Stable encapsulation and controlled release remain the main challenge in the development of many promising diagnostic and therapeutic modalities.[1,2] Here we describe encapsulation of a model compound in thermosenstive liposomes, which were then coated with gold to form plasmon-resonant shells with optical resonances tunable in the near-infrared range. When exposed to 1064 nm laser light, these liposomes released their content in a spectrally-dependent manner, i.e., liposomes with stronger resonance at the laser wavelength required less energy to facilitate full content release. This spectrally addressable release may find applications in the delivery of diagnostic and therapeutic agents or, more broadly, in developing a technological basis of nanomedicine.
Liposomes have a long history of development as carriers of agents for triggered release and continue to be actively investigated.[3] The metastable character of liposomes allows for applications in which leakage can be attained in response to specific stimuli, including pH,[4,5] enzymatic activity,[6–8] ultrasound,[9] and temperature.[10] The latter type of release is associated with increased permeability of lipid membranes at their phase transition temperatures, e.g., the main phase transition (transition from gel to liquid-crystalline state) in liposomes composed of dipalmitoylphosphatidyl choline at 41.4 °C.[11] However, in clinical applications, local modulation of temperature leading to spatial and temporal control of release is often difficult to achieve.
The driving force behind the extensive studies of photoresponsive liposomes is their potential for precise, on-demand content delivery within individual cells in vitro or, in combination with catheter or endoscopic light delivery, intervention or diagnostic tests in vivo. Research efforts utilizing photochemical processes to facilitate release[12–16] have not yet produced an efficient and fully biocompatible prototype, in part because of the limited selection of photosensitizing dyes in the near-infrared light range that is desirable for biomedical applications. Synthesis of plasmon resonant nanoparticles of gold with broadly tunable optical resonances in the visible to near-infrared range[17] enabled controlled release from a variety of carriers, including gels, polymeric shells and liposomes, via photothermal conversion rather than by a photochemical process.[18–23] Generally, tethered or embedded nanoparticles destabilize the carrier when illuminated with light at resonant wavelengths. These approaches rely on plasmon resonance, a characteristic of metal nanoparticles of certain size, shape and composition. However, these methods have a limited potential in biomedical applications, in part because of a lack of clearance routes for gold particles in the required range of sizes.[24,25]
We recently introduced a new type of composite material, gold-coated liposomes.[26] This material uniquely combines the spectrally tunable optical properties of plasmon resonant coating with the biodegradability afforded by a liposome template. Rather than a continuous metallic shell, this composite nanostructure is formed as a shell-shaped array of discrete gold clusters supported by a spherical metastable core. The diameter of individual gold clusters is in single nanometers[26] and, therefore, in the range potentially clearable by the kidneys. Individually, these clusters did not produce observable plasmon resonances; rather, they produced plasmon resonances only when assembled in the shell-like structure.[26] We have shown that this fundamentally new class of materials maintains optical properties similar to those of solid metallic shells and exhibit unique tunability invoking the Maxwell Garnet theory, whereby the position of plasmon resonance is shifted toward longer wavelengths as the density of the gold particles increases.[26] We now demonstrate that the plasmon resonant coating deposited on the surface of temperature-sensitive liposomes enables light-controlled release of payload through the photothermal conversion of energy of absorbed light (Figure 1).
Figure 1.
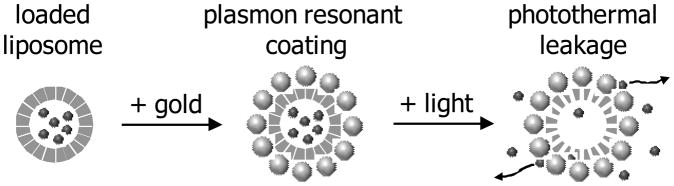
Schematic representation of the formation of plasmon resonant shell and subsequent content release. A water-soluble agent is encapsulated in thermosensitive liposomes. Gold coating is introduced by reduction of ionic gold in the presence of liposomes. The density with which gold clusters are produced on the liposome surface controls the spectral position of resulting plasmon resonance. When illuminated at the wavelength matching plasmon resonance, the energy of absorbed light is converted into heat so the thermosenstive liposomes release their content.
