Abstract
Reduced P3 amplitude to targets is an information-processing deficit associated with adult antisocial behavior and may reflect dysfunction of the temporal-parietal junction. This study aims to examine whether this deficit precedes criminal offending. From a birth cohort of 1,795 children, 73 individuals who become criminal offenders at age 23 and 123 noncriminal individuals were assessed on P3 amplitude. The two groups did not differ on gender, ethnicity, and social adversity. P3 amplitude was measured over the temporal-parietal junction during a visual continuous performance task at age 11, together with antisocial behavior. Criminal convictions were assessed at age 23. Reduced P3 amplitude at age 11 was associated with increased antisocial behavior at age 11. Criminal offenders showed significantly reduced P3 amplitudes to target stimuli compared to controls. Findings remained significant after controlling for antisocial behavior and hyperactivity at age 11 and alcoholism at age 23. P3 deficits at age 11 are associated with adult crime at age 23, suggesting that reduced P3 may be an early neurobiological marker for cognitive and affective processes subserved by the temporal-parietal junction that place a child at risk for adult crime.
The P3 (or P300) component of the event-related potential is a positive brainwave deflection evoked by infrequent, task-relevant events in a stimulus sequence.
The amplitude of P3 is considered an index of the allocation of neural resources and cognitive processing capability, with increasing amplitude reflecting enhanced processing capability. The latency of the P3 is thought to reflect stimulus evaluation time, with shorter latency indicating superior cognitive performance in allocating attentional resources for memory processing (Polich, 2003). As such, reduced P3 amplitude broadly reflects relatively poorer attentional processing of the target stimulus. P3 impairments have been associated with antisocial and psychopathic behavior in adolescents and adults (Bernat, Hall, Steffen, & Patrick, 2007; Costa et al., 2000; Gao & Raine, 2009; Kiehl, Hare, Liddle, & McDonald, 1999; Munro et al., 2007), and child conduct disorder (Bauer & Hesselbrock, 1999a, 1999b; Kim, Kim, & Kwon, 2001). Specifically, a recent meta-analysis of 38 studies has shown that antisocial individuals have reduced P3 amplitudes and longer P3 latencies to target stimuli (Gao & Raine, 2009). It was postulated that inefficient neural processing of salient environmental stimuli, as indicated by reduced P3 amplitude, may predispose a subgroup of individuals to antisocial and criminal behavior (Gao & Raine, 2009).
At the neuroanatomical level, emerging evidence from magnetoencephalography, functional magnetic resonance imaging, and lesion studies has indicated that the temporal-parietal junction may be critically involved in the generation of visual P3 to target stimuli (Iwaki, Sutani, Kou, & Tonoike, 2007; Kiehl, Laurens, Duty, Forster, & Liddle, 2001; Polich & Criado, 2006). The temporal-parietal junction has been implicated in a broad range of social cognition tasks and in representation of the mental states of others (Saxe & Kanwisher, 2003). The ability to represent the mental states of others has in turn been considered to be necessary for emotional empathy to occur (Batson, Fultz, & Schoenrade, 1987). Brain imaging studies have indicated that the key areas of the temporal-parietal junction (angular gyrus and the posterior superior temporal gyrus) are involved in moral decision making and empathy (Decety & Lamm, 2007; Moll, de Oliveira-Souza, & Eslinger, 2003; Raine & Yang, 2006), and a recent study has also suggested that temporal-parietal junction is involved in inhibition (Hedden & Gabrieli, 2010). In this context, antisocial and psychopathic behavior is associated with reduced empathy (Miller & Eisenberg, 1988) and, almost by definition, with disinhibition and immoral rule-breaking behavior (Blair, 1995). Consequently, reduced P3 amplitude may reflect dysfunction of the temporal-parietal junction in psychopathic and antisocial individuals. This could account for impaired development of morality, empathy, and behavioral inhibition, which in turn predisposes to adult offending. Reduced P3 amplitude relatively early in life may therefore increase the risk for criminal offending in adulthood.
Despite the relatively consistent evidence for a link between P3 impairments and antisocial behavior, prior work has almost exclusively utilized cross-sectional designs in late adolescence or adulthood. Because the brain is actively changing and developing during adolescence (Giedd et al., 1999), it is unclear whether the differences in P3 measures that confer increased risk for criminal behavior can be detected at an early age. Therefore the primary goal of the present study was to examine if reduced P3 amplitude early in life is associated with adult criminality. To the authors’ knowledge, only one study has prospectively investigated the relationship between P3 and criminality (Raine, Venables, & Williams, 1990). Raine et al. (1990) reported that P3 latency at age 15 years to be associated with criminal status at age 24 in a community sample of male subjects, but the direction of this effect (shorter latency in antisocial individuals) is inconsistent with findings in antisocial adults of longer P3 latencies (Gao & Raine, 2009). P3 latency has been interpreted as reflecting stimulus evaluation time and the time required to evaluate and categorize an event (Donchin & Coles, 1988). It was therefore proposed that the relationship between P3 latency and criminality indicates enhanced processing speed in criminals to task-relevant events (Raine et al., 1990).
However, two limitations of the study should be noted. First, the sample size was low (n = 17), thus resulting in instability and low power in estimates of the magnitude and direction of effects. Second, this study did not control for the potential confounding effect of alcohol use. Iacono, Carlson, Malone, and McGue (2002) found that reduced P3 amplitude in a visual oddball task predicted the development of substance use disorders at age 20 in a community sample of male youth. In fact, it has been proposed that P3 amplitude reduction is an index of genetic vulnerability for a broad spectrum of disinhibited psychiatric disorders, including childhood disruptive disorders, antisocial behavior, substance use disorders, and personality traits related to behavioral undercontrol (Iacono et al., 2002; Iacono, Malone, & McGue, 2003). Therefore, the secondary goal of the current study was to examine if the P3-criminality association remains significant after alcohol use is controlled for.
