Abstract
Hypertensive disorders of pregnancy (HDP, including gestational hypertension, preeclampsia, and eclampsia) have a substantial public health impact. Maternal exposure to high levels of air pollution may trigger HDP, but this association remains unclear. The objective of our report is to assess and quantify the association between maternal exposures to criteria air pollutants (ozone, carbon monoxide, nitrogen dioxide, sulfur dioxide, and particulate matter ≤ 10, 2.5 μm) on HDP risk. PubMed, EMBASE, MEDLINE, Current Contents, Global Health, and Cochrane were searched (last search: September, 2013). After a detailed screening of 270 studies, 10 studies were extracted. We conducted meta-analyses if a pollutant in a specific exposure window was reported by at least four studies. Using fixed- and random-effects models, odds ratios (ORs) and 95% CIs were calculated for each pollutant with specific increment of concentration.
Increases in risks of HDP (OR per 10 ppb = 1.16; 95% CI, 1.03-1.30) and preeclampsia (OR per 10 ppb = 1.10; 95% CI, 1.03-1.17) were observed to be associated with exposure to NO2 during the entire pregnancy, and significant associations between HDP and exposure to CO (OR per 1 ppm = 1.79; 95% CI, 1.31-2.45) and O3 (OR per 10 ppb = 1.09; 95% CI, 1.05-1.13) during the first trimester were also observed. Our review suggests an association between ambient air pollution and HDP risk. Although the ORs were relatively low, the population-attributable fractions were not negligible given the ubiquitous nature of air pollution.
Keywords: air pollution, pregnancy-induced hypertension, hypertensive disorders of pregnancy, gestational hypertension, preeclampsia, meta-analysis
1. Introduction
Hypertensive disorders of pregnancy (HDP) including chronic hypertension, gestational hypertension, preeclampsia, and eclampsia are prevalent, accounting for up to 10% of all pregnancies (Duley, 2009). These medical conditions among pregnant women are characterized by high blood pressure, usually after 20 weeks of gestation because blood volume change during pregnancy leads to higher stress on the cardiovascular system (Yoder et al., 2009). HDP is highly associated with increased neonatal and maternal morbidity and mortality (Duley, 2009; Lo et al., 2013). It causes pitting edema, endothelial abnormalities, liver and renal dysfunction, and increased risk of cardiovascular disease, stroke and Type II diabetes later in life of pregnant women (Bauer and Cleary, 2009; Bellamy et al., 2007; Duley, 2009; Wang et al., 2012). In addition, maternal HDP also put infants under higher risks of small for gestational age, preterm delivery, low birthweight, and hospitalization for a wide range of neonatal diseases (Allen et al., 2004; Wu et al., 2009a). For example, preeclampsia alone contributes to about 25% of all medically indicated preterm deliveries in the United States (Ananth and Vintzileos, 2006; Goldenberg et al., 2008).
The association between air pollution and increased risk of hypertension in the general population has been reported by many studies (Basile and Bloch, 2012; Coogan et al., 2012; Guo et al., 2010a; Guo et al., 2010b; Sorensen et al., 2012). Although the exact mechanisms underlying the effects of air pollution on blood pressure is yet to be determined, some plausible mechanisms have been suggested in previous studies (Brook and Rajagopalan, 2009). Briefly, there are three non-mutually exclusive pathways that may be responsible for hypertension following exposure to air pollution. The first pathway involves changes in autonomic system balance through interaction of air pollutants with the sympathetic nervous system, thereby increasing blood pressure. The second pathway is an indirect pathway, which involves circulating oxidative stress markers such as cytokines induced by affected body organs, particularly the lung cells. These stress markers may affect blood pressure through changes in endothelial and other hemodynamic function. Lastly, blood pressure may be affected directly by pollutants that enter the vascular system causing vasoconstriction and other vascular dysfunction.
Given the effects that air pollution may have on hypertension in the general population, it is plausible that exposure to air pollution during pregnancy may also increase the risk of HDP through the same mechanisms. Emerging studies have suggested that environmental exposures such as ambient air pollution during pregnancy may play a role in the development of HDP, including gestational hypertension and preeclampsia (Dadvand et al., 2013; Jedrychowski et al., 2012; Lee et al., 2013; Malmqvist et al., 2013; Mobasher et al., 2013; Olsson et al., 2013; Pereira et al., 2013; Rudra et al., 2011; van den Hooven et al., 2011; Vigeh et al., 2011; Vinikoor-Imler et al., 2012; Woodruff et al., 2008; Wu et al., 2009b; Wu et al., 2011; Xu et al., 2013; Zhai et al., 2012). However, inconsistencies and uncertainties remain concerning the effects of specific pollutants and critical exposure periods. To our knowledge, no review or meta-analysis examining the association between air pollution and HDP exists. Given the varied combinations of pollutants (i.e. NO2, SO2, PM2.5, PM10, O3, and CO) and exposure periods (i.e. month, trimester, periconception, and other periods), and treatment of exposure as both a continuous and categorical variable, a systematic review of these previous studies is needed. Therefore, we present a systematic review and meta-analysis of 10 studies examining associations between ambient air pollution and HDP. We provide summary estimates of effect by gestational period, quantify heterogeneity, evaluate publication bias, and conduct sensitivity analyses.
2. Materials and methods
2.1 Search methods
Studies were identified using electronic searches of bibliographic databases, and review of reference lists of all relevant papers. The following databases were searched: PubMed, EMBASE, MEDLINE, Current Contents, Global Health, and Cochrane. The Medical Subject Heading (MeSH) terms “hypertension, pregnancy-induced”, “preeclampsia”, “eclampsia”, “pregnancy”, “hypertension”, “air pollution”, “particulate matter”, “nitrogen dioxide”, “sulfur dioxide”, “ozone”, and “carbon monoxide” and non-MeSH terms “gestational hypertension”, and “hypertensive disorders of pregnancy” were used in the search. From this search, we selected articles which a) were published in English on/after January 1, 1980; b) were original epidemiologic studies on human live birth; c) had hypertensive disorders of pregnancy, gestational hypertension, preeclampsia, or eclampsia as outcome variables; d) investigated non-occupational non-accidental human prenatal exposure to outdoor air pollution. Since the majority of studies met the inclusion criteria examined exposure to criteria air pollutants, we excluded studies that only examined exposure measured by other surrogate methods such as traffic density data and ecologic assessment (e.g. high vs Low) because the results from these studies cannot be used for effect synthesis (Malmqvist et al., 2013; Vigeh et al., 2011). In addition, since this meta-analysis only focused on the effects of ambient air pollution exposure during pregnancy on HDP, studies with exposure window other than pregnancy period (e.g. periconception) were further excluded (Rudra et al., 2011). Studies available only in abstract form were also excluded as the abstract only provided very limited information about the study (Woodruff et al., 2008). Searches were last updated in September 2013. Relevance of citations for inclusion was evaluated independently by two investigators (SH and HH), differences between whom were resolved by consensus. At first, all identified publications were screened for the eligibility by reviewing the title and abstract based on the inclusion and exclusion criteria. Then, the remaining references were further evaluated by reviewing the full paper. As a result, a total of 270 unduplicated records were identified in the literature search. After review of the title and abstract, 149 studies were excluded due to irrelevant exposure and/or outcome, and 35 non-epidemiologic studies were also excluded. The remaining 86 studies were selected by reviewing the full paper to determine eligibility for inclusion. Nine of them were review, commentary and meeting report, and were further excluded. Sixty-five studies were not considered due to irrelevant exposures and/or outcomes. Finally, ten studies were identified and included in this meta-analysis (Figure 1).