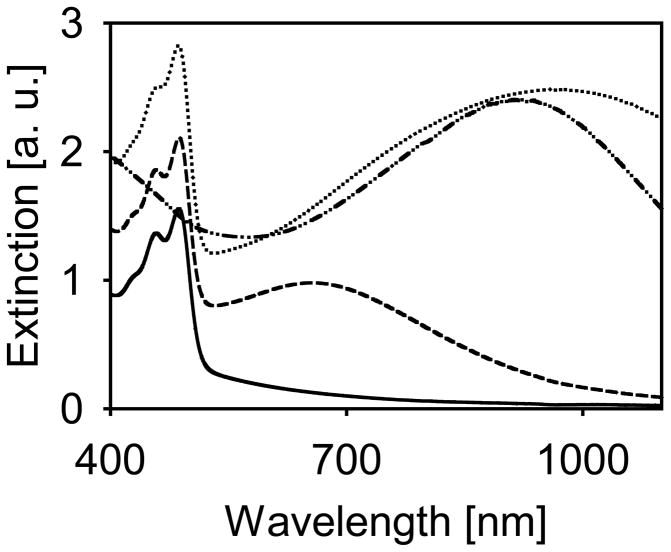
To monitor content release, fluorescein was encapsulated at self-quenching concentrations within the temperature sensitive liposomes. A lipid composition was chosen similar to one used for temperature controlled release.[27] The reduction of gold to the surface of liposomes yielded a final structure of 127 nm diameter, determined by quasi-elastic light scattering, and produced samples of distinct spectral characteristics (Figure 2). Samples with a broad extinction band centered at 971 nm were prepared to closely match the wavelength generated by the Nd:YAG source, 1064 nm, for on-resonance illumination. Samples for off-resonance illumination were prepared with a resonance peak at 655 nm, with minimal extinction at 1064 nm. The presence of fluorescein is evident by the narrow peak at 485 nm. The overall scattering level in samples containing gold was increased due to the increased scattering cross-section caused by plasmon resonance. By the nature of this type of particle, the tuning of the plasmon resonance is dependent on the quantity of gold reduced and, therefore, the extinction peak from the 655 nm sample is weaker than that of the 971 nm sample since suspensions were compared on the basis of equal lipid concentration. Similar to other shell-type structures, these nanoparticles have broad resonance peaks, especially in the infrared.
Figure 2.
Extinction spectra of liposome preparations. Bare liposome suspension loaded with fluorescein (solid), gold-coated liposome suspension resonant at 655 nm loaded with fluorescein (dashed), gold-coated liposome suspension resonant at 971 nm loaded with fluorescein (dotted), gold-coated liposome suspension without fluorescein (dash-dot).
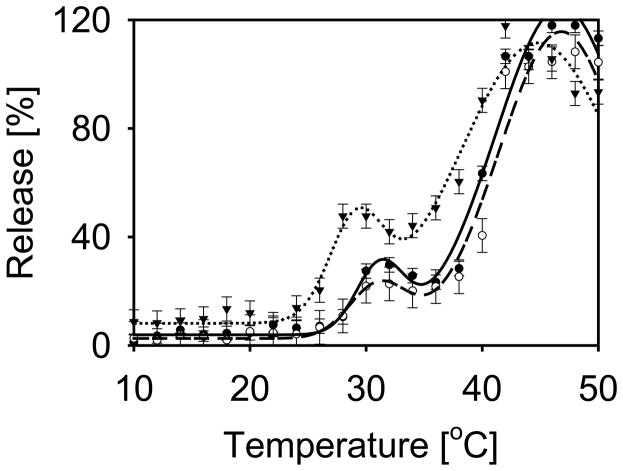
Temperature-induced content release was tested in both gold-coated and bare (prepared without gold coating) thermosensitive liposomes. Each experimental point in the graphs presented in Figure 3 represents an individual measurement obtained by exposing a separate liposome sample to a given temperature for 5 seconds. All liposome compositions tested here release content at two characteristic temperatures. Approximately 24% of content was released from gold-coated liposomes near 31 °C, and complete content release, 100%, was observed near 47 °C. These temperatures generally agree with the known phase transitions of the main lipid component of the liposomes, DPPC: a pretransition at 35.3 °C and the main phase transition at 41.4 °C.[11] This general behavior was also observed in liposomes prepared without gold, although liposomes prepared without gold initiated the release of their contents at slightly lower temperatures, 29 °C and 45 °C. The higher temperature required for the onset of release from gold-coated liposomes was consistent throughout testing.
