Abstract
Calcium phosphate (CaP) particles as a carrier in an injectable bone filler allows less invasive treatment of bony defects. The effect of changing granule size within a poloxamer filler on the osteointegration of silicate-substituted calcium phosphate (SiCaP) bone substitute materials was investigated in an ovine critical-sized femoral condyle defect model. Treatment group (TG) 1 consisted of SiCaP granules sized 1000–2000 μm in diameter (100 vol %). TG2 investigated a granule size of 250–500 μm (75 vol %), TG3 a granule size of 90–125 μm (75 vol %) and TG4 a granule size of 90–125 μm (50 vol %). Following a 4 and 8 week in vivo period, bone area, bone-implant contact, and remaining implant area were quantified within each defect. At 4 weeks, significantly increased bone formation was measured in TG2 (13.32% ± 1.38%) when compared with all other groups (p = 0.021 in all cases). Bone in contact with the bone substitute surface was also significantly higher in TG2. At 8 weeks most new bone was associated within defects containing the smallest granule size investigated (at the lower volume) (TG4) (42.78 ± 3.36%) however this group was also associated with higher amounts of fragmented SiCaP. These smaller particles were phagocytosed by macrophages and did not appear to have a negative influence on healing. In conclusion, SiCaP granules of 250–500 μm in size may be a more suitable scaffold when used as an injectable bone filler and may be a convenient method for treating bony defects. © 2013 The Authors. Journal of Biomedical Materials Research Part B: Applied Biomaterials Published by Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater 101B: 902–910, 2013
Keywords: bone regeneration, silicate-substituted calcium phosphate, particle size, animal model
INTRODUCTION
Silicate-substituted calcium phosphate has been used clinically in place of autograft and allograft and reported to further augment bone formation when compared with stoichiometric hydroxyapatite (HA).1 The purpose of a bone graft substitute material is to enhance bony regeneration within the defect area. This new bone should ideally penetrate and replace the graft through sequential remodelling, enabling the repair site to maintain an optimal balance between form, and function.2 A limitation with current CaP bone substitute materials is that they exist in a large granular form, limiting it's handling ability during surgery.3 Recently, injectable and moldable forms of bone substitute material have been developed as they offer many advantages including increased handling ability during surgery and due to their ability to completely fill contained defects of complex geometric shapes.4 In addition, with the development of minimally invasive surgical methods, directly injectable biomaterials are required.
The mechanisms behind bone's response to CaP have not yet been fully established however a number of studies have demonstrated that osteointegration, osteoinduction, and osteoconduction are influenced by morphology, crystallinity, porosity, surface roughness, chemistry, and calcium to phosphate ratio.5–7 Granule size is also thought to be important as it has an effect on the rate of resorption and the ability of the material to promote bone growth.8 Previous studies have shown that a minimum space of 100 μm between graft particles is essential to allow for neo-vascularisation and bone formation and that when the spaces become larger, bone growth increases.9–11 Particle sizes of about 380 μm in diameter will yield this minimal dimension of inter-particular space.12 A study by Yukna et al.11 reported that particle sizes between 300 and 500 μm provided adequate space between the particles and were optimum for the treatment of periodontal bone defects, however a study by Felipe et al.10 found that bioglass particles of smaller size range underwent faster resorption and were subsequently replaced by more new bone also shown in canine periodontal defects. Oonishi et al.13 evaluated the osteointegration of HA granules of 1–3 μm, 10 μm, and 100–300 μm in diameter and reported that a minimal size of 10 μm was necessary for direct bone contact between bone and the HA granules. A study by Kuroda et al.14 investigated bone defects filled with HA granules of varying sizes. They reported improved osteoconductive activity in the group with granules 100–300 μm in size when compared with larger sized particles of 1–2 mm. Sun et al.15 also investigated the effect of varying sizes of HA granules (from 0.5 to 841 μm) in osteoblast cultures and reported inhibitory effects for sizes ranging from 0.5 to 3 μm.
The aim of this study was to investigate injectable silicate-substituted calcium phosphate bone substitute materials and the influence of granule size on the regeneration of bone within cancellous critical-sized defects in an ovine model. We hypothesised that small particles of SiCaP implanted in a poloxamer paste would provide a larger surface area thereby inducing more bone formation both in direct contact with the SiCaP surface and within the spaces in between.