To address this research gap, the current study examines relationships between P3 measured at age 11 years and both antisocial behavior at age 11 and criminal offending at age 23. P3 amplitude and latency were measured over the temporal-parietal junction during a visual continuous performance task (CPT) at age 11. CPT is one of the most frequently used measures of sustained attention in both practice and research (Riccio, Reynolds, Lowe, & Moore, 2002). Involvement of the interaction of cortical (frontal, temporal, parietal), sub-cortical (limbic, basal ganglia), and functional systems including the pathways between the basal ganglia, thalamus, and frontal lobes has been suggested by studies (Riccio et al., 2002). Utilizing both cross-sectional and prospective approaches, we hypothesized that (a) reduced P3 amplitude at age 11 would be associated with increased antisocial behavior at age 11, and (b) adult criminal offenders would show reduced P3 amplitude at age 11. We also explored if the association between age 11 P3 amplitude and age 23 crime would remain significant after controlling for age 23 alcohol use, and age 11 antisocial behavior and hyperactivity, given high comorbidity between antisocial behavior and hyperactivity (Biederman, Newcorn, & Sprich, 1991). We did not hypothesize an effect on P3 latency, given the conflicting results of previous research (Gao & Raine, 2009; Raine et al., 1990). Finally, although some evidence suggests that psychopathic criminals exhibit reduced P3 asymmetry in response to visual stimuli (Kiehl et al., 1999), recent theorizing has not produced substantive lateralization theory of criminal behavior. Therefore the effect of hemisphere was examined but no prediction was made in the current study.
METHODS
Participants
Participants consisted of 73 criminals and 123 noncriminals (see next), who were among a birth cohort of 1,795 children from Mauritius, a tropical island in the Indian Ocean (Raine, Liu, Venables, Mednick, & Dalais, 2010). All children born in 1969 and 1970 in two towns (Quatre Bornes and Vacoas) were recruited at age 3 years (45.9% were female) on the basis of vaccination records. The ethnic makeup was as follows: 68.7% Indian, 25.6% African, and 5.6% other (Chinese, English, or French descent; Venables, 1978). A social adversity index was formed based on nine variables collected by social workers who visited the children's homes at age 3 years (Raine et al., 2001). One point was scored for each of the following variables: father uneducated (30%), mother uneducated (29.4%), semiskilled or unskilled occupation (55.5%), teenage mother (14.2%), single-parent status (2.1%), separation from parents (0.9%), large family size (30.0%), poor health of mother (3.3%), and overcrowded home (28.8%). Higher scores indicate higher adversity. Written informed consent and assent were obtained from parents and children.
Criminal Offending and Alcoholism at Age 23
Official court records for registration of offenses including property, drug, violence, and serious driving offenses were searched when the participants were aged 23 years. Seventy-three criminals and 123 noncriminal controls who had complete P3 and antisocial behavior data at age 11 were included in the current study. The two groups did not differ on gender, χ2(1) < 1, p = .84, ethnicity (p = .89), and social adversity, t = (194) = 1.30, p = .19. Demographic characteristics of the criminals and controls are listed in Table 1. A history of head injury (involving loss of consciousness) was also taken; criminals and controls did not differ on this measure, χ2(1) = 0.016, p = .90 Michigan. Alcoholism was assessed using the Alcoholism Screening Test (MAST), a commonly used screening instrument for alcoholism (Selzer, 1971). The use of MAST in this Mauritius sample has been validated in prior research (Luczak, Raine, & Venables, 2001).
TABLE 1.
Demographic Characteristics of Criminal Offenders and Comparison Subjects
| Criminalsa |
Controlsb |
||||
|---|---|---|---|---|---|
| Characteristics | M | SD | M | SD | p |
| Social Adversity Index | 1.63 | 1.49 | 1.96 | 1.39 | .12 |
| N | % | N | % | ||
| Male | 71 | 97.3 | 119 | 96.7 | 1.0c |
| Ethnicity | .90d | ||||
| Hindu | 28 | 38.4 | 37 | 30.1 | |
| Creole | 21 | 28.8 | 44 | 40.8 | |
| Moslem | 12 | 16.4 | 21 | 17.1 | |
| Tamil | 7 | 9.6 | 12 | 9.8 | |
| Chinese | 1 | 1.4 | 2 | 1.6 | |
| Other | 4 | 5.5 | 7 | 5.7 | |
Note.
n = 73.
n = 123.
Chi-square comparison.
Fisher's Exact Test.
Antisocial Behavior and Hyperactivity at Age 11
The Child Behavior Checklist (CBCL; Achenbach & Edelbrock, 1983) was completed by the parents when the children were aged 11 years. Aggression, Non-Aggressive Antisociality, and Total Antisociality scales were derived from the original CBCL Aggression and Delinquency subscales because (a) the original scales for male and female participants are somewhat different, and (b) the CBCL “Aggression” scale contains many items with no aggression component, whereas the “Delinquency” scale contains aggression items (Raine, Venables, & Mednick, 1997). As indicated by Achenbach, Conners, Quay, Verhulst, and Howell (1989), it is desirable to have common-item scales for both male and female individuals. Although such scales have been developed for Western cultures (Achenbach et al., 1989), none have been derived for Mauritius. Revised for the Mauritius population, these new scales have items common to both sexes and are more homogenous measures of aggression and nonaggressive antisocial behavior (see full detail in Raine, Venables, et al., 1997). Initial construct validity data for these scales indicate that they relate to measures of temperament and body size in theoretically expected directions, with specificity of larger body size and a more disinhibited temperament to aggressive behavior per se (Raine, Reynolds, Venables, Mednick, & Farrington, 1998). Brief details together with reliability data follow.
Aggression scale (10 items)
This scale consisted of items measuring both physical and verbal aggression (e.g., “Fights,” “Cruel,” “Swearing,” “Threatens”), which were common to both male and female participants. Coefficient alpha was .72 for male and .72 for female participants.
Non-aggressive antisociality scale (10 items)
This scale consisted of items related to antisocial, delinquent behavior that were not aggressive in nature (e.g., “Disobedient,” “Keeps Bad Friends,” “Lies,” “Cheats”) and that were common to both male and female individuals. Coefficient alpha was .64 for male and .68 for female participants.
