Abstract
Viruses rely upon host lipid metabolic pathways for successful replication, and there is increasing interest in these pathways as novel therapeutic targets for antiviral drug discovery. Despite this, relatively little is known about the impact of viral infection on cellular lipid metabolism, and the specific lipid metabolites utilized by viruses have not yet been examined. We have applied liquid chromatography-mass spectroscopy (LC-MS) based discovery metabolite profiling (DMP) to identify lipid metabolites whose steady-state abundance is significantly altered by replication of hepatitis B virus (HBV), a major human pathogen. DMP indicated that although major lipid classes were unaffected by HBV, an ion of 367 m/z was overabundant in HBV+ cells by 18-fold. As shown by ion fragmentation mass spectrometry and co-injection with standard, the identity of this ion is 7-dehydrocholesterol (7-DHC), an immediate dehydrogenated precursor to cholesterol. While cholesterol has previously been demonstrated to be essential in the replication of many viruses, this is the first to show that viral replication is associated with the selective accumulation of 7-DHC. Most virological studies to date have relied upon methods that deplete all sterols and preclude the observation of any selectivity in sterol utilization by viral pathogens. Our study suggests that HBV may selectively utilize 7-DHC versus other sterols and prompts experiments investigating the functional significance of this enrichment and the elucidation of the mechanism by which it is achieved. The results also highlight the value of DMP as a method for identifying critical metabolites for viral infection.
Cellular lipids play essential but understudied roles in the replication of mammalian viruses. For example, the replication and assembly of many viruses occurs on cellular membrane structures and the lipid bilayer of enveloped viruses is derived entirely from the host cell. Consequently, changes in lipid metabolism and signal transduction associated with viral infection and pathogenesis are of great interest as potential therapeutic targets for the treatment of viral diseases1–3. Significant advances in the use of quantitative mass spectrometry (MS) make this methodology ideal for interrogating virus-mediated alterations in host metabolism, as recently evidenced by the use of targeted MS methods to quantify changes in small molecule metabolites associated with cytomegalovirus infection4,5. Improvements in the detection and quantitative analysis of cellular lipids have, moreover, greatly increased the use of this methodology to characterize the lipid content of biological samples6,7. Recent advances in liquid chromatography-mass spectrometry (LC-MS) based untargeted metabolite profiling have enabled the identification of metabolites associated with specific disease states and would clearly complement existing virological methods that do not interrogate changes in low molecular weight metabolites. Towards this end, we have utilized liquid chromatography-mass spectroscopy (LC-MS) based discovery metabolite profiling8 (DMP) to examine changes in steadystate levels of lipids in cells replicating human hepatitis B virus (HBV), a small enveloped virus that infects the liver, the organ primarily responsible for lipid metabolism.
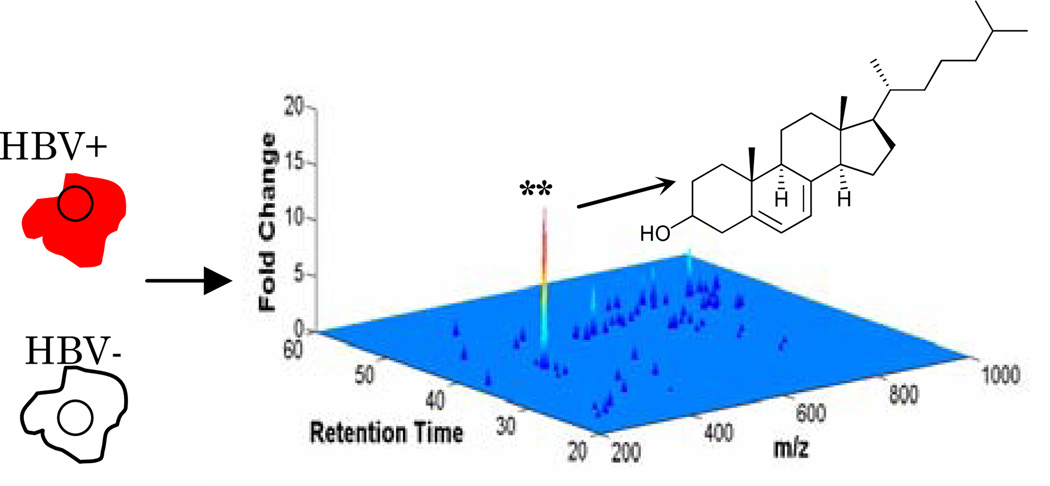
Cell line HepG2.1179 is a human hepatoma cell line engineered to stably replicate HBV in a tetracycline-controlled manner. Equal numbers of HBV-expressing (HBV+) and negative control (HBV−) cells from six independent cultures were homogenized in a 1:1:2 PBS-methanol-chloroform mixture, and total lipid in the chloroform phase was analyzed by positive mode and negative mode LC-MS. In all, over 10,000 individual mass ion intensities were compared between the HBV and control samples with the XCMS software package10 (Figure 1).
