Abstract
Background
Given numerous reports implicating involvement of the precuneus in cue-reactivity paradigms, the goal of this investigation was to examine the relationship between activation of the precuneus in response to drug cues and measures of subjective craving and severity of dependence in volunteers who were comorbid for alcohol and nicotine abuse.
Methods
Forty research participants, who all reported heavy drinking and daily smoking, were recruited (15 women; 70% Caucasian; mean age = 31.2 years) for a functional magnetic resonance imaging (fMRI) session involving a cigarette video-cues task and an alcohol taste-cues task. Mean precuneus activation from both tasks during cue presentation was subjected to bivariate correlation analyses with indices of dependence severity and subjective craving.
Results
Precuneus activation in the contrast of Cigarette Cues vs. Control Cues was positively correlated with scores on the Fagerström Test of Nicotine Dependence (r=0.389, p=0.016), and activation in the Alcohol Cues vs. Control Cues contrast was positively correlated with Alcohol Dependence Scale scores (r=0.338, p=0.038). No correlations with subjective craving were observed (ps>0.05).
Conclusions
These findings indicate that the precuneus is involved in cue reactivity for both cigarettes and alcohol, and that this involvement is moderated by severity of drug dependence. The precuneus may be a cortical locus for neuroplastic changes related to drug dependence.
Keywords: precuneus, cigarettes, alcohol use disorder, fMRI, cue-reactivity, craving
1. INTRODUCTION
Since the mid-1990s, numerous neuroimaging researchers have examined brain responses associated with cue-induced craving using variations of the cue-exposure paradigm (Grant et al., 1996). This paradigm is largely predicated on associative learning principles in that repeated pairing of drug cues with drug consumption produces conditioned reinforcement such that the drug cues become conditioned stimuli capable of eliciting craving (O’Brien et al., 1990), and that reactivity to drug cues plays an essential role in maintaining addictive behavior (e.g., Hogarth and Chase, 2011). Cigarette cue-exposure paradigms have historically involved pictorial cues (Bourque et al., 2013; King et al., 2010; McClernon et al., 2005, 2009), video cues (Culbertson et al., 2011), and even video cues paired with external cigarette-related stimuli (e.g., a cigarette placed in the hand; Brody et al., 2004; Franklin et al., 2007). As for alcohol, commonly employed versions of the cue-exposure paradigm in neuroimaging research include the use of alcohol-related pictures (Myrick et al., 2008; Vollstadt-Klein et al., 2010), alcohol taste cues (Filbey et al., 2008a; Myrick et al., 2008; Ray et al., 2013), and personalized scripts from alcohol-related situations (Seo et al., 2013). Due to their purported involvement in motivational value assignment and cognitive control, regions of focus in these investigations typically include the striatum, anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (dlPFC), and orbitofrontal cortex (OFC; e.g., Bonson et al., 2002; Brody et al., 2002; Claus et al., 2011; Filbey et al., 2008a). However, two recent meta-analyses have highlighted the role of the precuneus in alcohol and cigarette cue-reactivity. Specifically, the precuneus was identified, along with the posterior cingulate and superior temporal gyrus, as selectively affected by alcohol-cue presentation in samples of alcohol-dependent individuals (Schacht et al., 2013), and the precuneus was also implicated in response to smoking cues in samples of daily smokers (Engelmann et al., 2012). These findings suggest the precuneus may play a non-drug-specific role in cue-reactivity; however, no study to date has examined precuneus responses to alcohol and cigarette cues within the same research participants.
Located in the posteromedial portion of the parietal lobe, the precuneus has widespread connections with higher association cortical and subcortical structures. It has strong cortical interconnections to the prefrontal cortex as well as connections to the posterior cingulate and retrosplenial cortices, the dorsal premotor area, the supplementary motor area, and the anterior cingulate cortex. Major subcortical connections of the precuneus include the claustrum, the dorsolateral caudate nucleus, and the putamen (for a review see Cavanna and Trimble, 2006). Many of the same brain regions also have been implicated in reactivity to smoking and alcohol cues in the aforementioned meta-analyses. Activation of the precuneus within this larger network may reflect integration and relay of drug-cue information from the extended visual system to the systems involved in motivated behavior and choice (Engelmann et al., 2012). Further, the precuneus is putatively involved in self-centered, mental imagery strategies and successful episodic memory retrieval (Cavanna and Trimble, 2006), both of which are likely to play a role in drug cue-reactivity.
