Abstract
The ω-3 and ω-6 polyunsaturated fatty acids (PUFAs) are two major families of PUFAs present as essential cellular components which possess diverse bioactivities. The ω-3s, mainly found in seafood, are associated with many beneficial effects on human health, while the ω-6s are more abundant in our daily diet and could be implicated in many pathological processes including cancer development. Increasing evidence suggests that the adverse effects of ω-6s may be largely attributed to arachidonic acid (AA, a downstream ω-6) and the metabolite prostaglandin E2 (PGE2) that stems from its cyclooxygenase (COX)-catalyzed lipid peroxidation. On the other hand, two of AA’s upstream ω-6s, γ-linolenic acid (GLA) and dihomo-γ-linolenic acid (DGLA), are shown to possess certain anti-cancer activities, including inducing cell apoptosis and inhibiting cell proliferation. In this paper, we review the documented anti-cancer activities of ω-6 PUFAs, including the recent findings regarding the anti-cancer effects of free radical-mediated DGLA peroxidation. The possible mechanisms and applications of DGLA (and other ω-6s) in inducing anti-cancer activity are also discussed. Considering the wide availability of ω-6s in our daily diet, the study of the potential beneficial effect of ω-6 PUFAs may guide us to develop an ω-6–based diet care strategy for cancer prevention and treatment.
Keywords: anti-cancer activity, apoptosis, cyclooxygenase-catalyzed lipid peroxidation, dihomo-γ-linolenic acid, free radicals, ω-6 polyunsaturated fatty acids
Polyunsaturated fatty acids (PUFAs) are long-chain fatty acids with more than one C–C double bond in their backbones. According to the position of the first double bond in the structure, PUFAs can be classified into two major categories, namely ω-3 and ω-6, which are present as essential cellular components and possess diverse biofunctions. There is a great deal of variation in the sources and bioactivities between these two different classes of PUFAs. For example, the ω-3s, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are mainly found in seafood and have been shown to be associated with several beneficial effects in human health, including cancer suppression, cardiovascular disease prevention, and cognitive ability improvement.[1–10] On the other hand, the ω-6s, especially arachidonic acid (AA, a downstream ω-6), are much more abundant in our daily diet and are generally associated with many adverse effects on the human body, including cancer promotion. For instance, the high intake of ω-6s was found to correlate with a high risk of breast, prostate, and colon cancer incidence in many animal and human studies, and the ratio of ω-6s to ω-3s was suggested to be a predictor for cancer progression.[11–22] The pro-cancer effects may be mainly due to AA, the downstream and pro-cancer ω-6.[17–23] Given these differences, the bioactivities from ω-3s have been extensively studied for health improvement purposes, whereas the potential beneficial effects from ω-6s have received much less research attention.
Increasing evidence suggests that unlike the downstream ω AA, which has been associated with cancer development, the upstream ω-6s, such as linoleic acid (LA), γ-linolenic acid (GLA), and dihomo-γ-linolenic acid (DGLA), may possess anti-cancer effects, and thus could be a promising dietary source for cancer prevention and therapy.[24–39] However, the upstream ω-6s can be effectively converted into AA by a series of fatty acid metabolism enzymes. Upon uptake, LA (the precursor of ω-6s) will be converted into GLA in the presence of ∆-6 desaturase (D6D), followed by a two-carbon chain elongation by elongase to become DGLA, and finally be de-saturated by ∆-5 desaturase (D5D) to form AA [Figure 1]. Such a conversion could greatly restrict the availability and anti-cancer effects of upstream ω-6s. Thus, it seems critical to control ω-6 metabolism to favor upstream ω-6 synthesis while limiting AA production in order to elicit the anti-cancer activities from upstream ω-6s such as DGLA.
Figure 1.
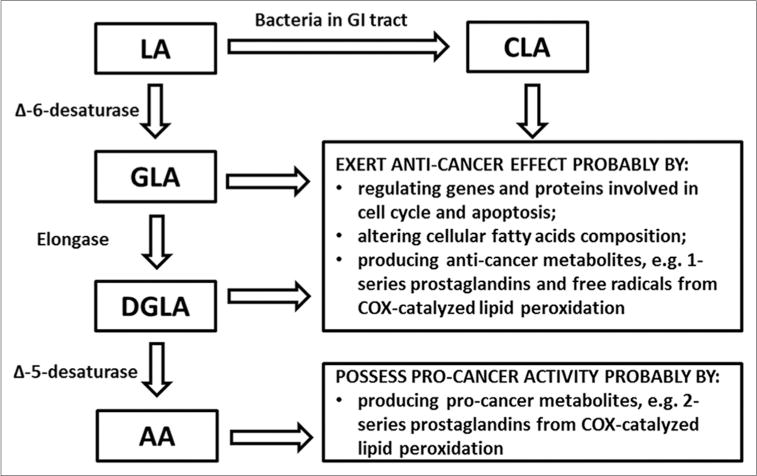
Overview of the metabolism of ω-6 PUFAs and their implications in cancer. Abbreviations: LA: Linoleic acid; GLA: γ-Linolenic acid; DGLA: Dihomo-γ-linolenic acid; AA: Arachidonic acid; CLA: Conjugated linoleic acid.
Through catalysis by cyclooxygenase (COX), a major lipid peroxidizing enzyme, ω-6s can undergo a free radical–mediated lipid peroxidation and produce various PUFA-derived metabolites. For instance, DGLA and AA, both major substrates for COX, can produce 1-series prostaglandins (PGs-1) and 2-series prostaglandins (PGs-2), respectively, during COX-catalyzed lipid peroxidation. Recent studies found that DGLA and AA could go through different free radical pathways during lipid peroxidation and produce distinct free radical metabolites.[40–42] It was recently proposed and demonstrated that the adverse effects from ω-6s may be mainly attributed to AA and its metabolite prostaglandin E2 (PGE2), while DGLA may exert an anti-cancer effect via the production of prostaglandin E1 (PGE1) and the exclusive free radical metabolites from its COX-catalyzed lipid peroxidation.[17–23,38,39,43–45]
In this paper, we will review the documented anti-cancer activities of ω-6 PUFAs, including the recent findings of the anti-cancer effect from COX-catalyzed DGLA peroxidation. The possible mechanisms and applications of anti-cancer effects induced by the ω-6s (especially DGLA) will also be briefly discussed.
