Abstract
Sex differences in circadian rhythms have been reported with some conflicting results. The timing of sleep and length of time in bed has not been considered, however, in previous such studies. The current study has three major aims: (1) replicate previous studies in a large sample of young adults for sex differences in sleep patterns and dim light melatonin onset (DLMO) phase; (2) in a subsample constrained by matching across sex for bedtime and time in bed, confirm sex differences in DLMO and phase angle of DLMO to bedtime; (3) explore sex differences in the influence of sleep timing and length of time in bed on phase angle. Three hundred and fifty-six (207 women) first year Brown University students ages 17.7–21.4 (mean=18.8, sd=.4) years were included in these analyses. Wake time was the only sleep variable that showed a sex difference. DLMO phase was earlier in women than men and phase angle wider in women than men Shorter time in bed was associated with wider phase angle in women and men. In men, however, a three way interaction indicated phase angles were influenced by both bedtime and time in bed, a complex interaction was not found for women. These analyses in a large sample of young adults on self-selected schedules confirm a sex difference in wake time, circadian phase, and the association between circadian phase and reported bedtime. A complex interaction with length of time in bed occurred for men but not women. We propose that these sex differences likely indicate fundamental differences in the biology of the sleep and circadian timing systems as well as in behavioral choices.
Introduction
Sex differences in circadian rhythms and sleep may occur due to differences in neurobiology, physiology, and/or behavior. Defining these sex differences and unraveling their origins is an area of active investigation that has implications for understanding interactions of basic biology and behavior. For instance, healthy women report that they require more hours of sleep for optimal functioning than men (Natale, Adan, & Fabbri, 2009; Tonetti, Fabbri, & Natale, 2008), which may reflect sex differences in the interaction of circadian and homeostatic oscillators (Wever, 1984). Sex differences in circadian rhythms may also influence clinical sleep disorders that differentially affect women and men, in part because these differences become amplified and more problematic when sleep and circadian rhythms are uncoupled or desynchronized (Wever, 1984),e.g., the observation that female patients report more problems with insomnia than their male counterparts (Phillips et al., 2008).
Sex differences in human circadian rhythms have been explored using a variety of methods and approaches with mixed findings. In general, subjective measures of circadian phase preference in large samples including the Horne and Östberg Morningness-Eveningness Questionnaire (MEQ; Horne & Ostberg, 1976) and Munich Chronotype Questionnaire (Roenneberg et al., 2004), have shown that women are more morning-type compared to men (Adan & Natale, 2002; Roenneberg et al., 2004; Taylor, Clay, Bramoweth, Sethi, & Roane, 2011). Consistent with these subjective data, several studies that measured endogenous circadian phase using the timing of core body temperature rhythm have demonstrated an earlier circadian phase position in women compared to men (e.g., Baehr, Revelle, & Eastman, 2000; Baker et al., 2001; Campbell, Gillin, Kripke, Erikson, & Clopton, 1989; Moe, Prinz, Vitiello, Marks, & Larsen, 1991)
Other studies have examined whether sex differences exist in the timing of melatonin rhythm under circumstances both free and experimentally fixed sleep/wake schedules. For instance, Mongrain and colleagues (2004) reported an earlier clock time of salivary dim light melatonin onset (DLMO) phase in women (21:14 ± 30 minutes) compared to men (22:49±39 mins) for 24 participants who kept fixed 8-hour (permitted range of 7–9 hours) per night sleep schedules based on their habitual sleep patterns for at least 7 days before the study (Mongrain, Lavoie, Selmaoui, Paquet, & Dumont, 2004). More recently, Cain and colleagues (2010) examined sex differences in a sample of 28 women and men matched based on their habitual bed and wake times. These participants kept 8-hour per night sleep schedules before an in-lab assessment of circadian rhythms. In this matched sample, women had significantly earlier DLMO (22:27 ± 97 mins) using a 10pg/ml threshold compared to men (23:16 ± 76 mins, respectively) and earlier time of minimum core body temperature rhythm than men (women=04:46±116 mins; men=06:11(±79 mins). In contrast, another study (Burgess & Eastman, 2005) examined the timing of salivary dim light melatonin onset (DLMO) in 60 participants on a free sleep schedule (participants chose their own schedule for 6 days) and 60 participants on a fixed 8-hour sleep schedule. No sex differences were observed in DLMO phase in participants on either schedule. Another study from this group assessed DLMO phase in 170 participants (85 women) (Burgess & Fogg, 2008) who completed a variety of study protocols requiring sleeping on an 8–9 hour per night fixed schedule for 3 to 15 days before the study. Again, no significant sex differences were observed for the timing of melatonin phase.
