Abstract
The microRNAs (miRNAs) are small noncoding RNAs that are potent regulators of gene expression and can regulate several diverse biological functions. This minireview provides an overview of recent studies that have examined the role and involvement of miRNAs in cholangiocarcinomas. These studies provide evidence for deregulated expression of miRNA and are providing new insights into the potential contribution of these in the pathogenesis of cholangiocarcinoma.
Keywords: Biliary tract cancers, Noncoding RNA, Epithelial–mesenchymal transition, Cell proliferation, Cell death
INTRODUCTION
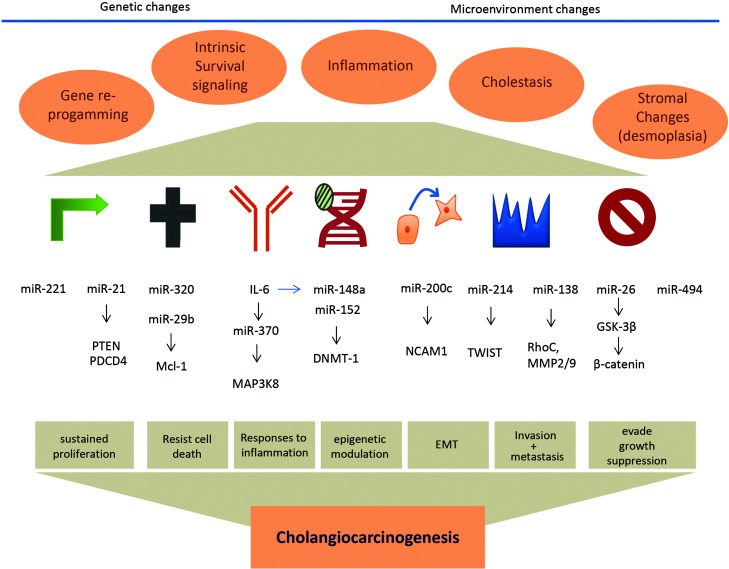
The microRNAs (miRNAs) are a group of small noncoding RNAs, approximately 22 nucleotides in size. Recent estimates indicate the presence of >1,700 miRNAs in humans. These small noncoding RNAs are potent regulators of gene expression, and according to some estimates, may contribute to regulation of ∼50% of the human genome (1). miRNAs can alter the expression of target genes by binding to sequence-defined sites within their 3′-untranslated regions, which results in target gene messenger RNA decay or inhibition of translation. The contribution of these genes to human liver diseases has been extensively reviewed (2). However, the specific roles and contributions to biliary tract disease are less well recognized. The first description of the involvement of miRNA in the biliary tract reported profiling studies of miRNA expression in malignant and nonmalignant biliary tract epithelia. Subsequent studies have reported expression of miRNA in cholangiocarcinomas (CCAs), cancers of the biliary tract. These findings are described in detail in this review, along with a description of how these miRNA may be involved in tumor pathophysiology and carcinogenesis. Similar to emerging data from other diseases, these studies provide evidence for the involvement of selected miRNA in cholangiocarcinogenesis (see Fig. 1).
Figure 1.
miRNA in molecular pathogenesis of cholangiocarcinoma. Deregulated expression of several miRNA in response to genetic or environmental changes can contribute to aberrant gene expression that can contribute to many different pathophysiological processes involved in the pathogenesis and behavior of cholangiocarcinoma. Selected examples of deregulated miRNA and their targets are shown.
miRNA IN BILIARY TRACT TUMORIGENESIS
Some miRNAs can contribute to tumorigenesis by acting as oncogenes or tumor-suppressor genes. In addition, deregulated expression of miRNA in malignant cells may contribute to the tumor phenotype through effects on diverse cellular processes.