Figure 1.
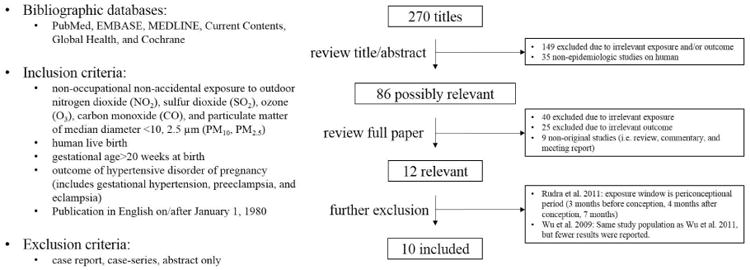
Data extraction from relevant studies was also conducted independently by two investigators (SH and HH). Each independent reviewer used a standardized data extraction sheet to extract relevant data from these studies. All data entries were confirmed and doubly checked for completeness and accuracy. The following study characteristics were extracted: study design, location, dates of data collection, sample size, outcome, number of cases, distribution of exposure, method of exposure characterization, statistical analysis methods, effect size estimates, covariate adjustment, and conduct of subgroup or sensitivity analyses. Study quality was assessed based on study design, exposure characterization and adjustment for covariates, and sensitivity analyses were conducted where feasible based on these factors. References were managed in Endnote (Thomson Reuters, CA).
2.2 Meta-analysis
We conducted meta-analyses if at least four studies reported the same pollutant and exposure window group. All studies reviewed excluded chronic hypertension in pregnancy from their outcomes, so in this study, we used the restricted definition of HDP which only includes gestational hypertension, preeclampsia, and/or eclampsia. Since different outcomes of HDP were reported in the included studies (i.e. HDP, gestational hypertension, and preeclampsia), we conducted two sets of meta-analysis: 1) for the association between air pollution and preeclampsia and 2) for the association between air pollution and HDP (including HDP, gestational hypertension, and preeclampsia). We used the risk estimates from the fully-adjusted and single-pollutant models presented in each study (Olsson et al., 2013; Xu et al., 2013), and risk estimates from sensitivity analyses were not used. There was one instance where results were reported for the same outcome, pollutant, and population in more than one paper by same authors (Wu et al., 2009b; Wu et al., 2011). We selected the latest result because it provides data of more comprehensive and updated information (Wu et al., 2011). In addition, Wu et al. intentionally reported risk estimates separately in their study based on two different study locations (i.e. Los Angeles County and Orange County, CA) because the study populations in these two locations were remarkably different in socio-demographic status and the land use regression models were originally developed based on measurements in Los Angeles County only. Given these reasons, we also treated them as two different studies. Two studies reported both gestational hypertension and preeclampsia as outcome (Lee et al., 2013; van den Hooven et al., 2011). We entered risk estimates from these publications together in the HDP meta-analysis, and we further conducted sensitivity analyses by entering them separately.
Since all the studies included used continuous exposures and only one reported both continuous and categorical exposures (Zhai et al., 2012), we only conducted meta-analyses for continuous exposure. For each pollutant, effect estimates were grouped by exposure periods (trimesters and entire gestational period, and were expressed in terms of pollutant increments equal to typical mean concentrations of pollutants in ambient air [10 μg/m3 PM10; 5 μg/m3 PM2.5; 10 parts per billion (ppb) O3; 1 parts per million (ppm) CO; 10 ppb NO2] to permit comparisons among different studies.
We used either fixed- or random-effects models to obtain the summary risk estimates depending on the heterogeneity assessed by Q-test (Cochran, 1954). Random-effects model was used when Q-test showed evidence for heterogeneity (p<0.1) (DerSimonian and Laird, 1986), and a fixed-effect analysis was conducted when no significant heterogeneity was observed (Mantel and Haenszel, 2004). Illustrative forest plots showing ORs from each of the individual studies and the summarized OR were also provided for pollutants by different exposure periods. Publication bias was examined using Egger's test (Sterne and Egger, 2001). We used Biostat Comprehensive Meta-Analysis version 2 (Englewood, NJ) and SAS version 9.3 (Cary, NC) for all analyses.
3. Results
Table 1 shows the characteristics of individual studies included for analyses. Most of these studies adopted a retrospective cohort design using administrative birth record data, while only one study used a case-control design. Individual studies were based on as few as 298 and as many as 222,775 subjects. Most studies using data collected from the late 1990s and early 2000s. Studies were carried out in 10 locations. Over half of the studies (n=6) were performed in North America, while the rest were from Europe (n=3) and Australia (n=1). One study was divided into two sub-studies based on different study locations (Wu et al., 2011). Four studies reported HDP as the only outcome, four studies reported preeclampsia as the only outcome, and the remaining two studies reported both gestational hypertension and preeclampsia as outcomes. These two studies were divided into two sub-studies based on two different outcomes reported (Lee et al., 2013; van den Hooven et al., 2011). Six studies assigned exposure based on central site monitoring data (Mobasher et al., 2013; Olsson et al., 2013; van den Hooven et al., 2011; Vinikoor-Imler et al., 2012; Xu et al., 2013; Zhai et al., 2012). Lee et al. performed a space-time ordinary kriging interpolation based on daily data from air monitors (Lee et al., 2013). Dadvand et al. and Pereira et al. used land use regression (LUR) models which take into account the information of air monitors as well as land uses, traffic indicators, population density, and geographic description of study area (Dadvand et al., 2013; Pereira et al., 2013). Wu et al. assessed exposure using a variety of models. In addition to ambient monitoring data, LUR models, and traffic density, they also used an air dispersion model to model local traffic emissions within 3km of each residence (Wu et al., 2011). Most studies focused on the criteria air pollutants: PM10 (seven studies), PM2.5 (six studies), NO2 (six studies), CO (four studies), and O3 (four studies). A few studies also reported NOx (three studies), SO2 (one study), and PM2.5-10 (one study). Table 2 presents the pollutant levels among primary studies. All studies adjusted for maternal age and parity as covariates, more than half adjusted for race, smoking, and season; while less than half adjusted for BMI, year of conception, or socioeconomic status. Few adjusted for maternal alcohol consumption, marital status, environmental tobacco smoke (ETS), noise, or prenatal care. All of the included studies examined these potential confounders by using several different multivariate models. Most studies also conducted other sensitivity analyses to assess the robustness of the results. Dadvand et al. performed a matched case-control analysis to address the potential biased exposure assessment due to the shorter duration of exposure during the third trimester among women diagnosed with preeclampsia (Dadvand et al., 2013). Mobasher et al. performed analyses restricted to women who had their first prenatal visit before 12 weeks gestation since this group consist of around 90% of the total sample (Mobasher et al., 2013). Vinikoor-Imler et al. checked the sensitivity of their results by restricting the analyses on women living within 10km and 5km of a monitor (Vinikoor-Imler et al., 2012). Wu et al. compared their results obtained from different exposure assessments (Wu et al., 2011).
Table 1.
