Abstract
Hepatitis C virus (HCV) vaccines may be able to increase viral clearance in combination with antiviral therapy. We analysed viral dynamics and HCV-specific immune response during retreatment for experienced patients in a phase Ib study with E1E2MF59 vaccine. Seventy-eight genotype 1a/1b patients [relapsers (30), partial responders (16) and nonresponders (32) to interferon-(IFN)/ribavirin-(RBV)] were randomly assigned to vaccine (V:23), Peg-IFNα2a-180-ug/qw and ribavirin 1000–1200-mg/qd for 48 weeks (P/R:25), or their combination (P/R + V:30). Vaccine (100 μg/0.5 mL) was administered intramuscularly at week 0-4-8-12-24-28-32-36. Neutralizing of binding (NOB) antibodies and lymphocyte proliferation assay (LPA) for E1E2-specific-CD4 + T cells were performed at week 0-12-16-48. Viral kinetics were analysed up to week 16. The vaccine was safe, and a sustained virological response (SVR) was achieved in 4 P/R + V and 2 P/R patients. Higher SVR rates were observed in prior relapsers (P/R + V = 27.3%; P/R = 12.5%). Higher NOB titres and LPA indexes were found at week 12 and 16 in P/R + V as compared to P/R patients (P = 0.023 and 0.025, P = 0.019 and <0.001, respectively). Among the 22 patients with the strongest direct antiviral effects of IFN (ε ≥ 0.800), those treated with P/R + V (10) reached lower HCV-RNA levels (P = 0.026) at week 16. HCV E1E2MF59 vaccine in combination with Peg-IFNα2a + RBV was safe and elicited E1E2 neutralizing antibodies and specific CD4 + T cell proliferation. Upon early response to IFN, vaccinations were associated with an enhanced second phase viral load decline. These results prompt phase II trials in combination with new antiviral therapies.
Keywords: HCV, immune response, interferon, neutralizing antibodies, vaccine, viral kinetics
Introduction
Hepatitis C virus (HCV) is an enveloped virus of the flaviviridae family which contains a single stranded plus-sense RNA genome with a single open reading frame [1]. The polyprotein is cleaved by host and viral proteases to produce structural proteins (core and envelope glycoproteins E1 and E2) and nonstructural (NS) components, among which NS3 (serine protease and RNA helicase), NS5A and NS5B (RNA-dependent RNA polymerase) represent the major targets of the recently developed direct acting antivirals (DAAs) [2–7].
Approximately 75% of acute infections become chronic, and 20% of these progress to cirrhosis [8,9], making HCV the most common viral disease in patients undergoing liver transplantation [10]. Due to the complex mechanism of HCV entry and the high variability of the E2 protein, the development of an effective vaccine against HCV is still underway. In the chimpanzee model, an adjuvanted prototype vaccine containing E1E2 envelope proteins was shown able to modify the natural course of the infection [11,12]. Animals which developed high antibody titres were protected against acute infection following homologous virus challenge and those with low anti-E1E2 antibody titres showed a significantly higher rate of viral clearance as compared to nonvaccinated controls [11,12]. Interestingly, protection against chronic evolution was not strongly correlated with the anti-E1E2 antibody titres, suggesting an immune control mediated by the combination of humoral and CD4 + T helper responses to E1E2 [13].
Despite the high rate of sustained virological response (SVR) obtained by the combination of DAAs with Pegylated interferons (Peg-IFN) and ribavirin (RBV) in naïve genotype one patients [14], retreatment with currently available triple therapy in prior partial or null responders to Peg-IFN/RBV does not warrant the same chances of SVR [15]. Indeed, in patients with insufficient response to IFN, the initial rapid antiviral effect of the DAAs is neutralized by the emergence of viral quasi-species with various degree of resistance responsible for treatment failure. Viral dynamics studies showed that whenever the block of HCV production is not complete, the immune-mediated clearance of the infected cells and virus infectivity become major determinants of the treatment outcome [16,17].
Recently, the E1-E2 HCV vaccine candidate was shown to be safe and generally well tolerated in the first clinical trial in healthy volunteers [18]. Based on the hypothesis that the humoral and cellular responses observed in noninfected individuals could contribute to HCV clearance in chronic patients, we performed a phase Ib trial aimed to assess the safety of this vaccine and to test whether the above responses could be elicited during the antiviral treatment in chronic hepatitis C patients.