Implications of ω-6s in cancer
LA and conjugated linoleic acid
Although all the ω-6s can be directly consumed from the daily diet, LA, the precursor of ω-6s, is more abundant in plant seeds and oils, and thus is considered to be the main dietary source of all ω-6s [Figure 1]. Research evidence shows that LA can be involved in both pro- and anti-cancer activities. For example, LA stimulates cell proliferation in the human breast cancer cell line BT-474 and the human lung cancer cell line A549 in vitro, and promotes colon and prostate tumorigenesis and tumor growth in animal models.[15,46–49] On the other hand, a high dose of LA inhibits proliferation of the colon cancer cell line Caco-2,[24] while a high intake of LA also shows a protective effect against cancer development.[50] LA is endogenously converted into various downstream ω-6 PUFAs and the corresponding metabolites by the enzymes D6D, elongase, and D5D. Thus, the observed effects of LA on cancer growth could actually be derived from a mixture of effects of its downstream products. In fact, various studies show that the lipid peroxidizing enzyme COX and the lipid peroxidation metabolite PGE2 are indeed involved in LA-induced cancer development.[46,51–56] The role of pure LA in cancer growth still remains to be investigated in the context of controlling PUFA metabolism.
Conjugated linoleic acids (CLAs) are a series of isomers of LA (mainly cis-9, trans-11 and trans-10, cis-12) with conjugated double bonds in their structures. Although chemically not belonging to the ω-6 family, CLAs can originate from the endogenous biohydrogenation of LA by gastrointestinal tract bacteria [Figure 1].[57–59] CLAs have been extensively studied and shown to possess anti-cancer effects in a number of cancer types both in vitro and in vivo. For instance, in vitro studies showed that CLA isomers could inhibit cell growth in diverse cancer cell lines including the breast cancer cell line MCF-7,[60–62] the colon cancer cell lines HT-29, DLD-1, and Caco-2,[63,64] the prostate cancer cell lines PC-3 and DU-145,[62,64–66] and the gastric cancer cell line SGC-7901.[67] Consistently, animal studies also show that a CLA-enriched diet reduces mammary epithelial mass, suppresses terminal end bud cell proliferation, and decreases premalignant lesions and tumor incidence in a methylnitrosourea-induced mammary tumor rat model.[68–71] A CLA supplement was also shown to reduce the tumor incidence and diameters in mice bearing forestomach tumors.[72] Although the anti-cancer effects varied among different CLA isomers and different cancer types, most of the existing evidence consistently indicates that CLAs could inhibit cancer development both in vitro and in vivo.
γ-Linolenic acid
LA can be desaturated and converted into GLA which is catalyzed by D6D enzyme [Figure 1]. Evidence shows that GLA is also associated with anti-cancer activities both in vitro and in vivo. For example, GLA inhibited cell growth of the human neuroblastoma cell lines GOTO, SK-N-DZ, NKP, and NCG, a rat C6 glioma cell line, and the rat carcinosarcoma cell line LLC-WRC256 in vitro.[25–27] A dietary supplement of GLA also reduced tumor growth in an implanted WRC256 rat model.[28] More interestingly, GLA-induced cytotoxicity was shown to exhibit high selectivity toward cancer cells with no significant effect on normal cell growth. For instance, a series of studies suggested that 3–7 days of incubation with GLA could selectively induce cell death in various human cancer cell lines, including the human breast cancer cell ZR-75–1, the lung cancer cell A549, and the prostatic cancer cell PC-3, without affecting normal cell growth.[29–36] GLA was shown to be cytotoxic to the malignant rat astrocytoma cell line 36B10 without affecting normal astrocytes. GLA also enhanced the radiation sensitivity of astrocytoma cells, but not normal astrocytes.[37] In the in vivo C6 glioma rat model, the infusion of GLA was shown to increase the frequency of cell apoptosis and regression in tumors, without influencing normal neural tissue and vasculature.[26] Therefore, GLA seems to be a promising cancer therapeutic agent with desirable characteristics, although the reason for the high selectivity in GLA-induced anti-tumor effect still remains to be investigated.
DGLA versus AA
Given the anti-cancer effects of GLA, it is anticipated that DGLA, the direct downstream ω-6 of GLA, may also possess similar anti-tumor effects [Figure 1]. In fact, it was observed that both GLA and DGLA inhibited cell proliferation in human cervical carcinoma cells (KB-3–1) in a dose-dependent manner. The potency of the cytotoxic effect of DGLA was shown to be equal to that of GLA.[38] In rats with 7,12-dimethylbenz(α) anthracene-induced mammary tumors, the ratio of tumor-bearing rats to total number of rats was lowest after 12 weeks of DGLA administration (by oral intubation, 0.15 g, twice a week) compared to groups treated with GLA and corn oil (which contains mainly LA).[39] However, some research groups also reported that DGLA may not influence or even promote cancer development. For instance, low doses of DGLA were shown to stimulate human breast carcinoma cell growth.[73] In a rat mammary tumor model, the tumor multiplicity in the DGLA treatment group was higher than the GLA and corn oil treatment groups.[39] The potential pro-cancer activity of DGLA observed in some studies may be due to the readily conversion of DGLA to AA (a downstream and pro-cancer ω-6) in cells, catalyzed by D5D. Such conversion could greatly restrict DGLA’s availability and its associated anti-cancer activity. So far, much less research attention has been paid to the implications of DGLA and D5D in cancer prevention and treatment.