Figure 1.
Untargeted lipid ion profiling of cells replicating HBV. Total lipid from HBV+ cells and negative control cells were quantified by LC-MS and comparisons were made with XCMS software. ** indicates a p-value of less than 0.0003 by students t–test.
The results from experiments performed in triplicate indicated that cells replicating HBV had small differences in the abundance of major lipid classes such as free fatty acids, phospholipids, and sphingolipids (Figure 1 and supporting). These small fold-changes may reflect significant changes in cellular composition in the case of highly abundant species. Notably, however, DMP indicated that HBV+ cells had a comparatively large overabundance of ion 367 m/z (M-OH) (Figure 1). Accurate mass calculations of this metabolite, A, indicate a formula of C27H44O, consistent with a dehydrogenated isomer of cholesterol. MS detection of A occurred at two elution times that presumably correspond to the free alcohol and ester forms. Notably, HBV replication was associated with a dose-dependent increase in the steady-state abundance of A (supporting). Using a deuterated cholesterol standard for absolute quantification in picomoles, we discovered 18-fold and 4-fold enrichment of the free alcohol and ester forms of A, respectively, in HBV+ cells (Figure 2). For comparison, cholesterol esters were decreased by 2-fold and cholesterol was unchanged in HBV+ cells versus negative controls (Figure 2).
Figure 2.
Quantification in biological triplicate of metabolite A, its ester, cholesterol, and cholesterol ester by normalization to deuterated cholesterol standard in HBV+ and HBV− cells.
Four lines of evidence support that the structure of A is 7-dehydrocholesterol. First, the LC-MS retention time of A most closely matches that of a 7-dehydrocholesterol standard versus other standards of the same mass (supporting). Second, coinjection of lipid extract from HBV+ cells with a 7-dehydrocholesterol standard resulted in a single peak for A whereas co-injection of the HBV+ extract with other standards, such as desmosterol, resulted in two peaks (supporting). Third, the peaks for A were consumed in a Diels-Alder reaction previously shown to specifically utilize 7-dehydrocholesterol11,12. Fourth, the ion fragmentation pattern of A matched that of the 7-dehydrocholesterol standard, but not the patterns of desmosterol, zymosterol, or vitamin D3 standards (supporting). Collectively, these data unequivocally demonstrate that the identity of A is 7-dehydrocholesterol (7-DHC), the penultimate intermediate in cholesterol biosynthesis.
Sterols have increasingly been implicated as essential for the entry and fusion activity of many enveloped viruses.13,14 Most previous studies have utilized cholesterol chelating agents or specific biosynthesis inhibitors15–19, both methods that deplete all intracellular sterols and that are therefore unable to resolve the role of individual sterols in the viral life cycle. Our results provide the first evidence that a virus is associated with accumulation of a specific lipid molecule, 7-DHC, and furthermore that this accumulation is selective versus all other cellular lipids detected by untargeted lipid metabolite profiling. The finding that viruses not only utilize cellular lipids but also have mechanisms for perturbing lipid metabolism highlights a growing appreciation of the mechanisms by which viruses exploit cellular resources to favor their own propagation. Coupled with increasing evidence from model systems that minor changes in sterol structure are associated with significant changes in function including membrane dynamics,20,21 lipid raft/domain formation,22,23 and raft-associated receptor signaling,24 our results provide strong motivation for on-going studies characterizing the functional role of 7-DHC in HBV biology. Finally, our results illustrate the power of untargeted analytical methods in identifying metabolites that are critical in disease states, including viral infection and pathogenesis.
Supplementary Material
Figure 3.
MS spectra for metabolite A from 2 aliquots of HBV+ total lipid extract. The peak corresponding to molecule A in HBV+ extracts (upper panel) disappears upon Diels-Alder reaction with 4-phenyl-1,2,4-triazoline-3,5-dione using a method previously reported for the selective derivatization of 7-DHC (lower panel)11,12.
Acknowledgment
We thank Dr James Cardia and Dr Ranitendranath Tagore for helpful discussion and Dr Michael Nassal for providing the HepG2.117 cell line. We gratefully acknowledge funding from The Alexander and Margaret Stewart Trust (P.L.Y.) and The Giovanni Armenise-Harvard Foundation (P.L.Y.), The Hellman Family Fund (P.L.Y.), and the Karnovsky Foundation (M.A.R). A.S. is a recipient of the Burroughs Wellcome Fund Career Award in the Biomedical Sciences.
