Abstract
High mammographic density (MD) is one of the main risk factors for development of breast cancer. To date, however, relatively few studies have evaluated the association between MD and diet. In this cross-sectional study, we assessed the association between MD (measured using Boyd's semiquantitative scale with five categories: <10%, 10–25%, 25–50%, 50–75% and >75%) and diet (measured using a food frequency questionnaire validated in a Spanish population) among 3,548 peri- and postmenopausal women drawn from seven breast cancer screening programs in Spain. Multivariate ordinal logistic regression models, adjusted for age, body mass index (BMI), energy intake and protein consumption as well as other confounders, showed an association between greater calorie intake and greater MD [odds ratio (OR) = 1.23; 95% confidence interval (CI) = 1.10-1.38, for every increase of 500 cal/day], yet high consumption of olive oil was nevertheless found to reduce the prevalence of high MD (OR = 0.86;95% CI = 0.76-0.96, for every increase of 22 g/day in olive oil consumption); and, while greater intake of whole milk was likewise associated with higher MD (OR = 1.10; 95%CI 1.00-1.20, for every increase of 200 g/day), higher consumption of protein (OR = 0.89; 95% CI 0.80-1.00, for every increase of 30 g/day) and white meat (p for trend 0.041) was found to be inversely associated with MD. Our study, the largest to date to assess the association between diet and MD, suggests that MD is associated with modifiable dietary factors, such as calorie intake and olive oil consumption. These foods could thus modulate the prevalence of high MD, and important risk marker for breast cancer.
Keywords: mammographic density, breast density, diet, calorie intake, olive oil
What's new?
Factors that influence mammographic density (MD), which is associated with breast cancer risk, could shed light on various aspects of breast malignancy. In this investigation of 3,548 Spanish women, a validated food frequency questionnaire identified an association between MD and elevated calorie intake. Even though more than 90% of the women consumed raw olive oil on a daily basis, higher olive oil consumption was associated with lower MD. The results support previous studies linking high caloric intake with MD and provide new evidence of an inverse association between MD and olive oil consumption.
Mammographic images are characterized by the presence of dense areas, which represent epithelial tissue and stroma, along with translucid areas corresponding to fat. In 1976, increased risk of breast cancer was first shown to be associated with higher mammographic density (MD), an association that has since been corroborated by subsequent studies.1–5 High MD is currently proposed as an “intermediate phenotype” for identifying women with higher risk of breast cancer.6,7
To a great extent, MD shares the same determinants as breast cancer, e.g., menarche, parity, benign, breast disease and hormonal replacement therapy (HRT) with estrogen and progestin.8–10 Some authors have investigated the influence of dietary-related exposures on MD. A study in Italy has shown a protective effect of consumption of vegetables and olive oil, as well as an increase in risk linked to consumption of meat.11 Dietary fats have been also related with higher MD in several studies,12–15 while other studies did not confirm these results.16,17 An inverse association between MD and consumption of calcium and vitamin D has been described,11,18 though this association was only evident among premenopausal women in another study.19 Alcohol would appear to increase breast density.11,18–21 Some studies have investigated the relationship between Mediterranean diet and MD with mixed results: while a German study found an inverse association with MD,22 another one reported this association only in smokers.23 Furthermore, two clinical trials have been undertaken to date, aimed at assessing the effect of a low-fat and high-carbohydrate diet, albeit with different results, i.e., whereas the intervention was observed to reduce MD in one of the trials,24 no differences with respect to the control group were observed in the most recent one.25
This study sought to investigate the association between dietary intake and MD among Spanish women participants in breast cancer screening programs.
Material and Methods
Study population
The DDM-Spain (Determinantes de la Densidad Mamográfica en España-Determinants of Mammographic Density in Spain) is a cross-sectional multicenter study based on 3,584 women recruited from seven specific screening centers within the Spanish breast cancer screening program network. Spanish programs are government-sponsored and cover the entire population of women aged 50–69 years or 45–69 years, depending on the region.
Recruitment was conducted from October 2007 through September 2008 at seven centers located in Zaragoza (Aragon); Palma de Mallorca (Balearic Isles); Burgos (Castile-Leon); Barcelona (Catalonia); Corunna (Galicia); Pamplona (Navarre) and Valencia (Valencia). The expected sample size was 500 women per center. Percentage participation in the study was 74.5% (range 64.7% in Corunna to 84.0% in Zaragoza). Further information can be consulted elsewhere.9,20,26
Questionnaire
Data on diet and the other study variables were obtained by personal interview conducted at each screening center by a trained interviewer using a structured questionnaire. The questionnaire gathered sociodemographic data and information on reproductive history, personal and family background, occupation, lifestyle and diet. In addition, each participant was weighed and measured twice by the interviewer, and a third time if the first two measures were not similar, using the same type of balance and stadiometer in all centers. Body mass index (BMI) was calculated using average values of weight and height.
Dietary intake was estimated using a 117-item semiquantitative food frequency questionnaire (FFQ) similar to that used by Willett in the US Nurses' Health Study27 and suitably adapted to and validated in several Spanish adult populations.28,29 The FFQ covers consumption of each food, specifying the use of standard portions or rations by means of nine frequency categories, ranging from “never or less than once per month” to “six or more times per day.” Based on the responses to each item, mean daily intakes of each nutrient were calculated for each woman, by multiplying the frequency of use of each food by the nutritional composition of the specified portion of that food, using the US Department of Agriculture Food Composition Tables30 and other tables published for Spanish foods31 as the primary source. Similarly, information for some nutrients was supplemented on the basis of scientific publications.32–34 Data on the use of vitamin or mineral supplements were also collected, thereby enabling this source of additional intake to be taken into account. The responses for each food were converted into mean intake per day for each study participant. Finally, the mean daily intakes were summed to calculate the daily intake for basic food groups (dairy products, eggs, white meat, red meat, processed meat, blue fish, white fish, vegetables, fruit, nuts, legumes, cereals and pasta, potatoes, bread, sweets, butter and olive oil). In our study, olive oil accounted for 92.3% of the consumption of all vegetable oils, so, rather than considering this food group as such, we decided instead to analyze olive oil alone.
Measurement of mammographic density
Mammograms were sent to a single center for density assessment. Four screening centers provided analog images while the other three had already implemented full-field digital mammography. MD was measured blindly and anonymously by a single radiologist on the left craniocaudal view of the left breast using Boyd's semiquantitative scale, which classifies density into 6 categories, namely, 0%, <10%, 10–25%, 25–50%, 50–75% and >75%.5 For quality control purposes, a random sample of 375 mammograms was analyzed in duplicate; the intrarater weighted Kappa was 91.7% (89.8–93.3).35 The consistency between first and second readings was similar in analog and digital images (weighted Kappa of 92% and 91%, respectively).35
Owing to the low number of women with MD = 0%, Boyd's first two categories (0% and <10%) were pooled and five density categories were considered in the statistical analysis.