Modulation of tumor cell proliferation is an established mechanism by which miRNA can contribute to tumor growth. Several studies have reported that miRNAs that are upregulated in CCA, such as miR-26a, miR-141, miR-210, miR-31, miR-21, and miR-421, can modulate cell proliferation through several different mechanisms (3–14). A role for miR-21 has been postulated in the carcinogenesis and metastasis of O. viverrini-associated CCA by suppressing the function of programmed cell death 4 (PDCD4) (12). miR-21 has been shown to be overexpressed in human CCA by a mechanism involving upregulation of arsenic resistance protein 2. miR-21 is implicated in many cancers and can act through effects of downstream targets such as phosphatase and tension homolog deleted on chromosome 10 (PTEN) and PDCD4 (11,15). Similarly, miRNAs such as miR-494, miR-148a, miR-152, miR-370, and miR-138 that are downregulated in CCA can also have effects on cell proliferation (16–21). For example, miR-494 can induce growth retardation through multiple targets involved in the G1/S transition and has been shown to be downregulated in CCA (16).
CCAs are highly resistant to chemotherapy (22). Indeed, several miRNAs such as miR-21, miR-200b, and miR-25 that are aberrantly expressed in CCA could contribute to chemoresistance through the activation of antiapoptotic pathways (4,9). miR-21 can modulate gemcitabine-induced apoptosis by phosphatase and tensin homolog deleted on chromosome 10 (PTEN)-dependent activation of PI 3-kinase signaling (4). miR-25, which is also overexpressed in CCA, can protect cells against TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis, whereas antagonism of miR-25 sensitizes cells in culture to apoptotic death (23). miR-200b can target the protein tyrosine phosphatase nonreceptor type 12 that is involved in cell growth, cell dedifferentiation, and oncogenic transformation (4). miRNAs that are decreased in CCA, such as miR-320, miR-29b, miR-204, miR-205, and miR-221 (6,24–27), could also contribute to chemotherapeutic responses. Exogenous expression of miR-320 or miR-204 can negatively regulate Mcl-1 or Bcl-2 expression and facilitate chemotherapeutic drug-triggered apoptosis (24). miR-29 is an endogenous regulator of Mcl-1 protein expression and thereby apoptosis (25). Thus, deregulated expression of miRNAs may contribute to the high chemoresistance of CCA. Targeting these miRNAs may offer the potential for improved response to therapeutic agents in CCA.
CCAs are frequently noted to arise in the setting of chronic biliary tract inflammation (22). The contribution of mediators of inflammation to cholangiocarcinogenesis is exemplified by the inflammation-associated cytokine interleukin-6 (IL-6), which contributes to the abnormal growth and survival of malignant cholangiocytes through both autocrine and paracrine mechanisms (28–30). By modulating miRNA such as miR-148a and miR-152, IL-6 can regulate the activity of DNMT-1 and expression of methylation-dependent tumor-suppressor genes (18). These observations provide a link between an inflammation-associated cytokine and miRNA-dependent oncogenic mechanisms in CCA.
The epithelial–mesenchymal transition (EMT) is a process whereby epithelial cells lose their cell polarity and cell–cell adhesion and can gain migratory and invasive properties to become mesenchymal cells (31). The activation of miR-200c can lead to a reduction of EMT with reduced cell migration and invasion in intrahepatic CCA (iCCA) cells (32). NCAM1, a known hepatic stem/progenitor cell marker, has been experimentally shown to be a direct target of miR-200c (32). Likewise, miR-214 may be important in regulating metastasis of iCCA because downregulation of this miRNA can promote EMT by directly targeting the Twist gene (33). These results suggest potential applications of therapies directed toward miRNA, such as miR-214 in the therapy of iCCA as an approach to reduce metastatic spread through modulation of EMT.