Despite the strong support for involvement of the precuneus in drug cue-reactivity, a proposed biological marker of cue-induced drug craving, few studies have observed correlations between self-reported, subjective craving and precuneus activation in response to drug cues. In a meta-analysis across drug-cue reactivity studies that included craving correlation analyses, only two of the 13 alcohol-cue studies (Park et al., 2007; Tapert et al., 2003), and one of the 15 nicotine-cue studies (Brody et al., 2007) identified by the authors contained a significant association between subjective craving and precuneus activation; furthermore, the meta-analysis itself failed to find this association (Chase et al., 2011). The absence of significant associations between self-reported craving and blood oxygen level-dependent (BOLD) response in many brain areas is not an uncommon finding in cue-exposure paradigms (e.g., Due et al., 2002; Filbey et al., 2008c; Heinz et al., 2004). It is possible that the precuneus, among other regions, plays a broader role in the phenomenon of substance dependence, functioning as an index of addiction further upstream from, or in parallel with, the experience of subjective craving per se. For example, the extent to which self-referential processing occurs during cue-reactivity may depend on one’s level of drug dependence severity (e.g., Broyd et al., 2009; Claus et al., 2013), which may or may not facilitate the subsequent experience of subjective craving in response to drug cues. Consistent with these interpretations, correlations between alcohol cue-elicited activation of the precuneus and a variety of measures of alcohol use disorder severity, including Alcohol Use Disorders Identification Test (AUDIT) score and years of heavy drinking have been previously reported (Claus et al., 2011).
In summary, the known involvement of the precuneus in self-centered mental imagery and episodic memory highlights a plausible mechanism in drug cue-reactivity. Based on the meta-analyses discussed, the recruitment of the precuneus in cue-reactivity paradigms appears to be largely consistent, although it remains unclear whether this region a non-drug specific index of addiction or responds selectively to reminders of one’s drug of choice. Further, the extent to which severity of dependence moderates precuneus reactivity to drug cues remains largely unknown. The goal of the present study, therefore, was to examine the role of the precuneus in drug cue reactivity, using both an alcohol- cue and cigarette-cue paradigm in a sample of heavy drinking, daily smokers. Specifically this study tests (a) precuneus activation in response to drug (alcohol and cigarette) vs. control cues, and (b) associations between precuneus activation during cue reactivity and measures of subjective craving and alcohol/nicotine dependence severity.
2. METHODS
2.1 Sample Characteristics
A community sample of non-treatment seeking individuals reporting daily cigarette smoking and heavy drinking was evaluated in the laboratory to investigate the effects of two medications (naltrexone and varenicline), alone and in combination, on subjective responses to nicotine and alcohol (n = 427). Recruitment occurred through community flyers and online advertisements. The protocol was approved by the University of California, Los Angeles Institutional Review Board. A total of 130 individuals were randomized to receive medication and, of those, a random sample of 40 heavy drinking smokers was invited to participate in the neuroimaging portion of the study. Inclusion criteria for the study were: 1) age between 21 and 55 years; 2) endorsement of smoking 10 or more cigarettes per day; 3) current status of heavy drinking according to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) guidelines (National Institute on Alcohol Abuse and Alcoholism, 1995): for men, >14 drinks per week or ≥5 drinks per occasion at least once per month over the past 12 months; for women, >7 drinks per week or ≥4 drinks per occasion at least once per month. Exclusion criteria were: 1) more than 3 months of smoking abstinence in the past year; 2) self-reported use of illicit drugs (other than marijuana) in the previous 60 days or positive urine toxicology result; 3) lifetime history of psychotic disorders, bipolar disorders, or major depression with suicidal ideation; 4) current symptoms of moderate depression (or higher), indexed by a score ≥20 on the Beck Depression Inventory-II (Beck, 1996); 5) ineligibility on physical exam and laboratory tests; and 6) MRI contraindications/constraints, including left-handedness.
2.2 Screening Procedures and Individual Difference Measures
Demographic information, including age, sex, ethnicity, and years of education, was collected from all participants. Also obtained were self-reports of cigarette and alcohol use, and indices of nicotine and alcohol dependence (Table 1). Independent t-tests or Pearson correlations were conducted on all demographic variables; no significant relationships between any demographic variables were observed (ps>0.05), except that FTND score was found to significantly correlate with years of education (r=0.321, p=0.044). Cigarette and alcohol use was assessed using the 30-day Timeline Follow-Back (TLFB; Sobell and Sobell, 1980), and dependence was assessed with the Fagerström Test of Nicotine Dependence (FTND; Heatherton et al., 1991) and Alcohol Dependence Scale (ADS; Skinner and Allen, 1982), respectively.
Table 1.
Demographic information, smoking behavior, and alcohol use.
| Variable | Mean (SD) or Frequency |
|---|---|
| Age | 31.17 (8.82) |
| Sex – Male/Female | 25/15 |
| Ethnicity | |
| - Caucasian | 28 |
| - African Am. | 7 |
| - Asian | 2 |
| - Latino | 3 |
| Education (years) | 14.55 (3.73) |
| Cigarettes Per Day | 14.69 (7.40) |
| Smoking Days per Month | 29.50 (1.13) |
| Drinking Days per Month | 20.77 (7.84) |
| Alcohol Drinks per Drinking Day | 66.18 (3.18) |
2.3 Neuroimaging Procedures
After screening, the 40 individuals selected for the neuroimaging portion of the study were randomized to one of four medication conditions: 25 mg/daily of naltrexone, 1mg/twice daily of varenicline, 25mg/daily and 1mg/twice daily of varenicline combined, or placebo (n=10 per group). Scanning occurred after 10–12 days on medication while at target dose. For the purposes of the present study, medication group represented a covariate of no interest and was controlled for in all fMRI analyses. The effects of the study medication on brain activation during drug cue presentation within this sample will be reported elsewhere. Participants were not required to abstain from smoking prior to the neuroimaging session; however, breath alcohol concentration (BrAC) was required to be 0.00g/dl.
