Abstract
Purpose
To compare the visual outcomes between PRK-MMC and phakic IOL in patients with more than 8 diopter (D) of myopia.
Methods
This comparative study was performed on 23 eyes under treatment with Artiflex (group A) and 23 eyes under treatment with PRK-MMC (group B). Artiflex phakic IOL (Ophtec BV) was used in group A, and the VISX STAR S4 Excimer Laser (Abbott) was used for PRK-MMC in group B.
Results
The safety index was 1.11±0.23 and 1.05±0.25 (P=0.100) and the efficacy index was 1.02±0.11 and 0.98±0.10 (P=0.266) in group A and B, respectively. At 1 year after surgery, the manifest refraction spherical equivalent was −0.17±1.18 and −0.25±0.18 D in group A and B, respectively (P=0.471). Mesopic CS showed no significant difference between the two groups in any spatial frequency. Total coma was 0.24±0.17 and 0.67±0.40 μm (P<0.001), spherical aberration was −0.11±0.11 and 0.41±0.18 μm (P<0.001), and RMS HOAT was 0.50±0.20 and 0.96±0.45 μm (P<0.001) in group A and B, respectively.
Conclusion
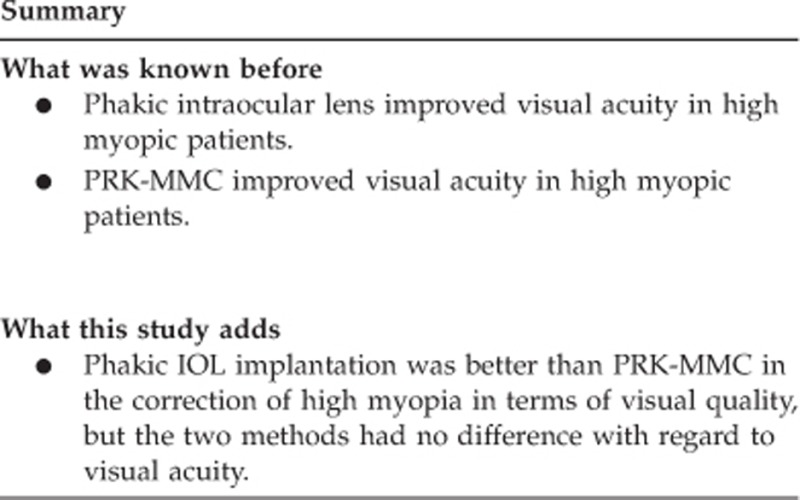
Phakic IOL implantation was better than PRK-MMC in the correction of high myopia in terms of visual quality, but the two methods had no difference with regard to visual acuity. Therefore, PRK-MMC can be used when the anterior chamber depth is a limiting factor in the implantation of phakic IOLs.
Introduction
In high myopic patients, phakic IOLs (PIOLs) or laser surgery can be used to correct refractive errors based on corneal thickness, anterior chamber depth (ACD), and the severity of the refractive error. Although there is report of the improvement of the vision and refractive error after laser-assisted in situ keratomileusis (LASIK) in high myopic patients,1 clinicians prefer the implantation of PIOLs in these patients for the possible risks of corneal opacity and decreased visual quality in corneal laser surgery. Numerous studies have shown that PIOL implantation results in favorable outcomes in high myopic patients2, 3, 4, 5, 6 and is preferred over LASIK.7 In the recent decade, among different types of refractive error surgery, photorefractive keratectomy (PRK) has regained attraction for the possibility of corneal ectasia in LASIK.8 Two long-term studies have shown that conventional PRK has acceptable results in high myopic patients, and improvement of the techniques to prevent the known complication of this procedure, that is, corneal haze, has been reemphasized.9, 10 Recent studies have indicated that mitomycin C (MMC) can decrease the incidence and severity of corneal haze, resulting in an acceptable visual acuity and quality.11, 12, 13, 14 A meta-analysis of 85 published articles compared PIOL and LASIK,7 and the comparative results of PIOL and PRK in mild and moderate myopia have been already reported.6, 15 Therefore, considering the increased safety of PRK-MMC, we decided to compare PRK-MMC and PIOL in correcting high myopia. The objective of the study was to find out which technique was superior in treating patients and had better results in terms of visual acuity and quality.
