Abstract
Objective Tested two family-based behavioral treatments for obesity in preschool children, one meeting the Expert Committee guidelines for Stage 3 obesity intervention criteria (LAUNCH-clinic) and one exceeding Stage 3 (LAUNCH with home visit [LAUNCH-HV]), compared with a Stage 1 intervention, pediatrician counseling (PC). Methods In all, 42 children aged 2–5 years with a body mass index (BMI) percentile of ≥95th were randomized. A total of 33 met intent-to-treat criteria. Assessments were conducted at baseline, Month 6 (posttreatment), and Month 12 (6-month follow-up). Results LAUNCH-HV demonstrated a significantly greater decrease on the primary outcome of change in BMI z-score (BMIz) pre- to posttreatment compared with PC (p = .007), whereas LAUNCH-clinic was not significantly different from PC (p = .08). Similar results were found for secondary outcomes. Conclusions LAUNCH-HV, but not LAUNCH-clinic, significantly reduced BMIz compared with PC by posttreatment, indicating the need for intensive behavioral intervention, including home visitation, to address weight management in obese preschool children.
Keywords: home visits, obesity, preschoolers, treatment
Dramatic increases in the prevalence of obesity among preschoolers parallels trends among school-age children and adolescents from the 1970s to its peak at 13.9% in 2003–2004 (Ogden et al., 2006). While epidemiological data since this time suggests a plateau, the prevalence of obesity among preschoolers continues to be 12.1% (Ogden, Carroll, Kit, & Flegal, 2012). Preschoolers with obesity have a greater incidence of health risks (Williams, Strobino, Bollella, & Brotanek, 2004), more behavior problems (Datar, Sturm, & Magnabosco, 2004), and lower health-related quality of life (Kuhl, Rausch, Varni, & Stark, 2012) than children who are ≤95th body mass index (BMI) percentile. Further, children who are obese during the preschool years are unlikely to “outgrow” excess weight. Two recent studies show that children who are ≥ the 95th percentile BMI between ages 2 and 5 years are 5 (if obese at age 2 years) to 47 times (if obese at age 5 years) more likely to remain overweight at later ages (Nader et al., 2006) compared with a 6% probability of being overweight for children who were at the 50th percentile BMI (Cunningham, Kramer, & Narayan, 2014). Explicitly targeting weight control in early childhood is crucial to modifying weight and health trajectories for preschoolers who are obese.
An expert committee representing 15 national health-care organizations was convened by the American Medical Association, Health Resources and Service Administration, and the Centers for Disease Control to develop practical guidelines for practitioners in the assessment and treatment of overweight and obesity in children aged 2–18 years (Barlow, 2007). Resulting guidelines recommended a staged intervention approach based on child age, BMI, parent weight status, and comorbidities (Barlow, 2007). Stage 1, Prevention Plus, is brief counseling on basic healthy lifestyle eating and activity delivered in the pediatrician office, where the family determines the lifestyle behaviors to prioritize and the frequency of follow-up visits. Stage 2, Structured Weight Management, is distinguished from Stage 1 by additional structure and support that may include specific eating and/or activity goals in addition to Stage 1 dietary guidelines and dietitian support. The number of visits are not specified, but suggested to occur monthly in the primary care office. Stage 3, Comprehensive Multidisciplinary Intervention, is designed to provide the maximum support and structure for families to make diet and activity changes (e.g., food monitoring, goal setting). Stage 3 is acknowledged to exceed the capacity of a pediatrician office and includes involvement of a behavioral counselor and 8–12 weekly visits followed by monthly visits.
A gap in the Expert Committee guidelines is that recommendations are informed by research conducted with older children and adolescents (Barlow, 2007). Several developmental barriers unique to preschoolers may challenge parental efforts to modify lifestyle behaviors and may warrant more intensive intervention. Specifically, food neophobia, or unwillingness to try new foods, peaks in this age-group (Addessi, Galloway, Visalberghi, & Birch, 2005) particularly for vegetables (Phillips & Kolasa, 1980). While it takes ∼10 (Birch & Marlin, 1982) to 15 (Sullivan & Birch, 1990) exposures for a child to accept a new food, parents typically only offer new foods 3–5 times (Carruth, Ziegler, Gordon, & Barr, 2004). Tantrumming is also unique to preschoolers. While caregivers control the food environment, research has shown that tantrumming for food is related to obesity in this age-group (Agras, Hammer, McNicholas, & Kraemer, 2004), indicating that parents may give in to their child’s food demands. As many parents of obese children have weight concerns themselves, we believe that preschooler’s food environment, while controlled by adults, likely contains unhealthy foods and beverages. Thus, changing the diet of the preschooler would necessitate changing parents’ interaction with their child and the home food environment.
To address these unique aspects of treating obesity in preschoolers, we developed and piloted a 6-month 18-session behavioral intervention for preschool obesity (Learning about Activity and Understanding Nutrition for Child Health [LAUNCH]; Boles, Scharf, & Stark, 2010). LAUNCH was modeled on successful behavioral family-based interventions for school-age children (Epstein, Paluch, Roemmich, & Beecher, 2007), but addressed the unique challenges of preschoolers. LAUNCH also included a home visit component to facilitate parents’ implementation of child behavior management strategies and recommendations for modifying diet, activity, and the home environment. Both the feasibility (Boles et al., 2010) and pilot study (Stark et al., 2011) showed promising outcomes for child weight without promoting restrictive feeding practices or authoritarian parenting, two parenting behaviors discouraged by the Expert committee because of their association with obesity-promoting eating behaviors and excess weight gain (Barlow, 2007).
However, the LAUNCH intervention exceeds Expert Committee recommendations for a Stage 3 intervention in terms of the number of sessions and inclusion of home visits. Thus, we sought to examine a version of LAUNCH that conforms to Expert Committee guidelines for Stage 3 intervention on the number of sessions (10 sessions), structure, and support compared with a condition similar to a Stage 1 intervention, a brief pediatrician counseling (PC) session provided by a primary care physician. Only one pilot study of LAUNCH with home visits (LAUNCH-HV) has been conducted, thus a replication of LAUNCH-HV as a promising Stage 3+ treatment compared with PC was included. Change in BMI z-score (BMIz) at posttreatment (6 months) was the primary endpoint, as BMIz allows for comparison across individuals who differ in age and gender, within an individual over time, and is sensitive to percent fat loss (Hunt, Ford, Sabin, Crowne, & Shield, 2007). Secondarily, we examined changes in diet, physical activity, and home environment, as these are hypothesized mechanisms of weight management. Change in BMIz at 6-month follow-up (12 months) was also examined to assess maintenance of treatment gains. In an exploratory manner, increases in restrictive feeding practices and authoritarian parenting were assessed as potential negative side effects of a structured weight management program for preschoolers (Barlow, 2007). Because of the small sample size, we were not powered to compare the two LAUNCH groups. Nevertheless, to facilitate power analysis for future studies, the effect size was calculated between the two groups on the primary outcome, posttreatment BMIz.