2.3.1 Cigarette Cues Task
Participants underwent a cigarette cues task which consisted of viewing videotaped cues, developed by Brody et al. (2002). Similar tasks have been widely used in neuroimaging studies of tobacco users (Brody, 2006; King et al., 2010; McClernon and Gilbert, 2004; McClernon et al., 2005, 2008). Stimuli consisted of first-person perspective color videos with smoking content (e.g., person smoking a cigarette) or control content (e.g., person writing in a journal), each lasting 45 seconds. The paradigm consisted of 12 video cue trials (6 cigarette and 6 control) pseudorandomly presented across participants (first video always control), followed by an urge-rating period for a maximum of 10 seconds or until key press, 1 second of response feedback, and a 10-second rest period before initiation of the next trial. During the urge-rating period, participants were instructed to rate their current urge to smoke using a scale of 1 (no urge at all) to 4 (very high urge) using a four-button response box placed in their right hand. The presentation of all stimuli and response collection were programmed using MATLAB (Mathworks, Natick, MA) and the Psychtoolbox (www.psychtoolbox.org) on an Apple MacBook running Mac OSX (Apple Computers, Cupertino, CA). Visual stimuli were presented using MRI-compatible goggles (Resonance Technologies, Van Nuys, CA).
2.3.2 Alcohol Cues Task
Participants completed an alcohol taste-cue paradigm previously reported to elicit blood oxygen level dependent (BOLD) response in mesocorticolimbic areas (Filbey et al., 2008a, 2008b). Alcohol and control (water) taste stimuli were delivered via Teflon tubing using a computer-controlled delivery system (Infinity Controller) as described by Filbey et al. (2008a). The paradigm consisted of 12 taste-cue trials (6 alcohol and 6 control trials) in which 1ml of liquid was delivered. Each trial consisted of a 24-second taste delivery period, followed by a 6-second rest period, a 12-second urge-to-drink rating period, and a 2-second delay before the initiation of the next trial. The words “Alcohol Taste” or “Control Taste” were visually presented during cue delivery. During the urge rating period, participants were instructed to rate their current subjective urge to drink alcohol using a scale of 1 (no urge at all) to 4 (very high urge) using a four-button response box placed in their right hand. Sauvignon Blanc wine was used as a standardized beverage option as it enhanced the feasibility of the taste delivery and had general appeal to research participants (no subject expressed a “dislike” of the wine. The presentation of visual stimuli and response collection were programmed using E-Prime (Psychology Software Tools, Inc., Sharpsburg, PA), and visual stimuli were presented using MRI compatible goggles (Resonance Technologies, Van Nuys, CA).
2.4 MRI Data Acquisition, Preprocessing, and Registration
Neuroimaging was conducted using a 3 Tesla Siemens Trio MRI scanner. The protocol began with initial structural scans followed by a series of three functional runs during which participants completed three different task paradigms, including the cigarette and alcohol cues tasks. A T2-weighted, high resolution, matched-bandwidth, anatomical scan (MBW) (TR, 5s; TE, 34ms; FOV, 192mm; matrix, 128x128; sagittal plane; slice thickness, 4mm; 34 slices) and a magnetization-prepared rapid-acquisition gradient echo (MPRAGE) were acquired for each subject to enable registration (TR, 1.9s; TE, 2.26ms; FOV, 250mm; matrix, 256×256; sagittal plane; slice thickness, 1mm; 176 slices). The orientation for MBW and echoplanar image (EPI) scans was oblique axial to maximize brain coverage. The cigarette- and alcohol-cues scans included 100 and 184 functional T2*-weighted EPIs, respectively (slice thickness, 4mm; 34 slices; TR, 2s; TE, 30ms; flip angle, 90°; matrix, 64 × 64; FOV, 192mm; voxel size, 3×3×4mm3). The first six volumes of each scan were discarded to allow for T1 equilibrium effects.
FSL 4.1 (FMRIB‧s Software Library; www.fmrib.ox.ac.uk/fsl) was used for the imaging analyses. EPI images were motion corrected using the Motion Correction Linear Image Registration Tool (McFLIRT, Version 5.0) with the estimated motion parameters entered as covariates in the general linear model (GLM). The images were high-pass filtered (100-second cutoff) in the temporal domain using a Gaussian-weighted straight line with the FMRI Expert Analysis Tool (FEAT, Version 5.63). Non-brain tissue/skull removal was conducted for both structural and functional images with the Brain Extraction Tool (BET). Computed contrast images (see Statistics) were first registered to the MBW, then to the MPRAGE using affine linear transformations, and finally into standard space (Montreal Neurological Institute, MNI avg152 template). Linear registrations using FSL’s FLIRT were refined with FNIRT nonlinear registration (Andersson et al., 2007). Data from two subjects were excluded from further analyses due to excessive motion on one or both tasks (exceeding 3mm of translation).