Unlike upstream ω-6s, the downstream ω-6 AA, produced directly from DGLA by D5D, is commonly associated with many adverse effects to human health [Figure 1]. Most results have built a substantial correlation between COX-catalyzed AA peroxidation (as well as AA metabolites, e.g. PGE2) and cancer development, including prostate, colon, and breast cancers.[17–23] For a long time, controlling COX-catalyzed AA metabolism by COX inhibition has received much research attention and become a conventional strategy for cancer therapy. There is only a little evidence to show that AA could inhibit cell proliferation in the human colon cancer cell line Caco-2 and in the human cervical carcinoma cell line KB-3-1.[24,38]
Given the contrasting activities in cancer development and the rapid conversion from DGLA to AA, it seems that the ratio of DGLA to AA is crucial in dictating their effects on cancer growth, and it is possible that preventing the conversion from DGLA to AA may represent an effective strategy for eliciting the anti-cancer activity of DGLA in vivo. Unfortunately, there have been very limited studies focusing on this topic. Recent research from Qian’s group found that DGLA’s free radical derivatives from lipid peroxidation could inhibit human colon cancer cell growth,[43] while direct treatment with DGLA had no effect on cell proliferation, probably due to effective D5D-catalyzed conversion of DGLA to AA. However, when this conversion was limited by D5D knockdown via siRNA transfection, DGLA treatment led to a significant inhibition in cell growth (unpublished research result from Qian’s group).
Mechanisms of the anti-cancer effects of ω-6 PUFAs
Inducing cell apoptosis and altering cellular fatty acid composition by ω-6s
The ω-6s have been shown to exert their anti-cancer proliferation effects by influencing gene and protein expression, thereby disrupting cell cycle progression and inducing apoptosis. For example, in rat carcinosarcoma cells (LLC-WRC256), GLA triggered cytochrome c release associated with changes in mitochondrial metabolism and increased caspase 3 activity, thereby finally leading to cell apoptosis.[27] In an implanted WRC256 rat model, GLA altered mitochondrial metabolism and structure by influencing mitochondrial membrane composition and decreasing hexokinase and carnitine palmitoyltransferase I activities, thus eventually leading to apoptosis.[28] Exogenous GLA treatment was also reported to induce apoptosis in human and rat glioma cell lines in vitro, while in the in vivo C6 glioma rat model, infusion of GLA increased the frequency of cell apoptosis, cell death, and regression in tumors.[26]
Treatment with CLAs, the derivatives of LA, caused G1 arrest in the DU-145 and HT-29 cell lines by up-regulating the protein expression of the cell cycle inhibitor p21 and decreasing the expression of cyclins A and D.[65,74] CLAs were also shown to promote cell apoptosis in various cancer cell lines, including Caco-2, HT-29, PC-3, SGC-7901, and dRLH-84, probably by increasing the expression and activity of pro-apoptotic proteins (e.g. caspase 3, caspase 9, and Fas), while decreasing the expression of pro-growth and anti-apoptotic proteins (e.g. ErbB3, phosphorylated Akt, bcl-2, c-myc, and Ki-67).[63–67,75–77]
Supplementation with ω-6s could also alter the lipid composition in cell membranes and lead to cell membrane dysfunction. For example, GLA was found to increase the triacylglycerol content in a WRC256 rat tumor model and alter the quantity of ω-6s in a triacylglycerol fraction.[28] In human neuroblastoma cell lines, GLA-induced cytotoxic effects were found to be associated with a significant increase in triglycerides and polyenoic acids in cell membrane phospholipids and decrease in monoenoic acids, suggesting that the anti-tumor effect of GLA may be attributed to fatty acid modification-induced cellular dysfunction.[25,78]
Involvement of PGs from COX-catalyzed lipid peroxidation
Through a free radical–mediated lipid peroxidation, COX can catalyze ω-6s to produce two types of PGs, the 1-series and 2-series PGs, which have been shown to possess diverse activities and are proposed to be responsible for the bioactivities of ω-6s. In fact, a number of studies suggest that ω-6s could regulate cancer cell growth depending on the COX level. For example, LA and AA are shown to dose-dependently decrease cell proliferation in cancer cell lines with high COX expression (e.g. Caco cells), but not in those with low COX expression (e.g. HT-29).[24] Other evidence, moreover, shows that the ω-6–induced cytotoxicity in cancer cells can be partially inhibited by a COX inhibitor, suggesting that COX-catalyzed lipid peroxidation and its corresponding metabolites may contribute to the anti-cancer bioactivity of ω-6s.[79]
Two major PGs, PGE1 and PGE2, derived from DGLA and AA, respectively, have been the most extensively studied due to their diverse implications in physio-pathological conditions. Interestingly, PGE2 is shown to promote inflammation and cancer development, while PGE1 is now accepted to exert beneficial effects on human health, including anti-cancer activity. For example, PGE1 was found to inhibit the growth of Hela cells in vitro.[44,79] In highly metastatic murine B16-F10 melanoma cells, 48 h and 72 h treatment with PGE1 inhibited cell growth and invasion, stimulated cell differentiation, and decreased matrix metalloproteinase (MMP)-2 and MMP-9 levels.[45] Infusion of PGE1 into the tail vein of peritoneal tumor–bearing rats was shown to increase the anti-cancer effect of cisplatin by increasing the platinum concentration in tumor masses while reducing the renal cytotoxicity.[80]
There is also other evidence that COX inhibitors do not influence the GLA-induced anti-proliferation effect in some cancer cell lines, while CLAs may exert an anti-cancer effect by inhibiting COX activity.[59,79] This is probably because the inhibition of COX could limit the production of PGE2, a pro-cancer factor derived from AA peroxidation. Thus, the opposing bioactivities from PGE1 versus PGE2 imply that COX-catalyzed lipid peroxidation in cancer diseases is indeed complicated, and the ratio of anti-cancer metabolites (PGE1) to pro-cancer metabolites (PGE2) is rather critical.
Implications of ω-6 free radicals from COX-catalyzed peroxidation in cancer
In addition to the PGs, a series of PUFA-derived free radical intermediates produced from COX-catalyzed lipid peroxidation may also be associated with the anti-cancer activity of ω-6s. In fact, evidence shows that the ω-6-induced inhibition of cell proliferation in Hela cells is a free radical–dependent process and can be blocked by the antioxidant vitamin E.[44] The dose-dependent inhibition of cell proliferation induced by GLA in rat carcinosarcoma LLC-WRC256 cells was correlated with an increase in lipid peroxide and reactive oxygen species.[27,28] In 36B10 cells, GLA treatment resulted in an increase in the cellular level of 8-isoprostane (which is an indicator of oxidative stress), whereas the antioxidant trolox blocked the GLA-induced inhibition of growth and enhanced the sensitivity to radiation.[37] In human neuroblastoma cell lines, treatment with antioxidants (e.g. coenzyme Q, alpha-tocopherol, and butylated hydroxytoluene) partially reduced the GLA-induced inhibitory effect on cell growth.[25] All these evidences indicate that the reactive PUFA-derived free radicals from COX-catalyzed lipid peroxidation are somehow responsible for the ω-6s’ anti-cancer activities. However, the individual PUFA-derived free radical species was not identified and studied for a long time due to the lack of an appropriate method.
