Abstract
Purpose
Subclassification of nodal stage may be of prognostic value in men with lymph node metastases (LNM) at the time of radical prostatectomy (RP). We explored the role of extranodal extension (ENE), size of the largest metastatic LN, size of the largest metastasis and LN density (LND) as predictors of biochemical recurrence (BCR).
Materials and Methods
We reviewed pathological material from 261 node-positive prostate cancer patients.
We examined the predictive value when adding the additional pathology findings to a base model including extraprostatic extension (EPE), seminal vesicle invasion (SVI), RP Gleason score, Prostate-Specific Antigen (PSA) and number of positive LNs, using Cox proportional hazards regression and Harrell's c-index.
Results
The median number of LNs removed was 14 (IQR 9, 20) and the median number of positive LNs was 1 (IQR 1, 2). With a median follow-up of 4.6 years (IQR 3.2-6.0 years), 155/261 patients experienced BCR; 5-year BCR-free survival 39% [95% CI 33%, 46%]. The median diameter of the largest metastatic LN was 9 mm (IQR 5, 16). On Cox regression, RP specimen Gleason score (>7 vs. ≤7), number of positive LNs (≥3 vs. 1-2), SVI and PSA were associated with significantly increased risks of BCR. In a subset analysis, size of the metastasis significantly improved the discrimination of the model (Harrell's C 0.700 vs. 0.655 for the base model, p=0.032).
Conclusion
Our study confirms that the number of positive LNs is a predictor of BCR in men with node-positive disease. The improvement in prognostic value of measuring the metastatic focus warrants further investigation.
Keywords: prostate cancer, radical prostatectomy, pelvic lymph node dissection, biochemical recurrence, prognosis, staging
Introduction
Accurate lymph node (LN) staging is important in the management of prostate cancer. It allows for reliably predicting prognosis and adjuvant therapy planning1. While the 1992 TNM classification included a subclassification for node-positive disease based on the size of single LN (N1: metastasis in single node ≤ 20mm in greatest dimension, N2: 20-50 mm or multiple nodes, N3: > 50mm), both the clinical and pathological version of the 2010 revision of the TNM system do not sub-stratify nodal metastasis and involves only three categories: NX – regional LNs were not assessed/sampled, N0 – no positive/regional LN metastases, N1 – metastases in regional LN(s)1.
Some researchers investigating prognostic factors influencing outcomes in prostate cancer patients with lymph node metastasis (LNM) have suggested including number of positive LNs, size of the largest metastasis as well as presence of micrometastasis in pN-substaging1-4. While, the role of number of positive LN and lymph node density as prognostic factors is well accepted2, 5-6, data regarding extranodal extension (ENE), size of the positive LN and size of the metastatic focus within the LN remains controversial and understudied1, 3, 7-8.
We sought to examine the prognostic role of detailed histopathological variables such as presence of ENE, size of the metastatic LN, size of the metastatic focus within the LN and lymph node density (LND) in men with node positive prostate cancer treated with radical prostatectomy (RP) without adjuvant hormonal therapy.
Methods
This study is an Institutional Review Board-approved analysis of data of patients treated for prostate cancer with RP + pelvic lymph node dissection (PLND) by one of 15 surgeons at our institution between January 2000 – December 2008. During this timeframe, 5,208 patients underwent RP + PLND of whom 296 patients were lymph node positive. Twenty-one patients who underwent salvage radical prostatectomy were excluded, as were patients with missing pathology (n=8 Gleason score, 2 extraprostatic extension, EPE, 4 seminal vesicle invasion, SVI) leaving 261 patients for analysis. Standard PLND at our institution included removal of the external iliac, obturator and hypogastric lymph node packets9, and none of these patients received immediate adjuvant hormonal therapy.
Postoperative surveillance included PSA measurement and physical examination at 6 weeks, every 6 months for 5 years and annually thereafter. Biochemical recurrence (BCR) was defined as a prostate specific antigen (PSA) level of ≥ 0.1 ng/mL with one confirmatory rise of detectable PSA. BCR is the outcome measured in this study.