Statistical analysis
In the initial descriptive analysis, we used the ANOVA test for independent samples, the Bonferroni post-hoc test for comparison of quantitative variables, and the chi-squared test for qualitative variables.
The association between macronutrients and food types (explanatory variable) and MD (dependent variable) was studied using mixed ordinal logistic regression models, with screening center being included as a random effects term. These models, also known as proportional-odds models, assume that odds ratios (ORs) remain constant, irrespective of the cut-off chosen to dichotomize the ordinal classification of MD in two groups: high versus low MD. The model simultaneously estimates as many equations as the number of categories in the dependent variable minus one. An OR greater than 1 indicates an increased probability for women with higher consumption of the corresponding dietary factor to be classified in a higher MD category and vice versa. The Brant test was used to verify the proportional-odds assumption. In a first stage, the association between calorie intake and each macronutrient with MD was separately assessed, adjusting for sociodemographic and lifestyle variables associated with MD in previous analyses: age, BMI, parity, menopausal status, smoking habit and alcohol consumption. Second, since calories and proteins were associated with MD, the association between specific food types and MD was explored adjusting for calorie intake and proteins and for the abovementioned confounders. In these analyses, each dietary item was included as a continuous variable. The procedure described by Benjamini and Hochberg was used to correct p-values for multiple testing.36
Finally, the OR associated with quartiles of consumption (or tertiles, if the distribution of consumption did not allow for calculation of quartiles) was quantified for all foods that displayed an association with MD with p < 0.100 in the previous models, adjusting for calorie intake, protein consumtion and the rest of potential confounders. The effect of each food on pre- and postmenopausal women was quantified. The possible heterogeneity of effect on the two groups was tested by ascertaining the statistical significance of the interaction term between menopausal status and the corresponding dietary variable. Furthermore, restricted cubic splines with four knots were used to explore departures from linearity in the shape of the dose–response curve for foods that showed a clear association with MD.37
Subgroup analyses were performed to estimate the effect of the number of calories and grams of olive oil per day on MD, by category of the following variables, i.e., age at screening, BMI, menopausal status, smoking habit, alcohol intake (no/yes), with both variables as well as the abovementioned confounders being included in the same model. All statistical analyses were performed using the Stata computer package (version SE/9.0; StataCorp LP, College Station, TX).
Results
Of the 3,583 women enrolled in the study, the following were excluded from the analysis: nine whose diet was regarded as implausible (energy intake of >4000 or <800 KCals/day); one who had been fed intravenously; ten who had prevalent breast cancer (development of breast cancer in the first 6 months after inclusion in the study); and 16 who had no mammogram available. This yielded a total sample of 3,548 women.
Table 1 shows the sociodemographic and lifestyle characteristics of all study participants by menopausal status. The mean age of study participants was 56.2 years (SD 5.5). Premenopausal women displayed higher percentages of BMI below 25 (p < 0.001), reported higher percentages of an educational level higher than secondary (p < 0.001), and were more frequently ranked in the medium-high or high socioeconomic levels (p < 0.001). Most of the women in the study had never undergone HRT. The percentage of women smokers was higher among premenopausal women (p < 0.001). Postmenopausal women registered a higher prevalence of osteoporosis and diabetes (p < 0.001). A total of 18% of postmenopausal and 41% of premenopausal women had a MD of over 50%. The distribution of these variables per categories of MD can be consulted in Supplementary material. As expected, age, BMI, menopausal status and parity were negatively associated with MD, while smoking and drinking presented a positive association. Table 2 presents the average intake of food and nutrients. As can be seen, there were statistically significant differences for intake of almost all food groups and macronutrients according to menopausal status.
Table 1.
Distribution of non dietary factors among study subjects according to menopausal status
| Variables | Total sample (n = 3,548) | Premenopausal (n = 811) | Postmenopausal (n = 2,737) | p-value | |
|---|---|---|---|---|---|
| Age, Mean (SD)1 (missing = 5) | 56 (5) | 50 (3) | 58 (5) | <0.001 | |
| BMI.2 N (%) (missing = 15) | <25 | 1,013 (28.7) | 330 (40.8) | 683 (25.1) | <0.001 |
| 25–29.9 | 1,479 (41.9) | 273 (33.8) | 1,206 (44.3) | ||
| ≥30 | 1,041 (29.5) | 205 (25.4) | 836 (30.7) | ||
| Menopausal status, N (%) | Premenopausal | 416 (11.7) | – | – | – |
| Perimenopausal | 395 (11.1) | – | – | ||
| Postmenopausal | 2,737 (77.1) | – | – | ||
| Education, N (%)(missing = 6) | <5th grade | 1,203 (34.0) | 133 (16.4) | 1,070 (39.2) | <0.001 |
| 5th-8th grade | 1,313 (37.1) | 298 (36.8) | 1,015 (37.2) | ||
| ≥8th grade | 1,026 (29.0) | 379 (46.8) | 647 (23.7) | ||
| Socioeconomic status, N (%)(missing = 15) | Low | 850 (24.1) | 155 (19.2) | 695 (25.5) | <0.001 |
| Medium | 2,507 (71.0) | 599 (74.3) | 1,908 (70.0) | ||
| High | 176 (5.0) | 52 (6.5) | 124 (4.5) | ||
| Nulliparous (N,%) | 317 (8.9) | 82 (10.1) | 235 (8.6) | 0.181 | |
| Number of births among parous women (mean, SD) | 2.3 (1.0) | 2.1 (0.9) | 2.4 (1.0) | <0.001 | |
| Hormone replacement therapy, N (%) | Never | 3,042 (85.7) | 790 (97.4) | 2,252 (82.3) | <0.001 |
| Current | 154 (4.3) | 19 (2.3) | 135 (4.9) | ||
| Past | 301 (8.5) | 2 (0.2) | 299 (10.9) | ||
| Raloxifen | 51 (1.4) | 0 (0.0) | 51 (1.9) | ||
| Diabetes, N (%) (missing = 6) | 195 (5.5) | 13 (1.6) | 182 (6.7) | <0.001 | |
| Osteoporosis, N (%) (missing = 56) | 463 (13.3) | 20 (2.5) | 443 (16.5) | <0.001 | |
| Physical activity (last year), N (%)(missing = 4) | Low | 223 (6.3) | 84 (10.4) | 139 (5.1) | <0.001 |
| Moderate | 1,620 (45.8) | 434 (53.9) | 1,186 (43.5) | ||
| High | 1,691 (47.8) | 287 (35.7) | 1,404 (51.4) | ||
| Smoking habit, N (%) | Never | 1,735 (48.9) | 331 (40.8) | 1,404 (51.3) | <0.001 |
| Current | 1,648 (46.4) | 440 (54.3) | 1,208 (44.1) | ||
| Former | 165 (4.7) | 40 (4.9) | 125 (4.6) | ||
| Drinking habit, N (%) | Never | 2,054 (57.9) | 344 (42.4) | 1,710 (62.5) | <0.001 |
| Current | 857 (24.2) | 257 (31.7) | 600 (21.9) | ||
| Former | 637 (18.0) | 210 (25.9) | 427 (15.6) | ||
| Mammographic density N (%) | 0–10% | 871 (24.5) | 107 (13.2) | 764 (27.9) | <0.001 |
| 10–25% | 732 (20.6) | 107 (13.2) | 625 (22.8) | ||
| 25–50% | 1,135 (32.0) | 267 (32.9) | 868 (31.7) | ||
| 50–75% | 623 (17.6) | 249 (30.7) | 374 (13.7) | ||
| >75% | 187 (5.3) | 81 (10.0) | 106 (3.9) |
SD: Standard deviation.