Total antisociality scale (20 items)
This scale comprised the addition of the 10 Aggression items with the 10 Non-Aggressive Antisociality items. Coefficient alpha was .81 for male and .83 for female participants. Aggressive and nonaggressive antisocial scores were highly correlated (r = .73), therefore a total antisociality score was used in the following analyses. Participants were also split into high (M antisociality score = 14.57, SD = 4.90, range 9–31) and low antisocial groups (M antisociality score = 4.34, SD = 2.48, range via = 0–8) via median split.
Finally, Hyperactivity consisted of the nine items from the Achenbach Hyperactivity scale that were common to both male and female participants (e.g., “Hyperactive,” “Poor Concentration,” “Impulsive”). Coefficient alpha was .57 for male and .56 for female participants.
Psychophysiological Assessment at Age 11
CPT (Raine et al., 2001)
Stimuli for the visual CPT consisted of the numerals 1 through 9 presented via a 22.5 × 33 mm red, seven-segment LED display unit (RS Components No. 586–807) situated one meter in front of the subject. The display unit was driven by an electronic control system programmed from a random access memory and designed to output trigger pulses (1 volt, 50 ms) 200 ms prior to stimulus presentation.
There were 250 trials. The number 5 was designated as the target and was presented 57 times. The remaining numerals were designated as nontargets and were presented 193 times. The probability of a target occurring was .23. Stimulus presentation was pseudo randomized with the constraint that no more than three consecutivetar-gets could occur. Interstimulus interval was 1.5 s with a stimulus display time of 100 ms. The task lasted 6.25 min.
Participants were told that numbers from 1 to 9 would be presented on the display unit and that their task was to press a response button (held in the preferred hand) as quickly as possible as soon as they saw the figure 5. It was stressed that only the number 5 should be responded to and that all other numbers should be ignored. Before the full test procedure, 20 practice trials were given to ensure that the participant understood the task.
P3 recording and data quantification
Event-related potentials were recorded during the CPT. Electroencephalographic (EEG) data were recorded using Beckman silver-silver chloride disc electrodes from bipolar leads T3-P3 and T4-P4 of the International 10–20 system (Jasper, 1958) with Fz as ground (Raine et al., 2001). Bipolar leads at more posterior sites were selected to minimize eye movement and other artifacts. Electrodes were filled with Cambridge electrode jelly (Camjel) and attached using collodion glue. The scalp was cleaned with acetone and abraded to keep all resistances below 5 Kohms.
Recordings were made on a Grass Model 79 Poly-graph using wide-band AC Grass 7P5 preamplifiers and 7DA DC driver amplifiers with a band pass of 0.3–100 Hz (–6 dB). Electrooculography (EOG) was recorded with a Grass Model 7DA driver amplifier and a 7P1 DC amplifier, with bipolar electrodes placed on the supra- and infraorbital ridge of the left eye. For the CPT, the longest time constant available on the amplifier (0.92 s) was used. EEG was amplified using a gain of 10,000 with a sampling rate of 512 Hz. A calibration sequence of 10 microvolt square-wave pulses were fed into each amplifier for calibration. All data were recorded on a Racal Store 4 FM tape recorder for off-line analysis.
After deletion of epochs containing EOG artifacts, EEG was sampled 200 ms prestimulus to 800 msec post stimulus, and the signal was adjusted to a 0 μV prestimulus baseline. Targets and nontargets were averaged separately, with digital filtering performed off-line using a 0.1–100 Hz band-pass filter. P3 peak amplitude (μV) and latency was identified as the maximum positive peak at each electrode between 300 and 800 msec poststimulus.
Statistical Analyses
For the behavioral data (response time in millisecond and number of correct responses) independent samples t tests were conducted. Repeated measures analysis of variance (ANOVA) with hemisphere (left, right) as the within-subject factor and criminal group as the between-subjects factor were conducted on P3 responses to targets and nontargets. Analysis of covariance (ANCOVA) was then conducted with the same within-and between-subjects factors and with age 11 antisocial behavior and hyperactivity and age 23 alcohol use as covariates. Similar t tests and ANOVA analyses with age 11 antisocial group as the between-subjects factor were also conducted. ANCOVA was conducted with age 11 hyperactivity as a covariate. Independent or paired sample t tests were conducted to break down interaction effects. Cohen's d (Cohen, 1988) and partial eta-square were reported for effect sizes.
RESULTS
Descriptive Statistics
Means, standard deviations, and correlations between variables are shown in Table 2. Left P3 amplitudes to both targets and nontargets were significantly and negatively correlated with the antisocial behavior score; increased antisocial behavior was associated with reduced P3 amplitudes. There was a trend that antisocial behavior was negatively associated with right P3 amplitude to nontargets (p = .08). P3 latencies were uncorrelated with antisocial behavior. Hyperactivity was associated only with right P3 latency to nontargets (r = .207, p = .005).
TABLE 2.
Means, Standard Deviations, and Correlations Between Study Variables
| P3 Amplitude |
P3 Latency |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AB. | MAST | Left, nT | Left, T | Right, nT | Right, T | Left, nT | Left, T | Right, nT | Right, T | ||
| MAST | .073 | 1 | |||||||||
| P3 Amplitude | Left, nT | –.166* | –.031 | 1 | |||||||
| Left, T | –.176* | .018 | .814*** | 1 | |||||||
| Right, nT | –.138† | –.076 | .769*** | .668*** | 1 | ||||||
| Right, T | –.100 | –.055 | .665*** | .744*** | .857*** | 1 | |||||
| P3 Latency | Left, nT | .024 | –.033 | .085 | .079 | .114 | .114 | 1 | |||
| Left, T | .041 | –.098 | –.038 | .017 | –.083 | –.052 | .257*** | 1 | |||
| Right, nT | .105 | –.075 | .017 | –.009 | .119† | .080 | .509*** | .037 | 1 | ||
| Right, T | –.087 | –.049 | –.049 | –.007 | –.004 | .064 | .150* | .315*** | .169* | 1 | |
| M | 10.275 | 2.367 | 5.153 | 6.273 | 4.955 | 6.462 | 0.467 | 0.608 | 0.435 | 0.552 | |
| SD | 6.532 | 3.592 | 2.810 | 3.344 | 2.583 | 3.155 | 0.088 | 0.080 | 0.088 | 0.094 | |
Note: AB. = antisocial behavior (age 11); MAST = Michigan Alcoholism Screening Test; nT = nontarget stimuli; T = target stimuli.
p < .10.
p < .05.
p < .001.