Materials and methods
This nonrandomized clinical trial was performed on high myopic patients in Noor Eye Hospital, Tehran, Iran, in 2010. Because it was not possible to conduct the study in a random manner, it was performed on two matched groups. Patients with an aqueous depth ACD of >3 mm (from epithelium to lens) and endothelial cell count of >2500 cell/mm2 and those whose corneal thickness was not sufficient for laser surgery were candidates for PIOL surgery, and patients whose depth of anterior chamber was not suitable for IOL implantation and those who were not willing to undergo intraocular surgery received PRK-MMC, provided that they had a residual bed thickness of >350 μm after the procedure considering the corneal thickness and the refractive correction. Inclusion criteria were myopia >8.00 diopter (D), cylinder power <2.00 D, and stability of the refraction in the past 12 months. Patients with ocular pathology or a history of ocular surgery were excluded from the study. If a patient used a contact lens, (s)he was required to stop using it 4 weeks before the procedure. In each group, 23 eyes were included.
Preoperative and postoperative examination
The patients were examined before the surgery and after 1, 6, and 12 months. Visual acuity (VA) was measured with a Snellen chart and reported as uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA). Manifest refraction spherical equivalent (MRSE) was evaluated using an auto refractometer (Topcon 8800, Tokyo, Japan).
Visual quality was evaluated in terms of contrast sensitivity (CS) and aberrometry. CS was measured with CVS-1000 grating charts (VectorVison Inc., Greenville, OH, USA) under mesopic conditions with best distance correction and without dilation. Aberrometry was performed using the Allegretto WaveLight analyzer (WaveLight Laser Technologie AG, Erlangen, Germany). The data of aberrometry were obtained from the 6 mm setting of the device. Three measurements were performed for each patient and the best measurement was selected. Of aberrometry indices, C6 (trefoil), C7 (vertical coma), C8 (horizontal coma), RMS comatotal, C9 (trefoil), C12 (spherical aberration/SA), and RMS higher-order aberrationtotal (RMS HOAT) were reported. The following equation was used to calculate RMS comatotal: √(C72+ C82).
Except for vision examinations and refraction that were repeated in all follow-ups, evaluation of CS and aberrometry was only performed in the last follow-up.
Surgical techniques
PRK-MMC
The corneal epithelium was mechanically scraped without alcohol under anesthesia with proparacaine hydrochloride 0.5%. Ablation was performed using the VISX STAR S4 Excimer laser (Abbott, North Chicago, IL, USA), software version 5.30, with a 6 mm ablation zone and a 1.25 mm blend zone. After laser, a sponge soaked in MMC 0.02% was applied on the ablated stroma for 10 s for each 1 D correction. After irrigation with 30 ml of balanced salt solution, a bandage contact lens (Air optix, Ciba Vision, Atlanta, GA, USA) was used. After the surgery, betamethasone 0.1% four times per day, levofloxacin eye drops 5 mg/ml four times per day, and artificial tears (Hypromellose, preservative free) were prescribed for patients. Daily examinations continued until complete epithelial healing was observed. On reepithelialization, the lens was removed and levofloxacin was discontinued but betamethasone and artificial tears were continued for another 2 weeks. After that, fluorometholone 0.1% was administered for 3 months in a tapering manner.
PIOL Implantation
First, topical anesthesia with i.v. sedation was performed by an anesthesiologist. Then, a 3.2 mm by 1.5 mm corneal incision was made at 12 o'clock with scissors, and a spherical Artiflex lens was enclavated in the eye horizontally. The PIOL formula in the IOL Master 500 (Carl Zeiss Meditec AG, Jena, Germany) with software 7.1 was used to determine the lens power. Peripheral iridotomy was performed at 12 o'clock and the incision was made watertight without suture. After the surgery, levofloxacin was instilled for 5 days and betamethasone drops were administered 4 times a day for 1 week. Then, fluorometholone 0.1% drops were administered for 2 month in a tapering manner. The patients were visited on days 1 and 3 after the surgery and were then followed-up 1, 6, and 12 months post operation.
Ethics
Informed consent was obtained from patients after they received information on the objective and the methodology of the study. The Institutional review Board approved the study protocol. The tenets of the Declaration of Helsinki were observed during the study.
