Abstract
Respiratory insufficiency is the major cause of morbidity and mortality in patients affected by cystic fibrosis. An excessive neutrophilic inflammation, mainly orchestrated by the release of IL-8 from bronchial epithelial cells and amplified by chronic bacterial infection with Pseudomonas aeruginosa, leads to progressive tissue destruction. The anti-inflammatory drugs presently utilized in cystic fibrosis patients have several limitations, indicating the need for identifying novel molecular targets. To address this issue, we preliminarily studied the association of 721 single-nucleotide polymorphisms from 135 genes potentially involved in signal transduction implicated in neutrophil recruitment in a cohort of F508del homozygous cystic fibrosis patients with either severe or mild progression of lung disease. The top ranking association was found for a nonsynonymous polymorphism of the phospholipase C beta 3 (PLCB3) gene. Studies in bronchial epithelial cells exposed to P.aeruginosa revealed that PLCB3 is implicated in extra cellular nucleotide–dependent intracellular calcium signaling, leading to activation of the protein kinase C alpha and beta and of the nuclear transcription factor NF-κB p65. The pro-inflammatory pathway regulated by PLCB3 acts by potentiating the Toll-like Receptors’ signaling cascade and represents an interesting molecular target to attenuate the excessive recruitment of neutrophils without completely abolishing the inflammatory response.
Introduction
Cystic fibrosis (CF), an autosomal-recessive disease manifesting progressive respiratory insufficiency, defective exocrine pancreatic secretion and elevated electrolyte concentration in the sweat, is related to loss-of-function mutations of the CF Transmembrane conductance Regulator (CFTR) gene encoding a chloride transporting protein (OMIM 219700).
The major cause of morbidity and mortality of CF patients is chronic lung disease. Innovative therapies to prevent the onset or reduce the pulmonary damage are under intensive pre-clinical and clinical investigation (1). Besides pharmaceutical approaches to correct the mutated CFTR protein and potentiate its ion transport function, novel anti-inflammatory drugs will be particularly relevant to those adolescent and adult CF patients who have already developed lung pathology (2). However, current anti-inflammatory drugs, such as corticosteroids, show limitations in terms of efficacy or occurrence of adverse effects in CF patients, suggesting the need of identifying novel molecular targets and more effective anti-inflammatory drugs specifically tailored for CF pulmonary pathophysiology (2).
A hallmark of lung pathology in CF is a neutrophil-dominated inflammation, which is disproportionate to the bacterial infection, typically by Pseudomonas aeruginosa. Excessive infiltrates of neutrophils, releasing proteases upon continuous activation by bacterial products, are presently thought to play a major role in the progressive destruction of the CF lung tissue (3, 4). The chemokine Interleukin (IL)-8 is the most abundant soluble mediator recruiting neutrophils in the bronchi of CF patients, being found in the airway secretions both in advanced and in early stages of the disease (5–7). Interestingly, IL-8 promoter variant alleles resulting in reduced expression of IL-8 protein are protective in respect to the severity of progression of lung disease in CF patients (8), supporting the concept that reduction of the excessive IL-8-driven neutrophil recruitment could be a relevant pharmacological objective to ameliorate CF pulmonary disease.
A major source of IL-8 release in CF lungs are the bronchial epithelial cells lining the conductive airways of CF patients (9, 10). Binding of bacterial components to pattern-recognition receptors expressed on epithelial cell surface, such as Toll-like receptors (TLRs)-2, 4 and 5, activates a series of kinases and adapters, ultimately triggering nuclear translocation of transcription factors and expression of pro-inflammatory genes (11). In addition, interaction of P.aeruginosa with AsialoGM1 receptor (ASGM1R), which colocalizes with TLR5, promotes sustained release of nucleotides (12, 13). Extra cellular ATP, through an autocrine binding to the seven-membrane spanning domain P2Y2 purinergic receptor, activates cytosolic calcium signaling and contributes, together with TLRs, to the expression of IL-8 (14).
Due to the high redundancy in receptors and downstream signaling components triggered by P.aeruginosa in bronchial epithelial cells, finding key targets to effectively reduce excessive inflammatory response represents a major challenge. To prioritize the investigation on key target molecule(s) intervening in this pro-inflammatory network, we performed a pilot genotype-phenotype association study between variants of genes involved in the innate immune response and the severity of lung disease in CF patients. So far, association studies have highlighted that the mannose-binding lectin 2 (MBL2), the Transforming Growth Factor - beta 1 (TGFB1) and the IFRD1 genes can modulate the progression of lung disease in CF (15–17). Thus, to start identifying critical components modulating the inflammatory signaling cascade triggered by P.aeruginosa in bronchial epithelial cells, we studied the distribution of allelic variants of 721 Single Nucleotide Polymorphisms (SNP) from a panel of 135 genes of innate immunity in two groups of CF patients characterized by severe or mild progression of lung disease. By ranking the association of each SNP with the progression of lung disease severity, we found on the top a non-synonymous polymorphism of the phospholipase C beta 3 (PLCB3) gene, leading to investigate firstly the role of the PLCB3 enzyme. Functional studies performed in human bronchial epithelial cells exposed to P.aeruginosa demonstrate that PLCB3, by regulating intracellular calcium transients, plays a relevant role in amplifying the expression and release of IL-8, the major chemokine recruiting neutrophils in CF airway lungs.
Materials and Methods
Materials
The calcium chelator BAPTA-AM was from Molecular Probes (Eugene, OR), the broad protein kinase C inhibitor bisindolylmaleimide (BIM)-I was from Merck KGaA (Darmstadt, Germany), all the other reagents were from Sigma, unless otherwise indicated.
Subjects
DNA samples were collected from CFTR F508del homozygous patients enrolled by the University of North Carolina at Chapell Hill, Chapel Hill (M.R.K.) and by Case Western Reserve University, Cleveland (M.L.D.). CF patients were classified as “mild” (n=300) or “severe” (n=208) with respect to the progression of lung disease according to the FEV1 measurements, according to the criteria reported previously by the North American Gene Modifier Study Group (GMSG) (16). The study was approved by the institutional review boards (IRB) of the participating institutions collecting the samples from patients affected by cystic fibrosis.
Selection of SNPs
Different types of SNPs have been chosen in candidate genes, using the HapMap database. In particular, two different types of polymorphisms have been chosen: 1) nonsynonymous coding SNPs (nsSNPs) that include a group of SNP having the highest impact on phenotype for their potential to directly affect the structure, function and interactions of expressed proteins and 2) Tag SNPs, which represent a subset of SNPs capturing most of the haplotype diversity of each haplotype block or gene-specific region. Tag SNPs have been selected using Tagger, a software implemented in Haploview (http://www.broad.mit.edu/mpg/haploview/). Genotype data in raw Hap Map format have been uploaded in order to specify the genomic regions of interest within which tag SNPs to be picked. As output, Tagger produced a list of tag SNPs and corresponding statistical tests to capture all variants of interest, and a summary coverage report of the selected tag SNPs.
Genotyping by Illumina technology
Analysis of SNPs has been firstly performed by Illumina technology. An overall number of 721 SNPs was selected within 135 genes and analyzed using the “Custom Golden-Gate Genotyping" (http://www.illumina.com) assay on ILLUMINA platform. This genotyping system consisted of an initial allele-specific extension reaction followed by PCR amplification. Amplified products were hybridized to an array matrix of bead-based probe sequences where each bead is coated with universal probes and represented multiple times for increased accuracy (average of 25 times).
Genotyping by Taqman technology
Genotype of the SNPs ranking on top of statistical significance have been verified with Taqman assays from Applied Biosystems. All reactions utilizing TaqMan MGB probes were run under standard condition on an ABI PRISM® 9700 HT system. Genotyping of phospholipase C SNPs in the human bronchial epithelial cell lines IB3-1 and CuFi-1 was performed by both Taqman technology and direct sequencing (see Supplemental Tables 5 and 6).
Human bronchial epithelial cell culture
IB3-1 cells (LGC Promochem Europe) are human bronchial epithelial cells immortalized with adeno12/SV40, derived from a CF patient with a mutant F508del/W1282X genotype (18). Cells were grown in LHC-8 basal medium (Biofluids, Rockville, MO) supplemented with 5% fetal bovine serum (FBS). All culture flasks and plates were coated with a solution containing 35 mg/ml bovine collagen (Becton-Dickinson, Franklin Lakes, NJ), 1 µg/ml BSA (Sigma-Aldrich, St. Louis, MO) and 1 µg/ml human fibronectin (Becton-Dickinson) as previously described. CuFi-1 cells were a generous gift from A. Klingelhutz, P. Karp and J. Zabner (University of Iowa, Iowa City, IO). CuFi-1 cells were derived from human bronchial epithelia from a patient with CF (CFTR mutant genotype F508del/F508del) and transformed by reverse transcriptase component of telomerase, hTERT, and human Papillomavirus type 16 (HPV-16), E6 and E7 genes (19). These cells were grown on human placental collagen type IV (Sigma-Aldrich, St. Louis, MO)-coated flasks in bronchial epithelial growth medium (BEGM) (Cambrex Bioscience, Walkersville, MD), as previously described (19). CuFi-1 cells were seeded onto cell culture inserts (pore size of 0.4 µm) in Falcon 24-well multitrays (BD Biosciences, Franklin Lakes, NJ) at a density of 7 × 105 cells/insert and grown in BEGM for 15 days. Transepithelial electrical resistance (TER) was measured with an epithelial voltometer (EVOM; World Precision Instruments, Sarasota, FL). The cell inserts were used for experiments when the cell monolayers reached a TER> 1000 Ω x cm2.
Infection with P.aeruginosa bacterial strains
The well-characterized motile nonmucoid laboratory strains of P.aeruginosa named PAO1, PAK, PAK FliC (recombinant P.aeruginosa strain PAK lacking expression of flagellin) have been kindly donated by A. Prince (Columbia University, New York, NY). Bacteria colonies from overnight cultures on trypticase soy agar (TSA) (Difco, Detroit, MI) plates were grown with shaking in 20 ml of trypticase soy broth (TSB) (Difco) at 37°C until an optical density (A660 nm wavelength), corresponding to 1 × 107 colony-forming units (CFU)/ml, was reached. Bacteria were washed twice with PBS and diluted in each specific serum-free medium before infection and added to cells at the concentration indicated as CFU/cell.