Criminals and controls did not differ on age 11 antisocial behavior, t(194) = 0.490, p > .05 (criminals M = 9.94, SD = 6.33; controls M = 10.43, SD = 6.61, d = 0.08); hyperactivity, t(194) = 1.349, p > .05 (criminals M = 4.07, SD = 2.82; controls M = 4.67, SD = 2.97, d = 0.21); and age 23 alcohol use, t(194) = 0.500, p > .05 (criminals M = 2.55, SD = 3.62; controls M = 2.25, SD = 3.59, d = 0.08).
Behavioral Performance on the CPT and Age 23
Criminal Offending
Criminals and controls did not differ on response time, t(194) = 0.457, p > .05 (criminals M = 368.3, SD = 79.1; controls M = 363.3, SD = 66.9, d = 0.07), or number of correct responses, t(194) = 0.710, p > .05 (criminals M = 55.1, SD = 3.1; controls M = 54.6, SD = 5.2, d = 0.12).
P3 at Age 11 and Criminal Offending at Age 23
P3 amplitude
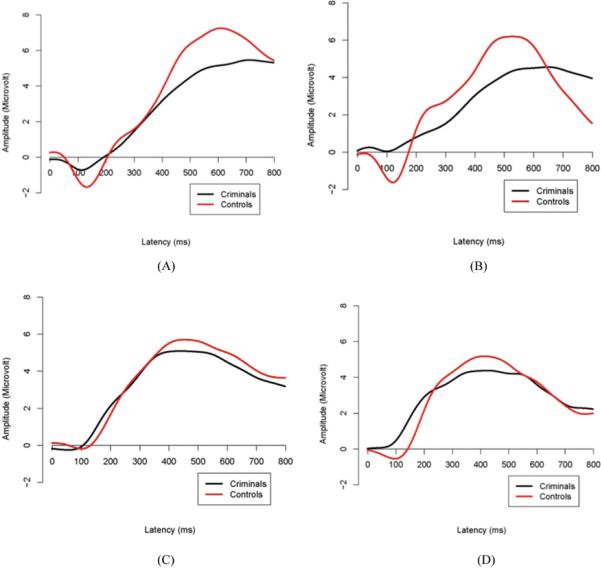
For targets, the criminal offenders had significantly smaller P3 amplitudes than the controls, F(1, 194) = 4.64, p = .03 (criminals M = 5.77, SD = 2.75; controls M = 6.72, SD = 3.15, d = 0.32; see Figure 1). No significant main effect of hemisphere or Group × Hemisphere interaction was found, F(1, 194) < .92, p > .05, η2 < .005. For nontargets, no significant main effects or interactions were observed, F(1, 194) < 2.64, p > .05, η2 < .013.
FIGURE 1.
Grand mean waveforms for the criminals and controls in each of the two stimulus types at each hemisphere. (A) Targets, Left Hemisphere; (B) Targets, Right Hemisphere; (C) Non-Targets, Left Hemisphere; (D) Non-Targets, Right Hemisphere. (Figure appears in color online.)
The aforementioned group effects for P3 amplitudes remained significant after age 11 antisocial behavior, hyperactivity, and age 23 alcoholism were controlled for. For targets, the main effect of criminal group remained significant, F(1, 191) = 3.87, p = .048, η2 = 0.028, with no significant hemisphere or Group × Hemisphere interactions (all p > .05, η2 < 0.018). For non-targets, no significant main or interaction effect was found (all p > .05, η2 < 0.012).. Furthermore, to further control for the effect of substance use on the aforementioned association, analyses were repeated after excluding eight criminals with substance-related offenses. The main effect of criminal group remained significant, F(1, 184) = 4.00, p = .047, η2 = 0.029.
P3 latency
No significant main effect of group or Group Hemisphere interaction was found for targets or nontargets, F(1, 194) < 1.63, p > .05, η2 < 0.008. However, participants showed shorter latencies in the right hemisphere than in the left hemisphere for both stimuli, F(1, 194) > 25.88, p < .001, η2 = 0.223 (for targets, right hemisphere M = 751.6, SD = 94.5; left hemisphere M = 808.1, SD = 79.7, d = 0.65; for nontargets, right hemisphere M = 635.3, SD = 88.5; left hemisphere M = 666.8, SD = 87.5, d = 0.36).
Behavioral Performance on the CPT and Age 11 Antisocial Behavior
High and low antisocial groups did not differ on response time, t(194) = 1.25, p > .05 (high antisocial group M = 573.8, SD = 80.8; low antisocial group M = 559.5, SD = 69.1, d = 0.19), or number of correct responses, t(194) = 0.93, p > .05 (high antisocial group M = 55.1, SD = 2.9, low antisocial group M = 54.5, SD = 5.4, d = 0.14).
P3 at Age 11 and Antisocial Behavior at Age 11
P3 amplitude
For targets, the high antisocial group had significantly smaller P3 amplitude than the low antisocial group, F(1, 194) = 14.13, p < .001, η2 = 0.073 (high antisocial group M = 5.664, SD = 2.60; low antisocial group M = 7.286, SD = 3.21, d = 0.56). No significant main effect of hemisphere or Group × Hemisphere interaction was found, F(1, 194) < .81, p > .05, η2 < 0.005. For nontargets, the high antisocial group showed significantly smaller P3 amplitude than the low group, F(1, 194) = 11.51, p = .001, η2 = 0.060 (high antisocial group M = 4.542, SD = 2.26; low antisocial group M = 5.748, SD = 2.51, d = 0.50). P3 amplitude on the left hemisphere (M = 5.181, SD = 2.76) was larger than that on the right hemisphere (M = 4.923, SD = 2.45, d = 0.10). The Group × Hemisphere interaction was not significant, F(1, 194) = 2.89, p = .09, η2 = 0.016. The aforementioned group effects for P3 amplitudes remained significant after age 11 hyperactivity was controlled for, F(1, 194) > 12.86, p < .001, η2 > 0.067.