Statistical analysis
The analysis of the study was performed in two parts. In the first part, based on the main objective of the study, the changes of the indices were compared between the two groups. In the second part, the trend of the changes in the indices was evaluated in each group. The χ2 and Fishers exact test were used to compare the two groups, and ANOVA and post hoc test were used to evaluate the trend of the changes in each group. The P-values of <0.05 were considered significant.
Results
The severity of myopia was −9.49±1.94 and −8.82±1.25 D in PIOL and PRK-MMC patients, respectively (P=0.871). Females comprised 73.9% of the patients in the PIOL and 60.9% of the patients in the PRK-MMC groups. The mean age of the participants was 27.74±5.28 in PIOL and 28.78±5.34 in the PRK-MMC group (P=0.509). In PRK-MMC group, corneal haze grade 1 was observed in 5 eyes (11.90%) in the first month post surgery that disappeared in month 6 of follow-up. No case of corneal haze was detected 1 year after the surgery.
At 1 year after the operation, the mean UCVA was 0.03±0.07 logMAR (20/21) in the Artiflex and 0.05±0.08 logMAR (20/22) in the PRK-MMC group (P=0.275). Approximately 73.90% of the patients in the Artiflex and 57.10% of the patients in the PRK-MMC group had a UCVA of 20/20 or better (0.241).
The mean BCVA was 0.01±0.02 logMAR (20/20) in the PIOL and 0.03±0.07 logMAR (20/21) in the PRK-MMC group (P=0.113). Approximately 95.7% and 76.20% of the patients in the PIOL and PRK-MMC group had a BCVA of 20/20 or better (P=0.088).
In the PIOL and PRK-MMC groups, the safety index was 1.11±0.23 and 1.05±0.25 (P=0.100) and the efficacy index was 1.02±0.11 and 0.98±0.10 (P=0.266), respectively. In the PIOL group, 15 eyes (65.20%) had no BCVA changes whereas others showed BCVA improvement of 1–5 lines. In the PRK-MMC group, 16 eyes (76.20%) had no BCVA changes, 2 eyes (9.50%) had 1 line decrease, and the remaining had BCVA improvement of 1–3 lines.
At 1 year after the operation, the mean spherical error was 0.02±0.10 and −0.13±0.42 D (P=0.132), the mean cylinder error was −0.39±0.34 and −0.24±0.31 D (P=0.143), and the mean MRSE was −0.17±0.18 and −0.25±0.41 D (P=0.471) in the Artiflex and PRK-MMC groups, respectively. In the end of the first year post operation, 95.7% of the patients in the PIOL group and 85.7% of the patients in the PRK-MMC group were within ±0.50 D of emmetropia. The two groups showed no significant difference in predictability (P=0.335).
The trends of the changes in the vision and refraction during 1 year after the operation are presented in Table 1, mesopic CS changes are depicted in Table 2, and HOAs are presented in Table 3.
Table1. The 1-year changes of visual acuity and refraction.
| Before surgery |
After surgery |
P-valuea | |||
|---|---|---|---|---|---|
| 1 Month | 6 Months | 12 Months | |||
| UCVA (logMAR) | |||||
| Artiflex | 1.83±0.24 | 0.03±0.05 | 0.04±0.06 | 0.03±0.07 | <0.001 |
| PRK+MMC | 1.84±0.29 | 0.23±0.18 | 0.07±0.10 | 0.05±0.08 | <0.001 |
| P-valueb | 0.871 | <0.001 | 0.420 | 0.275 | |
| BCVA (logMAR) | |||||
| Artiflex | 0.04±0.07 | 0.01±0.02 | 0.00±0.00 | 0.01±0.02 | 0.026 |
| PRK+MMC | 0.04±0.07 | 0.13±0.11 | 0.06±0.10 | 0.03±0.07 | 0.419 |
| P-valueb | 0.839 | <0.001 | 0.019 | 0.113 | |
| Sphere (diopter) | |||||
| Artiflex | −8.86±1.90 | 0.08±0.17 | 0.20±0.37 | 0.02±0.10 | <0.001 |
| PRK+MMC | −8.33±1.35 | 0.87±1.35 | 0.00±0.32 | −0.12±0.40 | <0.001 |
| P-valueb | 0.280 | 0.015 | 0.139 | 0.132 | |
| Cylinder (diopter) | |||||
| Artiflex | −1.27±0.66 | −0.53±0.47 | −0.36±0.81 | −0.39±0.33 | <0.001 |
| PRK+MMC | −0.99±0.53 | −0.94±0.62 | −0.30±0.32 | −0.24±0.31 | <0.001 |
| P-valueb | 0.118 | 0.018 | 0.807 | 0.143 | |
| MRSE (diopter) | |||||
| Artiflex | −9.49±1.94 | −0.19±0.25 | 0.02±0.60 | −0.17±0.17 | <0.001 |
| PRK+MMC | −8.82±1.25 | 0.40±1.22 | −0.15±0.37 | −0.24±0.39 | <0.001 |
| P-valuea | 0.168 | 0.041 | 0.329 | 0.471 | |
The difference of preoperative values and the last follow-up.