Figure 3.
Liposome content release induced by temperature. Data and fitted curves are shown for bare liposomes (inverted triangle, dotted), gold-coated liposomes resonant at 655 nm (open circle, dashed), and gold-coated liposomes resonant at 971 nm (closed circle, solid line). The data were modeled with a sum of two Gaussian functions yielding initial release at 29 and 31 °C for bare liposomes and coated liposomes, respectively. The level of initial release was 35, 24, and 19 % from uncoated, on-resonant, and off-resonant liposome suspensions respectively. Complete release was observed at 45 and 47 °C for bare liposomes and coated liposomes, respectively. Error bars represent average of the three standard deviations acquired at 6, 24 and 46 °C.
Content release from plasmon resonant liposomes was obtained by applying a 1064 nm wavelength light beam generated by the Nd:YAG laser (Figure 4, A–C). Each sample was illuminated multiple times and at increasing energies, and cumulative exposure is reported. For gold-coated liposomes having a plasmon resonance peak coincident with the illumination source, release of content by incident light occurs in a manner that resembles a step-wise function, with an energy threshold for release (i.e., energy causing 50% release) of 12 J. Illumination of liposomes with a plasmon resonance peak that is not coincident with the illumination source produced similar release characteristics, though the release threshold required greater cumulative energy, 82 J. The behavior of bare liposomes is different: the energy threshold is not as pronounced as in gold-coated liposomes, and release increases slowly over a broad range of energy, not reaching 100% in the range of energies tested. Photographs of samples obtained in each step of the preparative process and tested in light-induced release are presented in Supplementary Figure 1.
Figure 4.
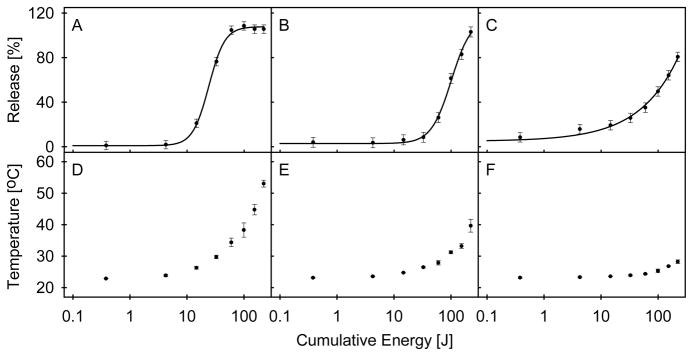
(A–C) Light-induced release of liposome contents. Data and fitted curves are shown for gold-coated liposomes resonant at 971 nm (A), 655 nm (B), and bare liposomes (C). The response of each liposome type to light was modeled with a four-parameter logistic function, with 50 % release observed at 12, 84, and 100 J of cumulative energy for on-resonant, off-resonant and non-coated liposome suspensions respectively. Error bars represent the average of the three standard deviations acquired at cumulative energy levels of 0.38, 14.65, and 221.9 J. (D–F) Peak temperatures recorded during light induced release, for gold-coated liposomes resonant at 971 nm (D), 655 nm (E), and bare liposomes (F) is shown. Error bars represent the standard deviations at cumulative energy levels of three trials.