With the use of a novel liquid chromatography/mass spectrometry/electron spin resonance (LC/MS/ESR) combined system along with spin-trapping technique, a recent series of studies by Qian’s group has successfully detected, identified, and characterized major free radical intermediates produced from COX-catalyzed DGLA versus AA peroxidation.[40–43,81,82] The contribution to the study on lipid peroxidation enables us for the first time to investigate the effect of individual ω-6 free radical metabolites and pathways in cancer development. These studies showed that in addition to a C-15 oxygenation shared by both DGLA and AA, there is a unique C-8 oxygenation pathway present during COX-catalyzed DGLA peroxidation, which can give rise to exclusive DGLA free radicals.[40–42] These studies also demonstrated that the exclusive DGLA free radical derivatives could induce significant inhibition of cell growth and significant cell cycle arrest and apoptosis in the human colon cancer cell line HCA-7 colony 29.[43] Interestingly, in a comparison experiment, PGE1 and PGE2 did not affect cell proliferation at the same concentration, suggesting that the free radicals, rather than PGs, may be more responsible for DGLA’s anti-cancer activity under physiological conditions.
A further mechanistic study from the Qian group suggests that the anti-cancer effect of the exclusive DGLA free radical derivatives may be due to the regulation of molecular targets involved in the cell cycle progression and the cell apoptotic pathway, such as p27, pro-caspase 9, and p53.[43] This work provided the first evidence suggesting that the distinct free radical pathway and metabolites from COX-catalyzed DGLA peroxidation are more likely than the PGs to account for the ω-6s’ anti-cancer activities under physiological conditions.
Potential applications of ω-6s in combination with other therapeutic drugs
Co-treatment with ω-6s and/or their metabolites was shown to enhance the efficacy of other chemo-drugs. For example, concurrent supplementation with GLA and paclitaxel or docetaxel inhibited cell growth in the human breast cancer cell lines MDA-MB-231, T47D, SK-Br3, and MCF-7, additively and/or synergistically.[83] The combination of DGLA and vincristine was shown to significantly enhance cell death in vincristine-resistant cells (KB-ChR-8–5) compared to DGLA or vincristine treatment alone.[38] Infusion of PGE1 into the tail vein of peritoneal tumor-bearing rats was shown to increase the anti-cancer effect of cisplatin while reducing the renal cytotoxicity.[80] Although they still remain unclear, the mechanisms by which ω-6s enhance the anti-cancer effects of chemo-drugs may include regulating the genes and proteins involved in apoptosis, modifying membrane composition, and altering drug uptake and efflux.[38,43,80,83]
A recent study from Qian’s group found that the exclusive DGLA free radical derivatives enhanced the cytotoxicity of 5-fluorouracil (5-FU) toward the human colon cancer cell line HCA-7 colony 29, probably by further promoting apoptosis triggered by pro-caspase 9 activation.[43] More interestingly, direct treatment with DGLA was found to further decrease the IC50 of 5-FU in HCA-7 cells in which D5D was knocked down via siRNA transfection (unpublished data from Qian’s group). These promising results suggest that regulating D5D to favor DGLA synthesis represents a potential novel strategy for cancer therapy in combination with other chemo-drugs. This strategy takes advantage of the high COX levels in colon cancer cells during cancer treatment. Thus, it might change the paradigm and outlook on COX inhibition and peroxidation in cancer biology.
Conclusions
The ω-6 PUFAs, widely available in the daily diet, are essential cellular components that play important roles in diverse physio-pathological processes. Although some studies have suggested that a high intake of ω-6s could be deleterious and promote cancer development, increasing evidence indicates that the upstream ω-6s are actually associated with anti-cancer growth effects. It is noteworthy that recent studies from Qian’s group show that the DGLA free radical metabolites from COX-catalyzed lipid peroxidation can induce cell growth inhibition, cell cycle arrest, and apoptosis in the human colon cancer cell line HCA-7 colony 29. These results provide a novel insight into the implications of COX-catalyzed lipid peroxidation in cancer prevention and treatment. It was also demonstrated in their studies that the regulation of PUFA metabolism enzymes (e.g. D5D) is an effective way to prevent beneficial upstream ω-6s (e.g. DGLA) from converting into AA, thus helping to elicit DGLA’s anti-cancer effect. In addition, ω-6 PUFAs and their metabolites (e.g. PGs and free radicals) were also shown to enhance the efficacy of various commonly used chemo-drugs in cancer cells, while preventing their cytotoxicity toward normal cells.
Given their wide availability and diverse anti-tumor effects, the ω-6 PUFAs, especially DGLA (the most under-investigated ω-6), should be able to become a promising and more common dietary source for cancer prevention and treatment. Regulating the ω-6 metabolic enzymes and taking advantage of the high COX levels in cancer cells to allow DGLA and the related beneficial free radical metabolites to accumulate may represent a potentially novel ω-6–based diet care strategy for cancer therapy and may challenge the current paradigm of COX inhibition in cancer treatment.
Acknowledgments
This work was supported by NIH grant 1R15CA140833.