Expression of phospholipase C isoforms and of IL-8
PLC and IL-8 transcripts were quantified Real-Time Polymerase Chain Reaction (qRT-PCR) as previously described (20). Briefly, total RNA from cells was isolated using High Pure RNA Isolation Kit (Roche, Mannheim, Germany). 2 µg of total RNA was reverse-transcribed to cDNA using the High Capacity cDNA Archive Kit and random primers (Applied Biosystems, Foster City, CA) in a 100-µl reaction. The cDNA (2 µl) was then amplified for 40 PCR cycles using the SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA) in a 10-µl reaction using 7900HT Fast Real-Time PCR apparatus (Applied Biosystems, Foster City, CA). The qRT-PCRs were performed in duplicates for both target and normalizer genes. Primer sequences and concentration are shown in Supplemental Table S4. Primer sets were purchased from Integrated DNA Technologies (Tema Ricerca s.r.l., BO, Italy). Relative quantification of gene expression was performed using the comparative threshold (CT) method as described by the manufacturer (Applied Biosystems User Bulletin 2). Changes in mRNA expression level were calculated after normalization to calibrator gene. The ratios obtained after normalization are expressed as fold changes over untreated samples for IL-8 mRNA and for the relative expression of the housekeeping gene Human Cytokeratin (HCK)-15 for the isoforms of phospholipase C. PLCB3 protein was detected by immunofluorescence. IB3-1 cells were seeded on eight-well chamber slides (Nunc, Naperville, IL) and pre-incubated with PLCB3 siRNA or scrambled duplexes for 24 hours in LHC-8 basal serum-free medium as indicated below in the silencing protocol. Cells were washed three times with PBS and fixed with 4% paraformaldehyde (wt/vol) in PBS for 20 minutes at room temperature. After three washes with PBS, cells were permeabilized with methanol at −20°C for 5 minutes and dried for 1 hour. Slides were incubated with 5% bovine serum albumin (BSA) in PBS for 90 minutes at room temperature and then subjected to three incubations at room temperature with: a) 1:200 dilution of rabbit polyclonal antibody anti-PLCB3 (sc-13958 from Santa Cruz Biotechnology, Santa Cruz, CA) in 5% BSA for 1 hour or with an irrelevant rabbit IgG antibody; b) 1:200 dilution of biotinylated goat anti-rabbit immunoglobulin G (Santa Cruz Biotechnology) in 1% BSA–PBS–0.1% Tween 20; c) 1:60 dilution of fluorescein isothiocyanate conjugated streptavidin (Sigma) in 1% BSA–PBS–0.1% Tween 20. Coverslips were mounted with Prolong Antifade (Molecular Probes, Eugene, OR) and stored at room temperature. Fluorescence was examined with a digital imaging system based on a Zeiss Axiovert 200 fluorescence microscope equipped with a back-illuminated CCD camera (Roper Scientific, USA), excitation and emission filter wheels (Sutter Instrument Company, USA) and piezoelectric motoring of the z stage (Physik Instrumente, GmbH & Co., Germany) for rapid focussing in the Z plane. The data were acquired and processed using the MetaMorph analyzing program (Universal Imaging Corporation, USA). The Z-steps were then turned into projections and the average intensity after background subtraction was determined. All intensity comparisons were determined from at least 10 different cells to minimise cell-to-cell staining variations. The levels of PLCB3-silencing were was expressed as % of arbitrary unit of fluorescence (F.A.U.), in respect to scrambled condition. IL-8 protein release was measured with an ELISA assay. IB3-1 and CuFi-1 cells were grown and infected as described, then supernatants were collected from each well and an enzyme-linked immunosorbent assay for the quantitative detection of human IL-8 was performed using the Human IL-8 Instant ELISA kit (Bender Med Systems, Vienna, Austria) according to the manufacturer’s protocol.
Silencing PLCB3 gene
To perform silencing experiments of PLCB3 gene a TriFECTa RNAi Kit (Integrated DNA technologies, Coralville, Iowa, IA) was used accordingly to the manufacturer’s instructions. IB3-1 cells were transiently transfected with specific small-interfering RNA (siRNA) for PLCB3 (sequence 1: AGAUGAGGGACAAGCAUAAGAAGGA; sequence 2: GCUCGAAAGAGGAACCGAAGCAUUUGUUCCU) or scrambled (sequence CUUCCUCUCUUUCUCUCCCUUGUGA) duplexes complexed with cationic liposomes Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Lipofectamine 2000 (4 µl) was diluted in 1 ml LHC-8 serum-free cell culture medium. PLCB3 siRNA or scrambled duplexes (10 nM) were added and incubated for 10 minutes. Liposome: duplexes complexes in LHC-8 serum-free medium (500 µl) were added to IB3-1 cells grown in 2-cm2 wells and incubated at 37°C/5% CO2 for 6 hours. Cells were washed twice with culture medium and left at 37°C/5% CO2 for a further 18 hours.
Activation of NF-kB
IB3-1 cell nuclear extracts were prepared as previously described (20). Briefly, IB3-1 cells were transfected with PLCB3 siRNA or scrambled duplexes for 24 hours and stimulated with P.aeruginosa, PAO1 strain (100 CFU/cell) for a further 1, 2 or 4 hours, washed twice with iced PBS and detached by trypsinization. Nuclear proteins were separated by hypotonic lysis followed by high-salt extraction treatment of nuclei. A quantitative NF-kB p65 subunit capture assay was performed by Trans AM NF-kB p65 Activation Assay (Active Motif, Carlsbad, CA) using 2.5 µg of nuclear extracts for each sample, according to the manufacturer’s instructions. Briefly, nuclear extracts were added to wells coated with an oligonucleotide containing a NF-kB consensus binding site. The binding was revealed by subsequent addition of a primary antibody directed against p65 and a secondary antibody conjugated to horseradish peroxidase and, after the addition of the substrate, absorbance was read at 450 nm wavelength.
Purification of flagellin and pilin from P.aeruginosa
Purification of flagellin was performed as described (21) starting from the PAK/NP recombinant P.aeruginosa strain lacking expression of pilin. Pilin proteins were isolated using the method of Castric (22), starting from the PAK/fliC recombinant P.aeruginosa strain lacking expression of flagellin.
Fura-2/AM measurements
The cytosolic free Ca2+ concentration was evaluated using the fluorescent Ca2+ indicator Fura-2 acetoxymethyl ester (Fura-2/AM; Molecular Probes). Briefly, cells were incubated in medium supplemented with 2.5 µM Fura-2/AM for 30 min, washed with KRB buffer to remove the extra cellular probe, supplied with preheated KRB buffer (supplemented with 1 mM CaCl2), and placed in a thermostated (37 °C) incubation chamber of an LS50 Perkin Elmer fluorometer (Perkin Elmer Ltd., Beaconsfield, UK). Fluorescence was measured every 100 ms with the excitation wavelength alternating between 340 and 380 nm and the emission fluorescence being recorded at 510 nm. The [Ca2+]i was calculated by the ratio method using the equation: [Ca2+]i= Kd*(R-Rmin)/(R-Rmax)*Sf2/Sf1 where Kd is dissociation constant of Fura-2/AM for (Ca2+) taken as 240 nM at 37 °C, R is ratio of fluorescence for Fura-2/AM at the two excitation wavelengths, F340/F380, Rmax is ratio of fluorescence in the presence of excess of calcium obtained by lysing the cells with 10 µM ionomycin (Sigma Aldrich), Rmin is ratio of fluorescence in the presence of minimal calcium obtained by lysing the cells and then chelating all the Ca2+ with 0.5 M EGTA, Sf2 is fluorescence of Ca2+ free form of Fura-2/AM at 380nm excitation wavelength and Sf1 is fluorescence of Ca2+ bound form of Fura-2/AM at 380 nm excitation wavelength. IB3-1 cells were transfected and stimulated with P.aeruginosa, PAO1 strain (100 CFU/cell), as reported in the figures.