The difference of the two groups.
Table 2. The 1-year logarithmic changes of mesopic contrast sensitivity.
| Before surgery | 12 Months after surgery | P-valuea | |
|---|---|---|---|
| C3 (CPD) | |||
| Artiflex | 1.70±0.08 | 1.71±0.09 | 0.539 |
| PRK+MMC | 1.68±0.11 | 1.62±0.24 | 0.100 |
| P-valueb | 0.527 | 0.092 | |
| C6 (CPD) | |||
| Artiflex | 1.89±0.08 | 1.94±0.11 | 0.034 |
| PRK+MMC | 1.79±0.20 | 1.80±0.30 | 0.388 |
| P-valueb | 0.074 | 0.084 | |
| C12 (CPD) | |||
| Artiflex | 1.52±0.11 | 1.56±0.19 | 0.327 |
| PRK+MMC | 1.44±0.25 | 1.40±0.31 | 0.660 |
| P-valueb | 0.160 | 0.083 | |
| C18 (CPD) | |||
| Artiflex | 1.04±0.19 | 1.07±0.20 | 0.602 |
| PRK+MMC | 0.93±0.31 | 0.97±0.36 | 0.516 |
| P-valueb | 0.136 | 0.247 | |
The difference of the preoperative values and the last follow-up.
The difference of the two groups.
Table 3. The 1-year changes of higher-order aberrations.
| Before surgery | 12 Months after surgery | P-valuea | |
|---|---|---|---|
| C6 trefoil (μm) | |||
| Artiflex | −0.08±0.18 | 0.11±0.17 | <0.001 |
| PRK+MMC | −0.03±0.22 | −0.08±0.20 | 0.280 |
| P-valueb | 0.421 | 0.001 | |
| C7 vertical coma (μm) | |||
| Artiflex | 0.14±0.11 | 0.15±0.18 | 0.701 |
| PRK+MMC | 0.18±0.11 | −0.00±0.35 | 0.095 |
| P-valueb | 0.903 | 0.071 | |
| C8 horizontal coma (μm) | |||
| Artiflex | −0.03±0.13 | 0.02±0.17 | 0.331 |
| PRK+MMC | −0.02±0.13 | 0.20±0.62 | 0.077 |
| P-valueb | 0.918 | 0.194 | |
| RMS comatotal (μm) | |||
| Artiflex | 0.19±0.10 | 0.24±0.17 | 0.263 |
| PRK+MMC | 0.22±0.18 | 0.67±0.40 | <0.001 |
| P-valueb | 0.412 | <0.001 | |
| C9 trefoil (μm) | |||
| Artiflex | −0.04±0.16 | −0.10±0.25 | 0.275 |
| PRK+MMC | 0.00±0.15 | −0.00±0.24 | 0.942 |
| P-valueb | 0.740 | 0.194 | |
| C12 spherical aberration (μm) | |||
| Artiflex | 0.04±0.09 | −0.11±0.11 | <0.001 |
| PRK+MMC | 0.05±0.10 | 0.41±0.18 | <0.001 |
| P-valueb | 0.945 | <0.001 | |
| RMS total HOA (μm) | |||
| Artiflex | 0.36±0.17 | 0.50±0.20 | 0.012 |
| PRK+MMC | 0.31±0.12 | 0.96±0.45 | <0.001 |
| P-valueb | 0.317 | <0.001 | |
Abbreviations: HOA, higher-order aberration; RMS, root mean square.