2.5 Statistical Analyses
Statistical analyses on the fMRI data were performed using a multi-stage approach to implement a mixed-effects model treating participants as a random-effects variable. Explanatory variables for the cigarette-cues task were created by convolving stick functions representing the onset of the cigarette- or alcohol-cue period for each trial type with a double-gamma hemodynamic response function (HRF) in FEAT. The events modeled included: Cigarette Cue and Control Cue exposure for the cigarette-cues task and Alcohol Cue and Control Cue exposure for the alcohol-cues task. The onset for each cigarette-cue event was set at the initiation of the video cue with duration of 45 seconds. The onset for each alcohol taste cue event was set at the first instruction to swallow (10 seconds after the initial taste cue was presented) with duration of 20 seconds plus the response time for the urge-to-drink rating. Temporal derivatives were included as covariates of no interest to improve statistical sensitivity. The Cigarette Cues vs. Control Cues and Alcohol Cues vs. Control Cues contrasts were computed1.
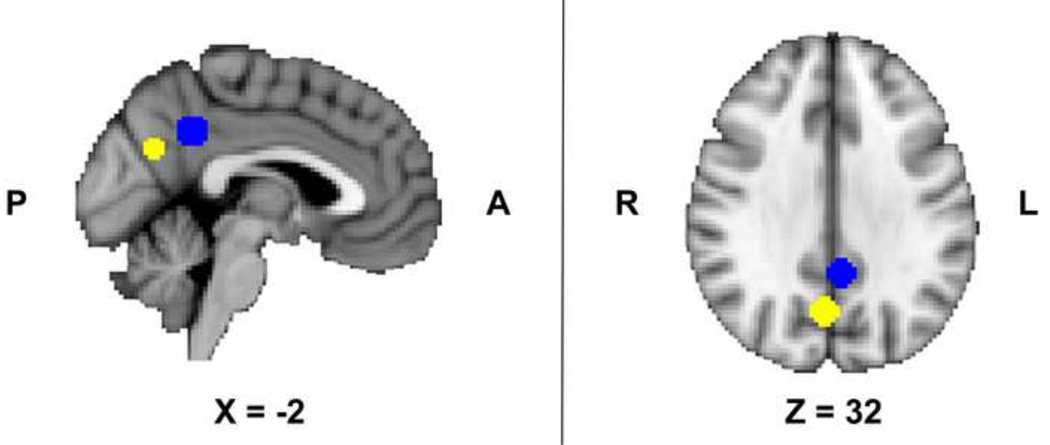
To enable an unbiased examination of the precuneus region of interest (ROI), we used a •leave-one-out• procedure, which allows definition of functional ROIs that are independent of the data submitted for analysis (Esterman et al., 2010). Thirty-eight whole-brain GLMs (using FEAT) were run for the Cigarette Cues vs. Control Cues and Alcohol Cues vs. Control Cues contrast images using medication group as a covariate of no interest. For each GLM, data from one participant was left out, and a spherical ROI (8mm radius) was defined around the peak voxel for each region. The left-out participant‧s data was extracted from this spherical ROI. An anatomically-defined mask from the Harvard-Oxford Probabilistic Brain Atlas was used to constrain the search space for finding the peak voxel within the precuneus. The mean peak voxels from the precuneus were located at the following MNI coordinates: x=2.4, y=−65.6, z=29.2 (Cigarette Cues vs. Control Cues) and x=−5, y=−47, z=37 (Alcohol Cues vs. Control Cues; Figure 1).
Figure 1.
Precuneus region of interest spheres (8mm radius) centered on the mean peak voxels produced using a “leave-one-out” procedure for the cigarette and alcohol cues tasks separately. The mean peak voxels from the precuneus were located at the following MNI coordinates: x=2.4, y=−65.6, z=29.2 (Cigarette Cues vs. Control Cues; depicted in yellow) and x=−5, y=−47, z=37 (Alcohol Cues vs. Control Cues; depicted in blue).
One-sample t-tests were conducted on the averaged ROI data from each drug vs. control cue contrast to determine statistical differences from 0. Bivariate Pearson correlations were run on the variables of interest: precuneus activation from the Cigarette Cues vs. Control Cues contrast (controlling for medication group), precuneus activation from the Alcohol Cues vs. Control Cues contrast (controlling for medication group), self-reported craving levels in response to cigarette cues, self-reported craving levels in response to alcohol cues, FTND total score, and ADS total score.