The temperature of all three liposome preparations increases with increasing energy exposure (Figure 4, D–F). However, the temperature recorded for gold-coated liposomes rose more steeply and reached a higher overall value than bare liposomes, with the steepest increase observed for liposomes with a resonance peak coincident with the illumination source. The sample temperature at the onset of light-induced release for the gold-coated liposomes corresponds to the temperature of the cusp of the first temperature-induced release peak shown in Figure 3 (approximately 28 °C), with rapid release occurring when the temperature approached the first observed phase transition temperature (approximately 31 °C). Bare liposomes demonstrated a similar tendency, with appreciable content leakage occurring at the base of the first phase transition peak (Figure 3).
A means to remotely control the release process is an important step in cellular delivery of therapeutic agents, or, more broadly, in developing a technological basis of nanomedicine. We recently demonstrated that liposome and plasmon resonant structure can be combined into an integrated, biodegradable material, opening the possibility for new multifunctional nanoparticles for medical applications. In this report, we employed a previously developed liposome composition that rapidly released content at temperatures slightly above the physiological range[27] as a template for plasmon resonant structures designed to strongly absorb near-infrared radiation.
We observed that the temperature-based permeability of this composition occurs at two temperatures consistent with the phase behavior of DPPC, which undergoes the pretransition at 35.3 °C and the main phase transition at 41.4 °C, with calorimetric enthalpies of 1.8 and 8.7 kcal/mol, respectively.[11] The lipid composition in this work varies mildly from that of Needham’s hyperthermic release model[27] in that the lipid supporting the PEG chain is slightly shorter; also, the temperature range below 30 °C and the pretransition content release were not characterized in that earlier work. As evident by the elevated temperature of release (by approximately 2 °C) and the decreased release quantity at this pretransition, the stability of gold-coated samples is greater relative to bare liposomes. A possible cause for this increase in stability and release temperature is the linking of multiple lipids in the process of coordinating multivalent gold to the surface of the liposome, as is required to facilitate metal-lipid complex formation.[28,29] This type of coordination requires two positively charged moieties, as found in the outer choline headgroups of DPPC, in order to form a bond with the gold ion.[28]
Full release of contents was achieved when the plasmon resonant liposomes were exposed to the laser light. Interaction of laser pulses with gold-coated liposomes produces measurable thermal changes in the sample volume. For the duration of the laser pulse, tp, thermal energy can diffuse over the thermal confinement zone of diameter d, approximately related as tp = d2/κ, where κ is the thermal diffusivity of water.[30] The 200 μs pulses used in this work result in a thermal confinement zone of approximately 5.1 μm diameter. With an estimated density of one liposome per cubic micron of a typical preparation described here, it follows that the gold-coated liposomes remain in thermal equilibrium with the entire sample volume. It appears that observed content release involves the photothermal conversion of light energy, a process that elevates the sample temperature to the threshold of thermal release. One of the hallmarks of thermosensitive content release from the liposomes is the transient change in permeability at the phase transition temperature, with no overall morphological change to the liposomes. Indeed, as shown in Supplementary Figure 2, there is little change in the position and intensity of plasmon resonance upon illumination with energies required for release, indicating that light-induced release does not produce appreciable changes in liposome morphology.