Biography

Dr. Steven Y. Qian
References
- 1.Chamras H, Ardashian A, Heber D, Glaspy JA. Fatty acid modulation of MCF-7 human breast cancer cell proliferation, apoptosis and differentiation. J Nutr Biochem. 2002;13:711–6. doi: 10.1016/s0955-2863(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 2.Serini S, Piccioni E, Merendino N, Calviello G. Dietary polyunsaturated fatty acids as inducers of apoptosis: Implications for cancer. Apoptosis. 2009;14:135–52. doi: 10.1007/s10495-008-0298-2. [DOI] [PubMed] [Google Scholar]
- 3.Spencer L, Mann C, Metcalfe M, Webb M, Pollard C, Spencer D, et al. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur J Cancer. 2009;45:2077–86. doi: 10.1016/j.ejca.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 4.D’Eliseo D, Manzi L, Merendino N, Velotti F. Docosahexaenoic acid inhibits invasion of human RT112 urinary bladder and PT45 pancreatic carcinoma cells via down-modulation of granzyme B expression. J Nutr Biochem. 2012;23:452–7. doi: 10.1016/j.jnutbio.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Kokura S, Nakagawa S, Hara T, Boku Y, Naito Y, Yoshida N, et al. Enhancement of lipid peroxidation and of the antitumor effect of hyperthermia upon combination with oral eicosapentaenoic acid. Cancer Lett. 2002;185:139–44. doi: 10.1016/s0304-3835(02)00262-8. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui RA, Harvey KA, Xu Z, Bammerlin EM, Walker C, Altenburg JD. Docosahexaenoic acid: A natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. Biofactors. 2011;37:399–412. doi: 10.1002/biof.181. [DOI] [PubMed] [Google Scholar]
- 7.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–49. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 8.Wen B, Deutsch E, Opolon P, Auperin A, Frascognal V, Connault E, et al. n-3 polyunsaturated fatty acids decrease mucosal/epidermal reactions and enhance anti tumour effect of ionising radiation with inhibition of tumour angiogenesis. Br J Cancer. 2003;89:1102–7. doi: 10.1038/sj.bjc.6601136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris WS, Dayspring TD, Moran TJ. Omega-3 fatty acids and cardiovascular disease: New developments and applications. Postgrad Med. 2013;125:100–13. doi: 10.3810/pgm.2013.11.2717. [DOI] [PubMed] [Google Scholar]
- 10.Haast RA, Kiliaan AJ. Impact of fatty acids on brain circulation, structure and function. Prostaglandins Leukot Essent Fatty Acids. 2014 doi: 10.1016/j.plefa.2014.01.002. [In Press] [DOI] [PubMed] [Google Scholar]
- 11.Williams CD, Whitley BM, Hoyo C, Grant DJ, Iraggi JD, Newman KA, et al. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr Res. 2011;31:1–8. doi: 10.1016/j.nutres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Brown MD, Hart C, Gazi E, Gardner P, Lockyer N, Clarke N. Influence of omega-6 PUFA arachidonic acid and bone marrow adipocytes on meta- static spread from prostate cancer. Br J Cancer. 2010;102:403–13. doi: 10.1038/sj.bjc.6605481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiebaut AC, Chajes V, Gerber M, Boutron-Ruault MC, Joulin V, Lenoir G, et al. Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int J Cancer. 2009;124:924–31. doi: 10.1002/ijc.23980. [DOI] [PubMed] [Google Scholar]
- 14.Pot GK, Geelen A, van Heijningen EM, Siezen CL, van Kranen HJ, Kampman E. Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: An endoscopy-based case-control study. Int J Cancer. 2008;123:1974–7. doi: 10.1002/ijc.23729. [DOI] [PubMed] [Google Scholar]
- 15.Sauer LA, Blask DE, Dauchy RT. Dietary factors and growth and metabolism in experimental tumors. J Nutr Biochem. 2007;18:637–49. doi: 10.1016/j.jnutbio.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Kimura Y, Kono S, Toyomura K, Nagano J, Mizoue T, Moore MA, et al. Meat, fish and fat intake in relation to subsite-specific risk of colorectal cancer: The Fukuoka Colorectal Cancer Study. Cancer Sci. 2007;98:590–7. doi: 10.1111/j.1349-7006.2007.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Zhao H, Wang Y, Zheng H, Yu W, Chai H, et al. Isoliquiritigenin induces growth inhibition and apoptosis through downregulating arachidonic acid metabolic network and the deactivation of PI3K/Akt in human breast cancer. Toxicol Appl Pharmacol. 2013;272:37–48. doi: 10.1016/j.taap.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Zhao X, Wu Z, Li Y, Zhu L, Cui B, et al. Polymorphisms in arachidonic acid metabolism-related genes and the risk and prognosis of colorectal cancer. Fam Cancer. 2013;12:755–65. doi: 10.1007/s10689-013-9659-2. [DOI] [PubMed] [Google Scholar]
- 19.Pender-Cudlip MC, Krag KJ, Martini D, Yu J, Guidi A, Skinner SS, et al. Delta-6-desaturase activity and arachidonic acid synthesis are increased in human breast cancer tissue. Cancer Sci. 2013;104:760–4. doi: 10.1111/cas.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakai M, Kakutani S, Horikawa C, Tokuda H, Kawashima H, Shibata H, et al. Arachidonic acid and cancer risk: A systematic review of observational studies. BMC Cancer. 2012;12:606. doi: 10.1186/1471-2407-12-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang P, Cartwright CA, Li J, Wen S, Prokhorova IN, Shureiqi I, et al. Arachidonic acid metabolism in human prostate cancer. Int J Oncol. 2012;41:1495–503. doi: 10.3892/ijo.2012.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amirian ES, Ittmann MM, Scheurer ME. Associations between arachidonic acid metabolism gene polymorphisms and prostate cancer risk. Prostate. 2011;71:1382–9. doi: 10.1002/pros.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CM, Hsieh HL, Lee CW. Intracellular signaling mechanisms underlying the expression of pro-inflammatory mediators in airway diseases. Chang Gung Med J. 2005;28:813–23. [PubMed] [Google Scholar]