Finally, the association of circadian phase position and sleep behavior as measured by phase angle has also been examined for sex differences. Mongrain et al., for example (2004), showed that DLMO was earlier in women relative to their bedtime versus men. Similarly, Cain and colleagues (Cain et al., 2010) reported a longer DLMO phase to bedtime interval in women (1.34 ± 0.96h) compared to men .75± .83h) using their sample of men and women matched on bed and wake times. The aforementioned study by Burgess and Eastman (2005) that showed no sex difference on DLMO phase, did, however, show a longer DLMO to bedtime and DLMO to sleep onset interval than men. Burgess and colleagues (2008), however, reported no sex differences in the interval between DLMO phase and bedtime in their sample of 170 participants on free schedules.
In summary, in studies where sex differences have been observed, women have demonstrated earlier circadian phase and wider phase angles, though sex differences have not been observed in every study. These conflicting findings may be due in part to methodological differences, such as experimentally constrained sleep schedules versus free sleep schedules. In addition, sex differences in DLMO phase and phase angle may also be related by a factor rarely assessed: sleep duration. For example, the light/dark cycle is the most potent stimulus for entrainment of endogenous rhythms’, and sleep is a major signal for the circadian clock in that it modulates or gates photic information to the master clock. Thus, DLMO phase, sleep timing, and time in bed are likely interconnected, and differences among studies may be influenced by the way sleep timing and sleep duration are controlled and/or evaluated.
Our data from a naturalistic study of a large sample of first-year college students provides the opportunity to examine sex differences in circadian rhythms and sleep variables from several different analytic perspectives in young adults living on their self-selected sleep schedules. We had three major aims: First, we used our full unconstrained and unmatched sample to assess sex differences in sleep and circadian variables similar to analyses by Burgess and Eastman (2005). Second, we used a sub-sample that we matched by sex for bedtime and time in bed to confirm differences in DLMO and phase angle shown by Cain et al. (2010) and extend their findings by evaluating sleep schedules across a diverse sample of sleep schedules (bedtimes ranging from 23:31 to 03:40; time in bed ranging from 5 hrs 43 mins to 9 hrs 58 mins). Finally, we used the full unmatched sample to explore sex differences in how sleep timing and length of time in bed (i.e., duration of darkness) influence phase angle.
Materials and Methods
Participants
First-year students ages 17.7–21.4 (mean=18.8, sd=. 4 years) enrolled in a study that took place across Week 1 to Week 8–10 of their first term at Brown University. All were invited to participate in a circadian phase assessment (Dim Light Melatonin Onset (DLMO)). Data from the 356 participants (207 women) who completed the DLMO protocol and daily online sleep/wake diaries the preceeding week were included in the current analyses. We also identified a subset of men and women (83 pairs) whom we were able to match for bedtime and time in bed. All participants provided informed consent and received monetary compensation for taking part in this study approved by the Lifespan Institutional Review Board for the Protection of Human Subjects.
Procedures
As part of a larger study, participants were asked to complete an on-line diary prompted by daily email for 8 to 9 weeks sleep diary from the start of term. They were given no instructions or feedback about their sleep schedules. Questions on the sleep diary focused on the major sleep episode in the previous 24 hours and included: “What time did you try to fall asleep?,” “Estimate how many minutes it took you to fall asleep?,” and “What time did you finally wake up?” Illume software (DatStat, Inc.) was used to create the diaries and collect the online data to a secure website.