The difference of the preoperative values and the last follow-up.
The difference of the two groups.
Discussion
Our study showed that PRK-MMC and PIOL implantation similarly corrected VA and refraction, and had similar efficacy, safety, and predictability. Their effects on CS were also similar. Regarding the effect of the two procedures on HOA, although both methods increase comaT, this increase is less in Artiflex when compared with PRK-MMC. In contrast to PRK-MMC, Artiflex implantation decreases SA.
Although the design of the study was nonrandomized, patients in both groups were similar in terms of the indices of visual acuity and quality before operation. In general and based on the 1-year changes, it could be said that IOL implantation was better than PRK-MMC in correcting high myopia. However, it should be noted that PIOL implantation is an intraocular surgery with known complications such as glaucoma, cataract, uveitis, and endophthalmitis.7 On the other hand, PRK might result in corneal haze, although its incidence has decreased recently with the application of modern lasers and mitomycin.11, 12, 14 Another important point is that we only evaluated the results of the first year post operation.
The efficacy index of Artiflex implantation (1.02) in our study was very similar to the results of a multicenter clinical trial (1.01),16 but the efficacy index of PRK-MMC in our study (0.98) was better than the results of studies conducted by Alio et al9 (0.90) and Rosman et al10 (0.80).The reason for the difference could be that conventional PRK was performed in the two aforementioned studies, whereas the application of Mitomycin improved our results.12 The decrease in the efficacy to <1 in our study was due to one patient with BCVA decrease of 1 line in the end of the first year when compared with that before the operation.
The safety index of IOL implantation was 1.11 in our study; the index has been reported to be 1.07 in a study conducted by Albarran-Diego et al3 and 1.11 in a multicenter clinical trial.16 The safety index following PRK-MMC was 1.05 in our study, and 1.16 (see Alio et al9) and 1.12 (see Rosman et al10) in other studies. It seems that both procedures in different populations similarly affected BCVA and had acceptable safety.
Both groups also showed acceptable predictability. Only one eye in the Artiflex group had MSRE of −0.62 D at 1 year after the procedure. Tehrani and Dick17 showed 91% of the eyes had refraction within ±0.50 D of emmetropia 6 months after surgery. In this study, similar to ours, astigmatism was <2.5 D, whereas sphere was <12.25 D. The reason for choosing cylinder power <2.00 D was the use of a spherical lens. In the PRK-MMC group of our study at 1 year after the procedure, two eyes had MRSE of −1.00 D and one eye had MRSE of −1.25 D. All these cases had overcorrection 1 month after the procedure. Studies have shown that predictability is acceptable even after conventional PRK,9, 10 although it was higher in our study.
According to our results, it could be stated that PRK-MMC can simultaneously correct astigmatism <2.00 D. On the other hand, as the incision was on the steep axis in our study, PIOL implantation also corrected astigmatism.
Measurement of mesopic contrast in both groups showed no significant changes before and after the procedures in all spatial frequencies. In laser-assisted refractive surgery, HOAs always increase noticeably because of the oblate change in the corneal shape, especially in high myopic patients that can result in decreased CS. In our study, although HOAs increased up to threefold in the PRK-MMC group, mesopic CS did not change obviously and this could be because of different pupil diameters during the measurement of HOA and CS. In a study performed by Peris-Martinez et al18 that reported CS in Artiflex and Artisan PIOLs, the patients in the Artisan group had better photopic CS and the patients in the Artiflex group had better mesopic CS.