P3 latency
No significant main effect of group or Group × Hemisphere interaction was found for targets or nontargets, F(1, 194) < 2.45, p > .05, η2 < 0.013. Participants showed shorter latencies in the right hemisphere than in the left hemisphere for both stimuli, F(1, 194) > 30.6, p < .001, η2 > 0.145 (for targets, right hemisphere M = 749.8, SD = 95.7; left hemisphere M = 806.5, SD = 80.8, d = 0.64; for nontargets, right hemisphere M = 634.6, SD = 88.0; left hemisphere M = 669.0, SD = 87.8, d = 0.39).
DISCUSSION
The key finding from this study is that reduced P3 amplitude at age 11 years was associated with criminal offending at age 23 years. This association was not explained by social adversity, ethnicity, gender, or history of head injury and remained significant after controlling for age 11 antisocial behavior and hyperactivity, or age 23 alcoholism. Reduced P3 at age 11 was also associated with antisocial behavior at age 11. Findings are the first to associate reduced evoked potential amplitudes in childhood to adult criminal behavior. Results suggest that reduced P3 amplitude in childhood reflects a process that increases the risk for the later development of criminal behavior and may reflect dysfunction of the temporal-parietal junction.
Consistent with our hypotheses, P3 reduction was associated with both antisocial behavior concurrently at age 11 and criminality at age 23. Although it is unknown if P3 is a marker associated with antisocial behavior or it precedes the development of antisocial behavior, Iacono et al. (2002)'s finding that reduced P3 amplitude at age 17 prospectively predicted the development of externalizing problems at age 20, even among individuals who did not evidence any externalizing problems at the time of P3 assessment supports the proposition that P3 decrements precede the onset of significant antisocial behavior. As previously mentioned, P3 to target stimuli is related to a number of cognitive processes including attention, context-updating operations, and subsequent memory processing (Polich, 2007). Attenuated P3 amplitude in criminals-to-be may reflect an inability or deficiency in sustaining attention or appropriately allocating attentional resources to task-relevant stimuli. This impairment could interfere with the children's abilities to cope with high situational demands, which in turn predispose them to school failure, occupational failure, and ultimately criminal offending.
An alternative perspective is that reduced P3 amplitude reflects an abnormality in other cognitive processes that are predicated on the temporal-parietal junction. As outlined earlier, the temporal-parietal junction is associated with the generation of P3 and it is critically involved in empathy and inhibition. In addition, studies have indicated that individuals with psychopathic traits (e.g., low empathy) do not necessarily exhibit prefrontal deficits (Blair, Mitchell, & Blair, 2005). Taken together, reduced P3 amplitude in childhood may reflect the impairment of temporal-parietal junction that is associated with the abnormal development of empathic and moral behavior, which in turn predisposes to criminal offending. Future longitudinal functional brain imaging studies that assess empathy and moral decision making in childhood are needed to further test this hypothesis.
Prior literature has also established that reduced P3 amplitude is associated with a variety of disorders in the externalizing spectrum besides psychopathic and antisocial behavior, including alcohol dependence, drug dependence, nicotine dependence (see review by Patrick et al., 2006). Furthermore, reduced P3 amplitude has been found to be associated not only with concurrent externalizing behavior but also with risk for the development of behavioral problems. For example, as previously mentioned, it has been found that reduced P3 amplitude at age 17 prospectively predicted the development of externalizing problems of various kinds at age 20, even among individuals who did not show any behavioral symptom at the time of P3 assessment (Iacono et al., 2002). It is therefore proposed that reduced P3 amplitude reduction may be a nonspecific marker of psychopathology and represent a quantitative endophenotype of externalizing vulnerability (Hicks et al., 2007; Patrick et al., 2006). Our findings are consistent with this hypothesis, although environmental influences cannot be ruled out. Because P3 was measured at age 11 and it is assumed that at this age alcohol use was virtually nonexistent in this sample, and because controlling for the effect of age 23 alcoholism and substance abuse did not eliminate the relationship, it is unlikely that findings are a confound of substance use. Although prospective research cannot conclusively demonstrate causal relationships, the present longitudinal findings are nevertheless consistent with the view that P3 amplitude reduction may be an early biomarker for processes that raise the risk of adult criminal offending.
Contrary to findings from one prior study of adolescents (Raine et al., 1990), we do not find abnormal P3 latencies in criminals. Given the tremendous amount of changes to the neural systems from preadolescence to adulthood, we cannot rule out the possibility that these changes may contribute to the different findings. In addition, P3 latency has been found to be heritable in the difficult visual oddball task, but not in the easy visual oddball task (Katsanis, Iacono, McGue, & Carlson, 1997). The CPT in our study may have been too easy to detect the reliable associations between P3 latency and antisocial behavior. Alternatively, this discrepancy in findings may be partly due to the different paradigms used in the two studies. In Raine et al.'s (1990) study, an auditory contingent negative variation paradigm was used. It consisted of 20 trials of an S1-S2-motor response sequence, and in each trial a warning stimulus (S1) was followed by an imperative stimulus (S2) of a tone of 105 dB intensity. To increase motivation levels, S2 could be terminated by the subject quickly pressing a response button. Some studies have also used the stop-signal or go=no-go task to examine the neurophysiological correlates of behavioral problems in children. These two types of tasks were designed to examine the inability to withhold or stop an ongoing response and have been commonly used in examining behavioral disinhibition in attention deficit hyperactivity disorder (Alderson, Rapport, & Kofler, 2007; Iaboni, Douglas, & Baker, 1995; Logan, Cowan, & Davis, 1984). In contrast, in the current study the CPT was used, and we focused on the ability to sustain attention. In fact, our failure to find P3 latency effect is consistent with a recent meta-analysis indicating that P3 latency effects may be more salient in older subjects (>18 years) and in auditory rather than visual tasks (Gao & Raine, 2009). Furthermore, somewhat different neural networks have been implicated in auditory and visual odd-ball tasks (Kiehl et al., 2001), and future studies are needed to examine if the current findings can be replicated using other modalities.