It can be estimated that light-absorbing spheres of diameter d = 127 nm suspended in water, such as the liposomes tested in this work, would require a pulse duration of tp = 124 ns or shorter to establish conditions of thermal confinement within these particles’ diameter.[30] It appears that 5–10 ns pulses, available from many q-switched lasers, could limit thermal changes to a sphere not larger than the gold-coated liposomes, therefore producing content release with minimal thermal changes outside the liposomes. This type of illumination strategy would minimize the required light dose and the resultant impact of the light source on tissues. It should be noted that the discussed mechanism of thermal release using microsecond and nanosecond pulses is different from that involving illumination using femtosecond pulses. In a system of liposomes with attached plasmon resonant nanoshells, femtosecond laser pulses produced release by pressure fluctuations and cavitations,[22] likely arising from the condition of stress confinement associated with such extremely short pulses.[30]
The significant gap between the energy required for content release from liposomes spectrally matched to the incident laser light and the much higher energy levels needed to cause release from liposomes not matched to the source may allow for spectrally selective release of contents. In an earlier example of thermally-induced release from polymeric nanoparticles, gold nanoparticles deposited on that spherical template produced a broad extinction with no distinct resonances in the visible and near infrared range; this implies that release from those optically addressable capsules could be accomplished using any of several laser sources available in that spectral range, with limited or no dependence on the wavelength of incident light.[19] More recently, polyelectrolyte capsules were functionalized using gold nanorods with different spectral positions of the plasmon resonance and, when illuminated by laser light, released their content in a wavelength-selective manner.[23] The preparation of gold-coated liposomes described in the present work results in pronounced and tunable plasmon resonances, which may, in turn, enable such spectrally addressable content release from the biodegradable liposomal carrier. This spectrally addressable system may allow a sequential release of multiple agents triggered by different wavelengths of light, in particular within the near-infrared, which enables further penetration into tissues with lower levels of phototoxicity than light of shorter wavelengths.
In conclusion, this research reveals a new method for light-induced content release from gold-coated liposomes. This plasmon resonant composite material has already demonstrated its capacity as a contrast agent in optical imaging techniques, including optical coherence tomography.[26] Plasmon resonant particles are often limited in their application by their in vivo retention caused by size or lack of ability to degrade.[24,25] The significant advantages of gold-coated liposomes is their demonstrated capacity to break down into particles that are in the range of sizes compatible with the requirements of renal clearance.[26] To date, this is the only plasmon resonant nanoparticle with demonstrated degradability, enabling the possible translation of this technology to the clinical setting. We now demonstrate that this degradable material can release its content when illuminated with a pulsed laser light matched to its resonance band. This release appears to involve photothermal conversion in the plasmon resonant coating with subsequent leakage from the thermosensitive liposomes (Figure 1), and is accompanied by mild temperature changes. The broad spectral tunability of such plasmon resonant liposomes is unsurpassed by release systems photosensitized by molecular dyes. The technology exists for the targeting of these types of materials to tissues and cells.[31] Such spectrally addressable content release may, therefore, find application in complex diagnostic tests and therapeutic intervention requiring sequential delivery of agents.
Experimental
Liposome Preparation
Dipalmitoylphosphatidylcholine (DPPC), monopalmitoylphosphatidylcholine (MPPC), and dipalmitoylphosphatidylethanolamine-[N-methoxy(polyethylene glycol)-2000] (DPPE-PEG2000) (Avanti Polar Lipids; Alabaster, AL), 90:10:4 ratio by weight, were dispersed in phosphate buffered saline (PBS, 10 mM phosphates, 140 mM NaCl, pH 7.4) containing fluorescein (5 mM) to achieve a 60 mM lipid concentration. Liposomes were prepared by the standard method of freeze-thaw cycles followed by extrusion through 100 nm polycarbonate membranes, described in detail earlier.[32] The preparation was dialyzed against PBS to remove excess fluorescein. The liposome diameter was determined by quasi-elastic light scattering, and reported based on intensity-weighted distribution (Malvern Zetasizer). Liposome preparations were stored at 4 °C.