Significant differences in comaT, SA, and HOAT were observed between the two procedures. After surface ablation procedures, HOAs increase because of refractive correction, ablation depth, and pupil diameter.19, 20 In a study by Serrao et al19 in which the mean SE was −6.30 D before operation, all three parameters doubled 1 year after the operation in the pupil diameter of 6 mm. In our study (preoperative SE=−8.0 D), a threefold increase was observed. However, as mentioned earlier, the effect of the pupil diameter on the results of aberrometry and CS should be considered, especially because the results of CS were concordant with VA but showed disagreement with the results of aberrometry. SA, which comprises a major proportion of HOAs and is of great significance, became more negative in the Artiflex and more positive in the PRK-MMC group 1 year after the operation. Toso and Morselli21 also reported a decrease in SA following phakic IOL. In their study, the Acrysof Cachet lens was used and SA decreased from −0.001 before the operation to −0.13 μm 6 months after the operation and became more negative. This finding is believed to result from the type of the implemented lens. In a study by Van Philips22 who used the Artiflex lens, SA was −0.05 μm after the procedure. This finding has been attributed to the negative aspheric profile of the Artiflex lens that has also been confirmed in laboratory analysis.23 Moreover, lack of comaT changes in the Artiflex group as compared with preoperation indicated more appropriate centration.
In general, it could be concluded that in high myopic patients with cylinder power of 2 D, phakic Artiflex IOL implantation and PRK-MMC have similar results in terms of refraction correction and visual acuity, but visual quality is better following Phakic Artiflex IOL implantation. Therefore, in high myopic patients whose anterior chamber depth is not suitable for IOL implantation, PRK-MMC can be performed with acceptable results. The results of this study course were limited to the first year after the procedures and longer follow-ups are required in this regard.
Acknowledgments
This study was funded by Noor Eye Hospital.
References
- Payvar S, Hashemi H. Laser in situ keratomileusis for myopic astigmatism with the Nidek EC-5000 laser. J Refract Surg. 2002;18:225–233. doi: 10.3928/1081-597X-20020501-03. [DOI] [PubMed] [Google Scholar]
- Akcay L, Eser I, Kaplan AT, Taskiran-Comez A, Dogan OK. Phakic anterior chamber lenses in very high myopia: an 18-month follow up. Clin Exp Ophthalmol. 2012;40:275–281. doi: 10.1111/j.1442-9071.2011.02632.x. [DOI] [PubMed] [Google Scholar]
- Albarran-Diego C, Munoz G, Ferrer-Blasco T, Garcia-Lazaro S, Belda-Salmeron L. Foldable iris-fixated phakic intraocular lens vs femtosecond laser-assisted LASIK for myopia between -6.00 and -9.00 diopters. J Refract Surg. 2012;28:380–386. doi: 10.3928/1081597X-20120508-01. [DOI] [PubMed] [Google Scholar]
- Ghoreishi M, Masjedi A, Nasrollahi K, Rahgozar A, Jenab K, Fesharaki H. Artiflex versus STAAR implantable contact lenses for correction of high myopia. Oman J Ophthalmol. 2011;4:116–119. doi: 10.4103/0974-620X.91266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerturk Y, Kubaloglu A, Sari ES, Koytak A, Capkin M, Akcay L, et al. Foldable iris-fixated phakic intraocular lens implantation for the correction of myopia: two years of follow-up. Indian J Ophthalmol. 2012;60:23–28. doi: 10.4103/0301-4738.91340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Philips LA. High and low contrast visual acuity after artiflex phakic intraocular lens implantation for the correction of myopia. J Refract Surg. 2010;26:423–429. doi: 10.3928/1081597X-20090710-05. [DOI] [PubMed] [Google Scholar]
- Huang D, Schallhorn SC, Sugar A, Farjo AA, Majmudar PA, Trattler WB. Phakic intraocular lens implantation for the correction of myopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:2244–2258. doi: 10.1016/j.ophtha.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Duffey RJ, Leaming D. US trends in refractive surgery: 2004 ISRS/AAO Survey. J Refract Surg. 2005;21:742–748. doi: 10.3928/1081-597X-20051101-14. [DOI] [PubMed] [Google Scholar]