Aequorin measurements
The probes employed (cytAEQ, erAEQmut and mtAEQ) are chimeric aequorins targeted to the cytosol, endoplasmic reticulum and mitochondria, respectively (23). For the experiments with cytAEQ and mtAEQ, cells were incubated with 5 µM coelenterazine for 1–2 h in modified KRB supplemented with supplemented with 1 mM CaCl2. Then the cover slip with transfected cells was placed in a perfused, thermostated chamber located in the close proximity of a low noise photomultiplier, with a built-in amplifier-discriminator. To reconstitute the erAEQmut with high efficiency, the luminal [Ca2+] of the ER first had to be reduced. This was achieved by incubating the cells for 1 h at 4°C in KRB supplemented with 5 µM coelenterazine, the Ca2+ ionophore ionomycin, and 600 µM EGTA. After this incubation, cells were extensively washed with KRB supplemented with 2% bovine serum albumin and then transferred to the perfusion chamber. All aequorin measurements were carried out in KRB supplemented with either 1 mM CaCl2 (cytAEQ and mtAEQ) or 100µM EGTA (erAEQmut). Agonist, as Histamine 100 µM, and other drugs were added to the same medium, as showed in the figures. The experiments were terminated by lysing the cells with 100 µM digitonin in a hypotonic Ca2+-containing solution (10 mM CaCl2 in H2O), thus discharging the remaining aequorin pool. The output of the discriminator was captured by a Thorn-EMI photon counting board and stored in an IBM-compatible computer for further analyses. The aequorin luminescence data were calibrated off-line into [Ca2+] values, using a computer algorithm based on the Ca2+ response curve of wildtype and mutant aequorins. Chemicals and reagents were from Sigma or from Merck except for coelenterazine and coelenterazine n, which were from Molecular Probes
Microscopic analysis of PKC translocation
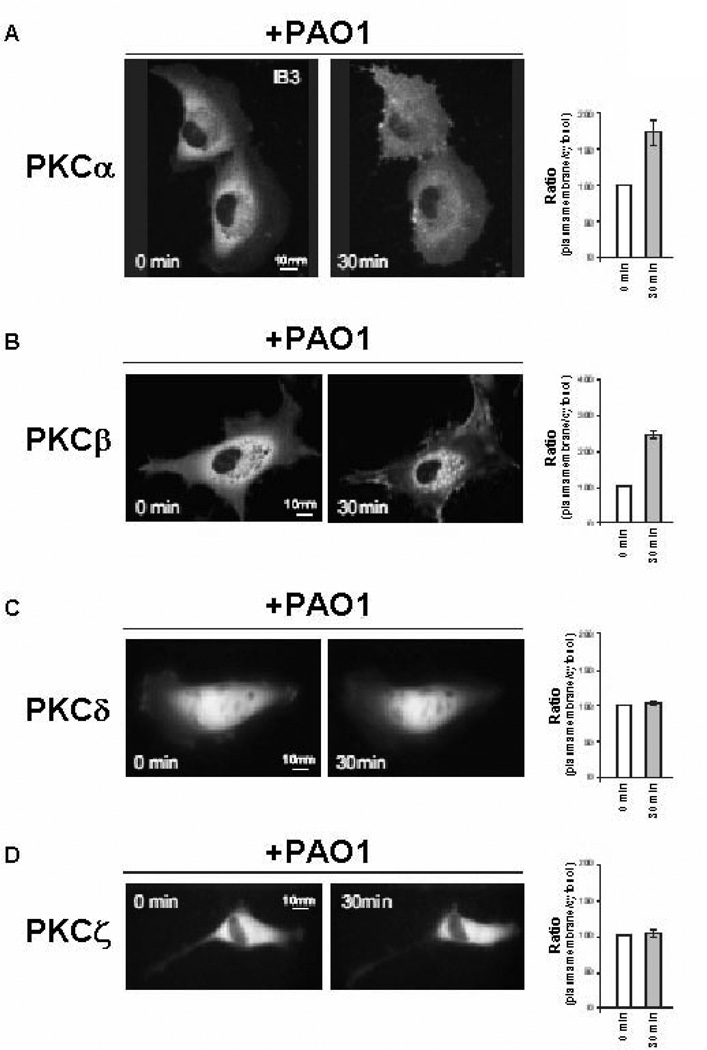
Images of PKC translocation were recorded using a digital imaging system based on a Zeiss Axiovert 200 fluorescence microscope. The data were acquired and processed using the MetaMorph analysis program (Universal Imaging Corporation, USA). For computational deblurring, a stack of images through the z plane was acquired (200 ms/image; 20 plans 0.5 µm apart) and processed using the EPR software developed by the Biomedical Imaging group of the University of Massachusetts Medical School (Worcester, MA, USA). Microscope analysis were performed 36 h after transfection. The medium was changed from LHC-8 basal medium + 5% FCS to KRB. P.aeruginosa strain, and other drugs (phorbol ester PMA 400nM and Apyrase 3 U/ml, respectively) were added to the buffer, as showed in the figures. The recruitment of the kinases are represented as plasma membrane translocation of different PKC–GFP chimeras, expressed as the increase in fluorescence ratio with respect to time 0 (calculated as the ratio of plasma membrane and cytosol average intracellular fluorescence, obtained from multiple regions inside the cytosol and on the cell membrane, measured on single cell). The graphs (figure 5) indicate the levels of PKC translocation, expressed as the fluorescence ratio, to plasma membrane (for PKCα, PKCβ and PKCδ) or nucleus (for PKCζ), on the average of cytosolic fluorescence intensity.
FIGURE 5.
P.aeruginosa activates conventional PKC isoforms alpha and beta. Fluorescence signal of Green Fluorescent Protein (GFP)-tagged PKC isoforms A, alpha, B, beta, C, delta and D, zeta before and after 30 min from the addition of PAO1 (100 CFU/cell) to IB3-1 cells. Histograms indicate the intracellular localization of PKC isoforms, as increase in fluorescence ratio with respect to time zero (ratio of translocation from cytosol to plasma membrane or nucleus) as mean ± S.E.M. for at least 7 single cells (see Materials and Methods). Averaging of % ratio: PKCα +76% ± 11.6; PKCβ +114% ± 11.4; PKCδ +5% ± 0.8; PKCζ +4% ± 1.0.
Statistical methods
For association between disease and single SNPs, test for allelic association (which compares frequencies of alleles in cases versus controls) have been utilized. For these analyses, permutation tests (ie, permuting the phenotypes) have been conducted in order to account for the large numbers of tests. Our analyses have been performed using PLINK (http://pngu.mgh.harvard.edu/~purcell/plink/) and R (http://www.r-project.org/). After validation of Illumina genotype screening by Taqman assay, allelic associations have been tested with chi-square test. Student’s t test has been applied to the experiments performed in human bronchial epithelial cells with statistical significance at p<0.05 (*) or p<0.01 (**) levels.
Results
Association of PLCB3 gene with the progression of lung disease in CF
A panel of genes of the innate immunity has been chosen as candidate modulators of the proinflammatory signaling elicited by P.aeruginosa in bronchial epithelial cells (Supplemental Table S1). They included 135 genes, such as those encoding chemokines and adhesion molecules, pattern-recognition receptors (PRRs) sensing the presence of bacteria in the lumen of the conductive airways and several components of the signaling network acting downstream the PRRs. To choose in each gene those allelic variants most probably contributing to changes in expression or function of the encoded proteins, due to the large series of Single Nucleotide Polymorphisms (SNPs) reported for each of these genes, we selected firstly all those SNPs resulting in non-synonymous changes in the coding regions and, secondly, those TagSNPs in intronic, 3’- and 5’- untranslated regions that mark the haploblocks composed of several single SNPs, for a total of 721 SNPs, as listed in the Supplemental Table S2. DNA samples were from a representative cohort of 508 CF patients selected by the North American Gene Modifier Study Group (GMSG) and classified as having either severe (n = 200) or mild (n = 308) progression of lung disease, according to the criteria described previously (16). Genotyping the 721 SNPs has been firstly performed using Illumina platforms with individual 508 DNA samples. The top-ranking group of SNPs showing significant genotype-phenotype association was subsequently validated with Taqman probe assays. By ranking the association between variant alleles and the severe or mild groups of CF patients, 26 SNPs showed a statistical significance with P values < 0.05, as calculated by permutation test (Supplemental Table S3). Interestingly, within the top-ranking list of the 721 SNPs, five of them were variants of phospholipase C (PLC) isoforms beta, namely a nonsynonymous SNP of the PLC isoform beta 3 gene (PLCB3), three intronic Tag SNPs of the PLC isoform beta 1 gene (PLCB1) and one intronic Tag SNP of the PLC isoform beta 4 gene (PLCB4). The C2534T variant (rs35169799) of the phospholipase C beta 3 (PLCB3) gene was found on top of the rank of the 721 SNPs. The minor allele T was found significantly associated with the milder CF phenotype group (Chi-square test value = 8.01, permutation test P value = 0.0046) (Table 1). Milder CF patients were associated with the minor 2534T allele with O.R. = 2.297 (C.I. 95% = 1.27 – 4.14), as shown in Table 2. The C2534T variant encodes a non-synonymous serine to leucine change in the amino acid residue at position 845, which is localized in the C-terminal tail in close proximity to the C2 domain of the PLCB3 protein (24, 25). These two domains are known to be involved in the interaction with Gaq/11 heterotrimeric GTPase protein and in the anchoring of PLCB3 to the plasma membrane through salt-like Ca2+ bonds, respectively (24, 25).
Table 1.
Prevalence of polymorphic genotypes according to the severe or mild phenotype of CF patients
| Gene SNP # |
Lung function |
Genotype CC Patients n (%) |
Genotype CT Patients n (%) |
Genotype TT Patients n (%) |
TOTAL patients n |
Chi- square value |
P value |
|---|---|---|---|---|---|---|---|
| PLCB3 rs35169799 |
Severe | 185 (92) | 15 (8) | 0 | 200 | 8.01 | 0.0046 |
| Mild | 260 (84) | 45 (15) | 3 (1) | 308 |
Table 2.