BMI: Body Mass Index.
Table 2.
Distribution of dietary factors among study subjects according to menopausal status
| Dietary variables | Total sample (n = 3,548) | Premenopausal (n = 811) | Postmenopausal (n = 2,737) | p-value |
|---|---|---|---|---|
| Calories (Kcal), Mean (SD1) | 2,053 (480) | 2,128 (485) | 2,031 (476) | <0.001 |
| Carbohydrates (g), Mean (SD1) | 226 (63) | 232 (63) | 224 (63) | 0.001 |
| Fats (g), Mean (SD1) | 85 (24) | 88 (26) | 83 (24) | <0.001 |
| Proteins (g), Mean (SD1) | 102 (24) | 105 (24) | 101 (24) | <0.001 |
| Alcohol (g), Mean (SD1) | 4.6 (8.7) | 4.5 (8.1) | 4.6 (8.9) | 0.777 |
| Dairy products (g), Mean (SD1) | 492 (245) | 489 (244) | 494 (246) | 0.610 |
| Whole milk (g), Mean (SD1) | 47 (138) | 60 (152) | 44 (133) | 0.003 |
| Semi-skimmed milk (g), Mean (SD1) | 130 (202) | 133 (207) | 129 (201) | 0.627 |
| Skimmed milk (g), Mean (SD1) | 106 (188) | 108 (192) | 106 (187) | 0.820 |
| Eggs (g), Mean (SD) | 19 (13) | 20 (11) | 18 (14) | 0.004 |
| White meat (g), Mean (SD1) | 34 (19) | 34 (19) | 33 (19) | 0.224 |
| Red meat (g), Mean (SD1) | 55 (36) | 64 (39) | 53 (34) | <0.001 |
| Processed meat (g), Mean (SD1) | 31 (20) | 35 (20) | 30 (20) | <0.001 |
| Blue fish (g), Mean (SD1) | 31 (24) | 30 (23) | 31 (24) | 0.186 |
| White fish (g), Mean (SD1) | 36 (21) | 33 (20) | 37 (22) | <0.001 |
| Vegetables (g), Mean (SD1) | 294 (129) | 282 (122) | 298 (131) | 0.002 |
| Fruit (g), Mean (SD1) | 431 (226) | 413 (212) | 436 (230) | 0.010 |
| Nuts (g), Mean (SD1) | 7.0 (10.3) | 6.8 (10.2) | 7.1 (10.3) | 0.369 |
| Legumes (g), Mean (SD1) | 33 (23) | 37 (24) | 32 (23) | <0.001 |
| Cereals and pasta (g), Mean (SD1) | 66 (40) | 69 (41) | 65 (40) | 0.004 |
| Potatoes (g), Mean (SD1) | 53 (32) | 49 (30) | 54 (32) | <0.001 |
| Sweets (g), Mean (SD1) | 33 (31) | 42 (38) | 30 (28) | <0.001 |
| Vegetable oil (g), Mean (SD1) | 26 (14) | 25 (15) | 26 (13) | 0.073 |
| Olive oil (g), Mean (SD1) | 24 (13) | 23 (15) | 24 (13) | 0.068 |
| Bread (g), Mean (SD1) | 98 (65) | 100 (64) | 97 (66) | 0.338 |
| Butter (g), Mean (SD1) | 0.3 (1.2) | 0.4 (1.6) | 0.2 (1.0) | <0.001 |
SD: Standard deviation.
2BMI: Body Mass Index.
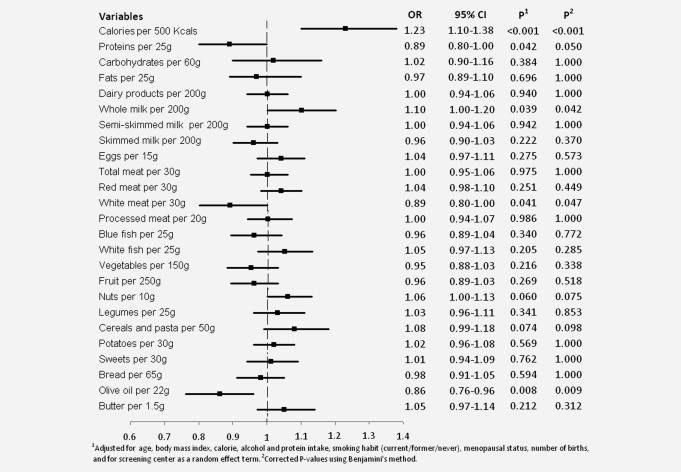
Figure 1 depicts the association between each nutrient and MD [OR and 95% confidence interval (CI)], based on models adjusted for age, BMI, parity, menopausal status, smoking habit, alcohol consumption and total caloric intake. Higher MD was associated with higher calorie intake (OR: 1.23; 95% CI: 1.10–1.38) and consumption of whole milk (OR: 1.10; 95% CI: 1.00–1.20). In contrast, protein intake (OR: 0.89; 95% CI: 0.80–1.00), consumption of white meat (OR: 0.89; 95% CI: 0.80–1.00), and consumption of olive oil (OR: 0.86; 95% CI: 0.76–0.96) showed a negative relationship with MD. Intake of nuts and that of cereals and pasta appeared to be associated with higher MD, albeit without reaching statistical significance. All vitamins and minerals were also studied but the data failed to indicate any association (data not shown).
Figure 1.
Association between daily intake of dietary variables and mammographic density among 3548 women from the DDM-Spain study.