Characteristics of primary studies.
| Reference | Pollutant | Outcome | Design | Location | Time period | Sample size | Exposure period | Exposure type | Covariates |
|---|---|---|---|---|---|---|---|---|---|
| Dadvand et al. 2013 | NOx, NO2, PM10, PM2.5, PM2.5-10 | Preeclampsia | Hospital-based Cohort | Barcelona, Spain | 01-Mar-2000- 30-Jun-2005 | 8,398 | Trimester, entire pregnancy | Continuous | Age, parity, race, smoking, BMI, alcohol consumption, season, year, SES, education, marital, diabetes |
| Lee et al. 2013 | PM10, PM2.5, O3 | GHT Preeclampsia | Hospital-based Cohort | Philadelphia, USA | 01-Jan-1997-31-Dec-2002 | 34,705 | First trimester | Continuous | Age, parity, race, smoking, season of birth, year of conception |
| Mobasher et al. 2013 | NO2, O3, CO, PM2.5, PM10 | HDP | Case-control | California and Nevada, USA | 01-Jan-1996-31-Dec-2008 | 298 | Trimester | Continuous | Age, parity, smoking, ETS, year of conception |
| Olsson et al. 2013 | NOx, O3 | HDP | Register-based Cohort | Greater Stockholm area, Sweden | 01-Jan-1998-31-Dec-2006 | 120,755 | First trimester | Continuous | Age, parity, education, area of origin, maternal asthma, season of conception, conception year |
| Pereira et al. 2013 | NO2 | Preeclampsia | Register-based Cohort | Perth, Australia | 01-Jan-2000-31-Dec-2006 | 23,452 | Trimester, entire pregnancy | Continuous | Age, parity, diabetes, aboriginal status, season of conception, smoking, socioeconomic index for areas score |
| van den Hooven et al. 2011 | NO2, PM10 | GHT Preeclampsia | Prospective Cohort | Rotterdam, Netherlands | 01-Jan-2001-31-Dec-2005 | 7,006 | Entire pregnancy | Continuous | Age, parity, race, education, height, weight, folic acid supplementation, smoking, alcohol consumption, noise exposure |
| Vinikoor-Imler et al. 2012 | PM10, PM2.5 | HDP | Register-based Cohort | North Carolina, USA | 01-Jan-2000-31-Dec-2003 | 222,775 | Entire pregnancy | Continuous | Age, parity, education, smoking, race, marital status, neighborhood deprivation index |
| Wu et al. 2011 | CO, NO, NO2, NOx, PM2.5, PM10 | Preeclampsia | Hospital-based Cohort | California, USA | 01-Jan-1997-31-Dec-2006 | 81,186 | Trimester, entire pregnancy | Continuous | Age, parity, race, prenatal care insurance type, poverty, season of conception, diabetes |
| Xu et al. 2013 | CO, NO2, SO2, O3, PM2.5, PM10 | HDP | Register-based Cohort | Florida, USA | 01-Jan-2004-31-Dec-2005 | 22,041 | Trimester, entire pregnancy | Continuous | Age, parity, race, education, marital, smoking, season, year, prenatal care, median household income |
| Zhai et al. 2012 | CO | Preeclampsia | Register-based Cohort | Ontario, Canada | 01-Jan-2004-31-Dec-2009 | 127,370 | Entire pregnancy | Quartiles, Continuous | Age, parity, smoking, previous cesarean section delivery, maternal health problem (chronic hypertension, diabetes, heart disease), income, education |
Abbreviations: NO-nitrogen monoxide, NO2-nitrogen dioxide, NOx-nitrogen oxide, SO2-sulfur dioxide, O3-ozone, CO-carbon monoxide, PM2.5-particulate matter of median diameter<2.5μm, PM2.5-10-particulate matter of median diameter between 2.5μm and 10μm, PM10-particulate matter of median diameter<10μm, GHT-gestational hypertension, HDP-hypertensive disorder of pregnancy, BMI-body mass index, SES-socioeconomic status, ETS-environment tobacco smoking.
Table 2. Mean/median pollutant concentration among primary studies.
| Full gestational period | Trimester 1 | Trimester 2 | Trimester 3 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO (ppm) |
NO2 (ppb) |
O3 (ppb) |
PM2.5 (μg/m3) |
PM10 (μg/m3) |
CO (ppm) |
NO2 (ppb) |
O3 (ppb) |
PM2.5 (μg/m3) |
PM10 (μg/m3) |
CO (ppm) |
NO2 (ppb) |
O3 (ppb) - |
PM2.5 (μg/m3) |
PM10 (μg/m3) |
CO (ppm) |
NO2 (ppb) |
O3 (ppb) |
PM2.5 (μg/m3) |
PM10 (μg/m3) |
|
| Dadvand et al. 2013 | - | 29.6 | - | 16.5 | 39.0 | - | 30.2 | - | 17.0 | 41.2 | - | 30.9 | - | 17.3 | 41.7 | - | 30.7 | - | 17.3 | 41.3 |
| Lee et al. 2013 | - | - | - | - | - | - | - | 21.7 | 15.6 | 24.8 | - | - | - | - | - | - | - | - | - | - |
| Mobasher et al. 2013 | - | - | - | - | - | 0.58 | 28.6 | 21.5 | 17.0 | 34.5 | 0.7 | 30.0 | 18.2 | 17.5 | 34.9 | 0.6 | 30.0 | 18.2 | 18.1 | 35.1 |
| Olsson et al. 2013 | - | - | - | - | - | - | - | 34.0 | - | - | - | - | - | - | - | - | - | - | - | - |
| Pereira et al. 2013 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| van den Hooven et al. 2011 | - | 21.2 | - | - | 30.3 | - | 21.5 | - | - | 30.9 | - | - | - | - | - | - | - | - | - | - |
| Vinikoor-Imler et al. 2012 | - | - | - | 14.5 | 22.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Wu et al. 2011 (Los Angeles) | 0.9 | 29.8 | 30.1 | 18.8 | 34.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Wu et al. 2011 (Orange County) | 0.6 | 20.4 | 40.5 | 16.0 | 31.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Xu et al. 2013 | 0.6 | 27.8 | 40.0 | 10.3 | - | 0.6 | 28.0 | 40.0 | 10.1 | - | 0.6 | 27.9 | 41.0 | 10.2 | - | - | - | - | - | - |
| Zhai et al. 2012 | 0.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Preeclampsia
Preeclampsia was analyzed in six studies, and it was defined as systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90mmHg during the second half of pregnancy, and accompanied by proteinuria after 20 weeks of gestation. Each study reported only one or a few statistically significantly increased risks of preeclampsia with increased exposure among the multiple exposure windows included: exposure to CO or O3 during the full gestational period and second trimester was related to higher risk of preeclampsia in one study (Wu et al., 2011), with ORs 1.22 (95% CI, 1.10-1.48) and 3.39 (95% CI, 1.97-5.69) per 1 ppm increase in CO, and ORs 1.19 (95% CI, 1.06-1.32) and 1.25 (95% CI, 1.04-1.54) per 10 ppb increase in O3, respectively. The study in Australia reported higher risks of preeclampsia in association with NO2 exposure during the entire pregnancy period and third trimester (Pereira et al., 2013), with ORs 1.22 (95% CI, 1.02-1.49) and 1.17 (95% CI, 1.04-1.32) per 10 ppb increase, respectively. A recent study also reported an association between increased risks of preeclampsia and exposure to PM2.5 in third trimester (OR per 5 μg/m3 = 1.33; 95% CI, 1.09-1.61) (Dadvand et al., 2013). Inverse associations were also observed for CO (Zhai et al., 2012) and PM2.5 (Wu et al., 2011).