On our primary endpoint, change in BMIz pre- to posttreatment, we hypothesized greater reductions in BMIz for the Stage 3 intervention (LAUNCH-clinic) compared with Stage 1 (PC), and greater reductions in BMIz for LAUNCH-HV (Stage 3+) compared with Stage 1 (PC). On secondary outcomes, we hypothesized that LAUNCH-clinic and -HV would show greater decreases in caloric intake and improvements in the home environment compared with PC. Given the lack of change in physical activity in our previous study, we did not hypothesize any difference between either LAUNCH-clinic or -HV compared with PC, but included this outcome given that it was a treatment recommendation. We hypothesized that there would be no change in exploratory outcomes of authoritarian parenting or use of restriction and both would be relatively low at all time points. This is the first study to examine interventions that approximate the components of Stage 1 and Stage 3 obesity intervention for preschoolers.
Methods
Design
This study was a pilot randomized controlled trial conducted at Cincinnati Children’s Hospital Medical Center from May 2009 to October 2011. The protocol was approved by the institutional review board, and parents provided informed consent for participation before data collection. Assessments were conducted at baseline, Month 6 (posttreatment for LAUNCH conditions), and Month 12 (6-month follow-up for LAUNCH conditions).
Study Participants
Participants were recruited from three large Midwestern pediatric practices. Inclusion criteria were (1) child age of 2–5 years; (2) child ≥95th percentile BMI (Kuczmarski etal., 2000), but <100% above the mean BMI; (3) one parent with a BMI ≥25; and (4) medical clearance from the child’s pediatrician. Exclusion criteria were (1) non-English speaking; (2) living ≥50 miles from the medical center; (3) disability or illness that would interfere with moderate physical activity; (4) medical condition/medication associated with weight gain; or (5) enrolled in a weight control program.
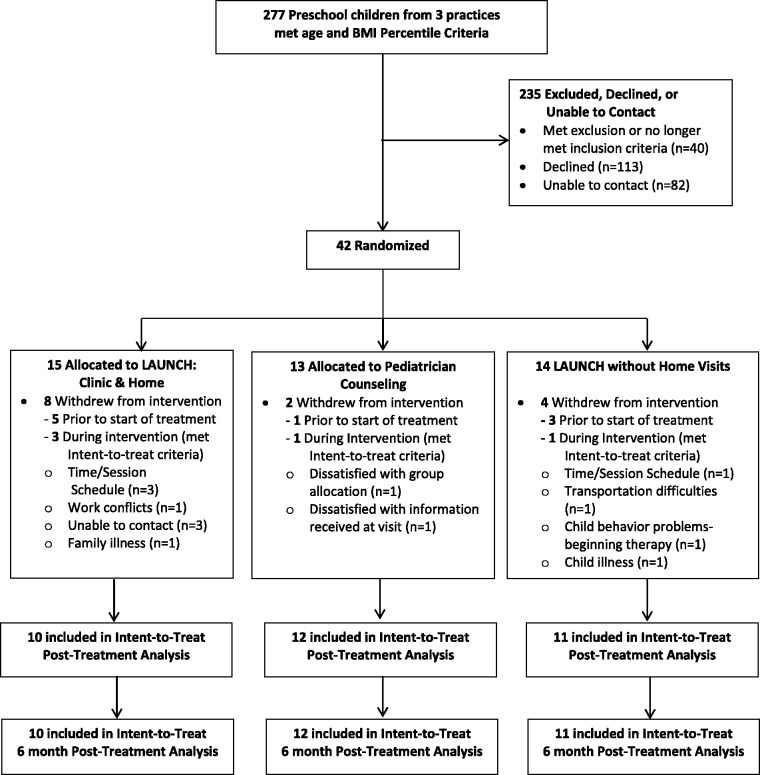
Recruitment. A systematic chart review of preschool-aged children was conducted through the pediatric practices. Children screened as eligible via chart review were sent a letter from the child’s pediatrician that introduced the study and included a “Do Not Contact” postcard (see Figure 1). Families who did not return the “Do Not Contact” postcard within 10 days of mailing were contacted by study staff to explain the study, further screen for eligibility, and invite participation. Baseline assessments were scheduled with eligible, interested families. Randomization was conducted using a random numbers table and was concealed until all baseline assessments were completed. Forty-two families were consented and randomized to treatment. There was no cost to the families, and they were reimbursed $50 for completing each assessment visit at baseline, 6 months, and 12 months.
Figure 1.
CONSORT flow diagram of participants to LAUNCH with home visits, LAUNCH-clinic, and pediatrician counseling at all time points.
Interventions
Enhanced Standard of Care
Pediatrician counseling was a manualized intervention designed to deliver dietary and physical activity recommendations outlined by the American Academy of Pediatrics (Spear et al., 2007). A board-certified pediatrician met each family individually for one 45 min visit to explain BMI, BMI percentiles and to review the child’s growth chart. Modeled on the Stage 1 Intervention “Prevention Plus” (Barlow, 2007), the pediatrician recommended (1) screen time ≤2 h daily; (2) active play ≥60 min daily; (3) eliminating soda and ≤4 ounces daily; (4) fruits and vegetables ≥5 servings daily; (5) limiting eating out; and (6) appropriate portion sizes for preschoolers. Each family was given a one-page healthy food and activity brochure created by the Collaboration for Healthy Ohio (Toolkit, 2007).
Clinic and Home-Based Behavioral Intervention
LAUNCH-HV was an 18-session manualized intervention designed to produce small decreases or stabilize the rate of child weight gain, consistent with current recommendations for treatment of preschool obesity (Barlow, 2007). The 6-month intervention consisted of two phases: Phase I (Intensive Intervention), 12 weekly sessions, alternating between group-based clinic sessions (parent and child concurrent groups), and individual home visits and Phase II (Maintenance), 12 weeks of every-other-week sessions, alternating between group clinic, and individual home sessions.