Table 3 analyzes the foods associated with MD with p < 0.10 in Figure 1, by quartile of consumption (or tertile, if the distribution of consumption did not allow for calculation of quartiles), for the study population, both overall and broken down by menopausal status. Daily calorie intake was associated with higher MD, especially among women in the upper quartile of consumption (OR: 1.34; 95% CI: 1.03-1.74). The inverse association between MD and protein consumption was observed in both groups, though it attained statistical significance only in postmenopausal women (OR: 0.87; 95% CI: 0.76–0.98). White meat was also inversely associated with MD in the whole set of women. Women who consumed more than 200 grams of whole milk per day had a higher MD than those who did not consume this food (OR: 1.30; 95%CI: 1.01-1.68). A higher intake of nuts seemed to be associated with a higher MD, although this association did not reach conventional statistical significance. Finally, an increase in olive oil consumption of two tablespoonfuls per day (22 g) was associated with a lower MD (OR: 0.72; 95% CI: 0.56–0.93), with no differences by menopausal status. Restricted cubic splines did not show any departure from linearity in the dose–response shape (results not shown).
Table 3.
Association between daily intake of dietary variables and Boyd's % breast density classification, among 3,548 women from the DDM-Spain study
| All women1 | Pre- and perimenopausal1 | Postmenopausal1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dietary variables | OR2 | 95% CI3 | p | OR2 | 95% CI3 | p | OR2 | 95% CI3 | p | p heterogeneity4 |
| Calories | ||||||||||
| <706 Kcals | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – | 0.299 |
| 1,706–2,018 Kcals | 1.02 | 0.85–1.23 | 0.838 | 0.77 | 0.51–1.17 | 0.229 | 1.15 | 0.93–1.41 | 0.201 | |
| 2,019–2,352 Kcals | 1.08 | 0.89–1.32 | 0.418 | 0.92 | 0.59–1.42 | 0.703 | 1.22 | 0.97–1.52 | 0.085 | |
| >2,352 Kcals | 1.34 | 1.03–1.74 | 0.027 | 1.16 | 0.68–2.00 | 0.584 | 1.50 | 1.11–2.03 | 0.008 | |
| per 500 Kcals5 | 1.23 | 1.10–1.38 | <0.001 | 1.23 | 0.97–1–54 | 0.082 | 1.23 | 1.08–1.40 | 0.001 | |
| Proteins | ||||||||||
| 85 g | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – | 0.122 |
| 85–99 g | 0.94 | 0.78–1.13 | 0.512 | 0.85 | 0.57–1.28 | 0.445 | 0.96 | 0.78–1.18 | 0.717 | |
| 100–116 g | 0.80 | 0.66–0.99 | 0.039 | 0.71 | 0.46–1.12 | 0.140 | 0.81 | 0.64–1.03 | 0.080 | |
| >116 g | 0.79 | 0.61–1.02 | 0.075 | 0.60 | 0.35–1.03 | 0.067 | 0.84 | 0.63–1.14 | 0.264 | |
| per 25 g5 | 0.89 | 0.80–1.00 | 0.042 | 0.93 | 0.74–1.17 | 0.534 | 0.87 | 0.76–0.98 | 0.023 | |
| Olive oil | ||||||||||
| <12 g | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – | 0.467 |
| 12–48 g | 0.85 | 0.73–0.98 | 0.026 | 0.87 | 0.65–1.16 | 0.339 | 0.86 | 0.73–1.01 | 0.062 | |
| >48 g | 0.72 | 0.56–0.93 | 0.010 | 0.63 | 0.40–0.99 | 0.045 | 0.76 | 0.57–1.02 | 0.069 | |
| per 22 g5 | 0.86 | 0.76–0.96 | 0.008 | 0.85 | 0.69–1.05 | 0.136 | 0.86 | 0.76–0.98 | 0.026 | |
| Whole milk | ||||||||||
| 0 g | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – | 0.609 |
| 1–200 g | 1.07 | 0.87–1.31 | 0.551 | 1.04 | 0.69–1.59 | 0.840 | 1.06 | 0.83–1.34 | 0.647 | |
| >200 g | 1.30 | 1.01–1.68 | 0.044 | 1.36 | 0.85–2.19 | 0.203 | 1.21 | 0.89–1.64 | 0.217 | |
| per 100 g5 | 1.10 | 1.00–1.20 | 0.039 | 1.14 | 0.96–1.36 | 0.124 | 1.06 | 0.96–1.18 | 0.258 | |
| Cereals and pasta | ||||||||||
| <43.8g | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – | 0.272 |
| 43.8–48.9 g | 0.95 | 0.74–1.22 | 0.694 | 1.00 | 0.60–1.68 | 0.990 | 0.93 | 0.70–1.24 | 0.623 | |
| 49.0–87.0 g | 1.00 | 0.86–1.17 | 0.951 | 1.12 | 0.81–1.56 | 0.479 | 0.97 | 0.82–1.16 | 0.769 | |
| >87.0 g | 1.14 | 0.96–1.36 | 0.123 | 1.17 | 0.84–1.63 | 0.346 | 1.11 | 0.93–1.34 | 0.252 | |
| per 40 g5 | 1.08 | 0.99–1.18 | 0.074 | 1.12 | 0.95–1.31 | 0.182 | 1.05 | 0.97–1.17 | 0.195 | |
| White meat | ||||||||||
| <19g | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – | 0.278 |
| 19–40 g | 1.04 | 0.88–1.21 | 0.668 | 1.03 | 0.74–1.44 | 0.860 | 1.06 | 0.89–1.27 | 0.491 | |
| 40.1–51.4 g | 0.89 | 0.73–1.10 | 0.293 | 1.04 | 0.67–1.61 | 0.852 | 0.89 | 0.70–1.12 | 0.326 | |
| >51.4 g | 0.93 | 0.76–1.14 | 0.489 | 1.19 | 0.79–1.80 | 0.402 | 0.96 | 0.77–1.20 | 0.728 | |
| per 20 g5 | 0.89 | 0.80–1.00 | 0.041 | 1.05 | 0.85–1.33 | 0.593 | 0.90 | 0.80–1.02 | 0.108 | |
| Nuts | ||||||||||
| 0 g | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – | 0.312 |
| 1–2 g | 1.06 | 0.89–1.25 | 0.521 | 0.95 | 0.66–1.37 | 0.798 | 1.05 | 0.87–1.28 | 0.592 | |
| 3–22g | 1.03 | 0.88–1.21 | 0.718 | 1.00 | 0.71–1.41 | 0.989 | 1.05 | 0.88–1.26 | 0.601 | |
| >23 g | 1.13 | 0.91–1.40 | 0.258 | 1.43 | 0.88–2.30 | 0.145 | 1.10 | 0.86–1.40 | 0.453 | |
| per 10 g5 | 1.06 | 1.00–1.13 | 0.060 | 1.14 | 1.00–1.30 | 0.046 | 1.05 | 0.97–1.17 | 0.195 | |
All the multivariate models were adjusted for daily calorie intake, proteins, alcohol intake, body mass index, smoking habit (current/former/never), menopausal status, number of births, and for screening center as a random effects term.