Pathological examination
RP specimens were serially sliced in 3 to 5 mm sections, whole mounted and entirely-submitted according to previously published methods10. All LN specimens were separately sent for permanent section pathologic analysis; frozen section analysis was not utilized. After fixation in 10% neutral buffered formalin, the LNs were dissected and manually counted by the pathologists. The number of positive LNs was recorded and size of the metastatic node measured in centimeter. The LNs were examined for presence of extranodal extension (ENE) that was defined as presence of prostate cancer cells outside the LN capsule infiltrating into peri-nodal tissue. Each identified node was cut when appropriate, embedded in paraffin, sectioned at 5 micrometers (μm), stained with hematoxylin and eosin, and examined under the microscope. No immunohistochemical stains for keratin or PSA were used.
Pathologic LN slides were re-reviewed for a subset of 96 patients, measuring the size of the metastatic LN in millimeter and also measuring the size of the metastatic focus within the LN. Two pathologists (S.W.F. and L.J.T.) evaluated the RP and LN specimens. They were blinded to the study outcome (BCR).
Statistics
Our aim was to determine if the additional pathological factors could improve the discrimination of a model predicting BCR as compared to a base model including well-known prognostic factors. We used Cox proportional hazards regression to test the marginal significance of the additional pathology findings – ENE (dichotomized as yes vs. no) and metastatic nodal size (continuous) – in a model containing other covariates known to be predictive of BCR: EPE, SVI, pathological Gleason score (6-7 vs. 8-10), pretreatment PSA and number of positive LNs (1-2 vs ≥ 3 LNs). We further investigated the role of LN density (LND) calculated as the number of positive nodes divided by the total number of LNs removed in percent (continuous), since recent studies have indicated its prognostic importance.1, 11-14
A subset analysis was performed for the 96 patients (complete data for 91) for whom the pathology slides were re-reviewed, also including size of the largest LN metastatic focus (continuous variable). The characteristics of this subset were compared to the overall cohort.
Harrell's c-index was calculated using 10-fold cross validation. Recurrence free survival was estimated by means of the Kaplan-Meier method. P-values < 0.05 were considered significant. All analyses were performed using Stata 12.0, (Stata Corp, College Station, TX USA).
Results
Patient characteristics are reported in Table 1. The median age at surgery was 61 years and the median preoperative PSA level was 7.9ng/mL. The majority (72%) of the patients had palpable tumors.
Table 1.
Patient characteristics.
| N=261 | |
|---|---|
| Age at surgery, yrs | 61 (56, 66) |
| Pre-operative PSA, ng/mL | 7.9 (5.2, 13.6) |
| Clinical T stage | |
| T1b | 1 (<1%) |
| T1c | 65 (25%) |
| T2 | 137 (52%) |
| T3 | 53 (20%) |
| T4 | 1 (<1%) |
| TX/missing | 4 (2%) |
| Pathological RP specimen Gleason score | |
| 6 | 2 (1%) |
| 7 | 124 (48%) |
| 8-9 | 135 (52%) |
| Extraprostatic extension | 239 (92%) |
| Seminal vesicle invasion | 112 (43%) |
| Positive surgical margins | 94 (36%) |
| Histopathological lymph node review | |
| Extranodal extension, n=194 | 89 (46%) |
| Number of lymph nodes removed (nodal yield) | 14 (9, 20) Range:2-48 |
| Number of positive lymph nodes | 1 (1, 2) Range: 1-18 |
| Positive lymph nodes, categorical | |
| 1 LN+ | 155 (59%) |
| 2 LN+ | 57 (22%) |
| ≥3 LN+ | 49 (19%) |
| Maximum diameter of largest metastatic LN, mm, n=188 | 9 (5, 16) |
| Lymph node density, %, n=261 | 11 %(7%, 19%) |
| Maximum diameter of largest metastatic focus in LN, mm, n=96 | 3 (2, 6) Range: 0.4-21 |
Values presented are median (IQR) or frequency (%). LN= lymph node
On histopathological exam of RP specimens, the majority (92%) had extraprostatic extension, and 36% had positive surgical margins. The median number of LNs removed was 14 (IQR 9, 20, range 2-48). The median number of positive LNs was 1 (IQR 1, 2, range 1-18) with 49 (19%) patients having more than 3 positive LNs. The median maximum diameter of largest metastatic LN was 9 mm. A total of 46% showed extranodal extension (Table 1).