Phase I. Months 1–3 of the LAUNCH-HV intervention targeted lifestyle behavior modification and improving parenting skills (see Supplementary Material online for treatment flow and session topics). The parent-group clinic sessions (90 min each) were conducted by a licensed clinical psychologist or second-year psychology postdoctoral fellow and included education on diet (Weeks 2–7), physical activity (Weeks 8–12), and parenting skills (all sessions to facilitate diet and activity goals). Parents kept 7-day diet diaries for themselves and their child (Weeks 1–12). During Phase I, parents were provided vegetables at each session for daily taste tests (14 days) between sessions (see protocol described by Wardle et al., 2003).
In a concurrent session, children participated in a manualized group-based intervention led by a pediatric psychology postdoctoral fellow and a research coordinator. Session topics paralleled the topics covered in the parent group and focused on education about healthy eating, opportunities to try new foods (vegetable taste test, healthy dinners), and engage in physical activity.
Home sessions (60–90 min each) were conducted by a pediatric psychology fellow following a manualized protocol to support generalization of clinic-taught skills to the home environment through instruction, therapist modeling and parent rehearsal of dietary changes, physical activity, and behavioral techniques. A “home clean-out” was conducted following each clinic session on diet. During the “home clean-out”, high-calorie low-nutrient foods and beverages were identified, and parents were given the choice to remove items from the home or have an “eating in moderation” plan affixed while keeping the items in the home.
Phase II. Months 4–6 focused on helping families maintain treatment gains by engaging parents in long-term planning, problem-solving around individual barriers, and use of parenting skills to promote maintenance of diet and activity changes.
Clinic-Based Intervention
LAUNCH-clinic intervention content was identical to LAUNCH-HV clinic sessions (see Supplementary Materials online), including providing parents with vegetables at each session to facilitate daily taste tests (14 days) between sessions, keeping a 7-day diet diary for themselves and their preschooler (Weeks 1–12). In lieu of home visits, parents were provided a “home clean-out” box to use on their own to eliminate high-calorie low-nutrient foods from the home. To match LAUNCH-HV on treatment duration (6 months), LAUNCH-clinic sessions were conducted every other week during Months 1–3 and monthly during Months 4–6 of the intervention, for 10 treatment sessions. This intervention conforms to the content, structure, and support recommended by the Expert Committee guidelines for a Stage 3 intervention (Barlow, 2007).
Measures
Demographic information was collected at baseline, and remaining measures were obtained at baseline, Month 6 (posttreatment for LAUNCH conditions), and Month 12 (6-month follow-up).
Primary Outcomes
Child and parent weight and height were measured in triplicate following standard anthropometric procedures (Cameron, 1986) by trained personnel from the General Clinical Research Center (GCRC) who were unaware of the child’s treatment assignment. Measurements were averaged to calculate the children’s BMIz and BMI percentile for sex and age using the Centers for Disease Control and Prevention growth curves (Kuczmarski et al., 2000).
Secondary Outcomes
Children’s dietary intake was assessed via three random 24-h dietary recalls (2 weekdays and 1 weekend) with the child’s parent over a 2-week period using the multiple-pass method (Guenther, DeMaio, Ingwersen, & Berline, 1995), which has been validated and deemed accurate among young children (Johnson, Driscoll, & Goran, 1996), by a GCRC dietician unaware of treatment assignment. Average daily caloric intake was calculated using the Minnesota Nutrition Data System for Research software versions 2009–2011 (Nutrition Data Systems, 2004).
Home food environment was adapted from validated self-report measures of the food and activity environment described in Boles, Scharf, Filigno, Saelens, and Stark (2013). It was modified to assess only the food environment based on the presence/absence of a predefined list of 18 fresh fruits and 14 vegetables, and 23 other foods/beverages predefined as unhealthy foods (e.g., potato chips; based on criteria adapted from the Traffic Light Diet; Epstein & Squires, 1988), and high-calorie beverages (e.g., sports drinks, soda). The coder recorded the presence of each food item located in the kitchen and secondary food storage areas (e.g., basement refrigerator), yielding number of foods present in three categories: fruits/vegetables, high-calorie foods, and high-calorie beverages. Interrater reliability conducted by an independent coder on 26% of randomly selected home assessments yielded alpha coefficients suggesting excellent interrater reliability (Nunnally & Bernstein, 1978) for high-calorie foods (0.95), high-calorie beverages (0.97), and fruits and vegetables (0.96).
Children’s physical activity was measured by the GT1M accelerometer, validated and calibrated for use with preschool children (Pate, Almeida, McIver, Pfeiffer, & Dowda, 2006). Accelerometers, with epochs set to 15 s (Trost, Sirard, Dowda, Pfeiffer, & Pate, 2003), were worn for 7 days, with a minimum of 3 days of data required for inclusion in analysis (Trost, Pate, Freedson, Sallis, & Taylor, 2000). A valid day was defined as the availability of data for 5 valid hours of wear time. Nonwear time was defined as ≥60 min of consecutive zeroes and ≤2 min of activity. Average daily minutes of moderate and vigorous activity (MVPA) were calculated for each assessment period (Pate et al., 2006). Only three children, all in the PC group, did not have a weekend day at one assessment point each (one child at Month 6 and two children at Month 12).
Exploratory Outcomes
Parenting Styles and Dimensions (Robinson, Mandelco, Olsen, & Hart, 1995), a 53-item questionnaire assessing three parenting styles (authoritarian, authoritative, and permissive), was included to explore hypothesized links between parenting styles and child nutritional intake (Faith, 2005). Parents rated each item on a 5-point Likert-type scale (“never” to “always”). Scores range from 1 to 5 with higher scores indicating higher endorsement.
Child Feeding Questionnaire (CFQ) (Birch et al., 2001) is a 31-item questionnaire assessing parental child feeding attitudes and practices. We used two subscales, Restriction and Pressure to Eat, that have been linked with child eating and weight status (Faith, 2005). Parents rated items on a 5-point Likert-type scale (“disagree” to “agree”) with higher scores indicating higher endorsement.
Data Analysis
SAS PROC MIXED analyses analogous to analyses of co-variances controlling for the baseline values for the dependent variable (Winkens, van Breukelen, Schouten, & Berger, 2007) were conducted on change from baseline to Month 6 and change from baseline to Month 12 for each outcome of interest using intent-to-treat (ITT) analyses comparing LAUNCH-HV with PC and LAUNCH-clinic with PC separately. Participants met ITT criteria if they were randomized to one of the three groups and attended at least one intervention session. If a participant dropped out of the study after attending one intervention session, we used all available data on a particular outcome from that participant within the statistical analyses. PROC MIXED using maximum likelihood (ML) estimation was used to account for any missing data due to dropout in the analyses under the missing at random (MAR) assumption (see, e.g., Schafer & Graham, 2002, for more detail on accounting for missing data with ML estimation and a conceptual understanding of MAR). All hypothesis tests used a two-tailed level of significance of .05. The standardized effect sizes of interest were calculated by using Cohen’s ds (Cohen, 1988). Because the PC and LAUNCH-HV were significantly different on BMIz at baseline (p = .049), we conducted the primary between-group analyses with and without baseline BMIz as a covariate. Because results from these analyses were not substantially different from one another, only results without controlling for baseline BMIz are reported.