Patients and Methods
Study design and conduct
This phase Ib open-label, randomized, multicentre, active-treatment-controlled study was aimed to evaluate safety, tolerability, viral kinetics and pattern of immune response to HCV vaccine alone or combined with Peg-IFNa2a plus ribavirin in patients chronically infected with HCV genotype 1a or 1b without signs of liver decompensation, portal hypertension or hepatocellular carcinoma. The study was approved by the phase I study commission of the Istituto Superiore della Sanità of the Italian Ministry of Health and from the central (Milano) and local Ethical Committees (Bologna, Palermo, Pisa, Roma and Napoli) of the participant centres. A written informed consent was obtained prior to any study procedure. Inclusion criteria required that patients who failed treatment could be classified according to the prior antiviral response as nonresponders (NR <2 log HCV-RNA decline at week 12), partial responders (PR >2 log HCV-RNA decline at week 12 but detectable HCV-RNA at week 24) or relapsers (REL = undetectable HCV-RNA at the end of therapy not maintained thereafter). Eligible subjects stratified for their prior antiviral response were randomized separately within each study centre in a 1:1:1 ratio to receive: Group 1 (V) eight 100 μg doses of HCV E1E2MF59 vaccine (0.5 mL total volume) by intramuscular (deltoid) injections at week 0 (baseline), 4, 8, 12, 24, 28, 32, 36; Group 2 (P/R): Peg-IFNa2a 180 μg weekly by subcutaneous injections and ribavirin 1000 mg (weight <75 kg) or 1200 mg (weight ≥75 kg) per os bid for 48 weeks and Group 3 (P/R + V): P/R combined with HCV E1E2MF59 vaccine as in Group 1.
At each study visit (screening, day: 0, 2 and 4, week: 1, 2, 3, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44 and 48 during therapy, week: 1, 2, 4, 8, 12 and 24 after therapy), blood samples were obtained for haematology, biochemistry, serology and (auto) immunology according to the protocol. Peg-IFNa2a and ribavirin in Groups 2 and 3 were discontinued in absence of EVR (week 12 HCV-RNA decline from baseline <2 log) or in presence of detectable HCV-RNA at week 24.
Assays
Alanine aminotransferase (ALT) and HCV-RNA serum levels were measured at each study visit during treatment and follow-up. Quantification of HCV-RNA for viral kinetics was obtained by Roche Cobas Taqman® assay (dynamic range: 15–70 000 000 IU/mL) testing all frozen sera (−80 °C) from one patient in a single run at the centralized reference laboratory (Hepatology Unit – University Hospital of Pisa).
The presence HCV antibodies with potential neutralizing activity was investigated by the NOB assay that estimates by cytofluorimetry the binding of serum gamma-globulins to human cells coated with HCV envelope glycoprotein 2 (E2) [19]. The E2 antigen employed in this binding assay was produced by the same system and purified in the same manner as the antigen used for immunization. Sera were tested at baseline, week 12, 16 and 48. A CD4 T cell lymphocyte proliferation assay (LPA) for E1E2-specific CD4+ T cells was performed at the same time points in a subgroup of Group 2 and 3 patients using a 3H thymidine incorporation assay [18]. CHO expressed HIV-gp120 and phytohemagglutinin (PHA) were used as negative and positive controls, respectively. The results were expressed as stimulation index (S.I.), the mean counts per minute (cpm) of duplicate stimulated cell cultures divided by mean cpm of duplicate unstimulated cell cultures. Stimulation index corresponding at value one is the lower limit of LPA.
Viral kinetics model
To analyse individual viral and infected cell dynamics, we applied a physic–mathematical model that fits both HCV-RNA and ALT declines during the first month of therapy [16]. A detailed explanation of the method used to compute parameters is available online (http://www.nature.com/clpt/journal/v84/n2/extref/clpt200821x1.doc) as supporting information of our previous publication [20].