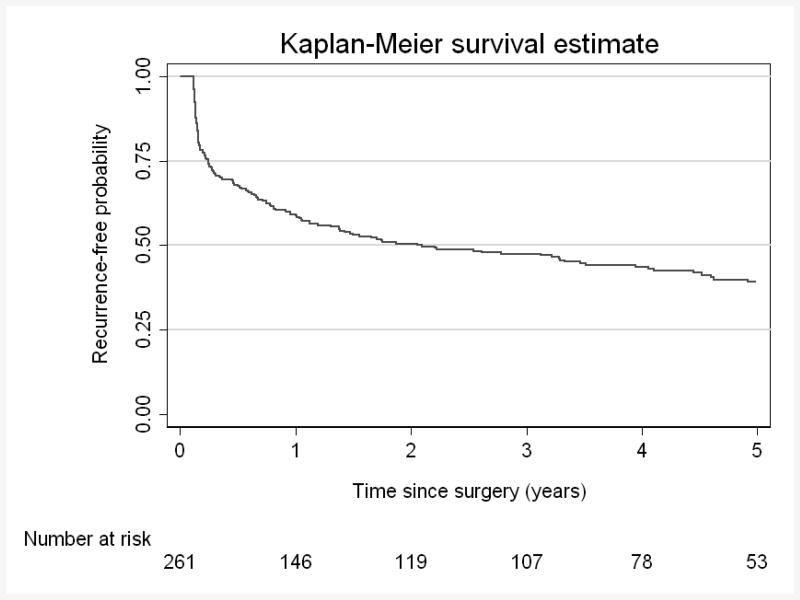
With a median follow-up of 4.6years (IQR 3.2-6.0), 155/261 patients experienced BCR, corresponding to a 5-year BCR-free survival of 39% [95% CI 33%46%] (Figure 1).
Figure 1. BCR-free survival for cohort.
5-year BCR-free survival 39% (95% CI 33%, 46%)
On Cox regression, RP specimen Gleason score (8-10 vs. 6-7), number of positive LNs (≥3 vs. 1-2), SVI and PSA were statistically significant independent predictors of BCR, increasing the risk) (Table 2). None of the additional predictors from the extended pathologic review significantly improved the discrimination of the model (Table 3). In a subset of patients for whom the pathology slides were re-reviewed (n=96), the median size of the largest metastatic focus in the LN was 3 mm (IQR 2, 6). In a subset analysis on this cohort, adding this variable led to a significant increment in Harrell's C to 0.700 as compared to 0.655 for the base model, p=0.032 (table 3). The characteristics for this subset of patients were no different from the overall cohort, except from a slightly lower rate of EPE (80% vs. 92%, data not shown).
Table 2. Cox regression (n=261).
| Covariate | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
|
| |||
| EPE (yes vs. no) | 1.38 | 0.69-2.74 | 0.4 |
| SVI | 1.46 | 1.04-2.04 | 0.027 |
| PSA | 1.01 | 1.00-1.02 | 0.026 |
| RP Gleason Score (8-10 vs. 6-7) | 1.92 | 1.36-2.72 | <0.001 |
| Number of positive lymph nodes (≥3 vs. 1-2) | 1.84 | 1.24-2.73 | 0.002 |
EPE=extraprostatic extension, SVI=seminal vesicle invasion, PSA=prostate-specific antigen, RP=radical prostatectomy
Table 3. Harrell's Concordance Index.
| Base model | Harrell's C | p-value** | |
|---|---|---|---|
|
| |||
| Base Model + ENE (yes vs. no) (n=194) | 0.657 | 0.667 | 0.078 |
| Base Model + LND (continuous) (n=261) | 0.667 | 0.676 | 0.19 |
| Base Model + Metastatic LN Size (continuous) (n=188) | 0.658 | 0.655 | 0.12 |
| Base Model + Size of metastasis (n=96) | 0.655 | 0.700 | 0.032 |
Base model includes EPE (yes vs. no), SVI (yes vs. no), RP specimen Gleason score (yes vs. no), PSA (continuous) and number of pos LNs (dichotomized as 1-2 vs.≥3 pos LNs).
Test for marginal significance of additional variable.