Results
Study Population
Forty-two families consented and were randomized to treatment, 15 to LAUNCH-HV, 14 to LAUNCH-clinic, and 13 to PC. Of these, 10 in LAUNCH-HV, 11 in LAUNCH-clinic, and 12 in PC met ITT criteria. This represents a recruitment rate of 15.2% for all children identified via screening of chart reviews and 27% of families verified as meeting inclusion criteria (see Figure 1). Three families in LAUNCH-HV and one in LAUNCH-clinic who dropped out before completing all treatment sessions and one PC family who withdrew after completing the PC session were included in ITT analysis. Treatment groups did not significantly differ on any demographic or outcome variables at baseline, with the exception of significantly lower BMIz at baseline for LAUNCH-HV compared with PC (see Table I).
Table I.
Baseline Characteristics of Children and Their Families in Pediatric Counseling (PC), LAUNCH With Home Visits (LAUNCH-HV), and LAUNCH-Clinic
| PC (N = 12) | LAUNCH-HV (N = 10) | LAUNCH-clinic (N = 11) | |
|---|---|---|---|
| Age | 4.8 (0.7) | 4.7 (1.3) | 4.2 (1.1) |
| Female (%) | 8 (67) | 8 (80) | 7 (64) |
| White (%) | 9 (75) | 9 (90) | 10 (91) |
| Weight (kg) | 26.1 (5.7) | 24.6 (4.8) | 26.6 (8.9) |
| Height (cm) | 111.2 (7.0) | 110.5 (10.6) | 109.2 (11.8) |
| Body mass index z-score for sex and age | 2.4 (0.4)** | 2.1 (0.2)** | 2.5 (0.8) |
| Family income (%) | |||
| Under $50,000 | 2 (17) | 1 (11) | 2 (18) |
| $50,000–99,999 | 7 (58) | 3 (33) | 6 (55) |
| $100,000 and over | 3 (25) | 5 (56) | 3 (27) |
| Hollingshead score | 45.8 (8.4) | 49.8 (8.5) | 43.3 (13.0) |
**p < .05.
Primary Outcome: BMIz Pre- to Posttreatment
LAUNCH-HV Compared With PC
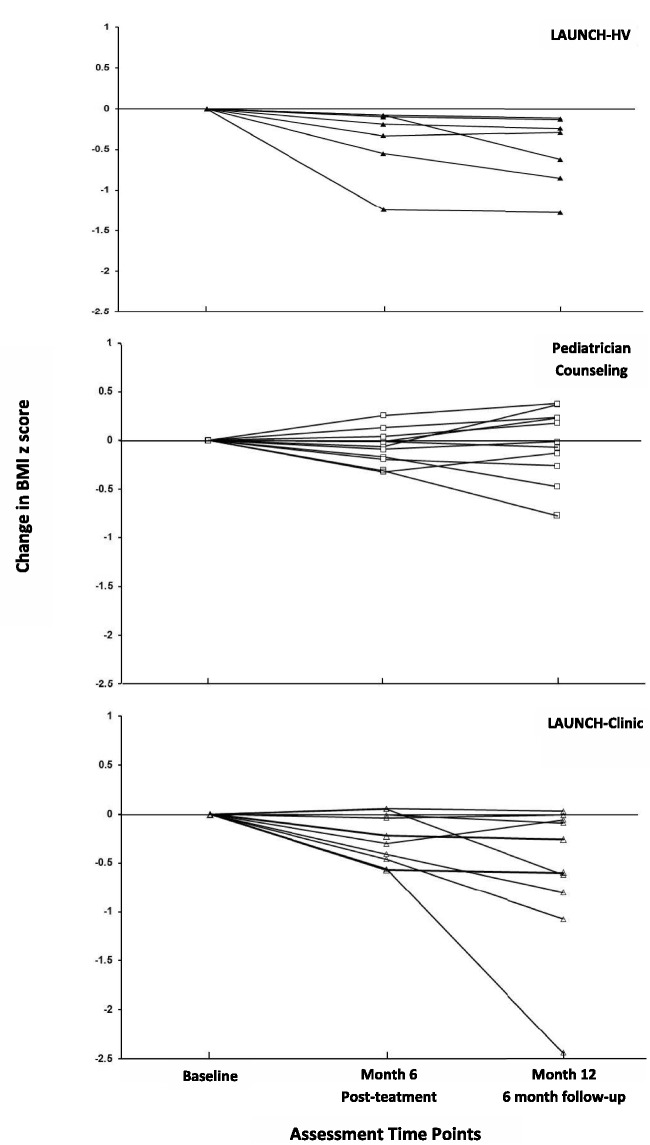
Children in LAUNCH-HV showed a significantly greater decrease on the primary outcome of change in BMIz (Month 6) pre- to posttreatment compared with PC (see Table II). They also showed significantly less weight gain and a greater decrease in BMI percentile than children in PC. All seven children who completed treatment showed decreases in baseline to Month 6 BMIz (as shown in Figure 2), and none met criteria for severe obesity (≥99th percentile BMI; Skelton, Cook, Auinger, Klein, & Barlow, 2009). In contrast, 5 of 11 children in PC showed BMIz increases and met criteria for severe obesity across this same time period. No child in either group achieved a BMI percentile <85th.
Table II.