Consistent with findings from recent P3 studies on criminals and psychopaths and children with oppositional defiant disorder, no group differences were observed on behavioral performance during the CPT task (Iacono et al., 2002; Kiehl, Bates, Laurens, Hare, & Liddle, 2006; Patrick et al., 2006). This probably is due to the low task difficulty which was reflected by high accuracy rates in both groups. Similarly, brain imaging studies have shown that murderers do not show behavioral underperformance on the CPT despite their reduced frontal activity compared to the controls (Raine, Buchsbaum, & LaCasse, 1997; Raine, Buchsbaum, et al., 1994). The fact that groups do not differ in behavioral performance indicates that reduced P3 amplitudes in criminals cannot be attributed to inability to perform the task or a motivation deficit in this group. In fact, their performance is relatively good for the 11-year-olds. It is possible that other cortical or subcortical brain regions not normally utilized on this task compensated for the temporal-parietal junction dysfunction in criminals, thus allowing them to perform as well as controls on the CPT. Future brain imaging studies are needed to test this hypothesis. Finally, although most studies that investigated the visual or auditory evoked P3 found a delay of latency and a decrease of amplitude for more difficult discrimination processes, especially when comparing target stimuli with nontarget stimuli (Polich, 1987; Senkowski & Herrmann, 2002), this effect of difficulty does not seem to interact with criminality.
It is worth noting that although P3 amplitude reduction is specific to detecting the target for adults, at age 11 it is generalized to both targets and nontargets. Although the target P3 is associated with a facilitatory updating of memory processes, the nontarget P3 is linked to an inhibitory updating (McCallum, Barrett, & Pocock, 1989). P3 deficits to both stimuli may reflect generalized information-processing deficits associated with delayed brain maturation in children. During childhood and adolescence, the human brain continues to mature and differentiate. It is possible that at age 23 this more generalized cognitive deficit disappears due to brain maturation, and P3 deficits become more specific to detecting target stimuli in adult criminals. Alternatively, strong genetic influences have been found for amplitudes for nontarget stimuli in children and adolescents, and suggestions are that the genetic factor influencing targets is the same as that influencing nontargets (Van Baal, De Geus, & Boomsma, 1998; Van Beijsterveldt, Molenaar, De Geus, & Boomsma, 1998). Given that the stability of P3 amplitudes over time is high and heritable (Carlson & Iacono, 2006), it is proposed that the association between antisocial behavior and P3 amplitudes and the underlying mechanisms remain unchanged across time. Longitudinal research is needed to test this proposition.
Only letters were involved in the current CPT, and it is unknown if findings generalize to emotional-relevant CPT. In one study, undergraduate students with high versus low psychopathic tendencies were compared when they detected rare emotional faces (fear, happy, etc.) among a series of neutral ones. It was found that the high psychopathy group showed delayed P3 latency to emotional faces compared to the low psychopathy group, although no effect on P3 amplitude was found (Campanella, Vanhoolandt, & Philippot, 2005). Given the important role of the temporal-parietal junction in emotional aspects such as empathy and moral decision making, and the view that antisocial behavior is characterized by deficient emotional processing, it is speculated that antisocial individuals show P3 deficits when a CPT with emotional components is employed.
Limitations of the current study need to be recognized. First, P3 data were not available in very early childhood due to the task requirement to respond to target stimuli. It has been found that age 3 electrodermal fear conditioning deficits are associated with crime at age 23 (Gao, Raine, Venables, Dawson, & Mednick, 2010), raising the question as to at what age the P3 abnormality is in place. Given that evidence has accumulated to suggest a neurodevelopmental basis to offending (Moffitt, 1993; Raine et al., 2004; Raine et al., 2003), the possibility that a P3 deficit among future criminals could be observed in preschool years needs to be tested using a passive P3 task. Second, this study was conducted in Mauritius and it needs to be ascertained whether this P3–crime association generalizes to Western countries. We have, however, repeatedly found that theories and empirical findings derived from Western cultures hold up in the Mauritian context (Raine et al., 2010). Third, a bipolar montage (T3-P3, T4-P4) was used in the current study, which courses along the temporal-parietal junction. Although the resulting amplitudes predominantly reflect the P3 event-related potentials at P3 and P4 sites (corresponding to the angular gyrus—a part of the temporal-parietal junction) as indicated by recent studies (Koessler et al., 2009; Okamoto et al., 2004), functional imaging in childhood is required to more directly assess dysfunction of these brain areas in relation to antisocial behavior. Fourth, it can be neither claimed nor concluded from our study that P3 deficits are specific to antisocial behavior because the internal reliability of the Hyperactivity scale was relatively low.
The clinical significance of these findings can be viewed at two levels. First, an important feature of these findings is that P3 measured at age 11 years predicts later criminal behavior. Two previous prospective studies had measured event-related potentials at age 15 years (Raine et al., 1990) and age 17 years (Iacono et al., 2002). Together with the behavioral genetic literature which suggests that P3 amplitude is an etiologically relevant process in externalizing behavior (Hicks et al., 2007), current findings that age 11 P3 is linked to both aggression at age 11 and also criminal behavior at age 23 suggest that P3 abnormalities may occur fairly early in life and may indirectly reflect a process of etiological significance. As such, findings push time of prediction back 4 years and have implications for the early prediction of antisociality in children. Second, given that previous studies have found that birth complications and maternal rejection at age 1 year (Raine, Brennan, & Mednick, 1994) and deficient electrodermal fear conditioning at age 3 years (Gao et al., 2010) are associated with adult violence, one practical implication of the current findings is that treatment and intervention studies need to begin much earlier in life than hitherto in order for success to be maximized in preventing violence.