miRNA IN BILIARY TRACT CANCERS
Specific patterns of miRNA expression can be useful for identifying specific types of cancers (34). Similarly, miRNA expression profiling could provide insights into the molecular basis of carcinogenesis or clinical behavior of CCA. Thus, studies to define specific clinical subtypes of CCA that correlate with clinical behavior and are based on miRNA expression should be considered. Based on published studies, several miRNAs have been identified that are upregulated (Table 1) or downregulated (Table 2) in CCA. Expression profiles based on cell lines show some heterogeneity of expression across cell lines, whereas differences in tissue expression have been noted to vary based on etiology of underlying cancer. Some deregulated miRNAs, such as miR-21, show consistencies in expression across cell lines and tissues. Studies in CCA cell lines have identified an increased expression of miR-21, miR-200b, and miR-141 and decreased expression of miR-214, miR-29b, and miR-494 (4,16,17,25–27,33,35). In liver tissues, aberrant upregulation of miR-21, miR-421, and miR-26A with downregulation of miR-370, miR-373, miR-200b/c, and miR-494 have been noted (3,4,6,8–14,16,17,19,20,33,36–41). There are, however, some differences noted in the expression of some miRNAs, such as miR-200b and miR-31, between cell lines and CCA tissues further emphasizing the need to have carefully validated expression studies that are correlated with clinical and pathological features (4,42).
Table 1.
miRNA With Increased Expression in Cholangiocarcinoma
| miRNA | Target | Tumor Cell Lines | Human CCA | Animal Models | References |
|---|---|---|---|---|---|
| miR-21 | PDCD4, PTEN, TIMP-3,RECK | KMCH-1, Mz-ChA-1, TFK, RBE, QBC939 | CCA | (4,6,8–13,36–38) | |
| miR-421 | FXR | HCCC-9180, SSP25, RBE | CCA | (14) | |
| miR-200b | PTPN12 | KMCH-1, Mz-ChA-1, TFK | (not described) | (4) | |
| miR-26a | GSK-3β | SG231, TFK, HuCCT1, CCLP-1 | CCA | (3) | |
| miR-141 | CLOCK | KMCH-1, Mz-ChA-1, TFK | (not described) | (4) | |
| miR-210(mouse) | Mnt | (not described) | (not described) | mouse CCA | (5) |
| miR-let7a | NF2 | KMCH-1, Mz-ChA-1 | (not described) | (44) | |
| miR-125a-5p | DUSP6 (putative) | HuH28, HuCCT1 | (not described) | (27) | |
| miR-31 | RASA1 | HCCC-9810 | iCCA | (6,7) | |
| miR-25 | TRAIL death receptor-4 | KMCH-1, Mz-ChA-1 | (not described) | (23) | |
| miR-151-3p | (not described) | CCA | (45) | ||
| miR-223 | (not described) | iCCA | (6) | ||
| miR-135b, miR-106b, miR-30e, miR-30b, miR-151-3p, miR-10a, miR-181a, miR-96, miR-221 | (not described) | CCA | (38) |
Table 2.