OR: Odds ratio.
CI: Confidence Interval.
Statistical significance of the interaction term between menopausal status and the corresponding dietary variable.
Linear trend.
Figure 2 shows the ORs and CIs for total caloric intake and olive oil consumption, by category of age, BMI, menopausal status, smoking habit and alcohol. Although there were no statistically significant differences in the risk estimators by reference to the different subgroups, the effect of both variables was less marked among younger women.
Figure 2.
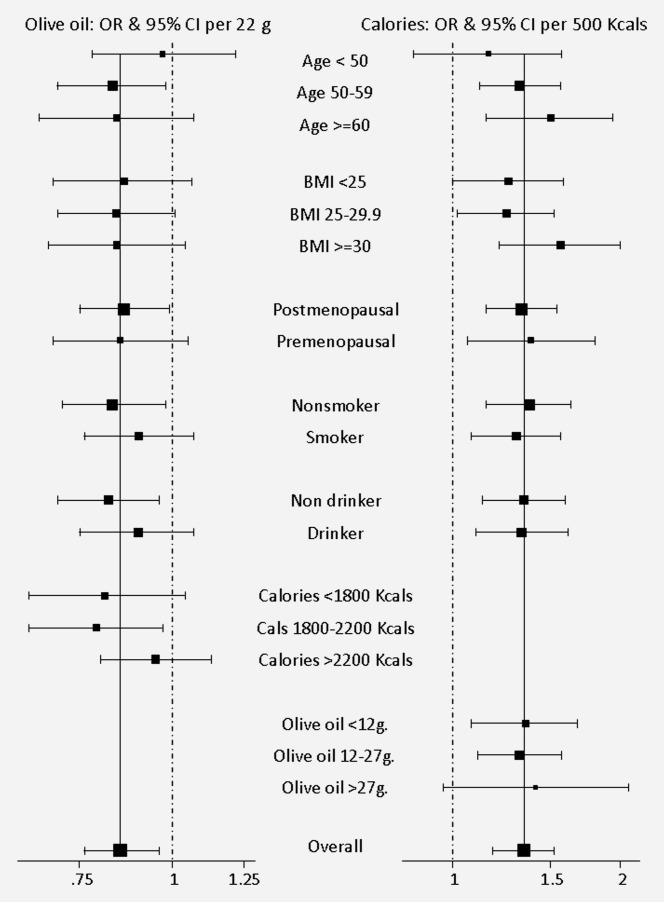
Association between calories, olive oil and mammographic density per category of explanatory variables (odds ratios and 95% confidence intervals). Estimators are adjusted for the rest of the variables presented in the graphic.
Discussion
This study provides additional evidence supporting the hypothesis that dietary factors may influence MD, a phenotype risk factor for breast cancer. Our results indicate an increased probability of having a high-risk mammographic pattern among women with a higher calorie intake, and a lower MD among participants having a high consumption of olive oil.
Our findings show a clear association between MD and calorie intake, a finding reported by a previous cross-sectional study,16 but not found in others.11,18,19,38 However, a prospective study on 1,161 British women indicated that greater caloric intakes during adult life were associated with higher MD.39 In addition, we have previously reported a positive relationship between weight gain in adult life and MD among DDM-Spain participants,26 but the inclusion of this factor in the model only slightly reduced the association between MD and total energy intake (OR = 1.19; 95% CI = 1.06–1.33). While the link between higher energy intake and MD still remains unclear, several studies nevertheless suggest an association between caloric intake and breast cancer through mechanisms such as alterations in the production of ovarian steroid hormones,40 changes in the availability of insulin growth factor-1, increasing cell proliferation41 or increasing tissue susceptibility to damaging carcinogens by an increasing DNA replication that reduces the rate of apoptosis.42 Moreover, studies with transgenic mice containing the human aromatase gene have shown that diet-induced weight gain preferentially stimulates local aromatase expression in the breast, which may lead to local estrogen excess and breast cancer risk.43 Conversely, calorie restriction has proven to reduce mammary tumors in MMTV-Her2/neu mice.44 An increase in MD could be an intermediate step in one of the biological mechanisms linking calorie intake with breast cancer risk.
Higher olive oil consumption was associated with lower MD in our study. Our results are in line with those obtained in the only study that has investigated this association.11 Olive oil is a key component of the Mediterranean diet, and this dietary pattern has been inversely related with MD in two previous studies,22,23 but neither of them explored this component separately. It is important to stress that, in our study, consumption of olive oil refers to consumption in its fresh or raw form, such as dressing for salads and other dishes on the table. This consumption pattern is very frequent in Spain, and thus accounts for the fact that 98% of our participants reported taking at least one tablespoonful of olive oil daily (11 g). The protective effect of olive oil consumption on breast cancer has already been highlighted in a case–control study undertaken in Spain, and confirmed in a recent meta-analysis.45 Experimental studies on animal and human models support this finding: the protective effect observed is presumably the result of molecular influences eliciting a balance between proliferation and apoptosis, shifted in favor of apoptosis or lower levels of DNA damage in tumors.46
The association between MD and consumption of protein and white meat found in our study is more unexpected. Despite the fact that white meat (chicken and game) is rich in protein, the correlation between these two variables is weak, albeit statistically significant (r = 0.378). Four previous studies examined the relation of proteins and MD: two reported a positive association,13,16 while no effect was found in the other two.11,38 One of them also presented a positive correlation between white meat and MD.16 Although a positive association between red meat and breast cancer has been suggested in recent years,47,48 very few studies have analyzed the role of white meat.49,50 It might be thought that women who ate white meat would report a lower consumption of red meat but we failed to find a negative correlation between these two variables in our population (r = 0.112). In a study conducted in 2002, a negative relationship was observed between white meat and incidence of breast cancer,50 though another study did not confirm this result.50 Furthermore, an inverse association between white meat and other types of cancer, such as hepatocellular carcinoma, has been described,51,52 though the mechanisms implicated are still unknown. With respect to proteins and breast cancer, however, recent research tends to point more to a positive relationship between higher protein consumption and higher risk of breast cancer.53
Insofar as nuts are concerned, higher consumption in the preceding year was associated with a higher MD in our study. This is the first time that this relationship has been observed, though a 2010 study analyzed the effect of consumption of nuts during adolescence and development of breast cancer 15 years later and found that this food group had a protective effect.54 Nevertheless, in view of the dearth of studies that have analyzed the effect of nuts on MD and/or breast cancer, more research is needed to corroborate or refute these hypotheses.