Prevalence of polymorphic alleles according to the severe or mild phenotype of CF patients
| Gene SNP # |
Lung function |
Allele | Allele n |
Allelic frequency | minor allele Odds Ratio (C.I. 95%) |
|---|---|---|---|---|---|
| PLCB3 rs35169799 |
Severe | C | 385 | 385/400 = 0.962 | allele T in mild CF pts 2.297 (1.27 – 4.14) |
| Mild | C | 565 | 565/616 = 0.917 | ||
| Severe | T | 15 | 15/400 = 0.037 | ||
| Mild | T | 51 | 51/616 = 0.083 |
Silencing PLCB3 reduces the expression of IL-8 chemokine
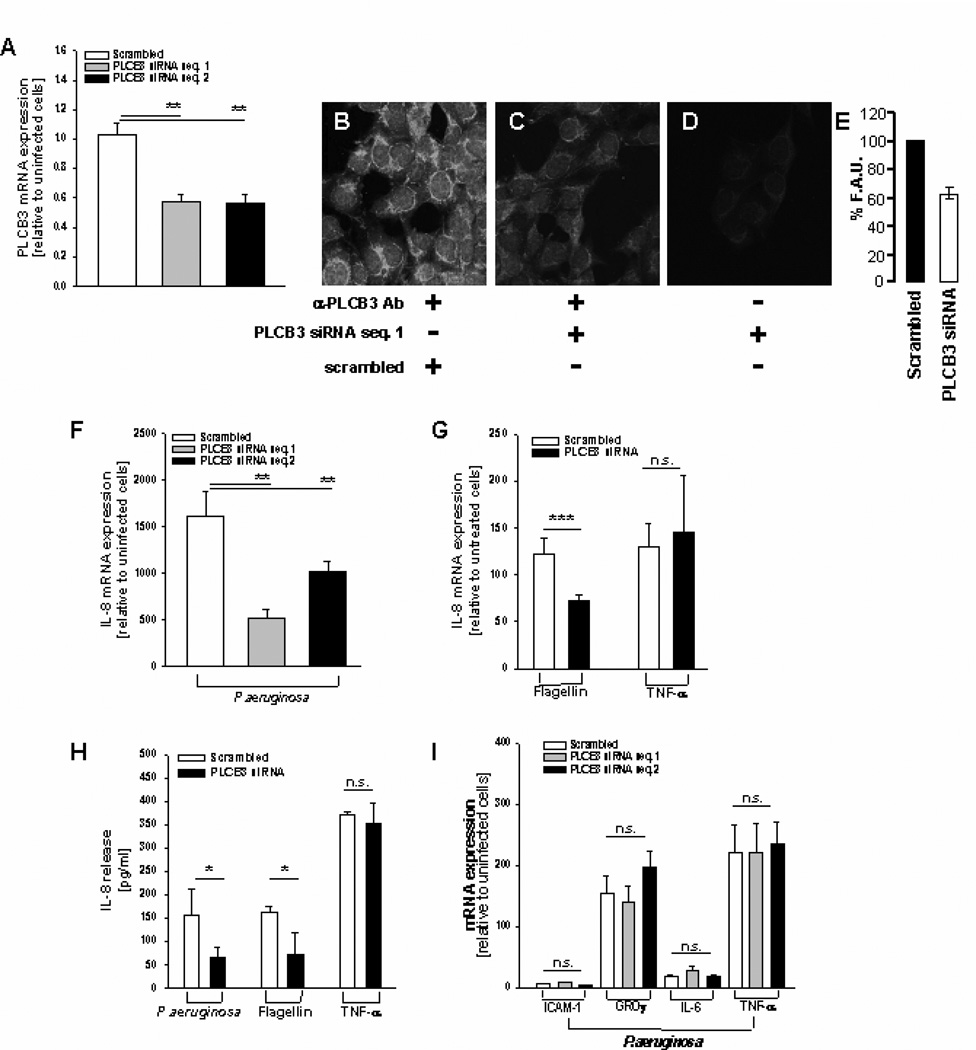
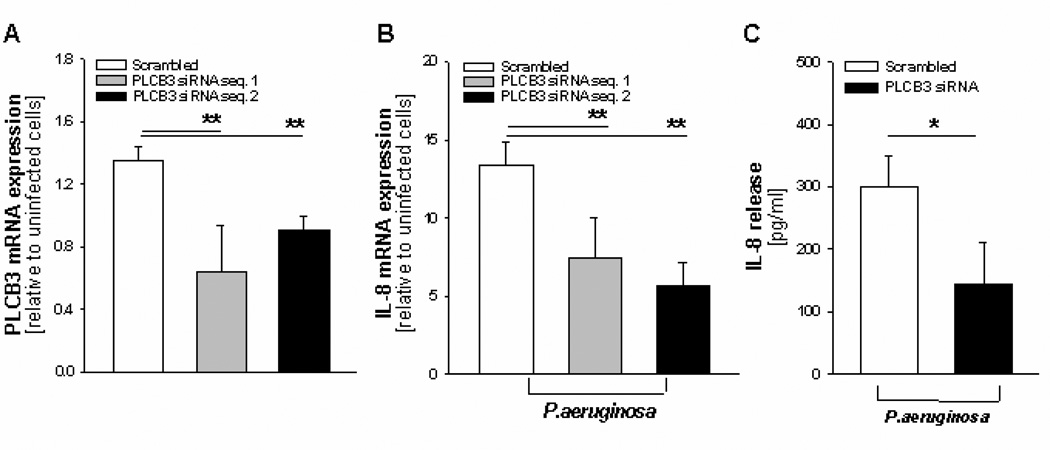
PLC beta isoforms are implicated in signal transduction by receptors for hormones, growth factors, neurotransmitters and other ligands involved in regulation of different cellular processes, including the immune response (25). Although human bronchial epithelial cells express, albeit at different levels, the transcripts of all the PLC beta isoforms, (Supplemental Figures S5 and S2), we focused our attention on PLCB3, since it is the most highly expressed within the beta isoforms and it is the top-ranking gene associated with clinical phenotype. To understand whether PLCB3 could be relevant in the induction of IL-8 in respiratory cells exposed to bacterial infection, we studied the transcription and release of IL-8 after silencing the expression of endogenous PLCB3 with siRNA oligonucleotides in human bronchial epithelial cells from cystic fibrosis patients exposed to P.aeruginosa. The bronchial cell lines utilized were all homozygous for the major allele of the PLCB3 C2534T variant (S845), as reported in Supplemental Table 5. Transfection of two different duplexes PLCB3 siRNA reduced significantly, albeit partially, the levels of expression of PLCB3 mRNA (Fig. 1A) and protein (Fig.1B–E), as detected by quantitative RT-PCR and confocal immunofluorescence, respectively. No significant reduction of transcript levels of PLC isozymes beta 1, 2 and 4 was observed with PLCB3 siRNA in IB3-1 cells (Supplemental Figure S1). Infection with P.aeruginosa did not change significantly the levels of PLCB3 mRNA. In the same experimental model, partial silencing of PLCB3 produced a parallel reduction of IL-8 transcription and release in IB3-1 cells exposed to P.aeruginosa (Fig. 1 F and H), without changing the basal IL-8 mRNA levels in uninfected cells. Silencing PLCB3 did reduce the IL-8 transcription and release induced by flagellin, a component of P.aeruginosa interacting with TLR5, but did not affect the TNF-α-dependent IL-8 expression (Fig. 1 G and H), suggesting that PLCB3 may have a role in downstream signaling of TLR5 but not TNFRs. The effect of silencing PLCB3 gene seems mainly restricted to IL-8, since the expression of other genes induced by P.aeruginosa in bronchial epithelial cells, such as ICAM-1, GRO-γ, IL-6 and TNF-α, is not reduced (Fig 1 I). The reduction of IL-8 expression after silencing PLCB3 was confirmed in the CF human bronchial epithelial cell lines CuFi-1 grown polarized on Transwell filters, after exposure to P.aeruginosa on the apical side (Fig. 2). These results provide the first evidence that PLCB3 could be one of the components of a signaling network involved in the expression of IL-8 in human bronchial epithelial cells exposed to P.aeruginosa.
FIGURE 1.
Silencing PLCB3 reduces P.aeruginosa-dependent expression and release of IL-8 in human bronchial epithelial IB3-1 cells. A, Quantitative expression of PLCB3 mRNA by qRT-PCR after transfection with PLCB3 siRNA or scrambled oligonucleotides sequences 1 and 2 for 24 hrs and subsequent infection with PAO1 (100 CFU/cell) for further 4 hrs. The mRNA expression reported in Y-axis is relative to scrambled-treated uninfected cells. Mean ± S.E.M. of 8 independent experiments performed in duplicate. B, Immunofluorescence signal of PLCB3 protein in IB3-1 cells transfected with scrambled oligonucleotide sequence 1 or C, PLCB3 siRNA oligonucleotide sequence, in the presence of primary anti-PLCB3 antibody D, or irrelevant antibody. E, Quantification of the fluorescence signal as % Fluorescence Arbitrary Units (F.A.U.) related to the expression of PLCB3 protein of IB3-1 cells treated with scrambled versus PLCB3 siRNA oligonucleotides. Percentual reduction of 36.8 ± 6.6 of the fluorescent signal, of 10 cells treated with PLCB3 siRNA and 10 cells treated with scrambled oligonucleotides, respectively. F, Quantitative expression of IL-8 mRNA after transfection with PLCB3 siRNA sequence 1 and 2 or scrambled oligonucleotides in IB3-1 cells as described for panel A. Mean + S.E.M. of 5 independent experiments performed in duplicate. G, Effect of PLCB3 siRNA (sequence 1) on IL-8 transcription induced by flagellin (10 µg/ml) and TNF-α (50 ng/ml). Mean + S.E.M. of 3 independent experiments performed in duplicate. H, Effect of PAO1 (100 CFU/cell), flagellin (10 µg/ml) and TNF-α (50 ng/ml) on IL-8 protein release, treated as in panel A with PLCB3 siRNA (sequence 1) or scrambled oligonucleotide. Mean ± S.E.M. of 3 independent experiments performed in duplicate. I, Effect of PAO1 (100 CFU/cell) in IB3-1 cells transfected with PLCB3 siRNA (sequences 1 or 2) on ICAM-1, GRO-γ, IL-6 and TNF-α mRNA transcription. Mean ± S.E.M. of 3 independent experiments performed in duplicate.
FIGURE 2.
Silencing PLCB3 reduces P.aeruginosa-dependent expression and release of IL-8 in polarized human bronchial CF CuFi-1 cells. A, Quantitative expression of PLCB3 mRNA by qRT-PCR after transfection with PLCB3 siRNA sequences 1 and 2 or scrambled oligonucleotides for 24 hrs and subsequent infection with PAO1 (100 CFU/cell) for further 4 hrs. The mRNA expression reported in Y-axis is relative to scrambled-treated uninfected cells. Mean ± S.E.M. of 4 independent experiments performed in duplicate. B, Levels of IL-8 mRNA in the same experiments reported in panel A. C, Release of IL-8 protein in the same experiments reported in panel A with PLCB3 siRNA sequence 1. IL-8 protein concentration refers to that collected from the apical side of the Transwell® insert filter. Mean ± S.E.M. of 3 separate experiments performed in duplicate.
PLCB3 is implicated in Ca2+-related signaling and activation of protein kinase C (PKC) alpha and beta
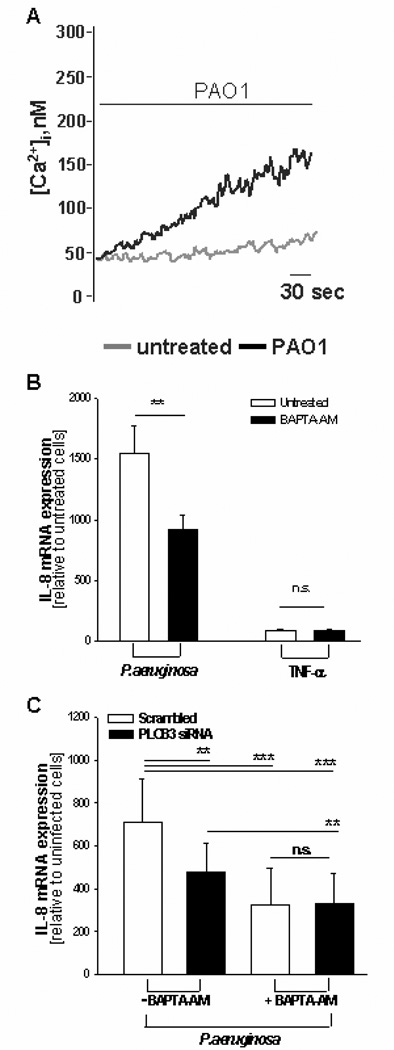
Like the other phospholipase C (PLC) isoforms, PLCB3 catalyzes the hydrolysis of phosphatidylinositol 4,5-biphosphate (PIP2) to generate two second messengers, diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which in turn activate intracellular calcium transients (24, 25). Notably, it has been recently shown that PLCB3 is a critical regulator of intracellular Ca2+ in murine macrophages (26). Exposure of IB3-1 cells to P.aeruginosa induced a sustained increase of cytosolic Ca2+ concentration ([Ca2+]c) as measured using fura-2 technique (Fig. 3A). Conversely, buffering the increase of [Ca2+]c with the intracellular Ca2+-chelator BAPTA reduced significantly the induction of IL-8 mRNA (Fig. 3B), as already reported by other investigators (13, 27). BAPTA further reduced the IL-8 mRNA expression in cells silenced for PLCB3 (Fig 3C), but in BAPTA-loaded cells PLCB3 silencing did not reduce IL-8 expression below the level detected in cells treated with the control siRNA. These findings suggest two set of conclusions. Firstly, that in our experimental model calcium signaling is not completely mediated by PLCB3. Notably, IB3-1 and CuFi-1 bronchial epithelial cells express different PLC isoforms besides the beta ones, in particular PLCγ2, PLCδ3 and PLCε1, which could participate in activation of calcium transients (Supplemental Figure S2). Secondly, that silencing of PLCB has an effect only because it reduces calcium signaling because in cells in which calcium is chelated by BAPTA no further reduction of IL-8 expression is induced by PLCB3 siRNA.