miRNA With Decreased Expression in Cholangiocarcinoma
| miRNA | Target | Tumor Cell Lines | Human CCA | Animal Models | References |
|---|---|---|---|---|---|
| miR-370 | MAP3K8,DNMT-1 | Mz-ChA-1, KMCH-1 | CCA | (19,20) | |
| miR-373 | MBD2 | (not described) | Extrahepatic CCA | (39–41) | |
| miR-200c | NCAM1, SUZ12 | TFK, HuCCT1,MEC, IHGGK | iCCA | (6,32,42) | |
| miR-200b | SUZ12 | (not described) | CCA | (4,42) | |
| miR-214 | Twist | TFK, HuCCT1,MEC | iCCA | (33,35) | |
| miR-320 | Mcl-1 | RBE, QBC939 | iCCA | (24) | |
| miR-204 | Bcl-1 | RBE, QBC939 | iCCA | (24) | |
| miR-29b | Mcl-1 | KMCH-1, HuH28, HuCCT1, KMCH-1 | (not described) | (25–27) | |
| miR-494 | CDK-6 | HuCCT1, TFK-1 | CCA | (16,17) | |
| miR-148a, miR-152 | DNMT-1 | KMBC-1, Mz-ChA-1, TFK | (not described) | (18) | |
| miR-376c | GRB2 | TFK, HuCCT1, HuH28 | (not described) | (46) | |
| miR-205 | ErbB3,VEGFA (putative) | HuH28, HuCCT1 | (not described) | (27) | |
| miR-221 | PIK3R1 (putative) | HuH28, HuCCT1 | (not described) | (6) | |
| miR-138 | RhoC | RBE, QBC939 | CCA | (21) | |
| miR-124 | SMYD3 | (not described) | HCV-related CCA | (47) | |
| miR-34a(mouse) | c-Myc | (not described) | (not described) | mouse CCA | (5) |
| miR-126 | (not described) | CCA | (45) | ||
| miR-145 | (not described) | iCCA | (6,38) | ||
| miR-122 | (not described) | iCCA | (6) | ||
| miR-222 | (not described) | iCCA | (6) | ||
| miR-125a | KMCH-1, Mz-ChA-1, TFK, HuCCT1,MEC, IHGGK | (not described) | (4,35) | ||
| miR-22, miR-127, miR-192, miR-199a, miR-214, miR-376a, miR-424 | TFK, HuCCT1,MEC, IHGGK | (not described) | (35) | ||
| miR-125b, miR-100, miR-127-3p, miR-139-5p | (not described) | CCA | (38) | ||
| miR-95 | KMCH-1, Mz-ChA-1, TFK | (not described) | (4) | ||
| miR-31 | KMCH-1, Mz-ChA-1, TFK | (not described) | (4) |
SELECTED miRNA AND THEIR ROLES IN CCA
miR-370
Reduced expression of miR-370 was reported in a large cohort of human CCA (19). A target of miR-370 is the mitogen-activated protein kinase kinase kinase 8 (MAP3K8), and its expression was decreased by 5-aza-CdR in CCA cells. Enforced expression of IL-6 reduced miR-370 expression and reinstated MAP3K8 expression in vitro as well as in tumor cell xenografts in vivo. Thus, modulation of miR-370 expression could contribute to IL-6-dependent effects on tumor growth (20). Moreover, enhanced miR-370 expression suppresses growth of malignant human cholangiocytes. The paternal allele of miR-370 is normally silenced through genomic imprinting, and overexpression of IL-6 in CCA effectively suppresses the expression of miR-370 from the maternal allele. These studies lend support to a classic two-hit mechanism for the suppression of miR-370 (19).
miR-21
miR-21 has been characterized as playing an important role in growth and metastasis of CCA. Similar to many other cancers, miR-21 is overexpressed in human CCA. Inhibition of miR-21 and miR-200b increased sensitivity to gemcitabine in CCA cell lines (9). miR-21 modulates gemcitabine-induced apoptosis by PTEN-dependent activation of PI 3-kinase signaling (4). Inhibitors of miR-21 increased protein levels of PDCD4 and tissue inhibitor of metalloproteinases 3 (TIMP3). Thus, miR-21 may be oncogenic, at least in part, by inhibiting PDCD4 and TIMP3 (8). miR-21 expression inversely correlates with that of its target RECK, a metastasis suppressor, in human CCA (10). miR-21 may negatively control the expression of tumor-suppressor PTEN leading to cell proliferation (4,11,43). Moreover, upregulation of Ars2 may contribute to overexpression of miR-21 in human CCA (3). The knockdown of Ars2 in CCA cells decreased miR-21 expression, increased PTEN and PDCD4, inhibited cell proliferation, and prevented tumor formation (11). Accordingly, miR-21 is related to cell proliferation, apoptosis, metastasis, and migration in CCA.