Gold coating
Similar to the technique previously reported,[26] an aqueous solution of gold chloride (100 mM) and an aqueous solution of ascorbic acid (500 mM) were prepared and added to a liposome preparation diluted with PBS (1 mL, 20 mM lipids). For resonance wavelengths matched to Nd:YAG (1064 nm), gold chloride solution (18 μL, 100 mM) was added, followed with the ascorbic acid solution (27 μL, 500 mM) and gentle swirling until characteristic color developed. Samples for the off-resonance illumination characterization were prepared using lower quantities of gold chloride solution (7 μL, 100 mM) and of ascorbic acid solution (10.5 μL, 500 mM) to yield a resonance peak at 655 nm. Each sample was then dialyzed against PBS. All samples were prepared and characterized with equal quantities of lipids in solution and thereby, presumably, an equal number of liposomes per unit volume. The extinction spectra were taken with a Cary 5 spectrophotometer on double beam mode against PBS. Samples were diluted to 5 mM lipids in order to match the spectrophotometric range of the instrument.
Temperature-induced content release
The liposome suspension was maintained at 4 °C. A PBS solution was stirred and its temperature gradually increased from 4 °C to 50 °C. Upon reaching the temperature for measurement, PBS (1.97 mL) was transferred to a 1 cm (square) pathlength cuvette containing the tested liposome solution (30 μL, 20 mM lipids) to produce a rapid change in the liposome temperature. The concentrations of lipids in the tested sample were consistent (0.3 mM). The mixture was incubated for 5 seconds before placing it in the fluorescence spectrometer. The cuvette holder in the fluorescence spectrometer was maintained at 4 °C to inhibit further release. The excitation wavelength was 488 nm and spectral widths were 1 nm for both excitation and emission. Fluorescence emission spectra were collected over the range of 505 to 560 nm, and the maximum value for each characterized temperature was recorded. The percent release was determined as %release = (I − I0)/(IT − I0), where I is the intensity of fluorescence emission (generally near 512 nm) for an individually measured sample, I0 is the fluorescence intensity for an untreated or minimum temperature sample, and IT is the fluorescence intensity accompanying complete release, obtained by adding Triton X-100.
Light-induced content release
A quantity of liposome suspension (0.5 mL, 20 mM lipid concentration) was retained in a 1 cm square pathlength cuvette maintained at 24 °C, the highest temperature observed to not cause measurable release from the liposome compartments. Light from the Nd:YAG laser, 1064 nm (Equilasers, Inc.; Santa Clara, CA) was directed toward the sample with a fiber optic cable terminating 1.6 cm above the sample, with a measured half-angle of beam divergence of 7°. Samples were illuminated for 10 seconds, with 200 μs pulse widths at 25 Hz, and subjected to increasing energies, ranging from 1.5 to 275 mJ/pulse as determined with a Molectron PM30-VI thermal sensor and PM5200 laser power meter (Coherent; Santa Clara, CA). For subsequent fluorescence measurements, a portion of the irradiated solution (30 μL, 20 mM lipids) was collected and added to PBS (1.97 mL) to dilute the sample (0.3 mM lipids) for measurement. All fluorescence measurements were initiated within 30 seconds of illumination. Sample temperature was monitored using a K-type wire thermocouple with a 4-channel thermometer (Sper Scientific; Scottsdale, AZ). The peak temperatures accompanying each successive illumination event were recorded.
Supplementary Material
Acknowledgments
This work was supported by Grant K25CA120350 (MR) from the National Cancer Institute of the National Institutes of Health. Supporting Information is available online from Wiley InterScience or from the author.