- 24.Dommels YE, Haring MM, Keestra NG, Alink GM, van Bladeren PJ, van Ommen B. The role of cyclooxygenase in n-6 and n-3 polyunsaturated fatty acid mediated effects on cell proliferation, PGE2 synthesis and cytotoxicity in human colorectal carcinoma cell lines. Carcinogenesis. 2003;24:385–92. doi: 10.1093/carcin/24.3.385. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara F, Todo S, Imashuku S. Antitumor effect of γ-linolenic acid on cultured human neuroblastoma cells. Prostaglandins Leukot Med. 1986;23:311–20. doi: 10.1016/0262-1746(86)90198-8. [DOI] [PubMed] [Google Scholar]
- 26.Leaver HA, Bell HS, Rizzo MT, Ironside JW, Gregor A, Wharton SB, et al. Antitumour and pro-apoptotic actions of highly unsaturated fatty acids in glioma. Prostaglandins Leukot Essent Fatty Acids. 2002;66:19–29. doi: 10.1054/plef.2001.0336. [DOI] [PubMed] [Google Scholar]
- 27.Colquhoun A, Schumacher RI. γ-Linolenic acid and eicosapentaenoic acid induce modifications in mitochondrial metabolism, reactive oxygen species generation, lipid peroxidation and apoptosis in Walker 256 rat carcinosarcoma cells. Biochim Biophysica Acta. 2001;1533:207–19. doi: 10.1016/s1388-1981(01)00136-6. [DOI] [PubMed] [Google Scholar]
- 28.Colquhoun A. Gamma-linolenic acid alters the composition of mitochondrial membrane subfractions, decreases outer mitochondrial membrane binding of hexokinase and alters carnitinepalmitoyltransferase I properties in the Walker 256 rat tumour. Biochim Biophys Acta. 2002;1583:74–84. doi: 10.1016/s1388-1981(02)00162-2. [DOI] [PubMed] [Google Scholar]
- 29.Das UN. Cis-unsaturated fatty acids as potential anti-mutagenic, tumoricidal and anti-metastatic agents. Asia Pac J Pharmacol. 1992;7:305–27. [Google Scholar]
- 30.Das UN. Tumoricidal and anti-angiogenic actions of gamma-linolenic acid and its derivatives. Curr Pharm Biotech. 2006;7:457–66. doi: 10.2174/138920106779116892. [DOI] [PubMed] [Google Scholar]
- 31.Begin ME, Das UN, Ells G, Horrobin DL. Selective killing of human cancer cells by polyunsaturated fatty acids. Prostaglandins Leukot Med. 1985;19:177–86. doi: 10.1016/0262-1746(85)90084-8. [DOI] [PubMed] [Google Scholar]
- 32.Das UN. Essential fatty acids and their metabolites and cancer. Nutrition. 1999;15:239–41. doi: 10.1016/s0899-9007(98)00189-0. [DOI] [PubMed] [Google Scholar]
- 33.Das UN. Tumoricidal action of cis-unsaturated fatty acids and their relationship to free radicals and lipid peroxidation. Cancer Lett. 1991;56:235–43. doi: 10.1016/0304-3835(91)90008-6. [DOI] [PubMed] [Google Scholar]
- 34.Das UN. Gamma-linolenic acid, arachidonic acid and eicosapentaenoic acid as potential anti-cancer drugs. Nutrition. 1990;6:429–34. [PubMed] [Google Scholar]
- 35.Sangeetha P, Das UN. Cytotoxic action of cis-unsaturated fatty acids on human cervical carcinoma (HeLa) cells in vitro. Prostaglandins Leukot Essen Fatty Acids. 1995;53:287–99. doi: 10.1016/0952-3278(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 36.Begin ME, Ell G, Das UN, Horrobin DF. Differential killing of human carcinoma cells supplemented with n-3 and n-6 polyunsaturated fatty acids. J Natl Cancer Inst. 1986;77:1053–62. [PubMed] [Google Scholar]
- 37.Vartak S, McCaw R, Davis CS, Robbins ME, Spector AA. Gamma-linolenic acid (GLA) is cytotoxic to 36B10 malignant rat astrocytoma cells but not to ‘normal’ rat astrocytes. Br J Cancer. 1998;77:1612–20. doi: 10.1038/bjc.1998.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das UN, Madhavi N. Effect of polyunsaturated fatty acids on drug-sensitive and resistant tumor cells in vitro. Lipids Health Dis. 2011;10:159. doi: 10.1186/1476-511X-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramchurren N, Karmali R. Effects of gamma-linolenic and dihomo-gamma-linolenic acids on 7,12-dimethylbenz (alpha) anthracene-induced mammary tumors in rats. Prostaglandins Leukot Essent Fatty Acids. 1995;53:95–101. doi: 10.1016/0952-3278(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 40.Yu Q, Purwaha P, Ni K, Sun C, Mallik S, Qian SY. Characterization of novel radicals from COX-catalyzed arachidonic acid peroxidation. Free Radic Biol Med. 2009;47:568–76. doi: 10.1016/j.freeradbiomed.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao Y, Gu Y, Purwaha P, Ni K, Law B, Mallik S, et al. Characterization of free radicals formed from COX-catalyzed DGLA peroxidation. Free Radic Biol Med. 2011;50:1163–70. doi: 10.1016/j.freeradbiomed.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu Y, Xu Y, Law B, Qian SY. The first characterization of free radicals formed from cellular COX-catalyzed peroxidation. Free Radic Biol Med. 2013;57:49–60. doi: 10.1016/j.freeradbiomed.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Y, Qi J, Yang XY, Wu E, Qian SY. Free radical derivatives formed from COX-catalyzed DGLA peroxidation can attenuate colon cancer cell growth and enhance 5-FU’s cytotoxicity. Redox Biol. 2014;2:610–8. doi: 10.1016/j.redox.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sagar PS, Das UN, Koratkar R, Ramesh G, Padma M, Kumar GS. Cytotoxic action of cis-unsaturated fatty acids on human cervical carcinoma (HeLa) cells: Relationship to free radicals, and lipid peroxidation and its modulation by calmodulin antagonists. Cancer Lett. 1992;63:189–98. doi: 10.1016/0304-3835(92)90260-3. [DOI] [PubMed] [Google Scholar]