Statistical analysis
The distribution of the variables according to treatment and outcome was analysed using corrected chi-square for qualitative categories. Analysis of variance (ANOVA), Mann–Whitney U-test or Kruskal–Wallis tests were used for the quantitative variables. All the other tests were default parameters of the statistical software package SPSS (version 19.0, SPSS Inc., Chicago, IL, USA). The associations found were considered statistically significant for P value <0.05.
Results
Safety
Overall, 78 patients were enrolled in this study from January 2005 to June 2008: 23 were randomized to receive the vaccine alone (Group 1), 25 to P/R (Group 2) and 30 to P/R + V (Group 3). Serious adverse events (SAE) occurred in four patients. Colon cancer and hepatocellular carcinoma were diagnosed after 4 and 12 weeks of therapy, respectively, in two Group 3 patients. Both SAEs were judged not related to the therapy, and patients were withdrawn from the study to start appropriate treatments. Two patients had pneumonia, one during the screening period, the other after 9 months of Peg-IFN/RBV therapy and seven vaccine injections. Complete resolution was obtained after 2 weeks by antibiotic treatment in both patients. In the latter, the SAE was considered possibly related to Peg-IFN and not to the vaccine by site investigators and medical monitor. During the 72-week study period, the prevalence of nonserious adverse events (percentage of visits with at least one AE reported) was similar in patients who received P/R (73%) or P/R + V (78%) and lower (35%) in those who received vaccine alone. Most frequently reported symptoms across all vaccinations included mild or moderate fever, discomfort, headache, myalgia and pain/tenderness at the vaccination site. Other typical local reactions (redness, pain or tenderness and warmth) and systemic reactions (fever, malaise, myalgia, arthralgia, headache, nausea and fatigue) were reported and attributed to Peg-IFN or RBV therapy. None of the patients treated with the vaccine alone or in combination with P/R had the induction of autoimmune phenomena.
Treatment response
Response to prior and investigational treatments is summarized in Table 1. None of the 23 patients who received HCV E1E2MF59 vaccine alone cleared HCV, nor showed >1 log HCV-RNA decline at week 24 and 48. Peg-IFN/RBV antiviral treatment was completed according to the protocol in 24/25 Group 2 patients (1 drop out) and in 24/30 Group 3 patients (2 SAE, 4 did not take the vaccine for a procedural error). Two (8%) patients treated with P/R (1 prior NR and 1 REL) and 4 (16%) patients treated with P/R + V (1 prior NR and 3 REL) became SVR. The rate of SVR among patients with a previous relapse was higher with P/R + V (27.3%) than with P/R alone (12.5%), although the difference did not reach statistical significance (P = 0.173).
Table 1.
Response to prior and investigational treatments
| Patients by study treatment | Per protocol response in patients who completed the antiviral therapy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Prior therapy outcome | V | P/R | P/R + V | P/R | EVR (%) | SVR (%) | P/R + V | EVR (%) | SVR (%) |
| NR | 11 | 11 | 9 | 10 | 6 (60%) | 1 (10%) | 9 | 3 (33%) | 1 (11%) |
| PR | 5 | 6 | 5 | 6 | 4 (66%) | 0 | 4 | 3 (75%) | 0 |
| REL | 7 | 8 | 12 | 8 | 7 (87.5%) | 1 (12.5%) | 11 | 9 (82%) | 3 (27%) |
| Total | 23 | 25 | 26 | 24 | 17 (71%) | 2 (8%) | 24 | 15 (63%) | 4 (17%) |
EVR, early virological response (undetectable or HCV-RNA decline ≥2 Log from baseline at week 12). SVR, sustained virological response.