3. RESULTS
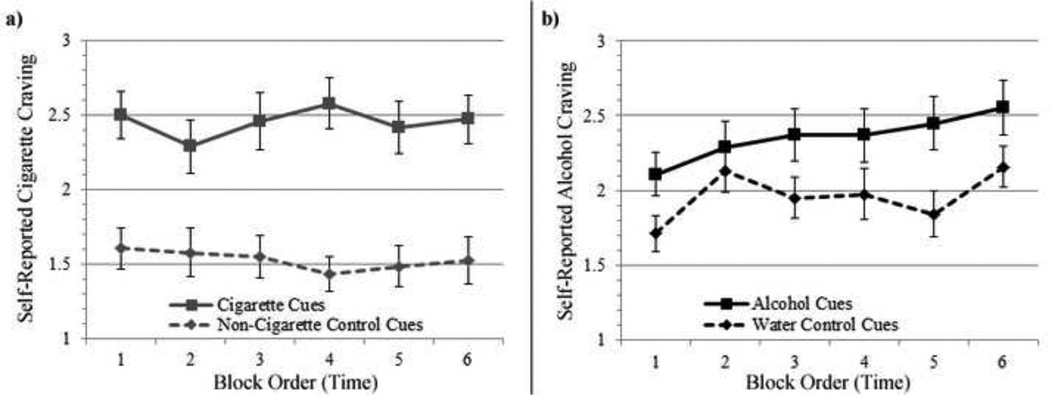
As expected, the alcohol and cigarette cues tasks were found to be effective in eliciting greater self-reported craving immediately following the respective drug cues, as compared to the neutral cues (Figure 2). Averaged across all subjects, activation of the precuneus in the relevant paradigms (controlling for medication group) was statistically significant (different from 0) in the Cigarette Cues vs. Control Cues contrast (t=4.71, p<.001), yet it did not reach statistical significance in the Alcohol Cues vs. Water Cues contrast (t=1.31, p=.198). Self-reported craving in response to alcohol and cigarette cues and years of education (which was positively associated with FTND score) were not associated with precuneus activation from either the alcohol or cigarette cue contrasts (ps>.05).
Figure 2.
Line plot of self-reported, subjective craving levels following a) cigarette and non-cigarette control cues, and b) alcohol and water control cues. *p <0.001.
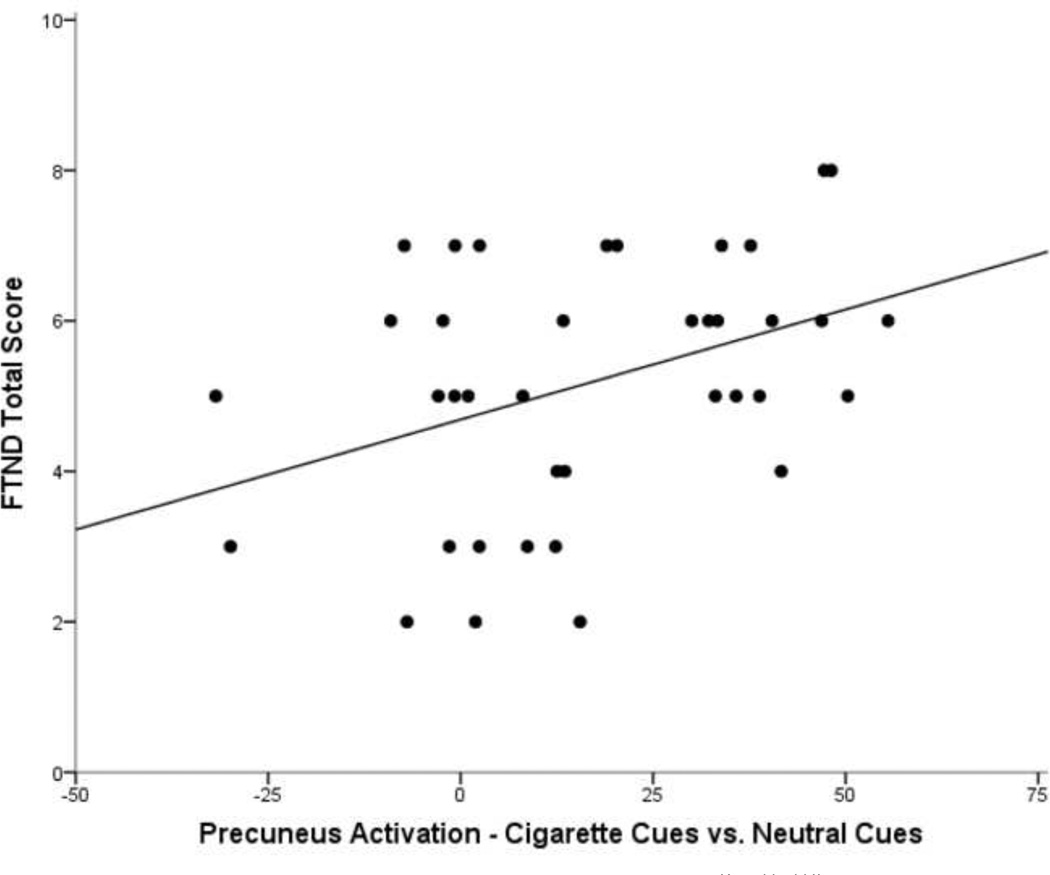
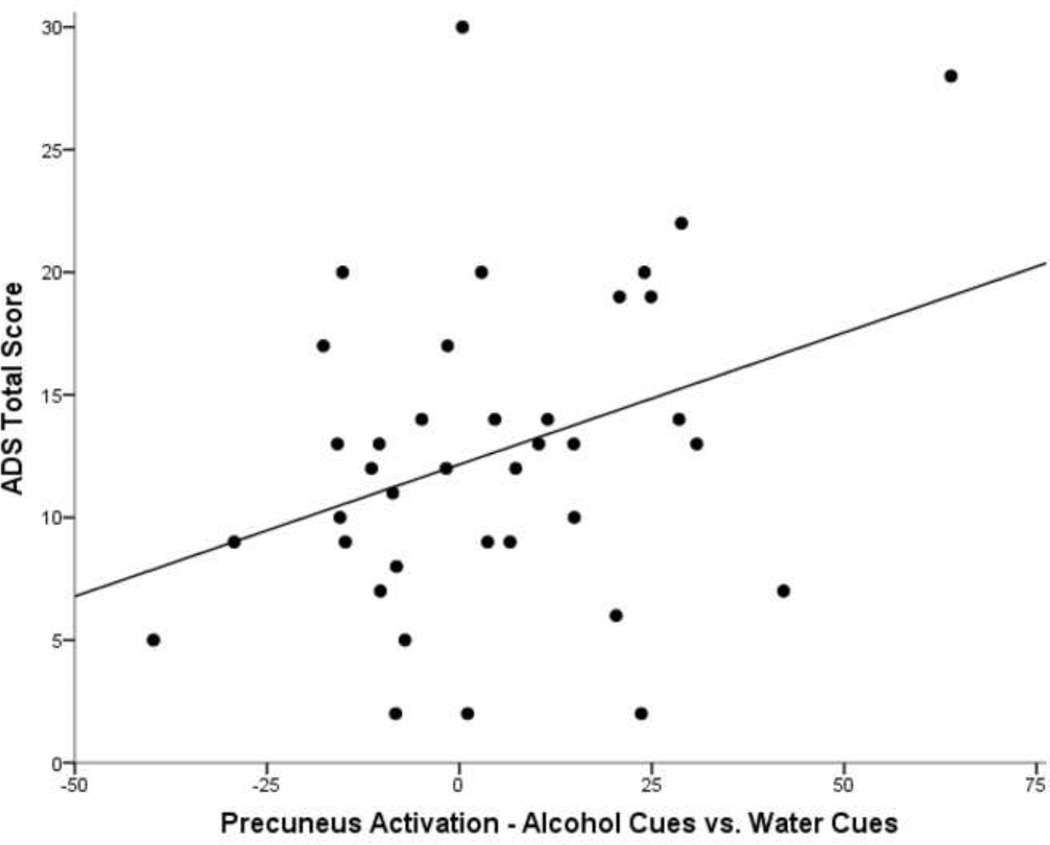
Precuneus activation from the cigarette and alcohol cue contrasts, however, revealed positive correlations with indices of nicotine dependence and alcohol dependence, respectively (Table 2). In the Cigarette Cues vs. Control Cues contrast, activation of the precuneus was significantly correlated with FTND total score (r=.389, p=.016; Figure 3), and precuneus activation in the Alcohol Cues vs. Control Cues contrast showed a significant correlation with ADS total score (r=.338, p=.038; Figure 4). ADS and FTND total scores were not significantly correlated with each other (p=.162). Further, ADS was not correlated with activation in the Cigarette Cues vs. Control Cues contrast, and FTND was not correlated with activation in the Alcohol Cues vs. Control Cues contrast (ps>.05), suggesting specificity of the relationship between severity and cue-reactivity to the substance in question (namely alcohol or cigarettes).
Table 2.
Correlations among dependence indices, self-reported craving measures, and precuneus activation from the Alcohol vs. Control and Cigarette vs. Control contrasts (controlling for medication group).