References
- 1.Langer R, Tirrel DA. Nature. 2004;428:487. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 2.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Nature Rev. 2007;6:443. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen TM, Cullis PR. Science. 2004;303:1818. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 4.Yatvin MB, Kreutz W, Horwitz BA, Shinitzky M. Science. 1980;210:1253. doi: 10.1126/science.7434025. [DOI] [PubMed] [Google Scholar]
- 5.Hafez IM, Cullis PR. Methods Enzymol. 2004;387:113. doi: 10.1016/S0076-6879(04)87007-1. [DOI] [PubMed] [Google Scholar]
- 6.Meers P. Adv Drug Deliv Rev. 2001;53:265. doi: 10.1016/s0169-409x(01)00205-8. [DOI] [PubMed] [Google Scholar]
- 7.Pak CC, Ali S, Janoff AS, Meers P. Biochem Biophys Acta. 1998;1372:13. doi: 10.1016/s0005-2736(98)00041-8. [DOI] [PubMed] [Google Scholar]
- 8.Davidsen J, Vermehren C, Frokjaer S, Mouritsen OG, Jorgensen K. Int J Pharm. 2001;214:67. doi: 10.1016/s0378-5173(00)00634-7. [DOI] [PubMed] [Google Scholar]
- 9.Huang S, MacDonald RC. Biochim Biophys Acta. 2004;1665:134. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Science. 1978;202:1290. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 11.Mabrey S, Sturtevant JM. Proc Natl Acad Sci USA. 1976;73:3862. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kano K, Tanaka Y, Ogmura M, Okahata Y, Kunitake T. Chem Lett. 1980:421. [Google Scholar]
- 13.Bisby RH, Mead C, Morgan CG. Biochem Biophys Res Comm. 2000;276:169. doi: 10.1006/bbrc.2000.3456. [DOI] [PubMed] [Google Scholar]
- 14.Thompson DH, Gerasimov OV, Wheeler JJ, Rui Y, Anderson VC. Biochim Biophys Acta. 1996;1279:25. doi: 10.1016/0005-2736(95)00210-3. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien DF, Armitage B, Benedicto A, Bennett D, Lamparski HG, Lee YS, Srisiri W, Sisson TM. Acct Chem Res. 1998;31:861. [Google Scholar]
- 16.Bondurant B, Mueller A, O’Brien DF. Biochim Biophys Acta. 2001;1511:113. doi: 10.1016/s0005-2736(00)00388-6. [DOI] [PubMed] [Google Scholar]
- 17.Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ. Chem Phys Lett. 1998;288:243. [Google Scholar]
- 18.Sershen SR, Westcott SL, Halas NJ, West JL. Biomed Mat Res. 2000;51:293. doi: 10.1002/1097-4636(20000905)51:3<293::aid-jbm1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Radt B, Smith TA, Caruso F. Adv Mater. 2004;16:2184. [Google Scholar]
- 20.Skirtach AG, Dejugnat C, Braun D, Susha AS, Rogach AL, Parak WJ, Mohwald H, Sukhorov GB. Nano Letters. 2005;5:1371. doi: 10.1021/nl050693n. [DOI] [PubMed] [Google Scholar]
- 21.Paasonen L, Laaksonen T, Johans C, Yliperttula M, Kontturi K, Urtti A. J Control Release. 2007;122:86. doi: 10.1016/j.jconrel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Wu G, Mikhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. J Am Chem Soc. 2008;130:8175. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skirtach AG, Karageorgiev P, De Geest BG, Pazos-Perez N, Braun D, Sukhorukov GB. Adv Mater. 2008;20:506. [Google Scholar]
- 24.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Nature Biotech. 2007;25:1165. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James WD, Hirsch LR, West JL, O’Neal PD, Payne JD. J Radioanal Nuclear Chem. 2007;271:455. [Google Scholar]
- 26.Troutman TS, Barton JK, Romanowski M. Adv Mater. 2008;20:2604. doi: 10.1002/adma.200703026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. Cancer Research. 2000;60:1197. [PubMed] [Google Scholar]
- 28.Warshawsky A, Upson DA. J Polymer Sci Part B: Polymer Phys. 1989;27:2963. [Google Scholar]
- 29.Ferrar WT, O’Brien DF, Warshawsky A, Voycheck CL. J Am Chem Soc. 1988;110:288. [Google Scholar]
- 30.Jacques SL. Appl Optics. 1993;32:2447. doi: 10.1364/AO.32.002447. [DOI] [PubMed] [Google Scholar]
- 31.Allen TM. Nature Rev. 2002;2:750. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 32.Romanowski M, Zhu X, Kim K, Hruby VJ, O’Brien DF. Biochim Biophys Acta. 2002;1558:45. doi: 10.1016/s0005-2736(01)00421-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.