In conclusion, these initial longitudinal findings indicate that reduced P3 amplitude at age 11 is associated with both antisocial behavior at age 11 and criminal offending at age 23. It is hypothesized that reduced P3 may be an early neurobiological marker for cognitive and affective processes predicated on the temporal-parietal junction (attention, empathy, moral decision making) that place a child at risk for adult antisocial and criminal behavior. Taken together with other early neurobiological markers that include fear conditioning (Gao et al., 2010), results are suggestive of a neurodevelopmental perspective of antisocial and criminal behavior.
Acknowledgments
Initial data collection was made possible by grants to the third author from the Medical Research Council (UK) and the Wellcome Trust (UK), and grants to the second author from NIH (R01 MH46435 and an Independent Scientist Award K02 MH01114). We thank all the local members of the Mauritius Joint Child Health Project and Siva Tian Tian (University of Houston) for help with data collection and cleaning.
Footnotes
All authors reported no financial interests or potential conflicts of interest.
Contributor Information
Yu Gao, Psychology Department, Brooklyn College, and The Graduate Center of the City University of New York.
Adrian Raine, Departments of Criminology, Psychiatry, and Psychology, University of Pennsylvania.
Peter H. Venables, Department of Psychology, University of York
Sarnoff A. Mednick, Department of Psychology, University of Southern California
REFERENCES
- Achenbach TM, Conners CK, Quay HC, Verhulst FC, Howell CT. Replication of empirically derived syndromes as a basis for taxonomy of child/adolescent psychopathology. Journal of Abnormal Child Psychology. 1989;17:299–323. doi: 10.1007/BF00917401. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Edelbrock CS. Manual for the Child Behavior Checklist and Revised Child Behavior Profile. University of Vermont, Department of Psychiatry; Burlington: 1983. [Google Scholar]
- Alderson RM, Rapport MD, Kofler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: A meta-analytic review of the stop-signal paradigm. Journal of Abnormal Child Psychology. 2007;35:745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Batson CD, Fultz J, Schoenrade PA. Adults’ emotional reactions to the distress of others. In: Eisenberg N, Strayer J, editors. Empathy and its development. Cambridge University Press; Cambridge, UK: 1987. pp. 163–185. [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: Implications for substance abuse risk and brain development. Biological Psychiatry. 1999a;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Subtypes of family history and conduct disorder: Effects on P300 during the Stroop test. Neuropsychopharmacology. 1999b;21:51–62. doi: 10.1016/S0893-133X(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Hall JR, Steffen BV, Patrick CJ. Violent offending predicts P300 amplitude. International Journal of Psychophysiology. 2007;66:161–167. doi: 10.1016/j.ijpsycho.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct disorder, depressive, anxiety, and other disorders. American Journal of Psychiatry. 1991;148:564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Blair RJR. A cognitive developmental approach to morality: Investigating the psychopath. Cognition. 1995;57:1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Mitchell D, Blair K. The psychopath: Emotion and the brain. Wiley-Blackwell; New York, NY: 2005. [Google Scholar]
- Campanella S, Vanhoolandt ME, Philippot P. Emotional deficit in subjects with psychopathic tendencies as assessed by the MMPI-2: An event-related potentials study. Neurosci. Lett. 2005;373:26–31. doi: 10.1016/j.neulet.2004.09.061. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG. Heritability of P300 amplitude development from adolescence to adulthood. Psychophysiology. 2006;43:470–480. doi: 10.1111/j.1469-8986.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Costa L, Bauer LO, Kuperman S, Porjesz B, O'Connor S, Hesselbrock VM, Begleiter H. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biological Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Gao Y, Raine A. P3 event-related potential impairments in antisocial and psychopathic individuals: A meta-analysis. Biological Psychology. 2009;82:199–210. doi: 10.1016/j.biopsycho.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raine A, Venables PH, Dawson ME, Mednick SA. Association of poor childhood fear conditioning and adult crime. American Journal of Psychiatry. 2010;167:56–60. doi: 10.1176/appi.ajp.2009.09040499. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Shared and selective neural correlates of inhibition, facilitation, and shifting processes during executive control. NeuroImage. 2010;51:421–431. doi: 10.1016/j.neuroimage.2010.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Bernat EM, Malone SM, Iacono WG, Patrick CJ, Krueger RF, McGue M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaboni F, Douglas VI, Baker AG. Effects of reward and response costs on inhibition in ADHD children. Journal of Abnormal Psychology. 1995;104:232–240. doi: 10.1037/0021-843X.104.1.232. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48 doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Iwaki S, Sutani K, Kou H, Tonoike M. Modeling multiple neuromagnetic activities during the visual infrequent target detection processing. International Congress Series. 2007;1300:535–538. [Google Scholar]
- Jasper HH. Report of committee on methods of clinical examination in EEG: Appendix: The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, McGue MK, Carlson SR. P300 event-related potential heritability in monozygotic and dizygotic twins. Psychophysiology. 1997;34(1):47–58. doi: 10.1111/j.1469-8986.1997.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Bates AT, Laurens KR, Hare RD, Liddle PF. Brain potentials implicate temporal lobe abnormalities in criminal psychopaths. Journal of Abnormal Psychology. 2006;115:443–453. doi: 10.1037/0021-843X.115.3.443. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Hare RD, Liddle PF, McDonald JJ. Reduced P300 responses in criminal psychopaths during a visual oddball task. Biological Psychiatry. 1999;45:1498–1507. doi: 10.1016/s0006-3223(98)00193-0. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF. An event-related fMRI study of visual and auditory odd-ball tasks. Journal of Psychophysiology. 2001;15:221–240. [PubMed] [Google Scholar]