FIGURE 3.
Intracellular calcium chelator BAPTA inhibits the transcription of IL-8 mRNA induced by P.aeruginosa. A, Cytosolic free Ca2+ concentration as measured with Fura-2/AM assay in IB3-1 cells exposed to PAO1 (black trace) or with tissue culture medium alone (grey trace). Traces are representative of 8 independent experiments. B, The intracellular Ca2+-chelator BAPTA-AM (5 mM) was pre-incubated with IB3-1 cells for 30 min at 37°C in order to obtain diesterification of the acetoxymethyl ester. Cells were exposed to PAO1 (100 CFU/cell) or Tumor Necrosis Factor (TNF)-α (50 ng ml) for further 4 hrs before extraction of total RNA and qRT-PCR. Data are mean ± S.E.M. of 3 separate experiments performed in duplicate. C, After pre-incubating IB3-1 cells with PLCB3 siRNA sequence 1 or scrambled oligonucleotides for 24 hrs, BAPTA-AM was added for 30 min at 37°C, to obtain intracellular partition and diesterification of the acetoxymethylester. Infection with PAO1 strain was performed for further 4 hrs, total RNA was extracted and IL-8 mRNA was quantified by qRT-PCR. Data are mean ± S.E.M. of 4 separate experiments performed in duplicate.
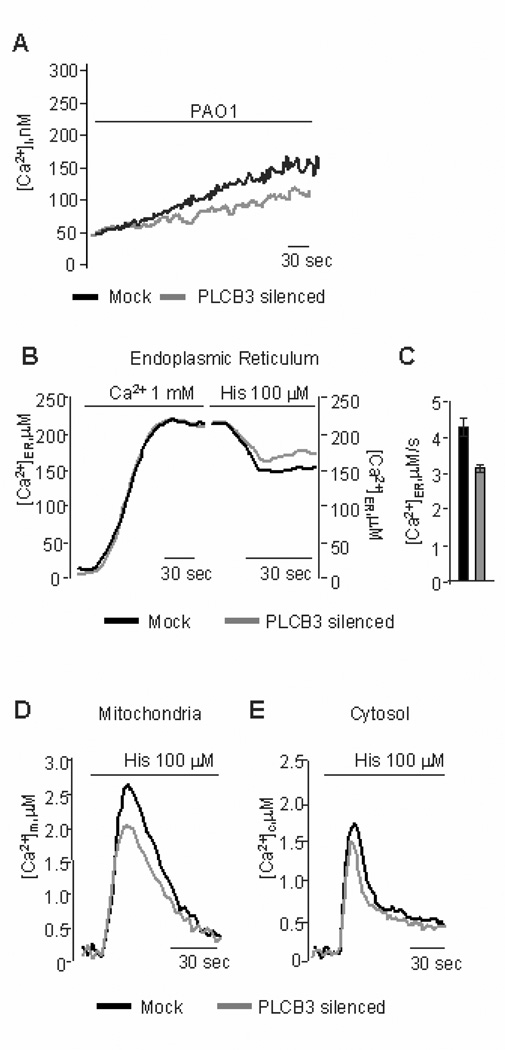
The role of PLCB3 in intracellular Ca2+ signaling upon interaction of P.aeruginosa with bronchial epithelial cells was studied in some details. The [Ca2+]c increase promoted by P.aeruginosa was reduced in IB3-1 cells pre-incubated with PLCB3 siRNA (Fig. 4A), although not completely, possibly due to a parallel partial reduction of PLCB3 expression. By inducing [Ca2+]c transients with histamine (causing the generation of inositol 1,4,5 trisphosphate and the consequent release of Ca2+ from the endoplasmic reticulum, ER) in IB3-1 cells expressing the intracellular organelle-targeted Ca2+-sensitive aequorins, no difference in ER Ca2+ uptake was observed between silenced PLCB3 and control cells. Indeed, both groups of cells showed very similar luminal ER Ca2+ concentrations ([Ca2+]er) at rest (see left part of Fig. 4B). On the contrary, we found that silencing PLCB3 reduces the release of Ca2+ from the ER (right part of Fig. 4B and C). Accordingly, the transient rises of [Ca2+]c and in the mitochondrial matrix ([Ca2+]m) elicited by the agonist were smaller in silenced PLCB3 cells compared to control cells (Fig. 4 D and E). Signals that elevate intracellular Ca2+ and DAG, such as those induced by PLCs, are known to activate conventional protein kinase C (PKC) isoforms (28). Activation of PKC was studied by observing the translocation of the recombinant GFP-tagged Ca2+- dependent conventional PKC isoforms alpha and beta to the plasma membrane in IB3-1 cells infected with P.aeruginosa. Bacterial exposure induced activation of the conventional PKC isoforms alpha and beta (Fig. 5 A and B), but not of the novel delta isoform (Fig. 5C) and of the atypical zeta isoform (Fig. 5D), the most striking effect being observed for PKC beta, where an average 56.1% of the cells showed membrane translocation. By silencing PLCB3 expression, the activation of PKC beta was abrogated in the majority of the IB3-1 cells (Fig. 6A and B), a residual PKC beta activation being observed only in an average 12.0 % of cells exposed to P.aeruginosa. The role of PKC activation in the P.aeruginosa-but not TNF-α-dependent signaling of our experimental model has been also confirmed by the effect of the broad PKC inhibitor bisindolylmaleimide (BIM)-I (Fig. 6C). It has been previously shown that P.aeruginosa PAO1 strain activates a Ca2+-dependent activation of the transcription factor NF-kB, which is critical in the regulation of IL-8 gene transcription also in human airway epithelial cells (27). Therefore, we tested the role of PLCB3 on the activation of NF-kB p65 induced by PAO1 in IB3-1 cells, with a time-course preceding the lapse of time of 4 hrs chosen to measure IL-8 mRNA levels. We confirm that PAO1 progressively activates NF-kB and that silencing PLCB3 significantly reduces the P.aeruginosa-induced activation of NF-kB p65 (Fig. 6D). Collectively, these results indicate that PLCB3 plays a relevant role in triggering free calcium transients induced by P.aeruginosa in human bronchial epithelial cells, thus regulating the activation of the PKCs alpha and beta and of the nuclear transcription factor NF-kB.
FIGURE 4.
Silencing PLCB3 affects the release of calcium from endoplasmic reticulum. IB3-1 cells were pre-treated with PLCB3 siRNA sequence 1 (grey traces) or scrambled (black traces) oligonucleotides, respectively, for 24 hrs before measuring intracellular Ca2+ transients. A, intracellular free [Ca2+]i with Fura-2/AM assay after addition of PAO1 (100 CFU/cell), n = 8. B, Endoplasmic reticulum calcium concentration ([Ca2+]er) by endoplasmic reticulum-targeted aequorin in the presence of 1 mM extra cellular calcium or with the addition of histamine (His, 100 mM) for the time indicated. Luminal ER Ca2+ concentration measurement was 212 ± 9.52 µM in mock vs. 215 ± 5.51 µM in PLCB3 silenced cells, n = 10. C, Variation of ER calcium release kinetic upon histamine addition expressed as mM/s: in mock 4.26 ± 0.33 vs 3.31 ± 0.13, p < 0.05, n = 10 PLCB3 silenced cells, respectively. D, Mitochondrial calcium concentration ([Ca2+]m) by mitochondrial-targeted aequorin with the addition of histamine, [Ca2+]m: mock 2.58 ± 0.19 µM vs. PLCB3 silenced 2.06 ± 0.23 µM, n = 10. E, cytosolic calcium concentration ([Ca2+]c) by native aequorin with the addition of histamine, [Ca2+]c: mock 1.65 ± 0.13 µM vs. PLCB3 silenced 1.50 ± 0.11 µM, n = 10.
FIGURE 6.
Silencing PLCB3 reduces the activation of PKC-beta and of NF-kB p65 induced by P.aeruginosa. A, IB3-1 cells were transfected with PLCB3 siRNA sequence 1 or B, scrambled oligonucleotides 24 hrs before the addition of PAO1 (100 CFU/cell). Images refer to representative experiments of expression of GFP-tagged PKC isoform beta, before and 30 min after exposure to PAO1. The PKC activator phorbol myristate acetate (PMA, 400 nM) was added to cells not responding to PAO1-dependent translocation, as an internal control. Translocation of PKC-beta from cytosol to membrane was observed in 56.1% of the IB3-1 cells transfected with scrambled sequence (on 57 scrambled cells 32 cells presented PKC-beta traslocation) and in 12.0% (on 25 PLCB3 siRNA cells only 3 cells presented PKC-beta traslocation) of cells transfected with PLCB3 siRNA sequence. The histograms indicate the intracellular localization of PKCβ, expressed as ratio of translocation from cytosol to plasma membrane. Averaging of % ratio in silenced cells: after 30 min to PAO +1% ± 10.3 and after treatment with PMA +275% ± 21.5 respect to time zero; in mock cells: after 30 min to PAO +78% ± 24.6. C, IB3-1 cells were pre-incubated with the PKCs inhibitor bisindolylmaleimide (BIM)-I (2 µM) for 30 mins and infected with PAO1 or stimulated with TNF-α for further 4 hrs, before quantitation of IL-8 mRNA by qRT-PCR. Data are mean ± S.E.M. of 3 separate experiments performed in duplicate. D, Activation of NF-kB p65 in IB3-1 cells transfected with either PLCB3 siRNA sequence 1 or scrambled oligonucleotide for 24 hrs before exposure to PAO1 (100 CFU/cell) or solvent alone in a lapse of time ranging from 1 to 4 hrs. Absorbance at 450 nm wavelength is proportional to the activation of NF-kB p65, as performed with the TransAM NF-kB p65 Activation Assay kit. Data are mean ± S.E.M. of 4 independent experiments performed in duplicate.