Among the six studies examined preeclampsia as the outcome, three studies investigated potential effect modifications, (Lee et al., 2013; Pereira et al., 2013; Wu et al., 2011) and only one of them reported whether the examination was pre-specified or conducted post hoc (Pereira et al., 2013). Lee et al. conducted stratified analyses by maternal race/ethnicity and smoking status, and found that PM10 affected preeclampsia more strongly in non-smokers compared to smokers, while PM2.5 affected preeclampsia in Caucasian but not African-American women (Lee et al., 2013). Pereira et al. performed pre-specified subgroup analyses for women with different socioeconomic status (SES), for women who did not change residence during pregnancy, for women who spent more time at home, and for women with circulatory or respiratory morbidity. They also conducted post hoc analyses for women with pre-existing or gestational diabetes, and for women aged ≤20 or ≥40 years (Pereira et al., 2013). This study found that NO2's effects on preeclampsia were lower among women who did not change residence or those who spent more time at home, while higher for women with preexisting or gestational diabetes. No differences were found between women with different SES or circulatory/respiratory morbidity. Wu et al. also examined effects modification by maternal age, infant sex, insurance type, parity, poverty, and race, but no differences were found in these stratified analyses. Thus, the results suggest that some subgroups may be at relatively elevated risk of HDP associated with ambient air pollution exposure, but the evidence is very limited.
Meta-analyses was conducted for 6 combinations of pollutants and exposure windows for which four or more studies (or sub-studies) published results (Supplemental Material, Table 1). The summary risk estimates from these meta-analyses were very weak, with a range of summary ORs from 0.98 to 1.10. No evidence of heterogeneity was found among the studies (p<0.10). We found statistically significantly increased summary risk estimates for NO2 exposure during the entire pregnancy period and risks of preeclampsia (OR per 10 ppb = 1.10; 95% CI, 1.03-1.17) (Table 3 and Figure 2). P-value for heterogeneity in this analysis showed limited evidence for heterogeneity (p=0.71). Sensitivity analyses excluding the study with the largest weight from this analysis showed that results were robust to this exclusion.
Table 3.
Summary of meta-analysis of studies on air pollutant exposures and hypertensive disorder of pregnancy.
| Pollutant and exposure window combination | Studies included | Total number of cases | Heterogeneity p-value | Summary OR (95% CI) |
|---|---|---|---|---|
| Outcome: Preeclampsia | ||||
| NO2 | per 10 ppb | |||
| Full gestational period | 1, 6, 8, 10, 11 | 3629 | 0.71 | 1.10 (1.03-1.17) |
| Trimester 1 | 1, 6, 10, 11 | 3488 | 0.79 | 1.03(0.97-1.10) |
| Trimester 2 | 1, 6, 10, 11 | 3488 | 0.67 | 1.05(0.98-1.12) |
| PM10 | per 10 μg/m3 | |||
| Full gestational period | 1, 8, 10, 11 | 2686 | 0.55 | 0.98(0.91-1.05) |
| Trimester 1 | 1, 3, 10, 11 | 3686 | 0.66 | 0.99(0.92-1.07) |
| PM2.5 | per 5 μg/m3 | |||
| Trimester 1 | 1, 3, 10, 11 | 2822 | 0.15 | 1.10(0.96,1.26) |
| Outcome: Hypertensive disorder of pregnancy (includes gestational hypertension and preeclampsia) | ||||
| CO | per 1 ppm | |||
| Full gestational period | 10, 11, 12, 13 | 5576 | <0.001 | 0.86(0.53-1.41) |
| Trimester 1 | 4, 10, 11, 12 | 3615 | 0.12 | 1.79(1.31-2.45) |
| Trimester 2 | 4, 10, 11, 12 | 3615 | 0.29 | 1.22(0.90-1.64) |
| NO2 | per 10 ppb | |||
| Full gestational period | 1, 6, 7, 8, 10, 11, 12 | 4916 | 0.047 | 1.16(1.03-1.30) |
| Trimester 1 | 1, 6, 7, 10, 11, 12b | 4775 | 0.029 | 1.16(1.02-1.31) |
| Trimester 2 | 1, 6, 8, 10, 11, 12c | 4666 | 0.031 | 1.15(1.02-1.30) |
| Trimester 1 | 1, 4, 6, 10, 11, 12 | 4661 | 0.42 | 1.05(0.99-1.12) |
| Trimester 2 | 1, 4, 6, 10, 11, 12 | 4661 | 0.39 | 1.03(0.96-1.11) |
| O3 | per 10 ppb | |||
| Trimester 1 | 2, 3, 4, 5, 10, 11, 12 | 10038 | 0.81 | 1.09(1.05-1.13) |
| 2, 4, 5, 10, 11, 12d | 8897 | 0.71 | 1.09(1.04-1.14) | |
| 3, 4, 5, 10, 11, 12e | 7960 | 0.73 | 1.09(1.04-1.15) | |
| Trimester 2 | 4,10,11,12 | 3615 | 0.006 | 1.12(0.90-1.39) |
| PM10 | per 10 μg/m3 | |||
| Full gestational period | 1, 7, 8, 9, 10, 11 | 15021 | 0.001 | 1.10(0.96-1.26) |
| 1, 7, 9, 10, 11b | 14880 | 0.001 | 1.09(0.94-1.25) | |
| 1, 8, 9, 10, 11c | 14771 | 0.004 | 1.06(0.93-1.20) | |
| Trimester 1 | 1, 2, 3, 4, 10, 11 | 5900 | 0.58 | 1.02(0.95-1.08) |
| 1, 2, 4, 10, 11d | 4759 | 0.44 | 1.02(0.95-1.09) | |
| 1, 3, 4, 10, 11e | 3822 | 0.84 | 0.99(0.92-1.06) | |
| Trimester 2 | 1, 4, 10, 11 | 2681 | 0.85 | 0.97(0.89-1.05) |
| PM2.5 | per 5 μg/m3 | |||
| Full gestational period | 1, 9, 10, 11, 12 | 15245 | <0.001 | 1.15(0.94-1.40) |
| Trimester 1 | 1, 2, 3, 4, 10, 11, 12 | 5207 | 0.01 | 1.18(0.98-1.41) |
| 1, 2, 4, 10, 11, 12d | 4508 | 0.007 | 1.18(0.94-1.48) | |
| 1, 3, 4, 10, 11, 12e | 3995 | 0.007 | 1.20(0.93-1.54) | |
| Trimester 2 | 1, 4, 10, 11, 12 | 3296 | <0.001 | 1.17(0.79-1.75) |
References are as follows: 1, Dadvand et al. 2013; 2, Lee et al. 2013 (Gestational Hypertension); 3, Lee et al. 2013 (Preeclampsia); 4, Mobasher et al. 2013; 5, Olsson et al. 2013; 6, Pereira et al. 2013; 7, van den Hooven et al. 2011 (Gestational Hypertension); 8, van den Hooven et al. 2011 (Preeclampsia); 9, Vinikoor-Imler et al. 2012; 10, Wu et al. 2011 (Los Angeles); 11, Wu et al. 2011 (Orange County); 12, Xu et al. 2013; 13, Zhai et al. 2012.
Sensitivity analysis excluding van den Hooven et al. 2011 (Preeclampsia).
Sensitivity analysis excluding van den Hooven et al. 2011 (Gestational Hypertension).
Sensitivity analysis excluding Lee et al. 2013 (Preeclampsia).