The current analyses assessed DLMO phase on a Thursday, Friday, or Saturday evening occurring between late October to mid November (DLMO phase was assessed before the end of DST for years 2010–2012 and 11 and 12 days after DST in 2009) of the Fall semester (No differences were detected between DLMOs measured in 2009 (mean = 23.3, sd=1.6) and those measured in 2010–2012 (mean=23.6, sd=1.4; t(354)=−1.65, p=.1)). Participants reported to a large auditorium on campus where light levels were set below 20 lux in line of sight. Timing of saliva sampling windows for the first 3 study years was determined for each participant based on their diary reported sleep schedules using an estimate of DLMO phase derived from the Burgess and Eastman (2005) free-sleep algorithm. A refined algorithm was used for the fourth year based on results from the first three years (unpublished). Based on these estimates, 6.5-hour sample collection windows were created surrounding the predicted time of DLMO phase. Saliva samples were collected every 30 minutes throughout the protocol using salivettes (Sarstedt, Germany), centrifuged and chilled immediately, and frozen at −20°C within 15 hours. Students were seated, permitted to study, talk quietly with friends, and use their laptops (all measured and set below 10 lux) throughout the sampling window. Melatonin concentration in saliva was determined using radioimmunoassay (RIA; Alpco) with sensitivity of .9pg/ml, intra-assay coefficient of variation (CV) 7.9% and inter-assay coefficient of variance 11.7%. Linear interpolation across times bracketing a 4pg/mL threshold was used to determine DLMO phase.
Sleep patterns were determined for each participant by averaging diary-reported bedtimes (BT), rise times (WT), and computed time in bed (TIB) across the 7 days preceding DLMO assessments. TIB was derived each day as the elapsed time from BT to WT. Phase angles were computed as minutes between DLMO phase and BT (negative values indicate DLMO occurred after BT).
Analytic approach
To replicate and extend previous findings, each circadian and sleep measure (DLMO, phase angle to BT, BT, WT, and TIB) was first evaluated to identify sex differences using Student’s t-tests. Next, TIB, BT, and WT were controlled using the matched subset of 83 pairs to test whether sex differences would persist after controlling for these factors. Differences in DLMO and phase angle in the matched pairs were evaluated using paired t-tests. Finally, a general linear model (GLM) was used for the full unconstrained sample to model sex differences in interrelationships among the circadian and sleep measures. The model included BT, TIB, and BT X TIB interaction as predictors of phase angle. In order to test whether these interrelationships differed between males and females, sex was included as a moderator. In other words, sex was allowed to interact with BT, TIB, and BT X TIB interaction. All analyses were performed using SPSS (Version 18, IBM®).
Results
Sex differences for DLMO phase, phase angle, BT, WT, and TIB in the full sample are presented in Table 1. Significant differences were observed for WT (t (354) = −2.49; p = .01; effect size (Cohen’s d) = .28), DLMO phase (t (354) = −2.76; p < .01; d = .28), and phase angle (t (354) =2.14; p =.03; d = .22). On average, women reported earlier WT and demonstrated earlier DLMO phase and longer phase angles between DLMO and BT. These full-sample analyses showed small effect sizes.
Table 1.
Age, Sleep, and Circadian Variables.