Evidence of the relationship between dairy products and lower MD has also been found in a number of studies on premenopausal women (24;42), and one paper even observed a negative relationship between cheese and MD.11 Our study found no association between consumption of dairy products and MD: curiously, however, when milk consumption was broken down by fat content, a positive association between consumption of whole milk and MD was suggested. The only paper that assessed whole milk in relation to MD found no association, though in this same paper a relationship between all dairy products and MD was observed.29 It has also been suggested that consumption of calcium and vitamin D, both present in dairy products, could reduce risk of breast cancer,55 though the association between vitamin D and calcium intake and MD is still controversial.11,15,18,19,38,56 In our study, none of these micronutrients was associated with MD. It must nonetheless be borne in mind that estimation of the micronutrient intake is subject to an important misclassification error, something that would bias the estimators of risk toward the null hypothesis.
The two clinical trials assessing the effect of a low-fat and high-carbohydrate diet showed mixed results: while a significant reduction of MD two years after the intervention was seen in the first trial,24 the most recent one with greater sample size and longer follow-up did not find any effect.25 In our study, after adjusting for energy intake, no association was seen either with fat or carbohydrate consumption.
These results are based in the largest epidemiologic study on MD and diet reported to date. However, our study has also a series of limitations that must be borne in mind. Visual assessment of MD implies a certain degree of subjectivity. In our case, the reader was a experienced radiologist with good reproducibility.35 Furthermore, a recent study by members of our team using the same classification confirmed an increased risk of subsequent breast cancer in women classified in higher categories of MD.5 MD was measured on the basis of a mammogram obtained the same day on which the questionnaire was administered, which means that the diet-MD relationship was assessed by taking nutritional intake in the preceding year into account. The study's cross-sectional design limits the possibility of collecting data on consumption of foods at earlier stages of life. Furthermore, food and nutrient consumption was self-reported. It has been reported that interviewees may overestimate the level of consumption of foods which are socially viewed as healthy and underestimate those which are socially less acceptable.57 Even so, the intakes obtained with the FFQ used in our study have shown a reasonable consistency with the results obtained on the basis of four, weekly dietary records.28 The use of a different interviewer at each screening center could also introduce inter-center differences in data-collection, including the information requested in the FFQ. However, the results obtained take this type of variability into account, by including the screening center as a random effects term. Moreover, the inclusion of women from different geographical settings in the study is also a strength, as is the high participation rate. Indeed, the women in our study display sociodemographic and lifestyle characteristics similar to those seen in the Spanish National Health Survey.58
In conclusion, our results show that higher calorie intake is associated with higher MD, a finding in line with the results obtained by other studies. Furthermore, high consumption of olive oil decreases the prevalence of high MD, and our study thus supports the protective role of olive oil vis-à-vis breast cancer. This result is interesting, bearing in mind that our results are based on a population of women among whom consumption of olive oil is habitual. The protective effect is evident in women who report a higher consumption, which goes to reinforce both the existence of a risk gradient, and the interest that lies in increasing the intake of this food, an essential constituent of the Mediterranean diet.
Acknowledgments
The authors thank the participants in the DDM-Spain study for their contribution to breast cancer research.
Other members of DDM-Spain: Pablo Fernández-Navarro1,4, Anna Cabanes1,4, Gonzalo López-Abente1,4, Maria Pilar Moreno5, Mercé Peris6 Dolores Salas7,8, Josefa Miranda7,8, Francisco Ruiz-Perales7,8, María Ederra9,4, Milagros García9,4, Carmen Pedraz-Pingarrón10, Francisca Collado11, Jose Antonio Vázquez-Carrete12.
Glossary
- BMI
body mass index
- DDM-Spain
Determinants of Density in Spain
- FFQ
food frequency questionnaire
- HRT
hormonal replacement therapy
- MD
mammographic density, OR: Odds ratio
- 95% CI
95% Confidence Intervals
- SD
Standard deviation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
References
- 1.Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230:29–41. doi: 10.1148/radiol.2301020870. [DOI] [PubMed] [Google Scholar]
- 2.Torres-Mejia G, De Stavola B, Allen DS, Perez-Gavilan JJ, Ferreira JM, Fentiman IS, dos Santos Silva I. Mammographic features and subsequent risk of breast cancer: a comparison of qualitative and quantitative evaluations in the Guernsey prospective studies. Cancer Epidemiol Biomarkers Prev. 2005;14:1052–9. doi: 10.1158/1055-9965.EPI-04-0717. [DOI] [PubMed] [Google Scholar]
- 3.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 4.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 5.Pollan M, Ascunce N, Ederra M, Murillo A, Erdozain N, Ales-Martinez JE, Pastor-Barriuso R. Mammographic density and risk of breast cancer according to tumor characteristics and mode of detection: a Spanish population-based case-control study. Breast Cancer Res. 2013;15:R9. doi: 10.1186/bcr3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6:798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 7.Assi V, Warwick J, Cuzick J, Duffy SW. Clinical and epidemiological issues in mammographic density. Nat Rev Clin Oncol. 2012;9:33–40. doi: 10.1038/nrclinonc.2011.173. [DOI] [PubMed] [Google Scholar]