Discussion
The present study aimed at identifying prognostic factors from detailed pathological examination of LNs in men undergoing RP + PLND for prostate cancer. We sought to explore the role of additional histopathological characteristics on the risk of BCR when added to other risk factors such as RP specimen Gleason score and pre-operative PSA.
However, the incremental value was found to be limited for ENE, LND and metastatic LN size, as we found no statistically significant increase in discrimination. We did observe a statistically significant improvement in predictive value of the size of the metastatic focus, and measurements like these could possibly be included into standard pathology report templates, similar to what is being done in breast and head and neck cancer.
Interestingly, we observed a rather small median size of the largest metastatic LN of 9 mm. This illustrates that the clinical presentation of node positive disease has also changed over time, unlike in historical series, LNM observed in our experience were in large part diagnosed microscopically and too small to be reliably detectable through intraoperative palpation or even preoperative cross sectional imaging. Until novel imaging targeting prostate cancer cells in the lymph node are fully integrated in clinical practice, the microscopic nature of the LNM diagnosed today make it difficult for the clinician to have a reliable preoperative staging.
Studies show disparate findings regarding the usefulness of extranodal extension (ENE), some show no association with outcome3, 15 whereas others found it to be predictive of cancer-specific survival16. The role of ENE as a marginal prognostic factor therefore remains uncertain and the cohorts have differed with regards to immediate ADT or hormone treatment-naive patients and with varying proportions of aggressive disease3, 15-16. We observed a proportion of ENE of 46% in the present study, whereas previous studies have shown a slightly higher rate (55-70%).3, 15-16 The observation of ENE as a predictor of BCR and survival has been suggested to co-exist/correlate with nodal tumor burden as measured by the size of the largest metastasis.3
In a subset analysis, we found an improvement in discrimination when size of the metastatic focus was added as a predictor. In breast cancer, the presence (vs. the absence) of occult micrometastases ≤ 2 mm in diameter detected on examination of a section of axillary LNs has been associated with poor overall survival in a review.17 Similar findings have been observed in prostate cancer. Fleischmann et al reported that presence of only micrometastasis (0.2-2 mm) as well as the diameter of the patient's largest metastasis had prognostic value.2 Men with micrometastases had the most favorable 5-year survival, whereas a man with one metastasis larger than >10 mm had a four-fold increased risk of prostate-cancer specific mortality as compared to a man with smaller metastases.2 However, the cutoff point between micro- and macrometastasis has varied in different studies.11
The median number of LN removed in our study was 14, which is in harmony with recent series in the literature18-19. von Bodman and Cheng reported that most patients with LN metastasis have only one or two positive nodes.5, 8, 20 The median number of positive LNs was 1 in our study. We observed that increasing number of positive LNs was associated with increased risk of BCR. Patients with 3 positive nodes or more had poorer outcome as compared to those with 1-2 positive nodes, which is in concordance with the literature.4, 6, 21
Several studies have reported that the number of positive nodes is predictive of recurrence and disease-specific death, and would be a simple way of stratifying patients with N1 disease (single versus multiple).5, 8, 20-21 Cheng et al have therefore proposed that a tumor with a single positive LN should be classified as N1 disease and two or more positive LNs as N2 disease.1 In a study of 703 N+ patients treated with RP and extended PLND and adjuvant therapy, Briganti et al reported that men with up to 2 positive nodes experienced favorable cancer-specific survival as compared to patients with 3 or more positive nodes at 15 years of follow-up – which the authors suggested reinforces a need for a stratification on number of positive nodes when revising the pathologic TNM classification.4
Men with node-positive disease can have variable clinical outcomes and prognosis; a proportion of men have excellent long-term survival and remain free of BCR for up to 10 years5-6, 22-24 whereas others never acquire an undetectable PSA value shortly after surgery(5) and for whom prognosis is typically poor.11 Identifying men at highest risk of poor prognosis would help selecting and deciding treatment management.1 Since the presence of LN metastasis is an important prognostic factor of recurrence, accurate N-staging is important.11 Cheng et al suggested that the TNM staging system for N-stage should involve subclassification based on nodal cancer volume, especially the diameter of the largest nodal metastasis as well as the number of positive nodes, as these were found to have prognostic information. They proposed the following subclassification: pN1a: a single positive LN with largest tumor metastasis ≤ 2 mm, pN1b=a single positive LN with largest tumor metastasis >2 mm and pN2=multiple positive LNs, i.e. two pos LNs or more.1 Our study suggests that measuring the size of the metastatic focus may be of value, however, more research is required to confirm or reject this finding and although this measurement may well be included in standard pathology report templates, pathologists do not routinely report on LN metastatic size for prostate cancer which would be required for such a subclassification.