Immune response
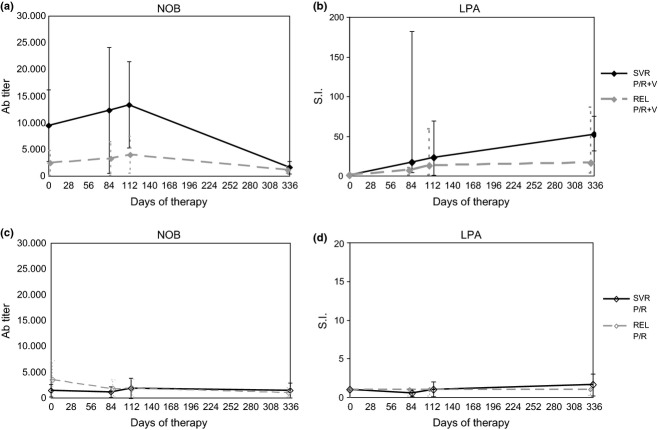
At baseline, the titre of anti-HCV E1E2 antibodies measured by the NOB assay, although higher in P/R + V (7263 ± 11130) than in P/R (3362 ± 5764) and V (3342 ± 4672) patients, was not significantly different between the three arms (ANOVA, P = 0.417). Considering only P/R and P/R + V patients, the median titre of the NOB assay at baseline was significantly higher in SVR (5545; range: 460–16200) and REL (2430; range: 130–37500) than in PR (440; range: 130–18320) and NR (260; range: 90–5930) (Kruskal–Wallis: P = 0.029). During therapy, the NOB titres decreased in P/R but not in P/R + V-treated patients, reaching significantly lower levels at week 12 (2172 ± 3109 vs 8979 ± 13228, P = 0.023) and 16 (2005 ± 2100 vs 7578 ± 11051, P = 0.025). At the end of therapy (week 48), the NOB titres decreased also in P/R + V patients to levels not significantly different from those of P/R-treated patients (Table 2). In vaccine alone-treated patients, the NOB titres did not show significant changes. The LPA index increased to significantly higher levels in P/R + V as compared to P/R alone-treated patients at week 12 (24.0 ± 40.7 vs 1.12 ± 0.79, P = 0.019) and 16 (34.1 ± 33.9 vs 2.43 ± 5.02, P < 0.001) (Table 2). The kinetics of NOB titre and LPA index during P/R + V therapy showed different profiles in SVR as compared to REL patients (Fig. 1); the difference, however, did not reach statistical significance due to the low number of cases and the high degree of the individual variability. On the contrary, in P/R-treated patients, no changes in NOB titres and LPA index were observed (Fig. 1).
Table 2.
Neutralizing antibodies by quantitative neutralization of binding (NOB) assay and specific E1E2-CD4 + T cells by lymphocyte proliferation assay (LPA) during therapy
| Group of patients (number) | Basal NOB titre | Week 12 NOB titre | Week 16 NOB titre | Week 48 NOB titre | Basal LPA index | Week 12 LPA index | Week 16 LPA Index |
|---|---|---|---|---|---|---|---|
| V (21) | |||||||
| Mean | 3362 | 2541 | 3602 | na | na | na | na |
| SD | 5764 | 2476 | 4524 | na | na | na | na |
| P/R (22) | |||||||
| Mean | 3342 | 2172 | 2005 | 1895 | 1.32 | 1.12 | 2.43 |
| SD | 4672 | 3109 | 2100 | 2207 | 1.06 | 0.79 | 5.02 |
| P/R + V (22) | |||||||
| Mean | 7263 | 8979 | 7578 | 3710 | 1.21 | 24.0 | 34.1 |
| SD | 11130 | 13228 | 11051 | 4788 | 0.54 | 40.7 | 33.9 |
| P/R vs P/R + V* | |||||||
| P value | 0.135 | 0.023 | 0.025 | 0.189 | 0.701 | 0.019 | <0.001 |
na, not available; *ANOVA.
Bold values indicate significant values (P < 0.05).
Fig. 1.
Kinetics of anti-HCV immune responses. Panels a and b show mean ± SD NOB antibody titre and median with range lymphocyte proliferation assay (LPA) stimulation index (S.I.) at baseline (day 0), week 12 (day 84), 16 (day 112) and 48 (day 336) in 4 SVR and in 10 REL patients treated with P/R + V. Panels c and d show mean ± SD NOB antibody titre and median with range lymphocyte proliferation assay (LPA) stimulation index (S.I.) at baseline (day 0), week 12 (day 84), 16 (day 112) and 48 (day 336) in 2 SVR and in 10 REL patients treated with P/R.