| Full Unmatched Sample | ||
|---|---|---|
| Women Mean (SD) | Men Mean (SD) | |
| N | 207 | 149 |
| Age (y) | 18.7(0.4) | 18.8 (0.5) |
| Bedtime (clock time) | 01:47 (71 mins) | 01:54 (68 mins) |
| Wake time (clock time)* | 09:12 (55 mins) | 09:28 (62 mins) |
| Time In Bed (hours) | 7.4 (1.0) | 7.6 (0.9) |
| DLMO (clock time)* | 23:25 (82 mins) | 23:50 (92 mins) |
| Phase angle (hours)* | 2.4 (1.4) | 2.1 (1.3) |
| Matched Subset Sample | ||
|---|---|---|
| Women Mean (SD) | Men Mean (SD) | |
| N | 83 | 83 |
| Age (y) | 18.7 (0.4) | 18.8 (0.5) |
| Bedtime (clock time) | 01:49 (53 mins) | 01:46 (50 mins) |
| Wake time (clock time) | 09:23 (46 mins) | 09:21 (51 mins) |
| Time In Bed (hours) | 7.6 (0.9) | 7.6 (0. 8) |
| DLMO (clock time)* | 23:21 (75 mins) | 23:59 (79 mins) |
| Phase angle (hours)* | 2.3 (1.2) | 1.8 (1.1) |
p<.05
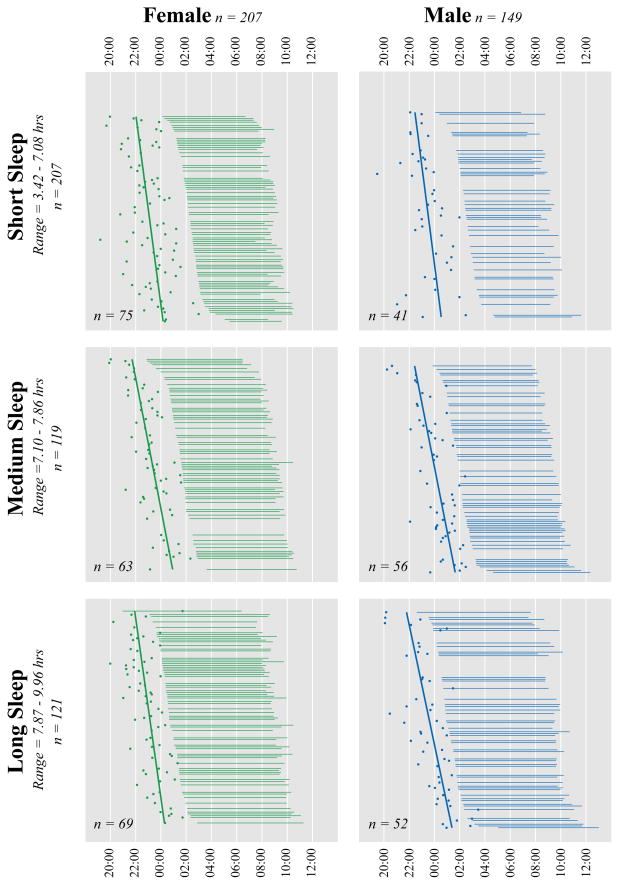
The sample included 83 pairs of women and men matched for BT and TIB identified independently by two of the investigators (EVR, MAC). Our goal was to match pairs within 30 minutes total difference of BT and TIB; in fact, we were able to match more closely such that the sum of the BT and TIB differences did not exceed 18 minutes for any pair. The means for the matched sample are reported in Table 1. Results from the matched sample indicate that women have an earlier DLMO phase (t (82) =3.07; p < .01; d = .49) and a longer interval from DLMO phase to BT (t (82) = −3.34; p < .01; d = .43) than men. The full, unmatched sample was used to evaluate whether the interactions among BT, WT, and TIB were differentially associated with phase angle in women versus men. The simple correlations among the variables are reported separately for women and men in Table 2. Results from the GLM indicated a significant higher-order interaction of sex, BT, and TIB (F (1, 348) = 8.43; p < .01 ; effect size (η2)= .024), indicating that the influence of BT and TIB duration on phase angle differs between males and females. To help visualize this interaction, Figure 1 illustrates the data where TIB was divided into three groups using a tertile split: participants within each group were rank ordered according to their bedtimes and then separated by sex.
Table 2.