| Mean | SD | (1) | (2) | (3) | (4) | (5) | |
|---|---|---|---|---|---|---|---|
| (1) FTND Score | 5.15 | 1.64 | |||||
| (2) ADS Score | 12.70 | 6.39 | −.231 | ||||
| (3) Craving Rating following Cig. Cues | 24.95 | 14.25 | −.322* | .317 | |||
| (4) Craving Rating following Alc. Cues | 21.61 | 6.35 | −.153 | .109 | .576** | ||
| (5) Precuneus - Cigarette vs. Control | 16.91 | 22.24 | .389* | −.228 | −.092 | .048 | |
| (6) Precuneus - Alcohol vs. Control | 4.35 | 20.46 | −.154 | .338* | .202 | .303 | .088 |
Note: FTND=Fagerström Test of Nicotine Dependence; ADS=Alcohol Dependence Scale;
p<.05,
p<.01.
Figure 3.
Scatterplot of precuneus activation (fMRI parameter estimates) from the Cigarette Cues vs. Control Cues contrast (controlling for medication group) and Fagerström Test of Nicotine Dependence (FTND) score. The fit line represents a R2 = 0.151.
Figure 4.
Scatterplot of precuneus activation (fMRI parameter estimates) from the Alcohol Cues vs. Control Cues contrast (controlling for medication group) and Alcohol Dependence Scale (ADS) score. The fit line represents a R2 = 0.114.
Study medication group (varenicline alone, naltrexone alone, varenicline + naltrexone, or placebo), considered here as a covariate of no interest, accounted for a significant amount of variance in precuneus activation from the Cigarette Cues vs. Control Cues contrast. Specifically, differences were observed when comparing the varenicline group (M=4.44, SD=17.11) to the naltrexone group (M=34.85, SD=19.59; p=.008, Bonferroni corrected). No other medication group differences on precuneus activation were observed from the cigarette or alcohol task contrasts. Furthermore, independent t-tests comparing Fisher r-to-z transformed correlation coefficients indexing fMRI precuneus activation from the drug (alcohol or cigarette) vs. control cue contrasts and dependence severity for the respective drug within the four medication groups revealed no significant differences between medication groups (ps>0.25); nevertheless, medication group was controlled for in all analyses.