- 8.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res. 2011;13:223. doi: 10.1186/bcr2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lope V, Perez-Gomez B, Sanchez-Contador C, Santamarina MC, Moreo P, Vidal C, Laso MS, Ederra M, Pedraz-Pingarron C, Gonzalez-Roman I, Garcia-Lopez M, Salas-Trejo D, Peris M, Moreno MP, Vázquez-Carrete JA, Collado F, Aragonés N, Pollán M. Obstetric history and mammographic density: a population-based cross-sectional study in Spain (DDM-Spain) Breast Cancer Res Treat. 2012;132:1137–46. doi: 10.1007/s10549-011-1936-x. DDM-Spain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Duijnhoven FJ, Peeters PH, Warren RM, Bingham SA, van Noord PA, Monninkhof EM, Grobbee DE, van Gils CH. Postmenopausal hormone therapy and changes in mammographic density. J Clin Oncol. 2007;25:1323–8. doi: 10.1200/JCO.2005.04.7332. [DOI] [PubMed] [Google Scholar]
- 11.Masala G, Ambrogetti D, Assedi M, Giorgi D, Del Turco MR, Palli D. Dietary and lifestyle determinants of mammographic breast density. A longitudinal study in a Mediterranean population. 2006;118:1782–9. doi: 10.1002/ijc.21558. [DOI] [PubMed] [Google Scholar]
- 12.Brisson J, Verreault R, Morrison AS, Tennina S, Meyer F. Diet, mammographic features of breast tissue, and breast cancer risk. Am J Epidemiol. 1989;130:14–24. doi: 10.1093/oxfordjournals.aje.a115305. [DOI] [PubMed] [Google Scholar]
- 13.Nagata C, Matsubara T, Fujita H, Nagao Y, Shibuya C, Kashiki Y, Shimizu H. Associations of mammographic density with dietary factors in Japanese women. Cancer Epidemiol Biomarkers Prev. 2005;14:2877–80. doi: 10.1158/1055-9965.EPI-05-0160. [DOI] [PubMed] [Google Scholar]
- 14.Nordevang E, Azavedo E, Svane G, Nilsson B, Holm LE. Dietary habits and mammographic patterns in patients with breast cancer. Breast Cancer Res Treat. 1993;26:207–15. doi: 10.1007/BF00665798. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi SA, Couto E, Hilsen M, Hofvind S, Wu AH, Ursin G. Mammographic density and intake of selected nutrients and vitamins in Norwegian women. Nutr Cancer. 2011;63:1011–20. doi: 10.1080/01635581.2011.605983. [DOI] [PubMed] [Google Scholar]
- 16.Sala E, Warren R, Duffy S, Welch A, Luben R, Day N. High risk mammographic parenchymal patterns and diet: a case-control study. Br J Cancer. 2000;83:121–6. doi: 10.1054/bjoc.2000.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng M, Byrne C, Evers KA, Daly MB. Dietary intake and breast density in high-risk women: a cross-sectional study. Breast Cancer Res. 2007;9:R72. doi: 10.1186/bcr1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berube S, Diorio C, Masse B, Hebert-Croteau N, Byrne C, Cote G, Pollak M, Yaffe M, Brisson J. Vitamin D and calcium intakes from food or supplements and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14:1653–9. doi: 10.1158/1055-9965.EPI-05-0068. [DOI] [PubMed] [Google Scholar]
- 19.Berube S, Diorio C, Verhoek-Oftedahl W, Brisson J. Vitamin D, calcium, and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2004;13:1466–72. [PubMed] [Google Scholar]
- 20.Cabanes A, Pastor-Barriuso R, Garcia-Lopez M, Pedraz-Pingarron C, Sanchez-Contador C, Vazquez Carrete JA, Moreno MP, Vidal C, Salas D, Miranda-Garcia J, Peris M, Moreo P, Santamariña MC, Collado-García F, Gonzalez-Román I, Ascunce N, Pollan M. Alcohol, tobacco, and mammographic density: a population-based study. Breast Cancer Res Treat. 2011;129:135–47. doi: 10.1007/s10549-011-1414-5. DDM-Spain. [DOI] [PubMed] [Google Scholar]
- 21.Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States) Cancer Causes Control. 2000;11:653–62. doi: 10.1023/a:1008926607428. [DOI] [PubMed] [Google Scholar]
- 22.Voevodina O, Billich C, Arand B, Nagel G. Association of Mediterranean diet, dietary supplements and alcohol consumption with breast density among women in South Germany: a cross-sectional study. BMC Public Health. 2013;13:203. doi: 10.1186/1471-2458-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng M, Sellers TA, Vierkant RA, Kushi LH, Vachon CM. Mediterranean diet and breast density in the Minnesota Breast Cancer Family Study. Nutr Cancer. 2008;60:703–9. doi: 10.1080/01635580802233991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knight JA, Martin LJ, Greenberg CV, Lockwood GA, Byng JW, Yaffe MJ, Tritchler DL, Boyd NF. Macronutrient intake and change in mammographic density at menopause: results from a randomized trial. 1999;8:123–8. [PubMed] [Google Scholar]
- 25.Martin LJ, Greenberg CV, Kriukov V, Minkin S, Jenkins DJ, Yaffe M, Hislop G, Boyd NF. Effect of a low-fat, high-carbohydrate dietary intervention on change in mammographic density over menopause. 2009;113:163–72. doi: 10.1007/s10549-008-9904-9. [DOI] [PubMed] [Google Scholar]
- 26.Pollan M, Lope V, Miranda-Garcia J, Garcia M, Casanova F, Sanchez-Contador C, Santamarina C, Moreo P, Vidal C, Peris M, Moreno MP, Vazquez-Carrete JA, Collado F, Pedraz-Pingarrón C, Ascunce N, Salas-Trejo D, Aragonés N, Pérez-Gómez B, Ruiz-Perales F. Adult weight gain, fat distribution and mammographic density in Spanish pre- and post-menopausal women (DDM-Spain) Breast Cancer Res Treat. 2012;134:823–38. doi: 10.1007/s10549-012-2108-3. DDM-Spain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 28.Vioque J, Weinbrenner T, Asensio L, Castelló A, Young IS, Fletcher A. Plasma concentrations of carotenoids and vitamin C are better correlated with dietary intake in normal weight than overweight and obese elderly subjects. 2007;97:977–86. doi: 10.1017/S0007114507659017. [DOI] [PubMed] [Google Scholar]
- 29.Vioque J, Navarrete-Munoz EM, Gimenez-Monzo D, Garcia-de-la-Hera M, Granado F, Young IS, Ramon R, Ballester F, Murcia M, Rebagliato M, Iniguez C. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J. 2013;12:26. doi: 10.1186/1475-2891-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Department of Agriculture ARSUNDL. 2007. USDA national nutrient database for standard reference, release 20.