Simple correlations among sleep parameters for women and men.
| Bedtime | Wake time | Time in Bed | DLMO | Phase Angle | |
|---|---|---|---|---|---|
| Bedtime | .601** | −.655** | .433** | .440** | |
| Wake time | .653** | .197** | .580** | −.055 | |
| Time in Bed | −.479** | .345** | .004 | −.575** | |
| DLMO | .526** | .580** | .018 | −.620** | |
| Phase Angle | .237** | −.115 | −.422** | −.701** |
Upper triangle: Women; Lower triangle: Men;
p<.01
Figure 1.
Participants were stratified by sex and time in bed (tertile split) and rank ordered by bedtime within each stratum. For each individual, Time in Bed is depicted by the length of the horizontal lines, DLMO is shown as a dot, and the solid regression lines illustrate the association between bedtime and DLMO for each sex by time in bed stratum.
For women, the analyses show that TIB is significantly related to phase angle (F (1, 348) = 43.23; p < .01; effect size (η2) = .11), such that shorter time in bed is associated with a wider phase angle. This effect can be seen in Figure 1 by observing the longer distance between DLMO and BT for short (Panel A; women) versus long TIB (Panel C; women). Neither a significant effect of BT on phase angle, nor an interaction of TIB and BT on phase angle was observed.
The analyses in men showed a similar association of TIB and phase angle (F (1, 348) = 24.22; p < .001; effect size (η2) = .07). In men, as in women, a shorter TIB is associated with a wider phase angle. This effect can be seen in Figure 1 by observing the longer distance between DLMO and BT for short (Panel A; men) versus long TIB (Panel C; men). Unlike in women, however, a small but significant effect was found for the interaction of TIB and BT on phase angle (F (1, 348) = 6.82; p = .01; effect size (η2) = .02). The interaction indicates that for men with a short TIB, as BT gets later phase angle gets wider (Figure 1, Panel A, males), whereas in men with a long TIB, as BT gets later phase angle narrows (Figure 1, Panel C, males)
Discussion
The current study examined sex differences in circadian rhythms and self-reported sleep in a large sample of first year college students. Using this large naturalistic sample, our results confirm previous findings that average DLMO phase is earlier in women than men (Burgess & Eastman, 2005; Cain et al., 2010; Mongrain et al., 2004) and that bedtimes chosen by women are later with respect to their melatonin onset than men (Cain et al., 2010; Mongrain et al., 2004). These results were found for both the full unmatched sample and matched subsample. The magnitude (about 35 minutes) and direction of these sex differences in phase angle are similar to Cain et al. (2010) and represent small to medium effect sizes. The effect sizes were small to moderate and indicate that the lack of sex differences found by some (e.g., Burgess & Eastman, 2005; Burgess & Fogg, 2008) may be due, in part, to insufficient power to detect modest differences. Other issues, such as seasonal confounds (due to changes in light exposure), employment status, and so forth may also minimize sex differences.
Our sample differed in important ways from previous studies, which allowed us to build upon previous findings: our sample was larger than previous samples, was obtained in individuals of a narrow age range and at a narrow seasonal window, and was derived from observational data that nevertheless included a wide range of sleep behaviors (i.e., we did not exclude individuals based on TIB). These sample characteristics allowed us to examine the interrelationships among sex, DLMO phase, phase angle, time in bed, and sleep timing, and we found that these interrelationships differed by sex. DLMO phase is earlier in women and phase angle is wider (both variables are correlated with sleep timing). Furthermore, in women and men shorter time in bed is associated with wider phase angle; however, in men phase angles also differed as a function of bedtime and time in bed. Thus, in the context of longer time in bed, men with later versus earlier bedtimes have narrow phase angles, whereas in the context of short time in bed, men with later versus earlier bedtimes have wide phase angles. This complex interaction was not found in women.
Circadian/sleep measures may differ between women and men due to sex differences in intrinsic circadian period length (Duffy et al., 2011). For example, Duffy and colleagues (2011) reported that women have shorter circadian periods (24 hours and 5 minutes) than men (24 hours and 11 minutes), which is consistent with an earlier phase in women. A developmental difference in the circadian timing system may also exist between women and men of the age group in our study. Unpublished data from our lab show that the intrinsic circadian period shortens across the second decade; as with other maturational events of adolescence, females may experience this change earlier contributing to the sex difference in phase we describe. The narrow range of ages of our current sample (17.7–21.4) limit exploration of this concept in our data set, and this mechanism is unlikely to explain sex differences in phase for older adults.