Viral dynamics
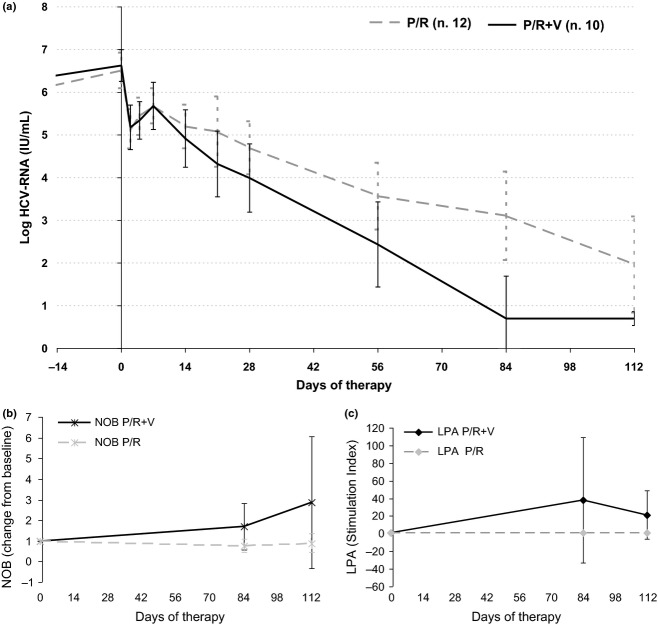
Viral kinetics could be analysed by the model in 44 patients who completed the protocol, 22 (91.7%) treated with P/R and in 22 (91.7%) treated with P/R + V (Table 3). Therapy outcome was significantly correlated with the values of the parameters ε (P = 0.010), π (P < 0.001) and γ (P = 0.001), describing the strength of the antiviral effects of Peg-IFN/RBV therapy during the biphasic decline in the viral load, and with the end of therapy estimates of HCV infected cells Ieot (P = 0.006) and of residual viral load Veot (P = 0.001). Given the major role played by the direct antiviral activity of Peg-IFN/RBV therapy, the potential impact of the first course of vaccinations (week 0-4-8-12) was analysed in patients with comparable effectiveness of Peg-IFN/RBV therapy. Dichotomization of Group 2 and 3 patients by the median value of ε (0.800) showed that in 22 patients (12 P/R and 10 P/R + V) with ε ≥0.800, the log HCV-RNA levels at week 16 were significantly lower in those treated with P/R + V than in those treated with P/R alone (Mann–Whitney U-test, P = 0.026). The kinetics of the viral load decline with the changes in the NOB titre and of the LPA index in these patients is shown in Fig. 2.
Table 3.
Baseline variables and model-computed parameters describing the dynamics of HCV infection during therapy according to treatment arm and response to antiviral therapy
| Group of patients (n.) | Basal ALT U/L | Basal HCV RNA Log IU/mL | ε | π | γ | δ0 | Log Ieot | Log Veot |
|---|---|---|---|---|---|---|---|---|
| P/R (22) | ||||||||
| Mean | 82.7 | 6.3 | 0.6611 | 0.1064 | 0.5562 | 0.0421 | 5.81 | 4.61 |
| SD | 50.2 | 0.6 | 0.5454 | 0.0934 | 1.0345 | 0.0337 | 6.17 | 5.14 |
| P/R + V (22) | ||||||||
| Mean | 98.7 | 6.5 | 0.7153 | 0.0924 | 0.5306 | 0.0346 | 5.21 | 4.14 |
| SD | 57.0 | 0.5 | 0.2667 | 0.0602 | 1.6283 | 0.0229 | 5.47 | 4.58 |
| P value* | 0.25 | 0.18 | 0.77 | 0.53 | 0.99 | 0.44 | 0.15 | 0.40 |
| NR (14) | ||||||||
| Median | 96 | 6.3 | 0.5950 | 0.0241 | 0.7400 | 0.0144 | 5.53 | 4.34 |
| Range | 52–185 | 5.2–6.9 | −0.09–0.94 | 0.004–0.07 | 0.023–8.07 | 0.003–0.068 | 3.3–6.6 | −2.6–5.8 |
| PR (9) | ||||||||
| Median | 61 | 6.9 | 0.7700 | 0.0691 | 0.0096 | 0.0399 | 3.11 | 1.64 |
| Range | 46–226 | 6.0–7.6 | 0.48–0.95 | 0.040–0.24 | 3·10−4–0.93 | 0.005–0.075 | 2.0–6.1 | −0.3–3.6 |
| REL (15) | ||||||||
| Median | 65 | 6.4 | 0.9050 | 0.1200 | 0.0032 | 0.0351 | 3.44 | −0.45 |
| Range | 35–234 | 5.2–7.2 | 0.52–0.999 | 0.064–0.23 | 2·10−5–0.98 | 0.001–0.14 | 0.3–6.7 | −3.4–3.0 |
| SVR (6) | ||||||||
| Median | 77.5 | 6.3 | 0.7404 | 0.1910 | 0.0002 | 0.0629 | 2.47 | −0.88 |
| Range | 38–220 | 5.5–7.4 | −1.58–0.94 | 0.085–0.44 | 4·10−6–0.81 | 0.007–0.10 | 1.7–5.5 | −4.1–2.4 |
| P value† | 0.35 | 0.083 | 0.010 | <0.001 | 0.001 | 0.080 | 0.006 | 0.001 |
ε = effectiveness of IFN in blocking viral production; π = 2nd phase HCV-RNA decay rate constant; γ = asymptotic value of the viral production coefficient during therapy; δ0 = infected cell clearance rate constant; I0 = computed infected cell number at baseline; Ieot = computed infected cell number at the end of therapy; Veot = computed viral load at the end of therapy; * ANOVA; † Kruskal–Wallis (comparison between the four categories).