Extra cellular ATP is not sufficient for IL-8 expression but acts in synergy with TLRs
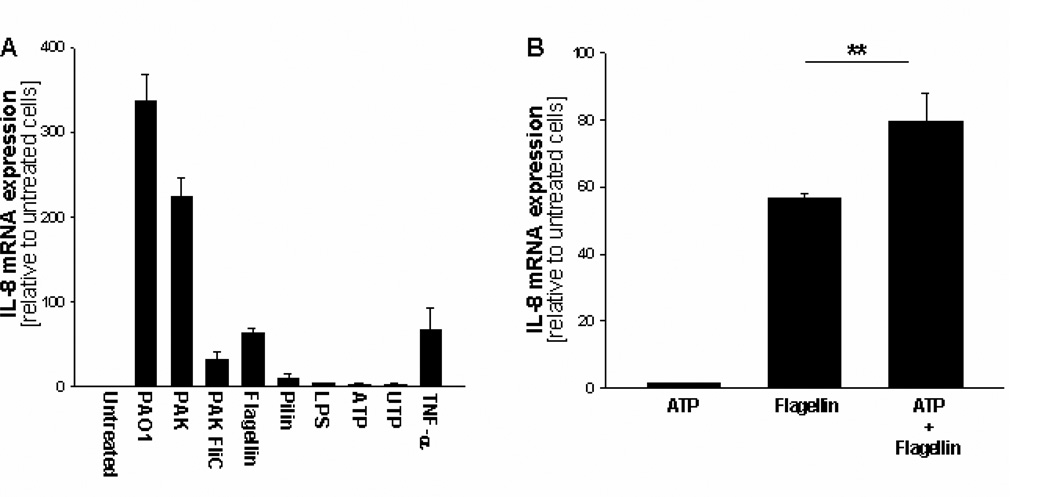
Interaction of P.aeruginosa with ASGM1R, colocalized with TLR5, is known to promote the release of nucleotides from epithelial cells, activating an autocrine loop with P2Y2 receptors (12, 13). Interestingly, PLCB3 has been shown to be selectively coupled to the P2Y2 receptor-dependent activation of intracellular Ca2+ transients in recombinant CHO cells (29). The role of different ligands on the expression of IL-8 has been preliminarily tested. IL-8 transcription was induced, albeit at different extents, after exposing IB3-1 cells to intact P. aeruginosa bacteria of strains PAO1 and PAK, to the purified P.aeruginosa bacterial components flagellin and pilin, and to the pro-inflammatory cytokine TNF-α (Fig. 7A). PAK FliC, a recombinant P.aeruginosa PAK strain lacking expression of flagellin, induces IL-8 expression at a level lower than that observed with PAK (Fig. 7A), suggesting a strong contribution of bacterial flagellum in this signaling pathway. On the contrary, no significant induction was obtained with classical ligands of TLR4 and P2Y2R, such as LPS and ATP/UTP, respectively (Fig. 7A). Since ATP-dependent induction of IL-8 in bronchial epithelial cells has been previously described only in association with ligands activating TLRs (30), we tested the effect of ATP on the expression of IL-8 upon stimulation with flagellin, which interacts with TLR5. We observed that ATP is not sufficient by itself to induce IL-8 expression, but it is able to potentiate the flagellin-induced one (Fig. 7B). In order to verify that P.aeruginosa induces an autocrine loop of release of nucleotides, we tested the effect of the ectonucleotidase apyrase in our model system. Pre-incubation of IB3-1 cells with apyrase before exposure to P.aeruginosa PAO1 strain, reduced the sustained increase of [Ca2+]c (Fig. 8A), the activation of PKC isoform beta (Fig. 8B and C) and, more relevantly, the IL-8 mRNA transcription and release of IL-8 protein (Fig. 8D and E). Interestingly, apyrase does not further reduce the P.aeruginosa-dependent IL-8 expression (Fig. 8F), suggesting that the contribution of the purinergic receptor-dependent IL-8 expression is mainly mediated its coupling with PLCB3. This confirms a role of extra cellular nucleotides, released upon interaction of P.aeruginosa with IB3-1 bronchial epithelial cells, in the pro-inflammatory signaling leading to the expression and secretion of IL-8 in our model system.
FIGURE 7.
ATP is not sufficient to activate IL-8 mRNA transcription but acts in synergy with TLR-dependent signaling. A, IB3-1 cells were exposed to the P.aeruginosa laboratory strains PAO1, PAK or PAK FliC (100 CFU/cell), to flagellin (10 mg/ml) and pilin (10 mg/ml) purified from PAK recombinant cells, to lipopolysaccharide (LPS, 10 mg/ml), to ATP and UTP (1 mM) or to TNF-α (50 ng/ml) for 4 hrs before extraction of total RNA and measurement of IL-8 mRNA. B, Similarly to panel A, IB3-1 cells were exposed to ATP or flagellin alone, or to both stimulants together, for 4 hrs before extraction of total RNA and measurement of IL-8 mRNA. Mean ± S.E.M. of 3 independent experiments performed in duplicate.
FIGURE 8.
The ectonucleotidase apyrase affects calcium signaling and IL-8 expression induced by P.aeruginosa. The ectonucleotidase apyrase (3 U/ml) was added to IB3-1 cells 2 hrs before and again together with the addition of PAO1 for further 4 hrs. A, Cytosolic Ca2+ transients by Fura-2/AM assay in IB3-1 cells exposed to PAO1 treated with apyrase (grey trace) or solvent alone (untreated, black trace), n = 8. B, Activation of PKC isoform beta in IB3-1 cells exposed to PAO1 treated either with apyrase or C, solvent alone (untreated). The PKC activator phorbol myristate acetate (PMA, 400 nM), a Ca2+-independent DAG-like agonist, was added to cells not responding to PAO1-dependent translocation, as an internal control. Translocation of PKC-beta from cytosol to membrane was observed in 56.1 % of the IB3-1 cells mock treated and in 25.9 % of cells treated with apyrase (on 27 pre-treated apyrase cells only 7 cells presented PKC-beta traslocation, after PAO1 exposition). Histograms show the intracellular localization of PKCβ isoform: averaging of % ratio in apyrase-treated cells: after 30 min to PAO +8% ± 2.6 and after PMA +89% ± 16.0 respect to time zero; in untreated cells: after 30 min to PAO +61% ± 15.2. D, IL-8 mRNA transcription was quantitated in IB3-1 cells exposed to PAO1 treated either with apyrase or solvent alone. E, As for panel C, the release of IL-8 protein was measured by ELISA in IB3-1 cells treated with apyrase or solvent alone. Mean ± S.E.M. of 3 independent experiments performed in duplicate. F, Apyrase (3 IU/ml) was incubated for 2 hrs at 37°C after a pre-incubation of 24 hrs with PLCB3 siRNA sequence 1 or scrambled oligonucleotides in IB3-1 cells. A further 4 hrs infection with PAO1 strain was performed and IL-8 transcription was quantified by qRT-PCR. Data are mean ± S.E.M. of 3 separate experiments performed in duplicate.
Discussion
Excessive inflammation in the lungs of patients affected by cystic fibrosis is considered a major cause of the lung tissue damage leading to respiratory insufficiency, and therefore anti-inflammatory drugs are included within the therapeutic pipeline of the innovative therapies to treat or cure CF lung disease (1). The identification of novel molecular targets in the pro-inflammatory signaling, which is firstly orchestrated by the bronchial epithelial cells on the surface of the conductive airways, is presently of paramount importance (2).
To prioritize the relevance of specific molecules within the large series of different receptors, kinases, phosphatases, phospholipases and adapters regulating the expression of inflammatory genes, we chose to apply an association study between genes of the innate immunity and the clinical progression of CF lung disease, by a genetic ranking approach. In this respect, the aim of our genetic analysis was not to find the strongest modifier gene(s) for CF lung disease, a task which should be presently pursued by Genome-Wide Association Studies (GWAS) in much larger cohorts of affected individuals, but to obtain hints on the relative clinical relevance within a list of genes selected in a family with homogeneous pathophysiological role, e.g. the signaling network of the innate immune response to bacteria in respiratory epithelial cells. Therefore, although the association of PLCB3 with clinical progression of lung disease that we found here is quite modest, our genetic ranking approach allowed us to focus on PLCB3 to demonstrate its role in regulating the expression of IL-8 elicited by P.aeruginosa in bronchial epithelial cells.
PLCs have been shown to be implicated in different cellular responses, due to their role in intracellular calcium homeostasis (for review see (25)). In particular, it was initially proposed that the PLCB3 isoform is involved in signal transduction triggered by hormones, growth factors and neurotransmitters (31). As far as its role in inflammatory processes is concerned, PLCB3 has been investigated in the context of leukocyte chemotaxis (32). PLC beta2- and beta3-dependent rise in intracellular calcium has been shown to regulate T lymphocyte chemotaxis (33). However, PLCB3-deficient neutrophils or macrophages, in which calcium transients were blunted, did not show reduction of chemotactic activity or migration (34, 35), suggesting that PLCB3 is required for the chemotaxis of T lymphocytes but not neutrophils or macrophages. Because T lymphocytes infiltrate the bronchial walls of CF patients, these early reports established already a possible link between PLCB3 and the progression of CF lung disease. Our findings strengthen this notion implicating PLCB3 in regulation of IL-8 expression by bronchial epithelial cells and hence neutrophil recruitment into the airways.