Change From Baseline on Child Weight Outcomes in Pediatric Counseling (PC), LAUNCH With Home Visits (LAUNCH-HV), and LAUNCH-Clinic
| Change from baseline, mean (SD) |
PC vs. LAUNCH HV, treatment effecta |
PC vs. LAUNCH-clinic, treatment effecta |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PC | LAUNCH-HV | LAUNCH-clinic | Mean [95% confidence interval] | d | p | Mean [95% confidence interval] | d | p | |
| Child body mass index (BMI) z-score | |||||||||
| Month 6 | −.07 (.18) | −.37 (.42) | −.25 (.25) | −.39 [−.65, −.12] | −1.50 | .007 | −.16 [−.34, .02] | −0.94 | .08 |
| Month 12 | −.03 (.36) | −.50 (.43) | −.59 (.75) | −.64 [−.95, −.34] | −1.64 | .0003 | −.50 [−.98, −.03] | −0.88 | .04 |
| Child BMI Percentile | |||||||||
| Month 6 | −0.1 (0.7) | −2.5 (3.5) | −0.8 (1.2) | −2.8 [−4.8, −0.7] | −1.22 | .01 | −0.7 [−1.6, 0.1] | −0.70 | .09 |
| Month 12 | 0.2 (1.1) | −4.0 (3.9) | −5.1 (11.3) | −4.6 [−6.9, −2.4] | −1.77 | .0003 | −5.4 [−12.3, 1.5] | −0.68 | .12 |
| Child weight (kg) | |||||||||
| Month 6 | 1.9 (0.9) | −0.1 (2.3) | 1.1 (2.4) | −2.2 [−3.7, −0.6] | −1.38 | .008 | −0.7 [−2.2, 0.9] | −0.41 | .37 |
| Month 12 | 5.2 (2.6) | 0.8 (2.5) | 2.3 (3.1) | −4.4 [−7.1, −1.8] | −1.69 | .002 | −3.0 [−5.5, −0.4] | −1.03 | .03 |
Note. aTreatment effect size estimates along with their corresponding 95% confidence intervals and p-values are computed using maximum likelihood estimation to account for missing data.
Significant p-values (p < .05) are denoted in boldface. d – standardized mean difference.
Figure 2.
Change in body mass index z-score for all children in LAUNCH with home visits, LAUNCH-clinic, and pediatrician counseling from baseline to Month 6 (posttreatment) and Month 12 (6-month follow-up).
LAUNCH-Clinic Compared With PC
Children in LAUNCH-clinic did not achieve a statistically greater decrease on the primary outcome of BMIz compared with the children in PC, pre- to posttreatment (Month 6; see Table II). Eight of the 10 LAUNCH-clinic children who completed treatment showed BMIz decreases at Month 6 (see Figure 2); however, 5 of these children also met criteria for severe obesity.
LAUNCH-HV and LAUNCH-Clinic
While the study was not powered to compare LAUNCH-HV and LAUNCH-clinic on changes in BMIz, Figure 2 provides a visual comparison of both conditions. All LAUNCH-HV children reduced their BMIz pre- to posttreatment, whereas LAUNCH-clinic children showed greater variability. The effect size is −0.21 on change in BMIz (Cohen’s d = 0.63) pre- to posttreatment between the two LAUNCH conditions.
Secondary Outcomes
Maintenance of Treatment Effects: BMIz 6 Months Posttreatment
As shown in Table II, BMIz continued to be significantly lower at Month 12 for LAUNCH-HV compared with PC, indicating maintenance and further improvement posttreatment. Of note, at Month 12, 6 of the 11 children in PC were ≥99th percentile (severely obese; Skelton, Cook, Auinger, Klein, & Barlow, 2009), whereas none of the children in LAUNCH-HV met criteria for this weight status. At Month 12, children in LAUNCH-clinic achieved significantly greater BMIz reductions compared with PC. While six of eight LAUNCH-clinic children maintained or further improved on BMIz including one child who achieved a BMI <85th percentile; three children also continued to meet criteria for severe obesity.
Calorie Intake and Home Food Environment
LAUNCH-HV demonstrated greater changes to the home environment compared with PC including statistically greater decreases in caloric intake at Month 6 (see Table III) that were maintained at Month 12, significantly greater decreases in number of high-calorie foods, but not beverages, at both Months 6 and 12, and significantly greater increases in number of fruits and vegetables in the home at Month 6, but at not Month 12. LAUNCH-clinic only showed a significantly greater decrease in high-calorie beverages at Month 6 and significantly greater decreases in average-caloric intake at Month 12 compared with PC.
Table III.
Change From Baseline in Caloric Intake, Home Food Environment, and Physical Activity for Children in Pediatric Counseling (PC), LAUNCH With Home Visits (LAUNCH-HV), and LAUNCH-Clinic
| Change from baseline, mean (SD) |
PC vs. LAUNCH-HV, treatment effecta |
PC vs. LAUCH-clinic, treatment effecta |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PC | LAUNCH-HV | LAUNCH-clinic | Mean [95% confidence interval] | d | p | Mean [95% confidence interval] | d | p | |
| Caloric intake | |||||||||
| Month 6 | −65 (724) | −465 (292) | −243 (567) | −566 [−888, −244] | 1.19 | .002 | −337 [−740, 67] | 0.51 | .10 |
| Month 12 | 3 (620) | −518 (315) | −249 (592) | −640 [−932, −348] | 1.35 | .0002 | −415 [−734, −97] | 0.68 | .01 |
| Home food environment | |||||||||
| Fruits and vegetables | |||||||||
| Month 6 | −1.0 (2.7) | 2.7 (3.1) | 0.6 (3.1) | 3.8 [0.9, 6.8] | 1.36 | .01 | 1.6 [−1.0,4.2] | 0.55 | .21 |
| Month 12 | 1.5 (3.0) | 1.3 (2.7) | 1.8 (3.0) | −0.2 [−3.2, 2.7] | −0.08 | .87 | 0.3 [−2.4,.3.0] | 0.09 | .84 |
| High-calorie foods | |||||||||
| Month 6 | 0.2 (1.1) | −1.7 (1.4) | −0.4 (1.1) | −2.0 [−3.0, −0.9] | −1.60 | .0009 | −0.6 [−1.5, 0.2] | −0.53 | .14 |
| Month 12 | −0.1 (1.1) | −1.3 (1.4) | −1.0 (1.6) | −1.4 [−2.4, −0.4] | −1.10 | .008 | −0.9 [−2.2, 0.3] | −0.65 | .13 |
| High-calorie beverages | |||||||||
| Month 6 | 0.0 (1.1) | −0.5 (0.5) | −0.8 (1.0) | −0.8 [−1.7, 0.1] | −0.84 | .07 | −1.4 [−2.2, −0.6] | −1.27 | .001 |
| Month 12 | −0.5 (1.0) | −0.7 (0.8) | −0.3 (1.2) | −0.4 [−1.3, 0.6] | −0.39 | .42 | −0.3 [−1.2, 0.6] | −0.27 | .50 |
| Physical activity | |||||||||
| Minutes moderate | |||||||||
| Month 6 | −6 (10) | −1 (9) | 4 (12) | 0.2 [−8.0, 8.4] | 0.02 | .96 | 1.5 [−5.1, 8.1] | 0.13 | .64 |
| Month 12 | −15 (16) | −4 (16) | 6 (16) | 3.1 [−8.0, 14.1] | 0.19 | .56 | 10.2 [−1.5, 22.0] | 0.61 | .08 |
| Minutes vigorous | |||||||||
| Month 6 | 1 (6) | 4 (6) | 4 (9) | 1.3 [−4.7, 7.4] | 0.23 | .65 | 0.5 [−5.7, 6.7] | 0.07 | .87 |
| Month 12 | −4 (8) | 4 (10) | 7 (8) | 4.3 [−2.3, 10.9] | 0.49 | .19 | 6.8 [−0.3, 13.9] | 0.81 | .06 |
Note. aTreatment effect size estimates along with their corresponding 95% confidence intervals and p-values are computed using maximum likelihood estimation to account for missing data.