Bold values indicate significant values (P < 0.05).
Fig. 2.
Viral kinetics and anti-HCV immune responses. Panel a shows median log HCV-RNA (±95% CI) decline during the first 16 weeks (day 112) of therapy in 12 P/R and 10 P/R + V patients with higher and homogeneous block of virus production (ε ≥0.800). For undetectable HCV-RNA levels (<15 IU/mL), the attributed value of 5 IU/mL (0.70 log) was used for computing the median value. Panel b shows mean ± SD NOB antibody titre variations (fold change from baseline) at week 12 and 16 in the same patients. Panel c shows mean ± SD LPA stimulation index (S.I.) variations at week 12 and 16 in the same patients.
Characterization of the IL28B polymorphism showed that the favourable CC genotype was present in only three patients per arm, and in none of the SVR patients (3 CT and 3 TT). In CC patients, there was a trend towards a more effective inhibition of viral replication (ε median value: CC = 0.945, CT = 0.880, TT = 0.680; P = 0.073, Kruskal–Wallis).
Discussion
The results of the phase Ib study of the candidate HCV E1E2 vaccine adjuvanted with MF59 in chronic hepatitis C patients who failed previous interferon and ribavirin therapy showed that HCV E1E2 vaccine is safe either alone or in combination with peginterferon-alpha-2a and ribavirin. Only mild–moderate local and/or systemic reactions related to the vaccine injections were reported. Given alone the vaccine did not induce significant changes in viral load, however, the combination of Peg-IFN/RBV (P/R) with the vaccine (V) was associated with a higher SVR rate (17% vs 8%). The SVR rate was influenced by the type of response to the previous treatment, being higher in prior relapsers (27.3% vs 12.5%). Although the outcome was twice as better in P/R + V than in P/R alone, this difference was not statistically significant (corrected chi-square: P = 0.173), possibly because of the low number of patients enrolled to study the safety and not the efficacy of HCV-E1E2 vaccine. Interestingly, the immunological assays showed relevant vaccine induced responses. he analysis of a surrogate assay for neutralizing antibody (NOB) in the overall cohort of patients receiving antiviral treatment showed that baseline NOB titre positively correlates with better outcomes. During therapy, NOB titres decreased in P/R-treated patients but not in P/R + V, reaching a significant difference between the two arms at week 12 and 16 (Table 2). In addition, HCV-specific CD4 responses significantly increased in the P/R + V-treated patients (Table 2) and reached the highest levels in SVR patients (Fig. 1). The difference between SVR and REL patients was not statistically significant, probably because of the high degree of individual variability observed and the low number of patients that could be studied. We can also hypothesize that the variability of the responses might be related to the abnormalities of the T cell functions found in chronically infected patients [22]. Indeed, HCV was shown to induce a state of T cell exhaustion that was attributed to a defective activation of the dendritic cells [23–25], not present in all patients [26].