miR-373
miR-373 is underexpressed in human CCA. miR-373 is a negative regulator of methyl CpG-binding domain protein 2 (MBD2) (39–41), and decreased expression of miR-373 leads to an increase in MBD2, which in turn suppresses methylation-mediated genes such as RASSF1A (40). Downregulation of miR-373 is associated with poor cell differentiation, advanced clinical stage, and shorter survival in hilar CCA (40,41).
miR-421
miR-421 expression is upregulated in biliary tract cancer, and it regulates the expression of farnesoid X receptor (FXR). FXR has been reported to be a tumor suppressor for hepatocellular carcinoma and breast cancer. Ectopic expression of miR-421 significantly decreased FXR protein concentration in tumor cells and promoted cell proliferation, colony formation, and migration in vitro. Furthermore, a decrease in miR-421 expression induces G0/G1 cell cycle arrest (14).
miR-200
Four miR-200 family members (miR-200a/b/c/429) are underexpressed in human CCA (6, 42). Inactivation of miR-200c can induce EMT, whereas activation of miR-200c can reduce EMT, cell migration, and invasion in iCCA cells (32). NCAM1 is a direct target of miR-200c. miR-200c and NCAM1 expression are negatively correlated, and their expression levels are predictive of survival in iCCA samples. Ectopic expression of miR-200 suppresses the multidrug resistance and metastasis of cancer. Moreover, ectopic miR-200b/200c inhibits the migration and invasion of CCA cells both in vitro and in vivo. miR-200b/c influenced the tumorigenesis of CCA cells, including their tumor-initiating capacity, sphere formation, and drug resistance. The mechanisms involved may involve direct targeting of rho-kinase 2 and SUZ12 (a subunit of a polycomb repressor complex) (6,42).
miR-214
Expression of miR-214 is decreased in metastatic iCCA tissues compared to nonmetastatic tissues. Experimental inhibition of miR-214 promotes metastatic behavior of human iCCA cells, along with an increased expression of the EMT-associated gene Twist transcript and decreased E-cadherin levels (33). By directly targeting Twist, downregulation of miR-214 can promote EMT. These results support an important role for miR-214 in regulating metastasis of iCCA (33).
miR-26a
miR-26a expression is upregulated in human CCA (3). Overexpression of miR-26a increased proliferation of CCA cells and colony formation in vitro. GSK-3β mRNA has been identified as a direct target of miR-26a. miR-26a-mediated reduction of GSK-3β results in activation of β-catenin and induction of several downstream genes, including c-Myc, cyclin D1, and peroxisome proliferator-activated receptor δ. Depletion of β-catenin partially prevents miR-26a-induced tumor cell proliferation and colony formation. Hence, miR-26a can promote CCA growth by inhibition of GSK-3β and subsequent activation of β-catenin (3).
miR-29b
miR-29b is underexpressed in CCA. Enforced expression of miR-29b restored gemcitabine sensitivity to HuH28(27), as well as reduced Mcl-1 protein expression in KMCH cells and sensitized tumor cells to TRAIL cytotoxicity. Consistent with these observations, transfection of nonmalignant cells that express high levels of miR-29 with a locked-nucleic acid antagonist of miR-29b increased Mcl-1 levels and reduced TRAIL-mediated apoptosis (25). A direct effect of miR-29 on Mcl-1 was identified based on negative regulation of expression of an Mcl-1 3′-untranslated region-based reporter construct by miR-29a. Modulation of miR-29b may therefore be a useful strategy to enhance chemotherapeutic responses.
miR-494
miR-494 is downregulated in human CCA. This miRNA is a major modulator of progression from G2 to M phase of the cell cycle. Upregulation of miR-494 induces tumor cell growth retardation through multiple targets involved in the G1/S transition (16). A direct target of this miRNA is cyclin-dependent kinase-6, and moreover, miR-494 has been shown to modulate the expression of several proteins involved in the G2/M transition, such as polo-like kinase 1, cyclin B1, cell-division cycle 2, cell-division cycle 20, and topoisomerase II α. Thus, miR-494 induces a significant arrest in G2/M in CCA cells and represents a key regulator of proliferation in CCA cells (17).