Bronchial epithelial cells do express different isoforms of PLC that could regulate intracellular calcium homeostasis and, in particular, IB3-1 and CuFi-1 cells express detectable transcript levels of PLC beta 1 and 4, gamma 2, delta 3 and epsilon 1, besides PLCB3 (Supplemental Figure S2). How can we explain the specific contribution of PLCB3 in the proinflammatory signaling induced by P.aeruginosa in bronchial epithelial cells ? Activation of gamma and epsilon isoforms is known to be dependent on tyrosine-kinase coupled receptors, of the delta isoforms on elevation of cytosolic calcium, of the beta isoforms on seven-membrane spanning domain receptors through GTP-binding proteins (25). As far as we know, among the surface receptors expressed in bronchial epithelial cell that are engaged by P.aeruginosa, TLRs and ASGM1R have not been described as coupled to GTP-binding proteins (36). However, it has been previously shown that the interaction of P.aeruginosa with bronchial epithelial cells induces the release of ATP in the extra cellular milieu, which binds to the seven-membrane spanning P2Y2 purinergic receptors (12, 13). Our results with the ectonucleotidase apyrase confirm a role of extra cellular ATP in the [Ca2+]c increase and report for the first time the activation of PKC alpha and beta by P. aeruginosa in an ATP-dependent manner (Fig. 8). Moreover, silencing experiments reducing cytosolic calcium increase and activation of PKC beta confirm the involvement of PLCB3 in the Ca2+ pathway activated by P.aeruginosa (Fig. 4 and 6). Thus, these results are consistent with the coupling of P2Y2 purinergic receptors with PLCB3, which is known to involve Gaq/11 heterotrimeric GTPase protein (29, 36). Because PLC beta 1 and 4 are also able to interact with seven-membrane spanning receptors, we can not definitely restrict to PLCB3 the role to modulate P.aeruginosa-dependent calcium transients in bronchial epithelial cells. Interestingly, in murine macrophages it has been shown that UDP can activate cytosolic calcium rise through purinergic receptors by using PLCB3 in parallel with PLC beta 4, whereas other ligands, such as C5a, utilize predominantly PLCB3 (26). Because, in our experimental model, selective silencing of PLCB3 resulted in a consistent, but not total, reduction of intracellular Ca2+-dependent expression of IL-8, we cannot exclude a partial contribution of the other PLC beta isoforms.
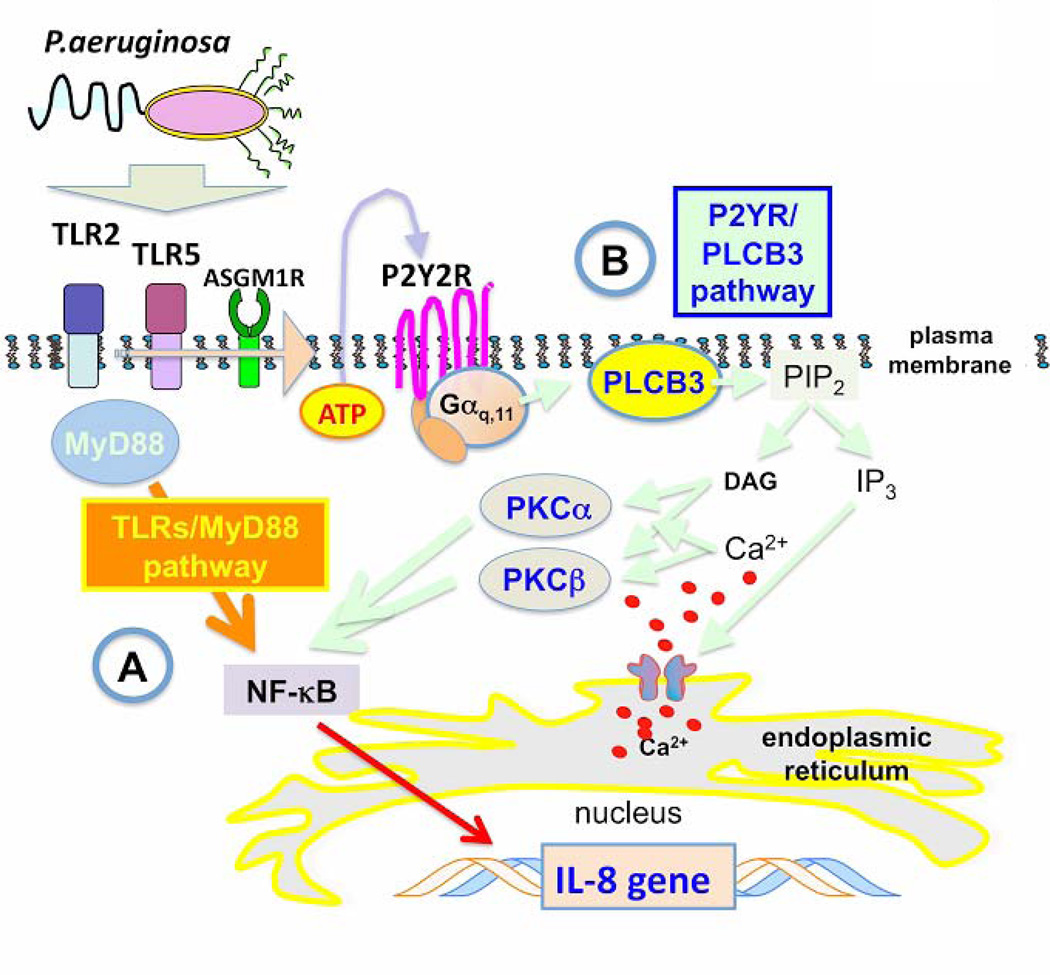
Silencing PLCB3 reduces only partially the P.aeruginosa-dependent expression of IL-8 (Fig. 1 and 2). This is not surprising at the light of the partial efficiency of PLCB3 silencing and of the evidence that P.aeruginosa activates the inflammatory response due to its capability to interact with multiple receptors, including TLRs and ASGM1Rs (37). Thus the ATP–P2Y2R autocrine loop that generates intracellular Ca2+-signaling should be considered only one of the pathways regulating IL-8 expression, in parallel with those elicited by TLRs via MyD88-dependent signals. We observed a significant reduction of IL-8 mRNA expression with the intracellular Ca2+ chelator BAPTA (Fig. 3), whereas direct stimulation of IB3-1 cells with P2Y2 ligands, such as ATP or UTP, that are known to stimulate directly cytosolic Ca2+ transients, were not sufficient to induce transcription of IL-8 mRNA (Fig.7), as previously observed by other investigators (30). This apparent discrepancy can be explained observing that the addition of ATP to the TLR5/2 and ASGM1R ligand flagellin increases IL-8 mRNA expression (Fig. 7), thus suggesting that the intracellular calcium signaling triggered by purinergic receptors upon release of ATP, albeit not sufficient by itself to completely activate the transcription machinery for IL-8 expression, works in synergy with TLRs-mediated signalling. As a further evidence that Ca2+ - signaling mediated by PLCB3 is indeed relevant to regulate IL-8 expression, we observed that silencing of PLCB3 significantly reduced the activation of the Nuclear Factor – kB (Fig. 6), which plays a critical role in the induction of IL-8 transcription (20, 29). Based on these and previous findings (12, 13), we conclude that in the CF airway tract chronically infected with P.aeruginosa, the Ca2+-dependent pathway induced by the release of nucleotides activates, through binding to P2Y2R, PLCB3 and amplifies the innate defense signaling based upon TLRs and ASGM1R. The cartoon reported in Fig. 9 summarizes our working hypothesis.
FIGURE 9.
Model of cooperation of PLCB3 in the P.aeruginosa-dependent signaling in bronchial epithelial cells. The cartoon depicts the signaling pathways elicited by TLRs/MyD88 (A) and P2Y2R/PLCB3 (B), based on previous reports from other investigators and the results presented here. A, Binding of P.aeruginosa surface components (flagellin, pilin) with TLR5 and TLR2 triggers a MyD88-dependent proinflammatory signaling cascade, eventually leading to nuclear translocation of NF-kB, which is a critical transcription factor for the expression of IL-8 gene, together with AP-1 and CHOP. TLRs/MyD88 pathway is sufficient to promote transcription of IL-8 gene. Besides exerting this direct effect, P.aeruginosa induces the extra cellular release of ATP, possibly via a cooperative interaction of TLR5, TLR2 and ASGM1R. B, Extra cellular ATP binds to P2Y2R that activates PLCB3 through the Gaq,11 heterotrimeric GTPase protein. By degrading PIP2, PLCB3 promotes IP3 release and DAG formation. IP3 triggers Ca2+ release from intracellular stores and rise of [Ca2+]c, together with DAG, promotes intracellular translocation of conventional PKC isoforms alpha and beta, which ultimately cooperate in activation of NF-kB. The P2Y2R/PLCB3 pathway is not sufficient to induce IL-8 transcription by itself but strongly act in synergy with the TLR/MyD88 signaling cascade.
The biological importance of intracellular calcium homeostasis in bronchial epithelial cells in the CF lung disease has been widely debated. For instance, it has been proposed that the defective chloride transport due to the mutated CFTR protein can be overcome by activating alternative Ca2+-dependent chloride channels, such as those recently identified (38, 39). Independently of the presence of a constitutive inflammation in CF bronchial epithelial cells, in which alteration of Ca2+ homeostasis might not play a role (39), when chronic bacterial infection intervenes in the first decades of life, the Ca2+ signaling in the bronchial epithelial cells greatly amplifies the recruitment and transepithelial migration of leukocytes, first of all polymorphonuclear neutrophils (40), that are considered unwilling actors of the progressive destruction of CF lung tissue (2). Thus, in the advanced stages of CF lung disease characterized by chronic bacterial infection, intracellular Ca2+ signals are known to induce endoplasmic reticulum Ca2+ store expansion leading to an overt hyper-inflammatory phenotype, with inappropriate release of chemokines and cytokines, that amplifies the recruitment and activation of leukocytes (41).
The inexorable decline of the lung function in patients affected by CF is presently faced with different approaches directed towards the mutated CFTR protein, alternative chloride channels, novel anti-inflammatory and anti-infective drugs (1). Consensus is growing on that effective causative therapies, when available, should be initiated as soon as possible, in order to prevent the onset of pulmonary complications. However, in patients who already developed bacterial infection, a novel anti-inflammatory approach directed towards the specific pathophysiology of infected CF lungs should be taken into consideration. In this respect, PLCB3, which relevantly regulates the extra cellular nucleotide-cytosolic Ca2+ signaling axis potentiating the Toll-like Receptors signaling cascade, represents a novel pharmacological target to attenuate the excessive recruitment of neutrophils without completely abolishing the inflammatory response.
Supplementary Material
Acknowledgements
We are indebt with the patients affected by CF who participated in this study and with the North American Gene Modifier Study Group (GMSG), that contributed in the collection of the DNA samples and the clinical classification of the CF patients, to A. Klingelhutz, P. Karp and J. Zabner (University of Iowa, Iowa City, IO) for CuFi cells, to A. Prince (Columbia University, New York, NY) for P.aeruginosa laboratory strains, to M. Cristina Dechecchi (Verona) for helpful discussion, to Federica Quiri and to Valentina Lovato (Verona) for excellent technical assistance.