- Kim M, Kim J, Kwon JS. Frontal P300 decrement and executive dysfunction in adolescents with conduct problems. Child Psychiatry and Human Development. 2001;32:93–106. doi: 10.1023/a:1012299822274. [DOI] [PubMed] [Google Scholar]
- Koessler L, Maillard L, Benhadid A, Vignal JP, Felblinger J, Vespignani H, Braun M. Automated cortical projection of EEG sensors: Anatomical correlation via the international 10–10 system. NeuroImage. 2009;46:64–72. doi: 10.1016/j.neuroimage.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time response: A model and a method. Journal of Experimental Psychology. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Raine A, Venables PH. Invariance of the MAST across religious groups. Journal of Studies on Alcohol. 2001;62:834–837. doi: 10.15288/jsa.2001.62.834. [DOI] [PubMed] [Google Scholar]
- McCallum WC, Barrett K, Pocock PV. Late components of auditory event-related potentials to eight equiprobable stimuli in a target detection task. Psychophysiology. 1989;26:683–694. doi: 10.1111/j.1469-8986.1989.tb03172.x. [DOI] [PubMed] [Google Scholar]
- Miller PA, Eisenberg N. The relation of empathy to aggressive and externalizing=antisocial behavior. Psychological Bulletin. 1988;103:324–344. doi: 10.1037/0033-2909.103.3.324. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ. Morals and the human brain: A working model. NeuroReport. 2003;14:299–305. doi: 10.1097/00001756-200303030-00001. [DOI] [PubMed] [Google Scholar]
- Munro GES, Dywan J, Harris GT, McKee S, Unsal A, Segalowitz SJ. Response inhibition in psychopathy: The frontal N2 and P3. Neuroscience Letters. 2007;418:149–153. doi: 10.1016/j.neulet.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, Dan I. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain imaging. NeuroImage. 2004;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. Task difficulty, probability, and inter-stimulus interval as determinants of P300 from auditory stimuli. Electroencephalography and Clinical Neurophysiology. 1987;68:311–320. doi: 10.1016/0168-5597(87)90052-9. [DOI] [PubMed] [Google Scholar]
- Polich J. Overview of P3a and P3b. In: Polich J, editor. Detection of change: Event-related potential and fMRI findings. Kluwer Academic; Boston, MA: 2003. pp. 83–98. [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. International Journal of Psychophysiology. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Raine A, Brennan PA, Mednick SA. Birth complications combined with early maternal rejection at age 1 year predis-pose to violent crime at age 18 years. Archives of General Psychiatry. 1994;51:984–988. doi: 10.1001/archpsyc.1994.03950120056009. [DOI] [PubMed] [Google Scholar]
- Raine A, Buchsbaum M, LaCasse L. Brain abnormalities in murderers indicated by Positron Emission Tomography. Biological Psychiatry. 1997;42:495–508. doi: 10.1016/S0006-3223(96)00362-9. [DOI] [PubMed] [Google Scholar]
- Raine A, Buchsbaum MS, Stanley J, Lottenberg S, Abel L, Stoddard J. Selective reductions in prefrontal glucose metabolism in murderers. Biological Psychiatry. 1994;36:365–373. doi: 10.1016/0006-3223(94)91211-4. [DOI] [PubMed] [Google Scholar]
- Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Bihrle S, Colletti P. Hippocampal structural asymmetry in unsuccessful psychopaths. Biological Psychiatry. 2004;55:185–191. doi: 10.1016/s0006-3223(03)00727-3. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Taylor K, Hellige HB, Bihrle S, LaCasse L, Colletti P. Corpus callosum abnormalities in psychopathic antisocial individuals. Archives of General Psychiatry. 2003;60:1134–1142. doi: 10.1001/archpsyc.60.11.1134. [DOI] [PubMed] [Google Scholar]
- Raine A, Liu J, Venables PH, Mednick SA, Dalais C. Cohort profile: The Mauritius Child Health Project. International Journal of Epidemiology. 2010;39:1441–1451. doi: 10.1093/ije/dyp341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Reynolds C, Venables PH, Mednick SA, Farrington DP. Fearlessness, stimulation-seeking, and large body size at age 3 years as early predispositions to childhood aggression at age 11 years. Archives of General Psychiatry. 1998;55:745–751. doi: 10.1001/archpsyc.55.8.745. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Dalais C, Mellingen K, Reynolds C, Mednick SA. Early educational and health enrichment at age 3–5 years is associated with increased autonomic and central nervous system arousal and orienting at age 11 years: Evidence from the Mauritius Child Health Project. Psychophysiology. 2001;38:254–266. [PubMed] [Google Scholar]
- Raine A, Venables PH, Mednick SA. Low resting heart rate age 3 years predisposes to aggression at age 11 years: Evidence from the Mauritius Child Health Project. Journal of American Academy of Child and Adolescent Psychiatry. 1997;36:1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Williams M. Relationships between N1, P300, and contingent negative variation recorded at age 15 and criminal behavior at age 24. Psychophysiology. 1990;27:567–574. doi: 10.1111/j.1469-8986.1990.tb01978.x. [DOI] [PubMed] [Google Scholar]
- Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Social, Cognitive, and Affective Neuroscience. 2006;1:203–213. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio CA, Reynolds CR, Lowe P, Moore JJ. The continuous performance test: A window on the neural substrates for attention? Archives of Clinical Neuropsychology. 2002;17:235–272. [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: The role of the temporo-parietal junction in “theory of mind.”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. American Journal of Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Senkowski D, Herrmann CS. Effects of task difficulty on evoked gamma activity and ERPs in a visual discrimination task. Clinical Neurophysiology. 2002;113:1742–1753. doi: 10.1016/s1388-2457(02)00266-3. [DOI] [PubMed] [Google Scholar]
- Van Baal GCM, De Geus EJC, Boomsma DI. Longitudinal study of genetic influences on ERP–P3 during childhood. Developmental Neuropsychology. 1998;14:19–45. [Google Scholar]
- Van Beijsterveldt CEM, Molenaar PCM, De Geus EJC, Boomsma DI. Individual differences in P300 amplitude: a genetic study in adolescent twins. Biological Psychology. 1998;47:97–120. doi: 10.1016/s0301-0511(97)00025-2. [DOI] [PubMed] [Google Scholar]
- Venables PH. Psychophysiology and psychometrics. Psychophysiology. 1978;15:302–315. doi: 10.1111/j.1469-8986.1978.tb01383.x. [DOI] [PubMed] [Google Scholar]