Sensitivity analysis excluding Lee et al. 2013 (Gestational Hypertension).
Figure 2.
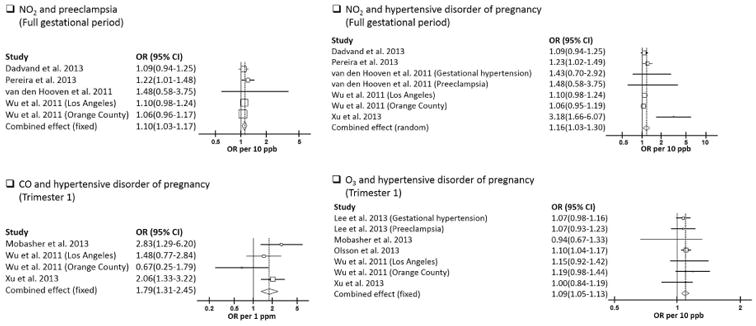
Hypertensive disorders of pregnancy
Four studies examined the association between air pollutants and risk of hypertensive disorders of pregnancy, which is the combination of cases with gestational hypertension and preeclampsia. Statistically significantly increased risks of HDP were observed in relation to CO during the entire pregnancy in one study (OR per 0.15 ppm = 1.12; 95% CI, 1.03-1.22) (Xu et al., 2013), and during the first trimester in two studies [OR per 1 ppm = 2.83; 95% CI, 1.29-6.2 (Mobasher et al., 2013); OR per 0.24 ppm = 1.19; 95% CI, 1.07-1.31 (Xu et al., 2013)]. Exposure to NO2 during the entire pregnancy (OR per 1.65 ppb = 1.21; 95% CI, 1.09-1.35) or the first trimester (OR per 5.39 ppb = 1.14; 95% CI, 1.01-1.29) was also reported to be associated with increased risks of HDP by one study (Xu et al., 2013). In addition, increased risks of HDP linked to O3 exposure during the first trimester [OR per 5 ppb = 1.05; 95% CI, 1.02-1.08 (Olsson et al., 2013)] or the second trimester [OR per 15 ppb = 2.05; 95% CI, 1.22-3.46 (Mobasher et al., 2013)] were also reported. One study reported increased risks of HDP with exposure to PM10 during the entire pregnancy [OR per 2.24 μg/m3 = 1.11; 95% CI, 1.08-1.15 (Vinikoor-Imler et al., 2012)]. Two studies observed associations between PM2.5 exposure and increased risks of HDP: one study found the association during the entire pregnancy (OR per 0.67 μg/m3 =1.24; 95% CI, 1.08-1.43) and second trimester [OR per 1.25 μg/m3 = 1.28; 95% CI, 1.13-1.46 (Xu et al., 2013)], while the other one found the association during first trimester [OR per 7 μg/m3 = 3.94; 95% CI, 1.82-8.55 (Mobasher et al., 2013)]. Only one study reported the effects of exposure to SO2 on HDP (Xu et al., 2013), and increased risks of HDP were observed with higher exposure to SO2 during entire pregnancy (OR per 2.55 ppb = 1.13; 95% CI, 1.01-1.25) and first trimester (OR per 3.73 ppb = 1.14; 95% CI, 1.03-1.26). No inverse associations were observed for HDP.
Two studies conducted stratified analyses to examine effect modifications by body mass index (BMI) and neighborhood deprivations, and neither of them reported whether the analyses were pre-specified or conducted post hoc (Mobasher et al., 2013; Vinikoor-Imler et al., 2012). Exposure to CO and PM2.5 in the first trimester was significant associated with increased odds of HDP among non-obese women, while no association was observed among obese women (Mobasher et al., 2013). In addition, the effects of exposure to PM2.5 and PM10 were stronger among women who resided in areas with higher neighborhood deprivation (Vinikoor-Imler et al., 2012).
Among the six studies reported preeclampsia as outcome, two studies also reported gestational hypertension as the second outcome (Lee et al., 2013; van den Hooven et al., 2011). In both studies, cases with preeclampsia were excluded from cases with gestational hypertension. Increased risks of gestational hypertension were observed with PM10 exposure during the entire pregnancy [OR per 10 μg/m3 = 1.72; 95% CI, 1.12-2.63 (van den Hooven et al., 2011)] and PM2.5 exposure during the first trimester [OR per 4 μg/m3 = 1.11; 95% CI, 1.00-1.23 (Lee et al., 2013)].
Given the limited number of studies reported HDP as outcome, and the common nature and shared mechanisms of HDP, gestational hypertension, and preeclampsia, we conducted meta-analyses for these outcomes comprehensively. For the four studies which only reported preeclampsia as outcome, we used the risk estimates from these studies as an estimation of the association between air pollutants and HDP which was not available in these studies. For the other two studies which reported both gestational hypertension and preeclampsia as outcomes, we first entered risk estimates from these publications together in the HDP meta-analyses, and we further conducted sensitivity analyses by entering them separately. A total of 14 combinations of pollutants and exposure window for which four or more studies (or sub-studies) published results were analyzed, and 10 sensitivity analyses were additionally conducted (for summary results, see Table 3; for full results, see Supplemental Material, Table 1). The summary risk estimates from these meta-analyses were generally close to one except for the CO-trimester1 combination, which has a summary OR of 1.79. Without this combination, the summary ORs ranged from 0.86 to 1.22. Heterogeneity tests showed evidence for heterogeneity among studies (p<0.10) in half of the analyses conducted, most consistently related to analyses of PM. We found statistically significantly increased summary risk estimates for CO exposure during the first trimester of pregnancy (OR per 1 ppm = 1.79; 95% CI, 1.31-2.45), for NO2 exposure during the full gestational period (OR per 10 ppb = 1.16; 95% CI, 1.03-1.30), and for O3 during the first trimester (OR per 10 ppb = 1.09; 95% CI, 1.05-1.13) (Table 3, Figure 2). Sensitivity analyses excluding the study with the largest weight from each meta-analysis showed that results for NO2 during the entire pregnancy and O3 during the first trimester of pregnancy were robust to this exclusion. The results for CO during the first trimester were marginally significant (OR per 1 ppm = 1.55; 95% CI, 0.99-2.42) after exclude the Xu et al.'s study (Xu et al., 2013).
Publication bias
Significant p-values from Egger test were found for only 4 of the 30 meta-analyses we conducted (Supplemental Material, Table 1), indicating that wide-scale publication bias is unlikely.
4. Discussion
This is the first systematic review to our knowledge to assess the quality and magnitude of the association between gestational exposure to major air pollutants and risks of hypertensive disorder of pregnancy. Increased risks of HDP/preeclampsia were reported in individual studies for some air pollutants in different exposure windows, mostly during the entire or first trimester of pregnancy. We demonstrated a significant association between increased risks of HDP/preeclampsia and exposure to NO2 during the entire pregnancy. In addition, first trimester exposure to CO or O3 was also significantly associated with increased risks of HDP. The significant associations observed in our study are consistent with evidence from experimental cellular, histological, animal studies. Although we based summary risk estimates on just a few studies (between 4 and 5), the total numbers of cases included were very large (between 2,681 and 15,245). We found no statistically significant increase in risk of other combinations of air pollutants and exposure windows in relation to HDP/preeclampsia.