- Alio JL, Ortiz D, Muftuoglu O, Garcia MJ. Ten years after photorefractive keratectomy (PRK) and laser in situ keratomileusis (LASIK) for moderate to high myopia (control-matched study) Br J Ophthalmol. 2009;93:1313–1318. doi: 10.1136/bjo.2007.131748. [DOI] [PubMed] [Google Scholar]
- Rosman M, Alio JL, Ortiz D, Perez-Santonja JJ. Comparison of LASIK and photorefractive keratectomy for myopia from -10.00 to -18.00 diopters 10 years after surgery. J Refract Surg. 2010;26:168–176. doi: 10.3928/1081597X-20100224-02. [DOI] [PubMed] [Google Scholar]
- Fazel F, Roshani L, Rezaei L. Two-step versus single application of mitomycin-C in photorefractive keratectomy for high myopia. J Ophthalmic Vis Res. 2012;7:17–23. [PMC free article] [PubMed] [Google Scholar]
- Gambato C, Miotto S, Cortese M, Ghirlando A, Lazzarini D, Midena E. Mitomycin C-assisted photorefractive keratectomy in high myopia: a long-term safety study. Cornea. 2011;30:641–645. doi: 10.1097/ICO.0b013e31820123c8. [DOI] [PubMed] [Google Scholar]
- Hashemi H, Fotouhi A, Foudazi H, Sadeghi N, Payvar S. Prospective, randomized, paired comparison of laser epithelial keratomileusis and photorefractive keratectomy for myopia less than -6.50 diopters. J Refract Surg. 2004;20:217–222. doi: 10.3928/1081-597X-20040501-04. [DOI] [PubMed] [Google Scholar]
- Hashemi H, Taheri SM, Fotouhi A, Kheiltash A. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy in high myopia: a prospective clinical study. BMC Ophthalmol. 2004;4:12. doi: 10.1186/1471-2415-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosse MV, Snoek C, Van Minderhout HM. Comparison of wavefront-guided photorefractive keratectomy and foldable iris-fixated phakic intraocular lens implantation for low to moderate myopia. J Cataract Refract Surg. 2011;37:370–377. doi: 10.1016/j.jcrs.2010.08.051. [DOI] [PubMed] [Google Scholar]
- Dick HB, Budo C, Malecaze F, Guell JL, Marinho AA, Nuijts RM, et al. Foldable Artiflex phakic intraocular lens for the correction of myopia: two-year follow-up results of a prospective European multicenter study. Ophthalmology. 2009;116:671–677. doi: 10.1016/j.ophtha.2008.12.059. [DOI] [PubMed] [Google Scholar]
- Tehrani M, Dick HB. Short-term follow-up after implantation of a foldable iris-fixated intraocular lens in phakic eyes. Ophthalmology. 2005;112:2189–2195. doi: 10.1016/j.ophtha.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Peris-Martinez C, Artigas JM, Sanchez-Cortina I, Felipe A, Diez-Ajenjo A, Menezo JL. Influence of optic quality on contrast sensitivity and visual acuity in eyes with a rigid or flexible phakic intraocular lens. J Cataract Refract Surg. 2009;35:1911–1917. doi: 10.1016/j.jcrs.2009.05.054. [DOI] [PubMed] [Google Scholar]
- Serrao S, Lombardo G, Ducoli P, Lombardo M. Long-term corneal wavefront aberration variations after photorefractive keratectomy for myopia and myopic astigmatism. J Cataract Refract Surg. 2011;37:1655–1666. doi: 10.1016/j.jcrs.2011.03.046. [DOI] [PubMed] [Google Scholar]
- Serrao S, Lombardo G, Ducoli P, Lombardo M. Optical performance of the cornea six years following photorefractive keratectomy for myopia. Invest Ophthalmol Vis Sci. 2011;52:846–857. doi: 10.1167/iovs.10-5905. [DOI] [PubMed] [Google Scholar]
- Toso A, Morselli S. Visual and aberrometric outcomes in eyes with an angle-supported phakic intraocular lens. J Cataract Refract Surg. 2012;38:1590–1594. doi: 10.1016/j.jcrs.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Van Philips LA. Higher-order aberrations after iris-fixated foldable phakic intraocular lens implantation and wavefront-guided photorefractive keratectomy for the correction of myopia. J Cataract Refract Surg. 2011;37:284–294. doi: 10.1016/j.jcrs.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Tahzib NG, MacRae SM, Yoon G, Berendschot TT, Eggink FA, Hendrikse F, et al. Higher-order aberrations after implantation of iris-fixated rigid or foldable phakic intraocular lenses. J Cataract Refract Surg. 2008;34:1913–1920. doi: 10.1016/j.jcrs.2008.07.014. [DOI] [PubMed] [Google Scholar]