miRNAs AS MARKERS OF BILIARY TRACT CANCERS
Biliary tract cancers can release RNA molecules such as miRNA into the circulation or in bile. Certain miRNAs are associated with biliary tract cancers and may provide the ability to detect the presence of biliary tract cancers. Circulating or biliary miRNA may be sequestered from degradation within extracellular vesicles such as exosomes. Exosomes have been identified from the bile of patients with biliary tract cancers, raising the potential for their isolation and analysis of their miRNA content to identify specific markers of disease.
CONCLUSIONS
The large number of miRNAs in humans, each of which is capable of targeting hundreds of target genes and modulating protein expression, can contribute to biliary tract carcinogenesis. Normal cellular physiological functioning is dependent on an intricate system that maintains homeostasis and that can involve miRNAs as regulatory molecules. CCA arises as a result of perturbations in cell signaling pathways that contribute to cardinal features of human cancers, and many of these pathways involve deregulation of miRNA-dependent signaling. Understanding critical deregulated miRNAs that contribute to CCA pathogenesis will be necessary in order to understand how these processes could be translated into effective approaches to diagnose, treat, or prevent these cancers.
ACKNOWLEDGMENT
Supported in part by Grant DK069370 from the National Institutes of Health.
REFERENCES
- 1. Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA 2004;101(9):2999–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Takahashi K, Yan I, Wen HJ, Patel T. microRNAs in liver disease: From diagnostics to therapeutics. Clin Biochem 2013;46(10–11):946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang J, Han C, Wu T. MicroRNA-26a promotes cholangiocarcinoma growth by activating beta-catenin. Gastroenterology 2012;143(1):246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 2006;130(7):2113–2129. [DOI] [PubMed] [Google Scholar]
- 5. Yang H, Li TW, Peng J, Tang X, Ko KS, Xia M, et al. A mouse model of cholestasis-associated cholangiocarcinoma and transcription factors involved in progression. Gastroenterology 2011;141(1):378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog 2013;52(4):297–303. [DOI] [PubMed] [Google Scholar]
- 7. Hu C, Huang F, Deng G, Nie W, Huang W, Zeng X. miR-31 promotes oncogenesis in intrahepatic cholangiocarcinoma cells via the direct suppression of RASA1. Exp Ther Med 2013;6(5):1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Selaru FM, Olaru AV, Kan T, David S, Cheng Y, Mori Y, et al. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology 2009; 49(5):1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hummel R, Hussey DJ, Haier J. MicroRNAs: Predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur J Cancer 2010;46(2):298–311. [DOI] [PubMed] [Google Scholar]
- 10. Namwat N, Chusorn P, Loilome W, Techasen A, Puetkasichonpasutha J, Pairojkul C, et al. Expression profiles of oncomir miR-21 and tumor-suppressor let-7a in the progression of opisthorchiasis-associated cholangiocarcinoma. Asian Pac J Cancer Prev 2012;13 Suppl:65–69. [PubMed] [Google Scholar]
- 11. He Q, Cai L, Shuai L, Li D, Wang C, Liu Y, et al. Ars2 is overexpressed in human cholangiocarcinomas and its depletion increases PTEN and PDCD4 by decreasing microRNA-21. Mol Carcinog 2013;52(4):286–296. [DOI] [PubMed] [Google Scholar]
- 12. Chusorn P, Namwat N, Loilome W, Techasen A, Pairojkul C, Khuntikeo N, et al. Overexpression of microRNA-21 regulating PDCD4 during tumorigenesis of liver fluke-associated cholangiocarcinoma contributes to tumor growth and metastasis. Tumour Biol 2013;34(3):1579–1588. [DOI] [PubMed] [Google Scholar]