A number of possible mechanisms for the associations observed in this study have been suggested. It is well-known that air pollution can aggregate the development and progression of atherosclerosis, which may potentially contribute to hypertension (Allen et al., 2012; Campen et al., 2012; Gill et al., 2011). Given the similarities between HDP and atherosclerotic cardiovascular diseases, they may share common pathways in relation to air pollutants (Duckitt and Harrington, 2005; Kaaja and Greer, 2005). Therefore, many of the hypothesized mechanisms between air pollution and cardiovascular diseases such as inflammation, oxidative stress, and endothelial dysfunction may also apply to HDP (Brook, 2008). In addition, direct links have been reported between air pollution and endothelial dysfunction, which is regarded as a pre-cursor associated with HDP (Bind et al., 2012; Brook and Rajagopalan, 2012; Steegers et al., 2010). Furthermore, the abnormal placentation and failed vascular remodeling caused by endothelial dysfunction may trigger preeclampsia (Powe et al., 2011). Hypoxia at the fetal-maternal interface due to impaired placentation has also been suggested to cause dissemination of free radicals that lead to HDP and preeclampsia in susceptible women (Roberts et al., 2003).
Both the results from this meta-analysis and the individual studies show that early pregnancy is a critical exposure window for air pollution's effects on HDP; however, since most of the reviewed studies used registry information, they were only able to obtain the residential address at birth rather than at the beginning of pregnancy. Without residential information in early pregnancy, the results from these studies may be biased. Previous studies reported that the residential mobility among pregnant women ranged from 12% to 35% (Brauer et al., 2008; Fell et al., 2004). However, the majority of these moves appear to be local and to areas with a similar socioeconomic make-up, and the characteristics of women who move are similar to those who do not (Canfield et al., 2006; Fell et al., 2004). Therefore, this is likely to be a non-differential misclassification, which may bias the estimate into the null (Canfield et al., 2006; Ritz et al., 2007). In addition, residential address-based air pollution measurement cannot take the time-location/activity patterns into account, thus unable to assess exposure from other locations. Future studies with personal monitored air pollution data are warranted. As the period from the conception to the onset of HDP is usually not very long, i.e. a few of months, it may be practically and economically feasible to use personal monitors to measure individual air pollution exposure in several pre-selected times of window. In the study design, they would need to start with a pregnancy cohort, which should be initialized before the onset of HDP and sufficiently large enough to observe a small effect, and personal monitors can be used to collect air pollution exposure data in the selected windows of time between the recruitment and the onset of HDP in these studies. In addition, information on residential history and time-location/activity patterns are warranted by using case-control surveys nested within birth cohorts (Ritz et al., 2007) or pregnancy cohorts such as the National Children's Study (Gilliland et al., 2005) and the Human Early-Life Exposome Project (HELIX) (Vrijheid et al., 2014).
Most of the reviewed studies used monitored air pollution data (Mobasher et al., 2013; Olsson et al., 2013; van den Hooven et al., 2011; Vinikoor-Imler et al., 2012; Xu et al., 2013; Zhai et al., 2012), and inverse-distance weighting is the most frequently method used by these studies. This exposure measurement method suffered from a number of limitations, especially the selection bias due to the poor spatial coverage of air monitors, and when assessing pollutants such as NO2 and CO which have great spatial variations, this method may be inappropriate. Lee et al. used a space-time ordinary kriging interpolation model. While it provided better spatial coverage, it still relied on ambient air monitors and ignored atmospheric influences on the dispersion of pollutants (Lee et al., 2013). Compared to these methods, the LUR model used by Dadvand et al. and Pereira et al. is able to characterize the small-scale within-city variation of pollutant levels, and it has been shown to be an effective tool in predicting long-term intra-urban variation of air pollution (Dadvand et al., 2013; Pereira et al., 2013). Despite these advantages it possesses, LUR models suffered from several limitations: 1) given the facts that variables included in LUR models are temporally stable, it is inferior to estimate air pollution at temporal scale; 2) LUR models are generally not transferable from one urban area to another; 3) the interpretation of LUR models may be influenced by the different buffering radius used in the model; and 4) the multi-pollutant aspects of air pollution is poorly addressed in LUR since it usually focuses on one pollutant at a time (Isakov et al., 2012). A Gaussian dispersion model was used by Wu et al. based on information including meteorology, roadway geometry and traffic activities, and vehicle emission factors (Wu et al., 2011). Compared to other methods discussed above, the dispersion model estimated local traffic-generated air pollutants with better spatiotemporal variability. However, the exposure estimates from this model may be influenced more strongly by residential mobility due to the more spatially resolved estimates compared to those obtained using other methods (Wu et al., 2009b). In addition to the dispersion model, Wu et al. also evaluated the validation of air pollution measured by different models (Wu et al., 2011), and they found comparable results between air pollution and preeclampsia when exposure were assessed using dispersion models, LUR models, or a more simplistic method such as nearest air monitor. Furthermore, only a small proportion of the reviewed studies used multiple-pollutant models in analyses. The applications of these models in future studies would be a step toward a further understanding the interactions of different air pollutants.
One big limitation in our analyses was the use of different outcomes of HDP in individual studies. Some studies only reported preeclampsia as the outcome, some reported both preeclampsia and gestational hypertension separately, while others reported HDP which combined preeclampsia and gestational hypertension cases. Although gestational hypertension, preeclampsia, and HDP all belong to pregnancy induced-hypertension, the different outcomes used in individual studies may cause heterogeneity in meta-analyses. The main reason for the use of different outcomes is that most of the included studies were based on registry information; however, data regarding HDP were collected by different definitions in different registries. Therefore, it is important for different registries to follow the same classification of pregnancy-related outcomes. This heterogeneity in defining outcomes is also a major issue in other pregnancy-related studies (Vrijheid et al., 2011). When possible, we encourage researchers who have data about gestational hypertension and preeclampsia to combine these two outcomes and report them as HDP. Another limitation related to many registry data is the lack of detailed date of HDP diagnosis. Only one study collected the diagnosis dates and investigated the potential different effects of air pollution on early- and late-onset HDP (Dadvand et al., 2013). Given the different severity and etiology between early- and late-onset HDP, future studies with detailed date of diagnosis are necessary to determine air pollution's effects on HDP.
Heterogeneity in the studies reviewed may also be due to inherent differences between the study areas/settings and analysis methods. Firstly, compositions of pollutant mixtures may dramatically differ in different areas, which may explain the consistent heterogeneity we observed for particulate matter. In addition, the underlying HDP risks, exposure levels and ranges were also different in these individual studies. As for study design, the assessments of exposure were relatively similar: same pollutant in same exposure window, and similar calculation methods were used. Since most of the reviewed studies used registry data, fixed cohort bias may be present (Strand et al., 2011). This selection bias is more likely to happen when shorter pregnancies are missed at the start of the study, and longer pregnancies are missed at the end. If the study period is longer and the study has a larger study population, the impact of fixed cohort bias will be negligible because the missed cases at the beginning or end of the study consist of a very small proportion of the study population. Therefore, given the facts that fixed cohort bias tends to decrease when the study has longer study period and/or when it has day and month of the start date just before day and month of the end date, the potential for this bias was reduced. Furthermore, covariates included in individual studies slightly differed and residual confounding structures may differ among the studies, which is another potential source of heterogeneity. However, the number of known risk factors for HDP is very limited, and none of these suspected risk factors can explain a large proportion of cases.