Which pathological factor is of most prognostic value is not clearly understood. Some studies have indicated that the size of the largest LN metastasis is the only factor with an independent impact on PSA recurrence, disease-specific survival and overall survival; by some suggested to be more important than the total number of positive LNs.1, 3, 25 Some authors have suggested a sub-classification of pN stage, based on the size of the largest metastasis in a single positive LN.1 Our subset analysis indicates an additive predictive value of the size of the metastasis, a finding that warrants further investigation. However, adding the number of positive lymph nodes (1 or 2 vs. 3 or more) to the standard N-staging may be sufficient.
Limitations of our current study include a rather short follow up limiting the analysis to BCR outcome instead of overall survival and cancer specific survival. Inconsistent findings between the different studies looking at this subject may be due to differences in the size of the study population, statistical methods applied, patients' characteristics and even possibly therapeutic differences. Strengths include a research quality pathological review dedicated to the purpose of the study, an adequate and homogeneous patient cohort uniformly treated with RP and PLND without immediate ADT, allowing for observation of the “natural course” of the disease in this patient population with regards to time to BCR and the role of histopathological LN characteristics. Further larger studies are needed to confirm or reject our conclusions.
Conclusion
Our study confirms that the number of positive LNs is a predictor of BCR in men with node-positive disease. The improvement in prognostic value of measuring the metastatic focus
Acknowledgments
This study was supported by funding from The Sidney Kimmel Center for Prostate and Urologic Cancers. S.C. is supported by grants from the Swedish Cancer Society, the Swedish Society for Medical Research (SSMF), the Sweden America Foundation and the Swedish Council for Working Life and Social Research.
Key of definitions of abbreviations
- LNM
lymph node metastases
- RP
radical prostatectomy
- ENE
extranodal extension
- LND
LN density
- BCR
biochemical recurrence
- EPE
extraprostatic extension
- SVI
seminal vesicle invasion
- PSA
Prostate-Specific Antigen
- IQR
Inter Quartile Range
- PLND
pelvic lymph node dissection
- ADT
Androgen Deprivation Therapy
References
- 1.Cheng L, Montironi R, Bostwick DG, et al. Staging of prostate cancer. Histopathology. 2012;60:87. doi: 10.1111/j.1365-2559.2011.04025.x. [DOI] [PubMed] [Google Scholar]
- 2.Fleischmann A, Schobinger S, Schumacher M, et al. Survival in surgically treated, nodal positive prostate cancer patients is predicted by histopathological characteristics of the primary tumor and its lymph node metastases. Prostate. 2009;69:352. doi: 10.1002/pros.20889. [DOI] [PubMed] [Google Scholar]
- 3.Fleischmann A, Schobinger S, Markwalder R, et al. Prognostic factors in lymph node metastases of prostatic cancer patients: the size of the metastases but not extranodal extension independently predicts survival. Histopathology. 2008;53:468. doi: 10.1111/j.1365-2559.2008.03129.x. [DOI] [PubMed] [Google Scholar]
- 4.Briganti A, Karnes JR, Da Pozzo LF, et al. Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur Urol. 2009;55:261. doi: 10.1016/j.eururo.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 5.von Bodman C, Godoy G, Chade DC, et al. Predicting biochemical recurrence-free survival for patients with positive pelvic lymph nodes at radical prostatectomy. J Urol. 2010;184:143. doi: 10.1016/j.juro.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bader P, Burkhard FC, Markwalder R, et al. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J Urol. 2003;169:849. doi: 10.1097/01.ju.0000049032.38743.c7. [DOI] [PubMed] [Google Scholar]