- 45.Tabolacci C, Lentini A, Provenzano B, Gismondi A, Rossi S, Beninati S. Similar antineoplastic effects of nimesulide, a selective COX-2 inhibitor, and prostaglandin E1 on B16-F10 murine melanoma cells. Melanoma Res. 2010;20:273–9. doi: 10.1097/CMR.0b013e328339d8ac. [DOI] [PubMed] [Google Scholar]
- 46.Mouradian M, Kikawa KD, Johnson ED, Beck KL, Pardini RS. Key roles for GRB2-associated-binding protein 1, phosphatidylinositol-3-kinase, cyclooxygenase 2, prostaglandin E2 and transforming growth factor alpha in linoleic acid-induced up regulation of lung and breast cancer cell growth. Prostaglandins Leukot Essent Fatty Acids. 2013;90:105–15. doi: 10.1016/j.plefa.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welsch CW. Relationship between dietary fat and experimental mammary tumorigenesis: A review and critique. Cancer Res. 1992;52:2040–8S. [PubMed] [Google Scholar]
- 48.Hudson EA, Beck SA, Tisdale MJ. Kinetics of the inhibition of tumour growth in mice by eicosapentaenoic acid-reversal by linoleic acid. Biochem Pharmacol. 1993;45:2189–94. doi: 10.1016/0006-2952(93)90188-3. [DOI] [PubMed] [Google Scholar]
- 49.Connolly JM, Coleman M, Rose DP. Effects of dietary fatty acids on DU145 human prostate cancer cell growth in athymic nude mice. Nutr Cancer. 1997;29:114–9. doi: 10.1080/01635589709514611. [DOI] [PubMed] [Google Scholar]
- 50.Horrobin DF, Ziboh VA. The importance of linoleic acid metabolites in cancer metastasis and in the synthesis and actions of 13-HODE. Adv Exp Med Biol. 1997;433:291–4. doi: 10.1007/978-1-4899-1810-9_61. [DOI] [PubMed] [Google Scholar]
- 51.Hillyard LA, Abraham S. Effect of dietary polyunsaturated fatty acids on growth of mammary adenocarcinomas in mice and rats. Cancer Res. 1979;34:4430–7. [PubMed] [Google Scholar]
- 52.Carter CA, Millholland RJ, Shea W, Ip MM. Effect of the prostaglandin synthesis inhibitor indomethacin on 7,12-dimethylbenz(a) anthracene-induced mammary tumorigenesis in rats fed different levels of fat. Cancer Res. 1983;43:3559–62. [PubMed] [Google Scholar]
- 53.Abou-El-Ela SH, Prasse KW, Carroll R, Wade AE, Dharwadkar S, Bruce OR. Eicosanoid syntheses in 7,12-dimethylbenz(a) anthracene-induced mammary carcinomas in Sprague-Dawley rats fed primrose oil, menhaden oil or corn oil. Lipids. 1988;23:948–54. doi: 10.1007/BF02536342. [DOI] [PubMed] [Google Scholar]
- 54.Kitigawa H, Noguchi M. Comparative effects of piroxicam and esculetin on incidence, proliferation and cell kinetics of mammary carcinomas induced by 7,12-dimethylbenz(a) anthracene on rats on high and low fat diets. Oncology. 1995;51:407–10. doi: 10.1159/000227374. [DOI] [PubMed] [Google Scholar]
- 55.Wymann MP, Schneiter R. Lipid signaling in disease. Nat Rev Mol Cell Biol. 2008;9:162–76. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 56.Noguchi M, Rose DP, Earashi M, Miyazaki I. The role of fatty acids and eicosanoid synthesis inhibitor in breast carcinoma. Oncology. 1995;52:265–71. doi: 10.1159/000227471. [DOI] [PubMed] [Google Scholar]
- 57.Kepler CR, Hirons KP, McNeill JJ, Tove SB. Intermediates and products of the biohydrogenation of linoleic acid by Butyrinvibriofbrisolvens. J Biol Chem. 1966;241:1350–4. [PubMed] [Google Scholar]
- 58.Ewaschuk JB, Walker JW, Diaz H, Madsen KL. Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J Nutr. 2006;136:1483–7. doi: 10.1093/jn/136.6.1483. [DOI] [PubMed] [Google Scholar]
- 59.Kelley NS, Hubbard NE, Erickson KL. Conjugated linoleic acid isomers and cancer. J Nutr. 2007;137:2599–607. doi: 10.1093/jn/137.12.2599. [DOI] [PubMed] [Google Scholar]
- 60.Chujo H, Yamasaki M, Nou S, Koyanagi N, Tachibana H, Yamada K. Effect of conjugated linoleic acid isomers on growth factor-induced proliferation of human breast cancer cells. Cancer Lett. 2003;202:81–7. doi: 10.1016/s0304-3835(03)00478-6. [DOI] [PubMed] [Google Scholar]
- 61.Tanmahasamut P, Liu J, Hendry LB, Sidell N. Conjugated linoleic acid blocks estrogen signaling in human breast cancer cells. J Nutr. 2004;134:674–80. doi: 10.1093/jn/134.3.674. [DOI] [PubMed] [Google Scholar]
- 62.De la Torre A, Debiton E, Durand D, Chardigny JM, Berdeaux O, Loreau O, et al. Conjugated linoleic acid isomers and their conjugated derivatives inhibit growth of human cancer cell lines. Anticancer Res. 2005;25:3943–9. [PubMed] [Google Scholar]
- 63.Beppu F, Hosokawa M, Tanaka L, Kohno H, Tanaka T, Miyashita K. Potent inhibitory effect of trans9, trans11 isomer of conjugated linoleic acid on the growth of human colon cancer cells. J Nutr Biochem. 2006;17:830–6. doi: 10.1016/j.jnutbio.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 64.Palombo JD, Ganguly A, Bistrian BR, Menard MP. The antiproliferative effects of biologically active isomers of conjugated linoleic acid on human colorectal and prostatic cancer cells. Cancer Lett. 2002;177:163–72. doi: 10.1016/s0304-3835(01)00796-0. [DOI] [PubMed] [Google Scholar]