- 13. Yang W, Wang X, Zheng W, Li K, Liu H, Sun Y. Genetic and epigenetic alterations are involved in the regulation of TPM1 in cholangiocarcinoma. Int J Oncol 2013;42(2):690–698. [DOI] [PubMed] [Google Scholar]
- 14. Zhong XY, Yu JH, Zhang WG, Wang ZD, Dong Q, Tai S, et al. MicroRNA-421 functions as an oncogenic miRNA in biliary tract cancer through down-regulating farnesoid X receptor expression. Gene 2012;493(1):44–51. [DOI] [PubMed] [Google Scholar]
- 15. Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007;133(2):647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olaru AV, Ghiaur G, Yamanaka S, Luvsanjav D, An F, Popescu I, et al. MicroRNA down-regulated in human cholangiocarcinoma control cell cycle through multiple targets involved in the G1/S checkpoint. Hepatology 2011;54(6):2089–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamanaka S, Campbell NR, An F, Kuo SC, Potter JJ, Mezey E, et al. Coordinated effects of microRNA-494 induce G(2)/M arrest in human cholangiocarcinoma. Cell Cycle 2012;11(14):2729–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Braconi C, Huang N, Patel T. MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocytes. Hepatology 2010;51(3):881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. An F, Yamanaka S, Allen S, Roberts LR, Gores GJ, Pawlik TM, et al. Silencing of miR-370 in human cholangiocarcinoma by allelic loss and interleukin-6 induced maternal to paternal epigenotype switch. PLoS One 2012;7(10):e45606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meng F, Wehbe-Janek H, Henson R, Smith H, Patel T. Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene 2008;27(3):378–386. [DOI] [PubMed] [Google Scholar]
- 21. Wang Q, Tang H, Yin S, Dong C. Downregulation of microRNA-138 enhances the proliferation, migration and invasion of cholangiocarcinoma cells through the upregulation of RhoC/p-ERK/MMP-2/MMP-9. Oncol Rep 2013;29(5):2046–2052. [DOI] [PubMed] [Google Scholar]
- 22. Patel T. Cholangiocarcinoma—Controversies and challenges. Nat Rev Gastroenterol Hepatol 2011;8(4):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Razumilava N, Bronk SF, Smoot RL, Fingas CD, Werneburg NW, Roberts LR, et al. miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma. Hepatology 2012;55(2):465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen L, Yan HX, Yang W, Hu L, Yu LX, Liu Q, et al. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J Hepatol 2009;50(2):358–369. [DOI] [PubMed] [Google Scholar]
- 25. Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 2007;26(42):6133–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem 2010;110(5):1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okamoto K, Miyoshi K, Murawaki Y. miR-29b, miR-205 and miR-221 enhance chemosensitivity to gemcitabine in HuH28 human cholangiocarcinoma cells. PLoS One 2013;8(10):e77623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park J, Tadlock L, Gores GJ, Patel T. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology 1999;30(5):1128–1133. [DOI] [PubMed] [Google Scholar]
- 29. Meng F, Yamagiwa Y, Ueno Y, Patel T. Over-expression of interleukin-6 enhances cell survival and transformed cell growth in human malignant cholangiocytes. J Hepatol 2006;44(6):1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blechacz B, Gores GJ. Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology 2008;48(1):308–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2(6):442–454. [DOI] [PubMed] [Google Scholar]