- 7.Boormans JL, Wildhagen MF, Bangma CH, et al. Histopathological characteristics of lymph node metastases predict cancer-specific survival in node-positive prostate cancer. BJU Int. 2008;102:1589. doi: 10.1111/j.1464-410X.2008.07904.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheng L, Bergstralh EJ, Cheville JC, et al. Cancer volume of lymph node metastasis predicts progression in prostate cancer. Am J Surg Pathol. 1998;22:1491. doi: 10.1097/00000478-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Touijer K, Rabbani F, Otero JR, et al. Standard versus limited pelvic lymph node dissection for prostate cancer in patients with a predicted probability of nodal metastasis greater than 1% J Urol. 2007;178:120. doi: 10.1016/j.juro.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Al-Ahmadie HA, Tickoo SK, Olgac S, et al. Anterior-predominant prostatic tumors: zone of origin and pathologic outcomes at radical prostatectomy. Am J Surg Pathol. 2008;32:229. doi: 10.1097/PAS.0b013e31812f7b27. [DOI] [PubMed] [Google Scholar]
- 11.Adams J, Cheng L. Lymph node-positive prostate cancer: current issues, emerging technology and impact on clinical outcome. Expert Rev Anticancer Ther. 2011;11:1457. doi: 10.1586/era.11.104. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg GD, Epstein JI, Piantadosi S, et al. Management of stage D1 adenocarcinoma of the prostate: the Johns Hopkins experience 1974 to 1987. J Urol. 1990;144:1425. doi: 10.1016/s0022-5347(17)39759-8. [DOI] [PubMed] [Google Scholar]
- 13.Palapattu GS, Singer EA, Messing EM. Controversies surrounding lymph node dissection for prostate cancer. Urol Clin North Am. 2010;37:57. doi: 10.1016/j.ucl.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Cai T, Nesi G, Tinacci G, et al. Clinical importance of lymph node density in predicting outcome of prostate cancer patients. J Surg Res. 2011;167:267. doi: 10.1016/j.jss.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Cheng L, Pisansky TM, Ramnani DM, et al. Extranodal extension in lymph node-positive prostate cancer. Mod Pathol. 2000;13:113–8. doi: 10.1038/modpathol.3880019. [DOI] [PubMed] [Google Scholar]
- 16.Griebling TL, Ozkutlu D, See WA, et al. Prognostic implications of extracapsular extension of lymph node metastases in prostate cancer. Mod Pathol. 1997;10:804–9. [PubMed] [Google Scholar]
- 17.de Boer M, van Dijck JA, Bult P, et al. Breast cancer prognosis and occult lymph node metastases, isolated tumor cells, and micrometastases. JNCI. 2010;102:410. doi: 10.1093/jnci/djq008. [DOI] [PubMed] [Google Scholar]
- 18.Briganti A, Chun FK, Salonia A, et al. Critical assessment of ideal nodal yield at pelvic lymphadenectomy to accurately diagnose prostate cancer nodal metastasis in patients undergoing radical retropubic prostatectomy. Urology. 2007;69:147. doi: 10.1016/j.urology.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Tokuda Y, Carlino LJ, Gopalan A, et al. Prostate cancer topography and patterns of lymph node metastasis. Am J Surg Pathol. 2010;34:1862. doi: 10.1097/PAS.0b013e3181fc679e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng L, Zincke H, Blute ML, et al. Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer. 2001;91:66–73. doi: 10.1002/1097-0142(20010101)91:1<66::aid-cncr9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Daneshmand S, Quek ML, Stein JP, Lieskovsky G, Cai J, Pinski J, et al. Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: long-term results. J Urol. 2004;172:2252. doi: 10.1097/01.ju.0000143448.04161.cc. [DOI] [PubMed] [Google Scholar]
- 22.Bianco FJ, Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005;66:83. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 23.Briganti A, Blute ML, Eastham JH, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009;55:1251. doi: 10.1016/j.eururo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Palapattu GS, Allaf ME, Trock BJ, et al. Prostate specific antigen progression in men with lymph node metastases following radical prostatectomy: results of long-term followup. J Urol. 2004;172:1860–4. doi: 10.1097/01.ju.0000139886.25848.4a. [DOI] [PubMed] [Google Scholar]
- 25.Sgrignoli AR, Walsh PC, Steinberg GD, et al. Prognostic factors in men with stage D1 prostate cancer: identification of patients less likely to have prolonged survival after radical prostatectomy. J Urol. 1994;152:1077. doi: 10.1016/s0022-5347(17)32507-7. [DOI] [PubMed] [Google Scholar]