Footnotes
Supporting Information Available: The relative ion abundances of major lipid classes in HBV+ cells as well as molecule identification data and protocols are provided. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Raulin J. Prog Lipid Res. 2002;41:27–65. doi: 10.1016/s0163-7827(01)00019-4. [DOI] [PubMed] [Google Scholar]
- 2.Barenholz Y. Subcell Biochem. 2004;37:167–215. doi: 10.1007/978-1-4757-5806-1_5. [DOI] [PubMed] [Google Scholar]
- 3.Ye J. PLoS Pathog. 2007;3:e108. doi: 10.1371/journal.ppat.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munger J, Bajad SU, Coller HA, Shenk T, Rabinowitz JD. PLoS Pathog. 2006;2:e132. doi: 10.1371/journal.ppat.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munger J, Bennett BD, Parikh A, Feng XJ, McArdle J, Rabitz HA, Shenk T, Rabinowitz JD. Nat Biotechnol. 2008;26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivanova PT, Milne SB, Forrester JS, Brown HA. Mol Interv. 2004;4:86–96. doi: 10.1124/mi.4.2.6. [DOI] [PubMed] [Google Scholar]
- 7.Ivanova PT, Milne SB, Byrne MO, Xiang Y, Brown HA. Methods Enzymol. 2007;432:21–57. doi: 10.1016/S0076-6879(07)32002-8. [DOI] [PubMed] [Google Scholar]
- 8.Saghatelian A, Trauger SA, Want EJ, Hawkins EG, Siuzdak G, Cravatt BF. Biochemistry. 2004;43:14332–14339. doi: 10.1021/bi0480335. [DOI] [PubMed] [Google Scholar]
- 9.Sun D, Nassal M. J Hepatol. 2006;45:636–645. doi: 10.1016/j.jhep.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Smith CA, Want EJ, O'Maille G, Abagyan R, Siuzdak G. Anal Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 11.Shimada K, Oe T. Analytical Sciences. 1990;6:461–463. [Google Scholar]
- 12.Batta AK, Salen G, Tint GS, Honda A, Shefer S. Steroids. 1997;62:700–702. doi: 10.1016/s0039-128x(97)00070-6. [DOI] [PubMed] [Google Scholar]
- 13.Rawat SS, Viard M, Gallo SA, Rein A, Blumenthal R, Puri A. Mol Membr Biol. 2003;20:243–254. doi: 10.1080/0968768031000104944. [DOI] [PubMed] [Google Scholar]
- 14.Teissier E, Pecheur EI. Eur Biophys J. 2007;36:887–899. doi: 10.1007/s00249-007-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finnegan CM, Rawat SS, Puri A, Wang JM, Ruscetti FW, Blumenthal R. Proc Natl Acad Sci U S A. 2004;101:15452–15457. doi: 10.1073/pnas.0402874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Hepatology. 2006;44:117–125. doi: 10.1002/hep.21232. [DOI] [PubMed] [Google Scholar]
- 17.Kapadia SB, Barth H, Baumert T, McKeating JA, Chisari FV. J Virol. 2007;81:374–383. doi: 10.1128/JVI.01134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funk A, Mhamdi M, Hohenberg H, Heeren J, Reimer R, Lambert C, Prange R, Sirma H. J Virol. 2008;82:10532–10542. doi: 10.1128/JVI.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bremer CM, Bung C, Kott N, Hardt M, Glebe D. Cell Microbiol. 2008 doi: 10.1111/j.1462-5822.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 20.Cao H, Tokutake N, Regen SL. J Am Chem Soc. 2003;125:16182–16183. doi: 10.1021/ja039172x. [DOI] [PubMed] [Google Scholar]
- 21.Shrivastava S, Paila YD, Dutta A, Chattopadhyay A. Biochemistry. 2008;47:5668–5677. doi: 10.1021/bi8001677. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, London E. Biochemistry. 2000;39:843–849. doi: 10.1021/bi992543v. [DOI] [PubMed] [Google Scholar]
- 23.Megha, Bakht O, London E. J Biol Chem. 2006;281:21903–21913. doi: 10.1074/jbc.M600395200. [DOI] [PubMed] [Google Scholar]
- 24.Pucadyil TJ, Shrivastava S, Chattopadhyay A. Biochem Biophys Res Commun. 2005;331:422–427. doi: 10.1016/j.bbrc.2005.03.178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.