Significant p-values (p < .05) are denoted in boldface. d – standardized mean difference.
Child Activity
LAUNCH-HV and LAUNCH-clinic did not differ from PC on changes in level of MVPA from baseline to Month 6 or to Month 12 (see Table III). Across all groups and time points, the children averaged ∼60 min of moderate activity and 20 min of vigorous activity a day.
Exploratory Outcomes
Parents in all three groups were relatively low on authoritarian (≤2) and permissive (≤2) parenting across all time points. However, by Month 12, parents in LAUNCH-clinic showed a significantly greater decrease in authoritarian parenting style (2.07–1.69) and permissive parenting (1.99–1.68) compared with parents in PC [2.0–1.90; t(22) = 3.07, p = .006] and [1.96–1.81; t(22) = 2.71, p = .01], respectively. From baseline to Months 6 and 12, CFQ restriction and pressure to eat remained relatively low (<2.3) across all time points with no significant changes between groups.
Discussion
There are currently no evidenced-based weight management interventions targeting preschoolers who meet the criteria for obesity. The current study piloted three interventions, two of which were based on Stages 1 and 3 of the Expert Committee for Assessing and Treating Obesity guidelines (Barlow, 2007), and one that exceeded the guidelines for intervention intensity (Stage 3+). Contrary to our hypothesis, the family-based behavioral intervention modeled on the Expert Committee Stage 3 intervention (Barlow, 2007), LAUNCH-clinic, did not demonstrate significantly greater BMIz reductions compared with children in PC by posttreatment (Month 6). However, consistent with our hypothesis, the more intensive intervention that included home visits, LAUNCH-HV (Stage 3+), demonstrated significant decreases in BMIz compared with PC by posttreatment. The decrease in BMIz (−.39) in the current study was very similar to the decrease seen in our prior pilot of this intervention (−.49; Stark et al., 2011). While we were not powered to examine statistical differences in change in BMIz between LAUNCH-HV and LAUNCH-clinic, the effect size of −0.21 for change in BMIz is considered clinically significant in older children and adolescents, as it has been associated with improvement in glucose tolerance and high blood pressure (Reinehr, Kleber, & Toschke, 2009). Collectively, our data provide preliminary evidence that Stage 1 interventions are likely insufficient for changing weight trajectories for already obese preschoolers and suggests that treatment intensity beyond the recommendations may be necessary to improve outcomes.
Similar to findings for BMIz, expected outcomes for the hypothesized mechanisms of weight change were also partially supported. Children in LAUNCH-HV showed significant decreases compared with PC on average daily caloric intake, high-calorie foods in the home, and increases in fruits and vegetables in the home by posttreatment. LAUNCH-clinic was not significantly different from PC on any of these posttreatment measures. As hypothesized neither LAUNCH group showed any significant differences from PC on MVPA. This may reflect a ceiling effect as children enrolled in our studies were already receiving the 60 min of recommended MVPA. Alternatively, it may indicate that increasing activity is difficult because parental supervision is necessary when preschoolers are outside or engaged in active play, as many of our participants anecdotally reported.
Surprising was the lack of significant pre- to posttreatment differences between a 10-session Stage 3 clinic-only model compared with a Stage 1 intervention, given the greater number of treatment sessions, structure, and support of LAUNCH-clinic. Barriers to promoting healthy weight gain in early childhood include greater availability and accessibility of unhealthy compared with healthy food options within the home food environment (Wyse, Campbell, Nathan, & Wolfenden, 2011), child resistance to trying new foods (Osborne & Forestell, 2012), and child tantrums over food (Agras et al., 2004). We hypothesize that the in-person guidance and support that parents received from the home therapists’ in removing high-calorie low-nutrient foods to improve the home food environment and generalization of clinic-taught child behavioral management strategies to the family’s natural environment through modeling, behavioral rehearsal, and coaching of parents in reinforcing positive food choices and managing child resistance to trying new foods, were key to better weight outcomes among children in LAUNCH-HV compared with PC. The current study would indicate that further examination of home visits is warranted.
In the current study, changes in BMIz continued to be significantly lower for LAUNCH-HV than PC at the 6-month follow-up (Month 12), and the difference in mean change between groups increased from −.39 at posttreatment to −.64 at follow-up. Given the lack of difference between LAUNCH-clinic and PC pre- to posttreatment, the significant decrease in BMIz for LAUNCH-clinic compared with PC at Month 12 was surprising. Despite this improvement for LAUNCH-clinic, the effect size for LAUNCH-HV compared with PC was almost twice the size (−1.64) of the LAUNCH-clinic to PC effect size (−0.88). The difference between LAUNCH-clinic and PC at Month 12 appears to be driven by the one child who achieved a healthy weight status by Month 12. This child appears to be an outlier for either intervention. While we do not have data on factors that contributed to this family’s magnitude of response, anecdotal data from group leader session notes indicate that in contrast to the majority of other families in either LAUNCH intervention, this family prepared most meals at home. The caloric contribution of home-prepared foods compared with commercially prepared foods was not systematically obtained in the current study, but may be an avenue for future research.
Perhaps the most concerning implication of our current and previous study is that without intensive behavioral intervention, 2–5-year-old children who are ≥95th percentile BMI are likely to continue to gain weight at an accelerated rate. This is consistent with the larger literature showing that obesity anytime between 2 and 5 years increases the likelihood of overweight or obesity at later years (Nader et al., 2006). Particularly concerning is the percentage of PC children (55%) with a BMI ≥99th percentile given the recent finding that 72% of 5 year olds at the 99th percentile BMI continued to be obese at age 14 years compared with 47 and 6% of those at the 95th and 50th percentile BMI, respectively (Cunningham et al., 2014). Pre- to posttreatment 100, 14, and 28% of children with severe obesity across LAUNCH-HV, LAUNCH-clinic, and PC, respectively, at baseline were no longer ≥99th% BMI. These findings highlight the importance of treating obesity during the early stages of child development.