This work was supported by the Italian Cystic Fibrosis Foundation (grants FFC # 3/2008, FFC # 8/2009, FFC # 13/2009, FFC # 18/2009, FFC # 12/2010 to P.G., G.C. and P.P.), the Italian Association for Cancer Research (AIRC), Telethon (GGP09128), local funds from the University of Ferrara, the Italian Ministry of Education, University and Research and the Italian Ministry of Health (to P.P.), NIH grants HL68890 and DK27651, and American Cystic Fibrosis Foundation grants DRUMM00A0, KNOWLES00A0 and RDPR026 (to M.L.D. and M.R.K.). V.B. is fellow of the Italian Cystic Fibrosis Research Foundation and E.N. of the “Azienda Ospedaliera Universitaria Integrata di Verona”.
Glossary
Abbreviations are
- TLR
Toll-like Receptor
- P2Y2R
purinergic receptor Y2, PLCB3, phospholipase C beta 3
- IL-8
interleukin 8
- ASGM1R
asialo-GM1 receptor
- AP-1
activator protein 1 nuclear transcription factor
- CHOP
CREB-HOmologous Protein transcription factor
- MyD88
Myeloid cell Differentiation adaptor protein induced by Inteleukin 1
- [Ca2+]c
cytosolic free calcium concentration
- PIP2
phosphatidylinositol 4,5-biphosphate
- DAG
diacylglycerol
- IP3
inositol 1,4,5-trisphosphate
Footnotes
The online version of this manuscript contains supplemental material
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Ashlock MA, Beall RJ, Hamblett NM, Konstan MW, Penland CM, Ramsey BW, Dalfsen JMVan, Wetmore DR, 3rd PWCampbell. A pipeline of therapies for cystic fibrosis. Semin. Respir. Crit. Care Med. 2009;30:611–626. doi: 10.1055/s-0029-1238919. [DOI] [PubMed] [Google Scholar]
- 2.Nichols DP, Konstan MW, Chmiel JF. Anti-inflammatory therapies for cystic fibrosis-related lung disease. Clin. Rev. Allergy Immunol. 2008;35:135–153. doi: 10.1007/s12016-008-8081-2. [DOI] [PubMed] [Google Scholar]
- 3.Welsh MJ, Ramsey BW, Accurso FJ, Cutting GR. Cystic Fibrosis. In: Scriver CR, Beaud AL, Sly WS, Valle D, et al., editors. The metabolic and molecular bases of inherited disease. McGraw-Hill; New York, NY: 2001. pp. 5121–5188. [Google Scholar]
- 4.Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest. 2008;133:489–495. doi: 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- 5.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 6.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 7.Noah TL, Black HR, Cheng PW, Wood RE, Leigh MW. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J. Infect. Dis. 1997;175:638–647. doi: 10.1093/infdis/175.3.638. [DOI] [PubMed] [Google Scholar]
- 8.Hillian AD, Londono D, Dunn JM, Goddard KA, Pace RG, Knowles MR, Drumm ML CF Gene Modifier Study Group. Modulation of cystic fibrosis lung disease by variants in interleukin-8. Genes Immun. 2008;9:501–508. doi: 10.1038/gene.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 10.Muhlebach MS, Reed W, Noah TL. Quantitative cytokine gene expression in CF airway. Pediatr Pulmonol. 2004;37:393–389. doi: 10.1002/ppul.20010. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 12.McNamara N, Khong A, McKemy D, Caterina M, Boyer J, Julius D, Basbaum C. ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9086–9091. doi: 10.1073/pnas.161290898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am. J. Respir. Cell. Mol. Biol. 2004;30:627–634. doi: 10.1165/rcmb.2003-0260OC. [DOI] [PubMed] [Google Scholar]
- 14.McNamara N, Gallup M, Sucher A, Maltseva I, McKemy D, Basbaum C. AsialoGM1 and TLR5 cooperate in flagellin-induced nucleotide signaling to activate Erk1/2. Am. J. Respir. Cell. Mol. Biol. 2006;34:653–660. doi: 10.1165/rcmb.2005-0441OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garred P, Pressler T, Madsen HO, Frederiksen B, Svejgaard A, Hoiby N, Schwartz M, Koch C. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J. Clin. Invest. 1999;104:431–437. doi: 10.1172/JCI6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, Zariwala M, Fargo D, Xu A, Dunn JM, Darrah RJ, Dorfman R, Sandford AJ, Corey M, Zielenski J, Durie P, Goddard K, Yankaskas JR, Wright FA, Knowles; MR Gene Modifier Study Group. Genetic modifiers of lung disease in cystic fibrosis. N. Engl. J. Med. 2005;353:1443–1453. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y, Harley IT, Henderson LB, Aronow BJ, Vietor I, Huber LA, Harley JB, Kilpatrick JR, Langefeld CD, Williams AH, Jegga AG, Chen J, Wills-Karp M, Arshad SH, Ewart SL, Thio CL, Flick LM, Filippi MD, Grimes HL, Drumm ML, Cutting GR, Knowles MR, Karp CL. Identification of IFRD1 as a modifier gene for cystic fibrosis lung disease. Nature. 2009;458:1039–1042. doi: 10.1038/nature07811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeitlin PL, Lu L, Rhim J, Cutting G, Stetten G, Kieffer KA, Craig R, Guggino WB. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am. J. Respir. Cell. Mol. Biol. 1991;4:313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]
- 19.Zabner J, Karp P, Seiler M, Phillips SL, Mitchell CJ, Saavedra M, Welsh M, Klingelhutz AJ. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:L844–L854. doi: 10.1152/ajplung.00355.2002. [DOI] [PubMed] [Google Scholar]
- 20.Bezzerri V, Borgatti M, Nicolis E, Lampronti I, Dechecchi MC, Mancini I, Rizzotti P, Gambari R, Cabrini G. Transcription factor oligodeoxynucleotides to NF-kappaB inhibit transcription of IL-8 in bronchial cells. Am. J. Respir. Cell. Mol. Biol. 2008;39:86–96. doi: 10.1165/rcmb.2007-0176OC. [DOI] [PubMed] [Google Scholar]
- 21.Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 2003;4:1247–1253. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 22.Castric P. PilO, a gene required for glycosylation of Pseudomonas aeruginosa 1244 pilin. Microbiology. 1995;141:1247–1254. doi: 10.1099/13500872-141-5-1247. [DOI] [PubMed] [Google Scholar]
- 23.Pinton P, Rimessi A, Romagnoli A, Prandini A, Rizzuto R. Biosensors for the detection of calcium and pH. Methods Cell. Biol. 2007;80:297–325. doi: 10.1016/S0091-679X(06)80015-4. [DOI] [PubMed] [Google Scholar]
- 24.Katan M. Families of phosphoinositide-specific phospholipase C: structure and function. Biochim. Biophys. Acta. 1998;1436:5–17. doi: 10.1016/s0005-2760(98)00125-8. [DOI] [PubMed] [Google Scholar]
- 25.Suh PG, Park JI, Manzoli L, Cocco L, Peak JC, Katan M, Fukami K, Kataoka T, Yun S, Ryu SH. Multiple roles of phosphoinositide-specific phospholipase C isozymes. B.M.B. Rep. 2008;41:415–434. doi: 10.5483/bmbrep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- 26.Roach TI, Rebres RA, Fraser ID, Decamp DL, Lin K-M-, Sternweis PC, Simon MI, Seaman WE. Signaling and cross-talk by C5a and UDP in macrophages selectively use PLCbeta3 to regulate intracellular free calcium. J. Biol. Chem. 2008;283:17351–17361. doi: 10.1074/jbc.M800907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratner AJ, Bryan R, Weber A, Nguyen S, Barnes D, Pitt A, Gelber S, Cheung A, Prince A. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J. Biol. Chem. 2001;276:19267–19275. doi: 10.1074/jbc.M007703200. [DOI] [PubMed] [Google Scholar]
- 28.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strassheim D, Williams CL. P2Y2 purinergic and M3 muscarinic acetylcholine receptors activate different phospholipase C-beta isoforms that are uniquely susceptible to protein kinase C-dependent phosphorylation and inactivation. J. Biol. Chem. 2000;275:39767–39772. doi: 10.1074/jbc.M007775200. [DOI] [PubMed] [Google Scholar]
- 30.Fu Z, Bettega K, Carroll S, Buchholz KR, Machen TE. Role of Ca2+ in responses of airway epithelia to Pseudomonas aeruginosa, flagellin, ATP, and thapsigargin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L353–L364. doi: 10.1152/ajplung.00042.2006. [DOI] [PubMed] [Google Scholar]
- 31.Lagercrantz J, Carson E, Phelan C, Grimmond S, Rosen A, Daré E, Nordenskjold M, Hayward NK, Larsson C, Weber G. Genomic organization and complete cDNA sequence of the human phosphoinositide-specific phospholipase C beta 3 gene (PLCB3) Genomics. 1995;26:467–472. doi: 10.1016/0888-7543(95)80164-h. [DOI] [PubMed] [Google Scholar]
- 32.Van Haastert PJ, Devreotes PN. Chemotaxis: signaling the way forward. Nat Rev Mol Cell Biol. 2004;5:626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]
- 33.Bach TL, Chen QM, Kerr WT, Wang Y, Lian L, Choi JK, Wu D, Kazanietz MG, Koretzky GA, Zigmond S, Abrams CS. Phospholipase Cbeta is critical for T cell chemotaxis. J. Immunol. 2007;179:2223–2227. doi: 10.4049/jimmunol.179.4.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Liu B, Wang P, Dong X, Fernandez-Hernando C, Li Z, Hla T, Li Z, Claffey K, Smith JD, Wu D. Phospholipase C beta3 deficiency leads to macrophage hypersensitivity to apoptotic induction and reduction of atherosclerosis in mice. J. Clin. Invest. 2008;118:195–204. doi: 10.1172/JCI33139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 37.Prince A. Flagellar activation of epithelial signaling. Am. J. Respir. Cell. Mol. Biol. 2006;34:548–551. doi: 10.1165/rcmb.2006-0022SF. [DOI] [PubMed] [Google Scholar]
- 38.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 39.Clunes MT, Boucher RC. Front-runners for pharmacotherapeutic correction of the airway ion transport defect in cystic fibrosis. Curr. Opin. Pharmacol. 2008;8:292–299. doi: 10.1016/j.coph.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chun J, Prince A. Ca2+ signaling in airway epithelial cells facilitates leukocyte recruitment and transepithelial migration. J. Leukoc. Biol. 2009;86:1135–1144. doi: 10.1189/jlb.0209072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones W, O'neal L, Boucher RC. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J. Biol. Chem. 2005;280:17798–17806. doi: 10.1074/jbc.M410618200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.