A unique feature of our analysis is that our data were collected from a large sample of participants on their self-selected sleep/wake schedules allowing us to match for sleep timing and length of time in bed, thus controlling for sleep behavior across a wide range of individual differences. In other words, this approach allowed us to examine our sex difference hypotheses while constraining bedtime and time in bed without imposing sleep schedules (and light/dark cycles) on our participants. Based on findings in the matched sample, it is unlikely that morning light exposure per se at an earlier time in women than men accounts for the sex differences in phase or phase angle. On the other hand, Cain et al., (2010) have proposed that men and women may differ in their sensitivity to the phase-shifting effects of light. Such a phase specific sex difference might explain the sex differences observed in circadian parameters and is a plausible explanation that merits further study.
In summary, findings of sex differences in human circadian phase and the pattern of sleep behavior relative to the circadian timing system are common though not universal. Our analysis of this issue in a large sample confirms sex differences in circadian phase and its association with reported bedtime. A complex interaction with length of time in bed occurred for men but not women. We propose that the sex differences likely involve fundamental differences in the biology of the sleep and circadian timing systems as well as differences in behavioral choices. Experiments that probe these issues are merited.
References
- Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiology international. 2002;19:709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. Journal of sleep research. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. The Journal of physiology. 2001;530:565–574. doi: 10.1111/j.1469-7793.2001.0565k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. Journal of sleep research. 2005;14:229–237. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PloS one. 2008;3:e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SBS, Santhi N, Duffy JF. Sex differences in phase angle of entrainment and melatonin amplitude in humans. Journal of biological rhythms. 2010;25:288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SS, Gillin JC, Kripke DF, Erikson P, Clopton P. Gender differences in the circadian temperature rhythms of healthy elderly subjects: relationships to sleep quality. Sleep. 1989;12:529–536. [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang A-M, Phillips AJK, Münch MY, Gronfier C, Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International journal of chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Moe KE, Prinz PN, Vitiello MV, Marks AL, Larsen LH. Healthy elderly women and men have different entrained circadian temperature rhythms. Journal of the American Geriatrics Society. 1991;39:383–387. doi: 10.1111/j.1532-5415.1991.tb02904.x. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in Morningness-Eveningness. Journal of biological rhythms. 2004;19:248–257. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- Natale V, Adan A, Fabbri M. Season of birth, gender, and social-cultural effects on sleep timing preferences in humans. Sleep. 2009;32:423–426. doi: 10.1093/sleep/32.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BA, Collop NA, Drake C, Consens F, Vgontzas AN, Weaver TE. Sleep disorders and medical conditions in women. Journal of women’s health (2002); Proceedings of the Women & Sleep Workshop, National Sleep Foundation; Washington, DC. March 5–6, 2007; 2008. pp. 1191–1199. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Current biology: CB. 2004;14:R1038–1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Clay KC, Bramoweth AD, Sethi K, Roane BM. Circadian phase preference in college students: relationships with psychological functioning and academics. Chronobiology international. 2011;28:541–547. doi: 10.3109/07420528.2011.580870. [DOI] [PubMed] [Google Scholar]
- Tonetti L, Fabbri M, Natale V. Sex difference in sleep-time preference and sleep need: a cross-sectional survey among Italian pre-adolescents, adolescents, and adults. Chronobiology international. 2008;25:745–759. doi: 10.1080/07420520802394191. [DOI] [PubMed] [Google Scholar]
- Wever RA. Sex differences in human circadian rhythms: intrinsic periods and sleep fractions. Experientia. 1984;40:1226–1234. doi: 10.1007/BF01946652. [DOI] [PubMed] [Google Scholar]