The results of this study are encouraging and indicate that a more intensive intervention (Stage 3+ that includes home visits) than currently recommended by the Expert Committee guidelines may be necessary to improve weight gain trajectories in already obese preschoolers. Conclusions drawn from the current study must be tempered by study limitations including the pilot nature and small sample size, the differential in treatment contacts across the three conditions, and the demographics of the sample (primarily Caucasian and middle class). It is noteworthy however, that an intensive behavioral intervention can be implemented without increasing authoritarian parenting styles or restrictive feeding behaviors by parents.
Finally, our enrollment rate of 27% adds to the literature demonstrating that many parents of preschoolers with obesity do not recognize their child’s weight as problematic. Barriers to recruiting families for obesity intervention programs identified in the larger literature include parents’ lack of concern about their child’s weight (Andersen, Christensen, & Sondergaard, 2013), discrepancies between real and perceived weight (Rietmeijer-Mentink, Paulis, van Middelkoop, Bindels, & van der Wouden, 2013), and beliefs that larger is healthier in early childhood (McGarvey et al., 2006). Understanding how to address these challenges will improve not only recruitment rates into studies but more importantly parental action to improve the health of young children once evidence-based treatments are developed.
Supplementary Data
Supplementary data can be found at: http://www.jpepsy.oxfordjournals.org/
Funding
The project described was supported by the National Institutes of Digestive Diseases and Kidney through grant D24 DK 059492 (L.J.S.) and National Center for Research Resources and the National Center for Advancing Translational Sciences through Grant 8 UL1 TR000077-04, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflicts of interest: None declared.
Supplementary Material
References
- Addessi E, Galloway A T, Visalberghi E, Birch L L. Specific social influences on the acceptance of novel foods in 2-5-year-old children. Appetite. 2005;45:264–271. doi: 10.1016/j.appet.2005.07.007. doi:10.1016/j.appet.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Agras W S, Hammer L D, McNicholas F, Kraemer H C. Risk factors for childhood overweight: A prospective study from birth to 9.5 years. Journal of Pediatrics. 2004;145:20–25. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Andersen M K, Christensen B, Sondergaard J. Child overweight in general practice—parents' beliefs and expectations – a questionnaire survey study. BMC Family Practice. 2013;14:152. doi: 10.1186/1471-2296-14-152. doi:10.1186/1471-2296-14-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow S E. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120(Suppl. 4):S164–S192. doi: 10.1542/peds.2007-2329C. doi:120/Supplement_4/S164 [pii] 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Birch L L, Fisher J O, Grimm-Thomas K, Markey C N, Sawyer R, Johnson S L. Confirmatory factor analysis of the Child Feeding Questionnaire: A measure of parental attitudes, beliefs and practices about child feeding and obesity proneness. Appetite. 2001;36:201–210. doi: 10.1006/appe.2001.0398. [DOI] [PubMed] [Google Scholar]
- Birch L L, Marlin D W. I don't like it; I never tried it: Effects of exposure on two-year-old children's food preferences. Appetite. 1982;3:353–360. doi: 10.1016/s0195-6663(82)80053-6. [DOI] [PubMed] [Google Scholar]
- Boles R, Scharf C, Stark L J. Developing a treatment program for obesity in preschool age children: Preliminary data. Children's Health Care. 2010;39:34–58. doi: 10.1080/02739610903455137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles R E, Scharf C, Filigno S S, Saelens B E, Stark L J. Differences in home food and activity environments between obese and healthy weight families of preschool children. Journal of Nutrition Education and Behavior. 2013;45:222–231. doi: 10.1016/j.jneb.2012.09.012. doi:10.1016/j.jneb.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N. The methods of auxological anthropometry. In: Falkner F, Tanner J M, editors. Human growth. 1986. (2nd ed. Vol. 3, pp. 3). New York: Plenum Press. [Google Scholar]
- Carruth B R, Ziegler P J, Gordon A, Barr S I. Prevalence of picky eaters among infants and toddlers and their caregivers' decisions about offering a new food. Journal of the American Dietetic Association. 2004;104(1 Suppl. 1):S57–S64. doi: 10.1016/j.jada.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cunningham S A, Kramer M R, Narayan K M. Incidence of childhood obesity in the United States. New England Journal of Medicine. 2014;370:403–411. doi: 10.1056/NEJMoa1309753. doi:10.1056/NEJMoa1309753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datar A, Sturm R, Magnabosco J L. Childhood overweight and academic performance: National study of kindergartners and first-graders. Obesity Research. 2004;12:58–68. doi: 10.1038/oby.2004.9. [DOI] [PubMed] [Google Scholar]
- Epstein L H, Paluch R A, Roemmich J N, Beecher M D. Family-based obesity treatment, then and now: Twenty-five years of pediatric obesity treatment. Health Psychology. 2007;26:381–391. doi: 10.1037/0278-6133.26.4.381. doi:2007-09406-001 [pii] 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L H, Squires S. The stop-light diet for children. Boston: Little Brown; 1988. [Google Scholar]
- Faith M S. Development and modification of child food preferences and eating patterns: Behavior genetics strategies. International Journal of Obesity. 2005;29:549–556. doi: 10.1038/sj.ijo.0802981. doi:0802981 [pii] 10.1038/sj.ijo.0802981. [DOI] [PubMed] [Google Scholar]
- Guenther P M, DeMaio T J, Ingwersen L A, Berline M. The multiple-pass approach for the 24-hour recall in the Continuing Survey of Food Intakes by Individuals (CSFII) 1994-1996. Boston, MA: Paper presented at the International Conference on Dietary Assessment Methods; 1995. [Google Scholar]
- Hunt L P, Ford A, Sabin M A, Crowne E C, Shield J P. Clinical measures of adiposity and percentage fat loss: Which measure most accurately reflects fat loss and what should we aim for? Archives of Disease in Childhood. 2007;92:399–403. doi: 10.1136/adc.2006.103986. doi:adc.2006.103986 [pii] 10.1136/adc.2006.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R K, Driscoll P, Goran M I. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. Journal of the American Dietetic Association. 1996;96:1140–1144. doi: 10.1016/S0002-8223(96)00293-3. doi:S0002-8223(96)00293-3 [pii] 10.1016/S0002-8223(96)00293-3. [DOI] [PubMed] [Google Scholar]