Caution should be taken when interpreting the results from this meta-analysis. We found significant results for four outcome-pollutant-period combinations; three of these were robust to the exclusion of the study with the largest weight, while the other one was marginally significant after exclusion. Since only one study reported results using categorical exposure, all our meta-analyses were based on continuous exposure assuming a log-linear relationship between air pollution and HDP, which may be inappropriate. Therefore, we encourage future studies to report both types of analyses when possible. Although the magnitude of association between air pollution and HDP is relatively small compared with other traditional risk factors. However, the population-attributable fraction (PAF) of each pollutant should not be overlooked since the majority of the population is exposed to air pollution, particularly in urban settings (World Health Organization, 2014). In addition, in developing countries where air pollution level is much higher than in developed countries, the impacts of air pollution on risks of HDP may be even stronger, leading to more severe impacts on maternal and neonatal health. Therefore, improvements in air quality, public perception, and behavioral changes in response to bad air quality (Semenza et al., 2008) may be beneficial in reducing risks of air pollution associated HDP.
Other limitations of this study also need to be noticed. First, although we observed low heterogeneity and no significant publication bias, we cannot rule out the possibility that the absence of publication bias in the study is due to the relatively small number of primary studies included. Second, due the limited number of studies conducted in this area, there are insufficient data to analyze more pollutants for different gestational periods. But even with the small number of studies available currently, we observed significant and consistent relationships between air pollution and HDP. Thirdly, most studies have used a single-pollutant model in spite of possible interactions between pollutants.
5. Conclusion
This meta-analysis is the first to our knowledge to evaluate the association between exposure to criteria air pollutants and the risks of hypertensive disorders of pregnancy. Based on the findings from 10 previous studies, our analyses suggest that ambient air pollution exposure during pregnancy may be associated with increased risk of hypertensive disorders of pregnancy and preeclampsia. Although the ORs were relatively low, the PAFs were not negligible given the facts that air pollution is ubiquitous. However, the findings remain inconclusive considering the limited number of studies available and methodological weaknesses in these existing studies such as the lack of individual air pollution measurement and/or time of HDP diagnosis. More studies with improved study designs and methodologies are needed in the field.
Supplementary Material
Table 1. Detailed results of meta-analyses: summary risk estimates and risk estimates from individual studies.
Table 1A. Carbon monoxide (CO).
Table 1B. Nitrogen dioxide (NO2).
Table 1C. Ozone (O3).
Table 1D. Particulate Matter (PM10).
Table 1E. Particulate Matter (PM2.5).
Highlights.
Effects of prenatal air pollution exposure on HDP were examined.
A meta-analysis was performed on studies published on/after 1980.
Exposure to NO2 during the entire pregnancy is associated with HDP and preeclampsia.
Exposure to Co and O3 during the first trimester is associated with HDP and preeclampsia.
In general, our review suggests an association between ambient air pollution and HDP risk.
Acknowledgments
The project described was supported by Grant Number K01ES019177 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen RW, Adar SD, Avol E, Cohen M, Curl CL, Larson T, Liu LJ, Sheppard L, Kaufman JD. Modeling the residential infiltration of outdoor PM(2.5) in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environmental health perspectives. 2012;120:824–830. doi: 10.1289/ehp.1104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen VM, Joseph K, Murphy KE, Magee LA, Ohlsson A. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: a population based study. BMC pregnancy and childbirth. 2004;4:17. doi: 10.1186/1471-2393-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. American journal of obstetrics and gynecology. 2006;195:1557–1563. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Basile JN, Bloch MJ. Exposure to air pollution increases the incidence of hypertension and diabetes in black women living in Los Angeles. Journal of clinical hypertension. 2012;14:819–820. doi: 10.1111/jch.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer ST, Cleary KL. Cardiopulmonary complications of pre-eclampsia. Seminars in perinatology. 2009;33:158–165. doi: 10.1053/j.semperi.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Bmj. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bind MA, Baccarelli A, Zanobetti A, Tarantini L, Suh H, Vokonas P, Schwartz J. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology. 2012;23:332–340. doi: 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environmental health perspectives. 2008;116:680. doi: 10.1289/ehp.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD. Cardiovascular effects of air pollution. Clinical science. 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. Journal of the American Society of Hypertension : JASH. 2009;3:332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S. Chronic air pollution exposure and endothelial dysfunction: what you can't see--can harm you. Journal of the American College of Cardiology. 2012;60:2167–2169. doi: 10.1016/j.jacc.2012.08.974. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Lund A, Rosenfeld M. Mechanisms linking traffic-related air pollution and atherosclerosis. Current opinion in pulmonary medicine. 2012;18:155–160. doi: 10.1097/MCP.0b013e32834f210a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield MA, Ramadhani TA, Langlois PH, Waller DK. Residential mobility patterns and exposure misclassification in epidemiologic studies of birth defects. Journal of Exposure Science and Environmental Epidemiology. 2006;16:538–543. doi: 10.1038/sj.jes.7500501. [DOI] [PubMed] [Google Scholar]
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, Burnett R, Palmer JR, Rosenberg L. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125:767–772. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand P, Figueras F, Basagana X, Beelen R, Martinez D, Cirach M, Schembari A, Hoek G, Brunekreef B, Nieuwenhuijsen MJ. Ambient Air Pollution and Preeclampsia: A Spatiotemporal Analysis. Environmental health perspectives. 2013 doi: 10.1289/ehp.1206430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. Bmj. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duley L. The global impact of pre-eclampsia and eclampsia. Seminars in perinatology. 2009;33:130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Fell DB, Dodds L, King WD. Residential mobility during pregnancy. Paediatric and perinatal epidemiology. 2004;18:408–414. doi: 10.1111/j.1365-3016.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- Gill EA, Curl CL, Adar SD, Allen RW, Auchincloss AH, O'Neill MS, Park SK, Van Hee VC, Diez Roux AV, Kaufman JD. Air pollution and cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Progress in cardiovascular diseases. 2011;53:353–360. doi: 10.1016/j.pcad.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland F, Avol E, Kinney P, Jerrett M, Dvonch T, Lurmann F, Buckley T, Breysse P, Keeler G, de Villiers T, McConnell R. Air pollution exposure assessment for epidemiologic studies of pregnant women and children: lessons learned from the Centers for Children's Environmental Health and Disease Prevention Research. Environmental health perspectives. 2005;113:1447–1454. doi: 10.1289/ehp.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Tong S, Li S, Barnett AG, Yu W, Zhang Y, Pan X. Gaseous air pollution and emergency hospital visits for hypertension in Beijing, China: a time-stratified case-crossover study. Environmental health : a global access science source. 2010a;9:57. doi: 10.1186/1476-069X-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Tong S, Zhang Y, Barnett AG, Jia Y, Pan X. The relationship between particulate air pollution and emergency hospital visits for hypertension in Beijing, China. The Science of the total environment. 2010b;408:4446–4450. doi: 10.1016/j.scitotenv.2010.06.042. [DOI] [PubMed] [Google Scholar]
