10.1128/jvi.72.2.1666-1670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannon P, Kim SH, Ulich C, Kim S. Analysis of Tat function in human immunodeficiency virus type 1-infected low-level-expression cell lines U1 and ACH-2. Journal of virology. 1994;68:1993–7. doi: 10.1128/jvi.68.3.1993-1997.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Folks TM, Justement J, Kinter A, Schnittman S, Orenstein J, Poli G, Fauci AS. Characterization of a promonocyte clone chronically infected with HIV and inducible by 13-phorbol-12-myristate acetate. Journal of immunology. 1988;140:1117–22. [PubMed] [Google Scholar]
- 50.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–82. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Kim AY, Chung RT. Coinfection with HIV-1 and HCV--a one-two punch. Gastroenterology. 2009;137:795–814. doi: 10.1053/j.gastro.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, Wolinsky SM, Detels R. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. The Journal of infectious diseases. 1999;179:859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 53.Bafica A, Scanga CA, Equils O, Sher A. The induction of Toll-like receptor tolerance enhances rather than suppresses HIV-1 gene expression in transgenic mice. Journal of leukocyte biology. 2004;75:460–6. doi: 10.1189/jlb.0803388. [DOI] [PubMed] [Google Scholar]
- 54.Bafica A, Scanga CA, Schito ML, Hieny S, Sher A. Cutting edge: in vivo induction of integrated HIV-1 expression by mycobacteria is critically dependent on Toll-like receptor 2. Journal of immunology. 2003;171:1123–7. doi: 10.4049/jimmunol.171.3.1123. [DOI] [PubMed] [Google Scholar]
- 55.Chen A, Boulton IC, Pongoski J, Cochrane A, Gray-Owen SD. Induction of HIV-1 long terminal repeat-mediated transcription by Neisseria gonorrhoeae. AIDS (London, England) 2003;17:625–8. doi: 10.1097/00002030-200303070-00019. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Li G, Bafica A, Pantelic M, Zhang P, Broxmeyer H, Liu Y, Wetzler L, He JJ, Chen T. Neisseria gonorrhoeae enhances infection of dendritic cells by HIV type 1. Journal of immunology. 2005;174:7995–8002. doi: 10.4049/jimmunol.174.12.7995. [DOI] [PubMed] [Google Scholar]
- 57.Bafica A, Scanga CA, Schito M, Chaussabel D, Sher A. Influence of coinfecting pathogens on HIV expression: evidence for a role of Toll-like receptors. Journal of immunology. 2004;172:7229–34. doi: 10.4049/jimmunol.172.12.7229. [DOI] [PubMed] [Google Scholar]
- 58.Ogawa Y, Kawamura T, Kimura T, Ito M, Blauvelt A, Shimada S. Gram-positive bacteria enhance HIV-1 susceptibility in Langerhans cells, but not in dendritic cells, via Toll-like receptor activation. Blood. 2009;113:5157–66. doi: 10.1182/blood-2008-10-185728. [DOI] [PubMed] [Google Scholar]
- 59.Thibault S, Tardif MR, Barat C, Tremblay MJ. TLR2 signaling renders quiescent naive and memory CD4+ T cells more susceptible to productive infection with X4 and R5 HIV-type 1. Journal of immunology. 2007;179:4357–66. doi: 10.4049/jimmunol.179.7.4357. [DOI] [PubMed] [Google Scholar]
- 60.Ding J, Rapista A, Teleshova N, Mosoyan G, Jarvis GA, Klotman ME, Chang TL. Neisseria gonorrhoeae enhances HIV-1 infection of primary resting CD4+ T cells through TLR2 activation. Journal of immunology. 2010;184:2814–24. doi: 10.4049/jimmunol.0902125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding J, Chang TL. TLR2 activation enhances HIV nuclear import and infection through T cell activation-independent and -dependent pathways. Journal of immunology. 2012;188:992–1001. doi: 10.4049/jimmunol.1102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thayil SM, Ho YC, Bollinger RC, Blankson JN, Siliciano RF, Karakousis PC, Page KR. Mycobacterium tuberculosis complex enhances susceptibility of CD4 T cells to HIV through a TLR2-mediated pathway. PloS one. 2012;7:e41093. doi: 10.1371/journal.pone.0041093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Novis CL, Archin NM, Buzon MJ, Verdin E, Round JL, Lichterfeld M, Margolis DM, Planelles V, Bosque A. Reactivation of latent HIV-1 in central memory CD4 (+) T cells through TLR-1/2 stimulation. Retrovirology. 2013;10:119. doi: 10.1186/1742-4690-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Victoria S, Temerozo JR, Gobbo L, Pimenta-lnada HK, Bou-Habib DC. Activation of Toll-like receptor 2 increases macrophage resistance to HIV-1 infection. Immunobiology. 2013;218:1529–36. doi: 10.1016/j.imbio.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 65.Wicovsky A, Muller N, Daryab N, Marienfeld R, Kneitz C, Kavuri S, Leverkus M, Baumann B, Wajant H. Sustained JNK activation in response to tumor necrosis factor is mediated by caspases in a cell type-specific manner. The Journal of biological chemistry. 2007;282:2174–83. doi: 10.1074/jbc.M606167200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of HCV core stimulation on MAP kinases phosphorylation in differentiated THP-1 cells. Differentiated THP-1 cells were left untreated, or stimulated with 5.0 μg/ml of HCV core or β-Gal protein for 20 and 60 minutes in the presence or absence of MAP kinases inhibitors 50 μM PD98059 and 10 μM UO126 (ERK1/2), 20 μM SB203580 (p38), and 50 μM SP600125 (JNK). Cell were pre-stimulated with inhibitors for 1 hour before HCV core stimulation. Cell lysates were prepared and immunoblot analysis was performed as described in Methods. Panels A and B show data after 20 minutes of stimulation with HCV core. (A) HCV core stimulates ERK1/2 phosphorylation and this effect is inhibited by its pharmacological inhibition (PD98059 and OU126) and augmented by SB203580, suggesting that p38 is a negative regulator of HCV core-mediated early ERK1/2 phosphorylation. JNK phosphorylation inhibition only slightly reduced ERK1/2 phosphorylation. (B) Effects of HCV core stimulation on p38 and JNK phosphorylation in THP-1 macrophages. Results are representative of two independent experiments with 4 replicates per experiment.