- Kuczmarski R J, Ogden C L, Grummer-Strawn L M, Flegal K M, Guo S S, Wei R, Mei Z, Curtin L R, Roche A F, Johnson C L. CDC growth charts: United States advance data from vital and health statistics; no. 314. Hyattsville, MD: National Center for Health Statistics; 2000. [PubMed] [Google Scholar]
- Kuhl E S, Rausch J R, Varni J W, Stark L J. Impaired health-related quality of life in preschoolers with obesity. Journal of Pediatric Psychology. 2012;37:1148–1156. doi: 10.1093/jpepsy/jss090. doi:10.1093/jpepsy/jss090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarvey E L, Collie K R, Fraser G, Shufflebarger C, Lloyd B, Norman Oliver M. Using focus group results to inform preschool childhood obesity prevention programming. Ethnicity and Health. 2006;11:265–285. doi: 10.1080/13557850600565707. doi:10.1080/13557850600565707. [DOI] [PubMed] [Google Scholar]
- Nader P R, O'Brien M, Houts R, Bradley R, Belsky J, Crosnoe R, Friedman S, Mei Z, Susman E J National Institute of Child Health, Human Development Early Child Care Research Network. Identifying risk for obesity in early childhood. Pediatrics. 2006;118:e594–e601. doi: 10.1542/peds.2005-2801. doi:118/3/e594 [pii] 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
- Nunnally J, Bernstein I. Psychometric theory. 2nd ed. New York: McGraw-Hill; 1978. [Google Scholar]
- Nutrition Data Systems (NDS) (Version 5.0) University of Minnesota, Division of Epidemiology; 2004. Minneapolis: Nutrition Data Systems Nutrition Coordinating Center. [Google Scholar]
- Ogden C L, Carroll M D, Curtin L R, McDowell M A, Tabak C J, Flegal K M. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Ogden C L, Carroll M D, Kit B K, Flegal K M. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. doi:jama.2012.40 [pii] 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne C L, Forestell C A. Physiology and Behavior. 106: 2012. Increasing children's consumption of fruit and vegetables: Does the type of exposure matter? pp. 362–368. doi:10.1016/j.physbeh.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Pate R R, Almeida M J, McIver K L, Pfeiffer K A, Dowda M. Validation and calibration of an accelerometer in preschool children. Obesity (Silver Spring) 2006;14:2000–2006. doi: 10.1038/oby.2006.234. doi:14/11/2000 [pii] 10.1038/oby.2006.234. [DOI] [PubMed] [Google Scholar]
- Phillips B K, Kolasa K K. Vegetable preferences of preschoolers in day-care. Journal of Nutrition Education. 1980;12:192–195. [Google Scholar]
- Reinehr T, Kleber M, Toschke A M. Lifestyle intervention in obese children is associated with a decrease of the metabolic syndrome prevalence. Atherosclerosis. 2009;207:174–180. doi: 10.1016/j.atherosclerosis.2009.03.041. doi:10.1016/j.atherosclerosis.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Rietmeijer-Mentink M, Paulis W D, van Middelkoop M, Bindels P J, van der Wouden J C. Difference between parental perception and actual weight status of children: A systematic review. Maternal and Child Nutrition. 2013;9:3–22. doi: 10.1111/j.1740-8709.2012.00462.x. doi:10.1111/j.1740-8709.2012.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C C, Mandelco B L, Olsen S F, Hart C H. Authoritative, authoritarian, and permissive parenting practices: Development of a new measure. Psychological Reports. 1995;77:819–830. [Google Scholar]
- Schafer J L, Graham J W. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Skelton J A, Cook S R, Auinger P, Klein J D, Barlow S E. Prevalence and trends of severe obesity among US children and adolescents. Academic Pediatrics. 2009;9:322–329. doi: 10.1016/j.acap.2009.04.005. doi:S1876-2859(09)00109-0 [pii] 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear B A, Barlow S E, Ervin C, Ludwig D S, Saelens B E, Schetzina K E, Taveras E M. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl. 4):S254–S288. doi: 10.1542/peds.2007-2329F. doi:10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- Stark L J, Spear S, Boles R, Kuhl E, Ratcliff M, Scharf C, Bolling C, Rausch J. A pilot randomized controlled trial of a clinic and home-based behavioral intervention to decrease obesity in preschoolers. Obesity (Silver Spring) 2011;19:134–141. doi: 10.1038/oby.2010.87. doi:oby201087 [pii] 10.1038/oby.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S A, Birch L L. Pass the sugar, pass the salt: Experience dictates preference. Developmental Psychology. 1990;26:546–551. [Google Scholar]
- Toolkit Ounce of prevention is worth a pound: Preventing childhood obesity starting from birth. 2007 Retrieved April 1, 2009. http://www.healthyohioprogram.org/healthylife/nutri2/nutrikids2/ounce.aspx. [Google Scholar]
- Trost S G, Pate R R, Freedson P S, Sallis J F, Taylor W C. Using objective physical activity measures with youth: How many days of monitoring are needed? Medicine and Science in Sports and Exercise. 2000;32:426–431. doi: 10.1097/00005768-200002000-00025. [DOI] [PubMed] [Google Scholar]
- Trost S G, Sirard J R, Dowda M, Pfeiffer K A, Pate R R. Physical activity in overweight and nonoverweight preschool children. International Journal of Obesity and Related Metabolic Disorders. 2003;27:834–839. doi: 10.1038/sj.ijo.0802311. [DOI] [PubMed] [Google Scholar]
- Wardle J, Cooke L J, Gibson E L, Sapochnik M, Sheiham A, Lawson M. Increasing children's acceptance of vegetables; a randomized trial of parent-led exposure. Appetite. 2003;40:155–162. doi: 10.1016/s0195-6663(02)00135-6. doi:S0195666302001356 [pii] [DOI] [PubMed] [Google Scholar]
- Williams C L, Strobino B, Bollella M, Brotanek J. Body size and cardiovascular risk factors in a preschool population. Preventive Cardiology. 2004;7:116–121. doi: 10.1111/j.1520-037x.2004.03224.x. [DOI] [PubMed] [Google Scholar]
- Winkens B, van Breukelen G J, Schouten H J, Berger M P. Randomized clinical trials with a pre- and a post-treatment measurement: Repeated measures versus ANCOVA models. Contemporary Clininical Trials. 2007;28:713–719. doi: 10.1016/j.cct.2007.04.002. doi:S1551-7144(07)00048-1 [pii]10.1016/j.cct.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Wyse R, Campbell E, Nathan N, Wolfenden L. Associations between characteristics of the home food environment and fruit and vegetable intake in preschool children: A cross-sectional study. BMC Public Health. 2011;11:938. doi: 10.1186/1471-2458-11-938. doi:10.1186/1471-2458-11-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.