4. DISCUSSION
The literature on neurobiology of addiction has generally ignored the potential role of the precuneus in drug cue-reactivity despite multiple reports of activation in this region in response to cue exposure (e.g., Claus et al., 2013; Park et al., 2007; Tapert et al., 2003). Part of this reluctance may be due to the lack of consistent support for an association between precuneus cue-reactivity and self-reported craving (Chase et al., 2011). By capitalizing on the range of dual substance use in a sample of heavy-drinking daily smokers, this study tested the extent to which precuneus activation during alcohol and cigarette cue-reactivity is moderated by subjective craving and/or severity of alcohol and nicotine dependence within the same individuals. Consistent with the majority of the literature, no correlations were observed between precuneus cue-reactivity and self-reported craving during exposure to either alcohol or cigarette cues. There were, however, significant positive associations between dependence severity and precuneus activation in response to the respective drug cues, in line with research indicating a moderating role of drug dependence severity on self-referential processing (e.g., Broyd et al., 2009; Claus et al., 2013), and suggesting that drug dependence severity may, at least in part, moderate this relationship via the precuneus.
Since cigarette and alcohol dependence frequently co-occur within the same individuals, it is possible that the observed associations between activation of the precuneus during cue-exposure and the indices of alcohol and nicotine dependence actually represent a deeper relationship between the precuneus and a common genetic basis of addiction. However, scores on the Alcohol Dependence Scale (ADS) and Fagerström Test of Nicotine Dependence (FTND) were not significantly correlated with one another, nor with precuneus activation in response to the alternate substance. This suggests that these indices of dependence are not indexing a single construct of dependence severity, and that precuneus activation is capturing unique variance associated with the relationship between dependence severity and cue-reactivity for each substance. This distinction is also supported by a recent report of precuneus involvement in cue-induced urges to game within a sample of individuals addicted to Internet gaming (Ko et al., 2013), a condition under investigation for inclusion as a disorder in the Diagnostic and Statistical Manual of Mental Disorders (DSM–5; American Psychiatric Association, 2013), and one that is less likely to share as much genetic etiology with drugs of abuse as compared to alcohol and nicotine dependence. Notably, the participants classified as addicted to Internet gaming exhibited comorbid nicotine dependence, yet no significant precuneus activation in response to cigarette cues was observed. Although this lack of association may be due to methodological differences in their cigarette cue-reactivity task as compared to that used in the present study, the distinct nature of precuneus involvement in cue-exposure paradigms across multiple drugs and behaviors of abuse is suggestive of the unique yet global involvement of the precuneus in the phenomenon of cue-reactivity.
Through processes such as self-referential processing and episodic memory retrieval (Cavanna and Trimble, 2006), the observed activation of the precuneus during cue-reactivity tasks may represent processes occurring further upstream from the experience of subjective craving. The precuneus, therefore, may represent more of the “biological experience of craving,” as compared to other brain regions (e.g., insula: Garavan, 2010) which may underlie the subjective interpretation of the craving experience and subsequently correlate more strongly with self-report measures (Drummond et al., 2000). Alternatively, precuneus involvement in drug cue reactivity may be subserving a habitual response to the cues in more severely dependent alcohol and cigarette users. This interpretation is consistent with the dual-process learning theory of addiction, which suggests that drug-seeking is mediated by parallel goal-directed and habitual processes, with habitual processes being more dominant in drug-cue contexts. Further, this account explains why cue-reactivity is impervious to drug satiety whereas subjective craving is not (Hogarth and Chase, 2011). The positive associations between precuneus activation and dependence severity observed in the present study are consistent with this hypothesis, as one would expect individuals with greater levels of dependence to experience higher levels of cue-reactivity. Further investigations of these reverse inferences are needed before conclusions can be drawn.
While strengths of the study include well-validated neuroimaging analysis methods and a unique sample comprised of alcohol and cigarette co-users, weaknesses include the lack of a non-alcohol abusing, non-smoking control group, and the administration of study medications (i.e., varenicline and/or naltrexone) to most of the participants. Although study medication effects were controlled for statistically, unforeseeable consequences associated with the medication effects warrant caution in interpreting the results. Previous work has observed effects of varenicline and naltrexone on several brain regions, primarily within the reward circuitry (Franklin et al., 2011; Myrick et al., 2008). Thus, future research is needed to validate these findings in un-medicated samples of substance users and non-users. Study designs that incorporate participants who use various substances, beyond alcohol and nicotine, while capturing the full spectrum of drug use would be well positioned to further investigate the role of the precuneus in drug cue-reactivity.
In conclusion, this study provides initial evidence that precuneus activation during cue-reactivity is moderated by severity of alcohol and nicotine dependence within the same individuals, independent of subjective craving. These findings advance our understanding of the role of this brain region in substance dependence through the pathway of drug cue-reactivity and suggest that the precuneus may be a locus of neuroplastic changes that arise with greater dependency on drugs of abuse.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the assistance of Eliza Hart, Pauline Chin, Andia Heydari, Ellen Chang, Jessica Web, and Katy Lunny to data collection and data management for this project. We would also like to thank the Staglin IMHRO Center for Cognitive Neuroscience.