Altogether these findings underlie the role of the specific immune responses during therapy and are conceivable with our model of viral dynamics [17,20,21], which simulates the interplay between the virus and the immune system during IFN/RBV therapy assuming that the reduction in the infected cell number during therapy leads to a relative reduction in the CTL clearance activity. In turn, this negative feedback may favour the persistence of infected cells that explain hepatitis recurrence after therapy in relapser patients. The new direct antiviral agents (DAAs) block viral replication more efficiently than IFN; however, they are not expected to enhance adaptive immune responses. Thus, patients in whom these drugs are not completely effective could benefit from the combination of a therapeutic vaccine.
To analyse the potential additional effect of the HCV E1E2 vaccine in patients with higher and homogeneous block of virus production, the subgroup of 22 patients (12 P/R and 10 P/R + V) with ε above the median value of 0.800 was analysed separately. Interestingly, the vaccine combination brought viral load to significantly lower levels at week 16, 4 weeks after the end of the first course of vaccine injections. The faster decline in the viral load, together with the evidence of a relative increase in NOB titre and LPA index (Fig. 2), supports the hypothesis of a potential effect of the vaccine in this subset of patients. Therefore, from the analysis of viral dynamics, it is possible to hypothesize that the increased titres of anti-HCV-E1E2 antibodies and the increased cellular responses observed in P/R + V-treated patients may translate in a more efficient control of the infection either by further reduction in target cell infections and/or residual viral production.
Finally, not surprisingly, the analysis of the rs12979860 SNP upstream to the IL28B gene [27] showed that the favourable CC allele was present in a small proportion (13%) of our treatment experienced patients. The CC allele was equally distributed in the two arms, and it was associated with a trend to a greater 1st phase direct antiviral effectiveness of Peg-IFN/RBV, but not with SVR. Nowadays, DAAs warrant an improved inhibition of viral replication but still need combination with P/R to prevent resistance. Our study let hypothesize that whenever IFN free schedules will be available, HCV vaccines might become a useful combination in a subgroup of patients to replace the nonspecific immune modulation activity of IFN with a more specific stimulation of anti-HCV adaptive responses. These results prompt future studies with candidate HCV vaccines in combination with new DAAs in difficult to treat patients.
Acknowledgments
We thank Dr. Massimo Sarracino for his support in providing the drugs Pegasys and Copegus (Roche, Monza, Italy), Dr. Antonella Colucci for her excellent assistance in the preparation and submission to regulatory authorities of the study protocol and in the administrative management of the clinical trial and all the patients for their contribution.
Glossary
- ALT
alanine aminotransferase
- HCV
hepatitis C virus
- IFN
interferon
- LPA
lymphocyte proliferation assay
- NOB
neutralizing of binding
- NR
nonresponders
- PR
partial responders
- RBV
ribavirin
- REL
relapsers
- SAE
Serious adverse events
- SVR
sustained virological response
Conflict of Interest
Authors who are not employees of Novartis have not any conflict of interest related to this study other that the financial support outlined below.
Financial Support
The study was supported in part by an educational grant of the Italian Ministry of Health ‘Viral hepatitis emergency: surveillance and management of antiviral resistance to therapy’- n.85 2006. Novartis provided the HCV E1E2-MF59 vaccine and Roche (Monza, Italy) provided the standard of care treatment, peginterferon-alpha-2a (Pegasys) and ribavirin (Copegus).