- 31.Centre d'Ensenyament Superior de Nutrició i Dietètica (CESNID) Madrid: McGraw-Hill; 2008. Tablas de composicion de alimentos por medidas caseras de consumo habitual en España. [Google Scholar]
- 32.Olivares AB, Bernal MJ, Ros G, Martinez C, Periago MJ. [Quality of data on folic acid content in vegetables included in several Spanish Food Composition Tables and new data on their folate content] Nutr Hosp. 2006;21:97–108. [PubMed] [Google Scholar]
- 33.Vicario IM, Griguol V, Leon-Camacho M. Multivariate characterization of the fatty acid profile of spanish cookies and bakery products. J Agric Food Chem. 2003;51:134–9. doi: 10.1021/jf0258297. [DOI] [PubMed] [Google Scholar]
- 34.Larqué E, Garaulet M, Pérez-Llamas F, Zamora S, Tebar Fj. Composición en ácidos grasos de las margarinas de mayor consumo en España y su importancia nutricional. Grasas y Aceites. 2003;54:65–70. [Google Scholar]
- 35.Garrido-Estepa M, Ruiz-Perales F, Miranda J, Ascunce N, Gonzalez-Roman I, Sanchez-Contador C, Santamarina C, Moreo P, Vidal C, Peris M, Moreno MP, Vaquez-Carrete JA, Collado-García F, Casanova F, Ederra M, Salas D, Pollán M. Evaluation of mammographic density patterns: reproducibility and concordance among scales. BMC Cancer. 2010;10:485. doi: 10.1186/1471-2407-10-485. DDM-Spain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 37.Harrell FE., Jr . General aspects of fitting regression models. relaxing linearity assumption for continuous predictors. In: Harrell FE Jr, editor. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. pp. 16–26. [Google Scholar]
- 38.Vachon CM, Kushi LH, Cerhan JR, Kuni CC, Sellers TA. Association of diet and mammographic breast density in the Minnesota breast cancer family cohort. Cancer Epidemiol Biomarkers Prev. 2000;9:151–60. [PubMed] [Google Scholar]
- 39.Mishra GD, dos Santos Silva I, McNaughton SA, Stephen A, Kuh D. Energy intake and dietary patterns in childhood and throughout adulthood and mammographic density: results from a British prospective cohort. Cancer Causes Control. 2011;22:227–35. doi: 10.1007/s10552-010-9690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2:133–40. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 41.Fair AM, Montgomery K. Energy balance, physical activity, and cancer risk. Methods Mol Biol. 2009;472:57–88. doi: 10.1007/978-1-60327-492-0_3. [DOI] [PubMed] [Google Scholar]
- 42.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 43.Chen D, Zhao H, Coon JS, Ono M, Pearson EK, Bulun SE. Weight gain increases human aromatase expression in mammary gland. Mol Cell Endocrinol. 2012;355:114–20. doi: 10.1016/j.mce.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizuno NK, Rogozina OP, Seppanen CM, Liao DJ, Cleary MP, Grossmann ME. Combination of intermittent calorie restriction and eicosapentaenoic Acid for inhibition of mammary tumors. Cancer Prev Res (Phila) 2013;6:540–7. doi: 10.1158/1940-6207.CAPR-13-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pelucchi C, Bosetti C, Negri E, Lipworth L, La VC. Olive oil and cancer risk: an update of epidemiological findings through 2010. Curr Pharm Des. 2011;17:805–12. doi: 10.2174/138161211795428920. [DOI] [PubMed] [Google Scholar]
- 46.Escrich E, Solanas M, Moral R, Escrich R. Modulatory effects and molecular mechanisms of olive oil and other dietary lipids in breast cancer. 2011;17:813–30. doi: 10.2174/138161211795428902. [DOI] [PubMed] [Google Scholar]
- 47.Ferrucci LM, Cross AJ, Graubard BI, Brinton LA, McCarty CA, Ziegler RG, Ma X, Mayne ST, Sinha R. Intake of meat, meat mutagens, and iron and the risk of breast cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Br J Cancer. 2009;101:178–84. doi: 10.1038/sj.bjc.6605118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho E, Chen WY, Hunter DJ, Stampfer MJ, Colditz GA, Hankinson SE, Willett WC. Red meat intake and risk of breast cancer among premenopausal women. Arch Intern Med. 2006;166:2253–9. doi: 10.1001/archinte.166.20.2253. [DOI] [PubMed] [Google Scholar]
- 49.Missmer SA, Smith-Warner SA, Spiegelman D, Yaun SS, Adami HO, Beeson WL, van den Brandt PA, Fraser GE, Freudenheim JL, Goldbohm RA, Graham S, Kushi LH, Miller AB, Potter JD, Rohan TE, Speizer FE, Toniolo P, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Hunter DJ. Meat and dairy food consumption and breast cancer: a pooled analysis of cohort studies. Int J Epidemiol. 2002;31:78–85. doi: 10.1093/ije/31.1.78. [DOI] [PubMed] [Google Scholar]
- 50.Ronco AL, DeStéfani E, Fabra A. White meat intake and the risk of breast cancer: a case-control study in Montevideo, Uruguay. Nutr Res. 2003;23:151–62. [Google Scholar]
- 51.Freedman ND, Cross AJ, McGlynn KA, Abnet CC, Park Y, Hollenbeck AR, Schatzkin A, Everhart JE, Sinha R. Association of meat and fat intake with liver disease and hepatocellular carcinoma in the NIH-AARP cohort. J Natl Cancer Inst. 2010;102:1354–65. doi: 10.1093/jnci/djq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talamini R, Polesel J, Montella M, Dal Maso L, Crispo A, Tommasi LG, Izzo F, Crovatto M, La Vecchia C, Franceschi S. Food groups and risk of hepatocellular carcinoma: a multicenter case-control study in Italy. Int J Cancer. 2006;119:2916–21. doi: 10.1002/ijc.22267. [DOI] [PubMed] [Google Scholar]
- 53.Prentice RL, Shaw PA, Bingham SA, Beresford SA, Caan B, Neuhouser ML, Patterson RE, Stefanick ML, Satterfield S, Thomson CA, Snetselaar L, Thomas A, Tinker LF. Biomarker-calibrated energy and protein consumption and increased cancer risk among postmenopausal women. Am J Epidemiol. 2009;169:977–89. doi: 10.1093/aje/kwp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su X, Tamimi RM, Collins LC, Baer HJ, Cho E, Sampson L, Willett WC, Schnitt SJ, Connolly JL, Rosner BA, Colditz GA. Intake of fiber and nuts during adolescence and incidence of proliferative benign breast disease. Cancer Causes Control. 2010;21:1033–46. doi: 10.1007/s10552-010-9532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;167:1050–9. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- 56.Bertone-Johnson ER, McTiernan A, Thomson CA, Wactawski-Wende J, Aragaki AK, Rohan TE, Vitolins MZ, Tamimi RM, Johnson KC, Lane D, Rexrode KM, Peck JD, Chlebowski RT, Sarto G, Manson JE. Vitamin D and calcium supplementation and one-year change in mammographic density in the women's health initiative calcium and vitamin D trial. Cancer Epidemiol Biomarkers Prev. 2012;21:462–73. doi: 10.1158/1055-9965.EPI-11-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hebert JR, Hurley TG, Peterson KE, Resnicow K, Thompson FE, Yaroch AL, Ehlers M, Midthune D, Williams GC, Greene GW, Nebeling L. Social desirability trait influences on self-reported dietary measures among diverse participants in a multicenter multiple risk factor trial. 2008;138:226S–34S. doi: 10.1093/jn/138.1.226S. [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Arenzana N, Navarrete-Munoz EM, Peris M, Salas D, Ascunce N, Gonzalez I, Sanchez-Contador C, Santamarina C, Moreo P, Moreno MP, Carrete JA, Collado-Garcia F, Pedraz-Pingarrón C, Ederra M, Miranda-García J, Vidal C, Aragonés N, Pérez-Gómez B, Vioque J, Pollán M. Diet quality and related factors among Spanish female participants in breast cancer screening programs. Menopause. 2012;19:1121–9. doi: 10.1097/gme.0b013e3182544925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information