- 32. Oishi N, Kumar MR, Roessler S, Ji J, Forgues M, Budhu A, et al. Transcriptomic profiling reveals hepatic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology 2012;56(5):1792–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li B, Han Q, Zhu Y, Yu Y, Wang J, Jiang X. Down-regulation of miR-214 contributes to intrahepatic cholangiocarcinoma metastasis by targeting Twist. FEBS J 2012;279(13):2393–2398. [DOI] [PubMed] [Google Scholar]
- 34. Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature 2005;435(7043):834–838. [DOI] [PubMed] [Google Scholar]
- 35. Kawahigashi Y, Mishima T, Mizuguchi Y, Arima Y, Yokomuro S, Kanda T, et al. MicroRNA profiling of human intrahepatic cholangiocarcinoma cell lines reveals biliary epithelial cell-specific microRNAs. J Nippon Med Sch 2009;76(4):188–197. [DOI] [PubMed] [Google Scholar]
- 36. Huang Q, Liu L, Liu CH, You H, Shao F, Xie F, et al. MicroRNA-21 regulates the invasion and metastasis in cholangiocarcinoma and may be a potential biomarker for cancer prognosis. Asian Pac J Cancer Prev 2013;14(2):829–834. [DOI] [PubMed] [Google Scholar]
- 37. Liu CZ, Liu W, Zheng Y, Su JM, Li JJ, Yu L, et al. PTEN and PDCD4 are bona fide targets of microRNA-21 in human cholangiocarcinoma. Chin Med Sci J 2012;27(2):65–72. [PubMed] [Google Scholar]
- 38. Collins AL, Wojcik S, Liu J, Frankel WL, Alder H, Yu L, et al. A differential microRNA profile distinguishes cholangiocarcinoma from pancreatic adenocarcinoma. Ann SurgOncol 2014; 21(1):133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen Y, Gao W, Luo J, Tian R, Sun H, Zou S. Methyl-CpG binding protein MBD2 is implicated in methylation-mediated suppression of miR-373 in hilar cholangiocarcinoma. Oncol Rep 2011;25(2):443–451. [DOI] [PubMed] [Google Scholar]
- 40. Chen Y, Luo J, Tian R, Sun H, Zou S. miR-373 negatively regulates methyl-CpG-binding domain protein 2 (MBD2) in hilar cholangiocarcinoma. Dig Dis Sci 2011;56(6):1693–1701. [DOI] [PubMed] [Google Scholar]
- 41. Chen YJ, Luo J, Yang GY, Yang K, Wen SQ, Zou SQ. Mutual regulation between microRNA-373 and methyl-CpG-binding domain protein 2 in hilar cholangiocarcinoma. World J Gastroenterol 2012;18(29):3849–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peng F, Jiang J, Yu Y, Tian R, Guo X, Li X, et al. Direct targeting of SUZ12/ROCK2 by miR-200b/c inhibits cholangiocarcinoma tumourigenesis and metastasis. Br J Cancer 2013; 109(12):3092–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stutes M, Tran S, DeMorrow S. Genetic and epigenetic changes associated with cholangiocarcinoma: From DNA methylation to microRNAs. World J Gastroenterol 2007;13(48):6465–6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meng F, Henson R, Wehbe-Janek H, Smith H, Ueno Y, Patel T. The MicroRNA let-7a modulates interleukin-6-dependent STAT-3 survival signaling in malignant human cholangiocytes. J BiolChem 2007;282(11):8256–8264. [DOI] [PubMed] [Google Scholar]
- 45. McNally ME, Collins A, Wojcik SE, Liu J, Henry JC, Jiang J, et al. Concomitant dysregulation of microRNAs miR-151-3p and miR-126 correlates with improved survival in resected cholangiocarcinoma. HPB (Oxford) 2013;15(4):260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Iwaki J, Kikuchi K, Mizuguchi Y, Kawahigashi Y, Yoshida H, Uchida E, et al. MiR-376c down-regulation accelerates EGF-dependent migration by targeting GRB2 in the HuCCT1 human intrahepatic cholangiocarcinoma cell line. PLoS One 2013;8(7):e69496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zeng B, Li Z, Chen R, Guo N, Zhou J, Zhou Q, et al. Epigenetic regulation of miR-124 by hepatitis C virus core protein promotes migration and invasion of intrahepatic cholangiocarcinoma cells by targeting SMYD3. FEBS Lett 2012;586(19):3271–3278. [DOI] [PubMed] [Google Scholar]