References
- 1.Choo QL, Richman KH, Han JH, et al. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA. 1991;88:2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartenschlager R, Frese M, Pietschmann T. Novel insights into hepatitis C virus replication and persistence. Adv Virus Res. 2004;63:71–180. doi: 10.1016/S0065-3527(04)63002-8. [DOI] [PubMed] [Google Scholar]
- 3.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 4.Weiner AJ, Brauer MJ, Rosenblatt J, et al. Variable and hypervariable domains are found in the regions of HCV corresponding to the flavivirus envelope and NS1 proteins and the pestivirus envelope glycoproteins. Virology. 1991;180(2):842–848. doi: 10.1016/0042-6822(91)90104-j. [DOI] [PubMed] [Google Scholar]
- 5.Weiner AJ, Geysen HM, Christopherson C, et al. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc Natl Acad Sci USA. 1992;89(8):3468–3472. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato N, Ootsuyama Y, Sekiya H, et al. Genetic drift in hypervariable region 1 of the viral genome in persistent hepatitis C virus infection. J Virol. 1994;68(8):4776–4784. doi: 10.1128/jvi.68.8.4776-4784.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunetto MR, Suzuki T, Aizaky H, et al. Variations in the hypervariable region 1 of the envelope region E2 of hepatitis C virus RNA appear associated with virus persistence independently of liver disease. Ital J Gastroenterol. 1996;28(9):499–504. [PubMed] [Google Scholar]
- 8.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 9.Fattovich G, Giustifna G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterol. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 10.Kim WR, Terrault NA, Pedersen RA, et al. Trends in waiting list registration for liver transplantation for viral hepatitis in the United States. Gastroenterology. 2009;137(5):1680–1686. doi: 10.1053/j.gastro.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choo QL, Kuo G, Ralston R, et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci. 1994;91:1294–1298. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrignani S, Houghton M, Hsu HH. Perspectives for a vaccine against hepatitis C virus. J Hepatol. 1999;31(S1):259–263. doi: 10.1016/s0168-8278(99)80413-9. [DOI] [PubMed] [Google Scholar]
- 13.Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436:961–966. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- 14.Hoofnagle JH. A step forward in therapy for hepatitis C. N Engl J Med. 2009;360(18):1899–1901. doi: 10.1056/NEJMe0901869. [DOI] [PubMed] [Google Scholar]
- 15.McHutchison JG, Manns MP, Muir AJ, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362(14):1292–1303. doi: 10.1056/NEJMoa0908014. [DOI] [PubMed] [Google Scholar]
- 16.Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-a therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 17.Colombatto P, Civitano L, Oliveri F, et al. Sustained response to interferon-ribavirin combination therapy predicted by a model of hepatitis C virus dynamics using both HCV RNA and alanine aminotransferase. Antivir Ther. 2003;8(6):519–530. [PubMed] [Google Scholar]
- 18.Frey SE, Houghton M, Coates S, et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine. 2010;28(38):6367–6373. doi: 10.1016/j.vaccine.2010.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosa D, Campagnoli S, Moretto C, et al. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc Natl Acad Sci USA. 1996;93(5):1759–1763. doi: 10.1073/pnas.93.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colombatto P, Ciccorossi P, Maina AM, et al. Early and accurate prediction of Peg-IFNs/ribavirin therapy outcome in the individual patient with chronic hepatitis C by modeling the dynamics of the infected cells. Clin Pharmacol Ther. 2008;84(2):212–215. doi: 10.1038/clpt.2008.21. [DOI] [PubMed] [Google Scholar]
- 21.Brunetto MR, Colombatto P, Bonino F. Bio-mathematical models of viral dynamics to tailor antiviral therapy in chronic viral hepatitis. World J Gastroenterol. 2009;15(5):531–537. doi: 10.3748/wjg.15.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torresi J, Johnson D, Wedemeyer H. Progress in the development of preventive and therapeutic vaccines for hepatitis C virus. J Hepatol. 2011;54(6):1273–1285. doi: 10.1016/j.jhep.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 23.Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97(10):3171–3176. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 24.Dolganiuc A, Chang S, Kodys K, et al. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177(10):6758–6768. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- 25.Shiina M, Rehermann B. Cell culture-produced hepatitis C virus impairs plasmacytoid dendritic cell function. Hepatology. 2008;47(2):385–395. doi: 10.1002/hep.21996. [DOI] [PubMed] [Google Scholar]
- 26.Piccioli D, Tavarini S, Nuti S, et al. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J Hepatol. 2005;42(1):61–67. doi: 10.1016/j.jhep.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Lindh M, Lagging M, Arnholm B, et al. IL28B polymorphisms determine early viral kinetics and treatment outcome in patients receiving peginterferon/ribavirin for chronic hepatitis C genotype 1. J Viral Hepat. 2011;18(7):325–331. doi: 10.1111/j.1365-2893.2010.01425.x. [DOI] [PubMed] [Google Scholar]