- 65.Kim EJ, Shin HK, Cho JS, Lee SK, Won MH, Kim JW, et al. Trans-10, cis-12 conjugated linoleic acid inhibits the G1-S cell cycle progression in DU145 human prostate carcinoma cells. J Med Food. 2006;9:293–9. doi: 10.1089/jmf.2006.9.293. [DOI] [PubMed] [Google Scholar]
- 66.Ochoa JJ, Farquharson AJ, Grant I, Moffat LE, Heys SD, Wahle KW. Conjugated linoleic acids (CLAs) decrease prostate cancer cell proliferation: Different molecular mechanisms for cis-9, trans-11 and trans-10, cis-12 isomers. Carcinogenesis. 2004;25:1185–91. doi: 10.1093/carcin/bgh116. [DOI] [PubMed] [Google Scholar]
- 67.Liu JR, Chen BQ, Yang YM, Wang XL, Xue YB, Zheng YM, et al. Effect of apoptosis on gastric adenocarcinoma cell line SGC-7901 induced by cis-9, trans-11-conjugated linoleic acid. World J Gastroenterol. 2002;8:999–1004. doi: 10.3748/wjg.v8.i6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ip C, Banni S, Angioni E, Carta G, McGinley J, Thompson HJ, et al. Conjugated linoleic acid-enriched butter fat alters mammary gland morphogenesis and reduces cancer risk in rats. J Nutr. 1999;129:2135–42. doi: 10.1093/jn/129.12.2135. [DOI] [PubMed] [Google Scholar]
- 69.Banni S, Angioni E, Murru E, Carta G, Melis MP, Bauman D, et al. Vaccenic acid feeding increases tissue levels of conjugated linoleic acid and suppresses development of premalignant lesions in rat mammary gland. Nutr Cancer. 2001;41:91–7. doi: 10.1080/01635581.2001.9680617. [DOI] [PubMed] [Google Scholar]
- 70.Ip C, Dong Y, Ip MM, Banni S, Carta G, Angioni E, et al. Conjugated linoleic acid isomers and mammary cancer prevention. Nutr Cancer. 2002;43:52–8. doi: 10.1207/S15327914NC431_6. [DOI] [PubMed] [Google Scholar]
- 71.Lavillonniere F, Chajes V, Martin JC, Sebedio JL, Lhuillery C, Bougnoux P. Dietary purifed cis-9, trans-11 conjugated linoleic acid isomer has anticarcinogenic properties in chemically induced mammary tumors in rats. Nutr Cancer. 2003;45:190–4. doi: 10.1207/S15327914NC4502_08. [DOI] [PubMed] [Google Scholar]
- 72.Chen BQ, Xue YB, Liu JR, Yang YM, Zheng YM, Wang XL, et al. Inhibition of conjugated linoleic acid on mouse forestomach neoplasia induced by benzo(a) pyrene and chemopreventive mechanisms. World J Gastroenterol. 2003;9:44–9. doi: 10.3748/wjg.v9.i1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robinson KM, Botha JH. Effects of gamma-linolenic acid, dihomo-gamma-linolenic acid and ethanol on cultured human mammary carcinoma cells. Prostaglandins Leukot Med. 1985;20:209–21. doi: 10.1016/0262-1746(85)90011-3. [DOI] [PubMed] [Google Scholar]
- 74.Cho HJ, Kim EJ, Lim SS, Kim MK, Sung MK, Kim JS, et al. Trans-10, cis-12, not cis-9, trans-11, conjugated linoleic acid inhibits G1-S progression in HT-29 human colon cancer cells. J Nutr. 2006;136:893–8. doi: 10.1093/jn/136.4.893. [DOI] [PubMed] [Google Scholar]
- 75.Cho HJ, Kim WK, Jung JI, Kim EJ, Lim SS, Kwon DY, et al. Trans- 10, cis-12, not cis-9, trans-11, conjugated linoleic acid decreases ErbB3 expression in HT-29 human colon cancer cells. World J Gastroenterol. 2005;11:5142–50. doi: 10.3748/wjg.v11.i33.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamasaki M, Chujo H, Koga Y, Oishi A, Rikimaru T, Shimada M, et al. Potent cytotoxic effect of the trans10, cis12 isomer of conjugated linoleic acid on rat hepatoma dRLh-84 cells. Cancer Lett. 2002;188:171–80. doi: 10.1016/s0304-3835(02)00459-7. [DOI] [PubMed] [Google Scholar]
- 77.Song HJ, Sneddon AA, Heys SD, Wahle KW. Induction of apoptosis and inhibition of NF-kappaB activation in human prostate cancer cells by the cis-9, trans-11 but not the trans-10, cis-12 isomer of conjugated linoleic acid. Prostate. 2006;66:839–46. doi: 10.1002/pros.20351. [DOI] [PubMed] [Google Scholar]
- 78.Fujiwara F, Todo S, Imashuku S. Fatty acid modification of cultured neuroblastoma cells by gamma linolenic acid relevant to its antitumor effect. Prostaglandins Leukot Med. 1987;30:37–49. doi: 10.1016/0262-1746(87)90023-0. [DOI] [PubMed] [Google Scholar]
- 79.Das UN. γ-linolenic acid therapy of human glioma-a review of in vitro, in vivo, and clinical studies. Med Sci Monit. 2007;13:RA119–31. [PubMed] [Google Scholar]
- 80.Ikeguchi M, Maeta M, Kaibara N. Cisplatin combined with prostaglandin E1 chemotherapy in rat peritoneal carcinomatosis. Int J Cancer. 2000;88:474–8. doi: 10.1002/1097-0215(20001101)88:3<474::aid-ijc22>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 81.Xu Y, Gu Y, Qian SY. An advanced electron spin resonance (ESR) spin-trapping and LC/(ESR)/MS technique for the study of lipid peroxidation. Int J Mol Sci. 2012;13:14648–66. doi: 10.3390/ijms131114648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qian SY, Xu Y, Gu Y. Combination of spin-trapping, LC/ESR and LC/MS technique in characterization of PUFA-derived free radicals in lipid peroxidation. Acta Biophys Sinica. 2012;28:355–72. [Google Scholar]
- 83.Shaikh IA, Brown I, Wahle KW, Heys SD. Enhancing cytotoxic therapies for breast and prostate cancers with polyunsaturated fatty acids. Nutr Cancer. 2010;62:284–96. doi: 10.1080/01635580903407189. [DOI] [PubMed] [Google Scholar]