Role of Funding Source
This research was supported grants from the California Tobacco Related Disease Research Program (TRDRP 18KT-0020) and from the National Institute on Drug Abuse (DA030898) to LAR. Support for this study was also provided by a grant from the UCLA Clinical and Translational Science Institute (CTSI), grants UL1RR033176 and UL1TR000124. KEC was supported by the UCLA Training Program in Translational Neuroscience of Drug Abuse (T32 DA024635). None of the funding sources had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Exploratory whole-brain results are presented as supplementary materials and can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:….
Contributors
Author LAR designed the original study and wrote the protocol. EDL consulted on study procedures and on critical editing of the final manuscript. KEC and DGG conceived of the hypothesis and undertook data analysis, and KEC wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
LAR is a paid consultant for GSK. All other authors declare that they have no conflicts of interest.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Andersson J, Jenkinson M, Smith S. Non-linear Registration. FMRIB Technical report TR07JA2. 2007 [Google Scholar]
- Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bourque J, Mendrek A, Dinh-Williams L, Potvin S. Neural circuitry of impulsivity in a cigarette craving paradigm. Front. Psychiatry. 2013;4:67. doi: 10.3389/fpsyt.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL. Functional brain imaging of tobacco use and dependence. J. Psychiatr. Res. 2006;40:404–418. doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, Jarvik ME, London ED. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Res. 2004;130:269–281. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch. Gen. Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol. Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci. Biobehav. Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol. Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology. 2013;38:2363–2372. doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ray LA. Subjective response to alcohol in the lab predicts neural response to alcohol cues. J. Stud. Alcohol Drugs. 2014;75:124–135. doi: 10.15288/jsad.2014.75.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson CS, Bramen J, Cohen MS, London ED, Olmstead RE, Gan JJ, Costello MR, Shulenberger S, Mandelkern MA, Brody AL. Effect of bupropion treatment on brain activation induced by cigarette-related cues in smokers. Arch. Gen. Psychiatry. 2011;68:505–515. doi: 10.1001/archgenpsychiatry.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC, Litten RZ, Lowman C, Hunt WA. Craving research: future directions. Addiction. 2000;2(95 Suppl):S247–S255. doi: 10.1080/09652140050111816. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am. J. Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Tamber-Rosenau BJ, Chiu YC, Yantis S. Avoiding non-independence in fMRI data analysis: leave one subject out. Neuroimage. 2010;50:572–576. doi: 10.1016/j.neuroimage.2009.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F, Claus E, Audette A, Niculescu M, Banich M, Tanabe J, Du Y, Hutchison K. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008a;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F, Ray L, Smolen A, Claus E, Audette A, Hutchison K. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcohol. Clin. Exp. Res. 2008b;32:1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008c;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, Childress AR. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch. Gen. Psychiatry. 2011;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Garavan H. Insula and drug cravings. Brain Struct. Funct. 2010;214:593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am. J. Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Chase HW. Parallel goal-directed and habitual control of human drug-seeking: implications for dependence vulnerability. J. Exp. Psychol. Anim. Behav. Process. 2011;37:261–276. doi: 10.1037/a0022913. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- King A, McNamara P, Angstadt M, Phan KL. Neural substrates of alcohol-induced smoking urge in heavy drinking nondaily smokers. Neuropsychopharmacology. 2010;35:692–701. doi: 10.1038/npp.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Liu GC, Yen JY, Yen CF, Chen CS, Lin WC. The brain activations for both cue-induced gaming urge and smoking craving among subjects comorbid with Internet gaming addiction and nicotine dependence. J. Psychiatr. Res. 2013;47:486–493. doi: 10.1016/j.jpsychires.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Gilbert DG. Human functional neuroimaging in nicotine and tobacco research: basics, background, and beyond. Nicotine Tob. Res. 2004;6:941–959. doi: 10.1080/14622200412331337394. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl.) 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch. Gen. Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. The Physicians’ Guide to Helping Patients with Alcohol Problems. Bethesda, MD: National Institutes of Health; 1995. [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict. Behav. 1990;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- Park M-S, Sohn J-H, Suk J-A, Kim S-H, Sohn S, Sparacio R. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol Alcohol. 2007;42:417–422. doi: 10.1093/alcalc/agl117. [DOI] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Hutchison KE, Mackillop J, Galvan A, Ghahremani DG. Initial evidence that OPRM1 genotype moderates ventral and dorsal striatum functional connectivity during alcohol cues. Alcohol. Clin. Exp. Res. Epub ahead of print. 2013 doi: 10.1111/acer.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol. Biochem. Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict. Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J. Abnorm. Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Convergent validity: an approach to increasing confidence in treatment oucome conclusions with alcohol and drug abusers. In: Sobell LC, Sobell MB, Ward E, editors. Evaluating Alcohol and Drug Abuse Treatment Effectiveness: Recent Advances. Elmsford, NY: Pergamon Press; 1980. pp. 177–209. [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch. Gen. Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.