- Isakov V, Johnson M, Touma J, Özkaynak H. Development and evaluation of land-use regression models using modeled air quality concentrations, Air Pollution Modeling and its Application XXI. Springer; 2012. pp. 717–722. [Google Scholar]
- Jedrychowski WA, Perera FP, Maugeri U, Spengler J, Mroz E, Flak E, Stigter L, Majewska R, Kaim I, Sowa A, Jacek R. Prohypertensive effect of gestational personal exposure to fine particulate matter. Prospective cohort study in nonsmoking and non-obese pregnant women. Cardiovascular toxicology. 2012;12:216–225. doi: 10.1007/s12012-012-9157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA : the journal of the American Medical Association. 2005;294:2751–2757. doi: 10.1001/jama.294.21.2751. [DOI] [PubMed] [Google Scholar]
- Lee PC, Roberts JM, Catov JM, Talbott EO, Ritz B. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in Allegheny County, PA. Maternal and child health journal. 2013;17:545–555. doi: 10.1007/s10995-012-1028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Current opinion in obstetrics & gynecology. 2013;25:124–132. doi: 10.1097/GCO.0b013e32835e0ef5. [DOI] [PubMed] [Google Scholar]
- Malmqvist E, Jakobsson K, Tinnerberg H, Rignell-Hydbom A, Rylander L. Gestational Diabetes and Preeclampsia in Association with Air Pollution at Levels below Current Air Quality Guidelines. Environmental health perspectives. 2013;121:488–493. doi: 10.1289/ehp.1205736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. The Challenge of Epidemiology: Issues and Selected Readings. 2004;1:533–553. [PubMed] [Google Scholar]
- Mobasher Z, Salam MT, Goodwin TM, Lurmann F, Ingles SA, Wilson ML. Associations between ambient air pollution and Hypertensive Disorders of Pregnancy. Environmental research. 2013;123:9–16. doi: 10.1016/j.envres.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson D, Mogren I, Forsberg B. Air pollution exposure in early pregnancy and adverse pregnancy outcomes: a register-based cohort study. BMJ open. 2013;3 doi: 10.1136/bmjopen-2012-001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G, Haggar F, Shand AW, Bower C, Cook A, Nassar N. Association between pre-eclampsia and locally derived traffic-related air pollution: a retrospective cohort study. Journal of epidemiology and community health. 2013;67:147–152. doi: 10.1136/jech-2011-200805. [DOI] [PubMed] [Google Scholar]
- Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Wilhelm M, Hoggatt KJ, Ghosh JK. Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. American journal of epidemiology. 2007;166:1045–1052. doi: 10.1093/aje/kwm181. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Pearson GD, Cutler JA, Lindheimer MD, National Heart L, Blood I. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension in pregnancy : official journal of the International Society for the Study of Hypertension in Pregnancy. 2003;22:109–127. doi: 10.1081/PRG-120016792. [DOI] [PubMed] [Google Scholar]
- Rudra CB, Williams MA, Sheppard L, Koenig JQ, Schiff MA. Ambient carbon monoxide and fine particulate matter in relation to preeclampsia and preterm delivery in western Washington State. Environmental health perspectives. 2011;119:886–892. doi: 10.1289/ehp.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza JC, Wilson DJ, Parra J, Bontempo BD, Hart M, Sailor DJ, George LA. Public perception and behavior change in relationship to hot weather and air pollution. Environmental research. 2008;107:401–411. doi: 10.1016/j.envres.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Sorensen M, Hoffmann B, Hvidberg M, Ketzel M, Jensen SS, Andersen ZJ, Tjonneland A, Overvad K, Raaschou-Nielsen O. Long-term exposure to traffic-related air pollution associated with blood pressure and self-reported hypertension in a Danish cohort. Environmental health perspectives. 2012;120:418–424. doi: 10.1289/ehp.1103631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. Journal of clinical epidemiology. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- Strand LB, Barnett AG, Tong S. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC medical research methodology. 2011;11:49. doi: 10.1186/1471-2288-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hooven EH, de Kluizenaar Y, Pierik FH, Hofman A, van Ratingen SW, Zandveld PY, Mackenbach JP, Steegers EA, Miedema HM, Jaddoe VW. Air pollution, blood pressure, and the risk of hypertensive complications during pregnancy: the generation R study. Hypertension. 2011;57:406–412. doi: 10.1161/HYPERTENSIONAHA.110.164087. [DOI] [PubMed] [Google Scholar]
- Vigeh M, Yunesian M, Shariat M, Niroomanesh S, Ramezanzadeh F. Environmental carbon monoxide related to pregnancy hypertension. Women & health. 2011;51:724–738. doi: 10.1080/03630242.2011.633599. [DOI] [PubMed] [Google Scholar]
- Vinikoor-Imler LC, Gray SC, Edwards SE, Miranda ML. The effects of exposure to particulate matter and neighbourhood deprivation on gestational hypertension. Paediatric and perinatal epidemiology. 2012;26:91–100. doi: 10.1111/j.1365-3016.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- Vrijheid M, Martinez D, Manzanares S, Dadvand P, Schembari A, Rankin J, Nieuwenhuijsen M. Ambient air pollution and risk of congenital anomalies: a systematic review and meta-analysis. Environmental health perspectives. 2011;119:598–606. doi: 10.1289/ehp.1002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, Slama R, Robinson O, Chatzi L, Coen M, van den Hazel P, Thomsen C, Wright J, Athersuch TJ, Avellana N. The Human Early-Life Exposome (HELIX): Project Rationale and Design. Environmental health perspectives. 2014 doi: 10.1289/ehp.1307204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IK, Tsai IJ, Chen PC, Liang CC, Chou CY, Chang CT, Kuo HL, Ting IW, Lin CC, Chuang FR, Huang CC, Sung FC. Hypertensive disorders in pregnancy and subsequent diabetes mellitus: a retrospective cohort study. The American journal of medicine. 2012;125:251–257. doi: 10.1016/j.amjmed.2011.07.040. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Morello-Frosch R, Jesdale B. Air Pollution and Preeclampsia Among Pregnant Women in California, 1996-2004. Epidemiology. 2008;19:S310. doi:310.1097/1001.ede.0000340396.0000314178.0000340381. [Google Scholar]
- World Health Organization. Burden of disease from Ambient Air Pollution for 2012. World Health Organization; Geneva: 2014. [Google Scholar]
- Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Health of children born to mothers who had preeclampsia: a population-based cohort study. American journal of obstetrics and gynecology. 2009a;201:269 e261–269 e210. doi: 10.1016/j.ajog.2009.06.060. [DOI] [PubMed] [Google Scholar]
- Wu J, Ren C, Delfino RJ, Chung J, Wilhelm M, Ritz B. Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environmental health perspectives. 2009b;117:1773–1779. doi: 10.1289/ehp.0800334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wilhelm M, Chung J, Ritz B. Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environmental research. 2011;111:685–692. doi: 10.1016/j.envres.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Hu H, Ha S, Roth J. Ambient air pollution and hypertensive disorder of pregnancy. Journal of epidemiology and community health. 2013 doi: 10.1136/jech-2013-202902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder SR, Thornburg LL, Bisognano JD. Hypertension in pregnancy and women of childbearing age. The American journal of medicine. 2009;122:890–895. doi: 10.1016/j.amjmed.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Zhai D, Guo Y, Smith G, Krewski D, Walker M, Wen SW. Maternal exposure to moderate ambient carbon monoxide is associated with decreased risk of preeclampsia. American journal of obstetrics and gynecology. 2012;207:57 e51–59. doi: 10.1016/j.ajog.2012.03.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Detailed results of meta-analyses: summary risk estimates and risk estimates from individual studies.
Table 1A. Carbon monoxide (CO).
Table 1B. Nitrogen dioxide (NO2).
Table 1C. Ozone (O3).
Table 1D. Particulate Matter (PM10).
Table 1E. Particulate Matter (PM2.5).