MATERIALS AND METHOD
Thirty-two implants were inserted into one of four cylindrical critical-sized defects created in the medial femoral condyle of 10 skeletally mature female commercially cross-bred sheep weighing between 65 and 80 kg and aged between 2 and 5 years. Positions were rotated such that no implant was placed in the same location in each sheep more than once. Repeats of four implants in each group at both 4 and 8 weeks were investigated. All procedures were carried out following Ethics approval granted by the Royal Veterinary College and in compliance with United Kingdom Home Office regulations [Animal Scientific Procedures Act (1986)]. Porous ceramic foams with varied strut porosity were manufactured via a foaming route where ceramic slurries comprising SiCaP powders, polyvinyl alcohol, and double distilled water were mechanically foamed in a ball mill. The resulting foamed slip was then cast into a mould and dried in air at a temperature of 40–50°C for up to 48 h. The dried green castings were then sintered at temperatures of 1200–1300°C for 2 h. The sintered porous monoliths were removed from the sintering furnaces and mechanically crushed between two ceramic-coated paddles. The sintered material was subsequently crushed and sieved to make irregular granules of different size that were of exactly the same chemistry and morphology. Therefore SiCaP scaffolds in each experimental group were identical and differed only in the size of granules produced. Prior to insertion, each of the granules in TG2, TG3, and TG4 were combined with poloxamer 407 to allow easier handling of the granules and to prevent their migration out of the defect. In TG1, a volume of 100% granules (no carrier) was inserted into defects. All granules were supplied by ApaTech, (Centennial Park, Elstree, UK). The four treatment groups investigated are shown in Table I.
TABLE I.
A Table Showing Details of the Four Treatment Groups Investigated
| Test Material | Abbrev. | Volume of granules in Poloxamer 407 | SiCaP Granule Size (μm) |
|---|---|---|---|
| Treatment Group 1 | TG1 | 100% (no carrier) | 1000–2000 |
| Treatment Group 2 | TG2 | 75% | 250–500 |
| Treatment Group 3 | TG3 | 75% | 90–125 |
| Treatment Group 4 | TG4 | 50% | 90–125 |
The silicate-substituted calcium phosphate (substituted with 2.6 wt % silicate or 0.8 wt % Si) used in this study was phase-pure with a total macroporosity of 80% and a microporosity of 10%. The average pore diameter of the macropores was 300 μm, and micropore diameter within the strut porosity was less than 50 μm where pores typically ranged between 1 and 10 μm. The volume of closed strut pores was <10%. Phase purity of the materials was confirmed by X-ray diffraction (XRD) as described previously.16 The calcium, phosphorus, and silicon contents were determined by X-ray fluorescence (XRF) also as previously described.16
Surgery
Animals were placed in ventral recumbence and an incision ∼3 cm in length made over the right femoral condyle. The muscle was exposed and parted by blunt dissection and two critical size 8 × 15 mm2 holes made.17 Following irrigation, the appropriate test material was applied using a syringe and gently pressed into place. In TG1, granules were gently poured into the defect. The wound was then closed. Sites were allocated to treatment group and test materials rotated between animals such that no animal had two test materials of the same type. This procedure was repeated on the contralateral side resulting in a total of four implants inserted in each animal. Following surgery, animals were allowed immediate postoperative mobilisation as tolerated. Antibiotic and analgesic prophylaxis was administered daily with subcutaneous injections of Baytril (Enrofloxacin 5 mg/kg; Bayer AG Leverkusen) and Finadyne (Flunixin Meglumine 2 mg/45 kg; Schering-Plough) for 3 days post-surgery. Animals were kept in individual pens for 1 week post operatively before being group housed. Implants remained in vivo for 4 and 8 weeks.
Histological analysis
On retrieval, condyles were immediately placed in 4% paraformaldehyde solution for 3 days before being processed for undecalcified histology. After dehydration in alcohol and defatting in chloroform, specimens were embedded in hard grade acrylic resin (LR White, London Resin Company, Reading, UK) following dehydration in serial dilutions of alcohol. A longitudinal thin section (∼60 μm thick) was prepared through the centre of each defect using grinding and polishing techniques (EXAKT, Norderstedt, Germany) and sections stained with Toluidine Blue and Paragon respectively. A total of 8 images of each defect were captured using a 5× objective lens onto the image analysis system (Axiovision 4.5; Carl Zeiss, Jena, Germany) (Figure 1). The line intercept method was used and a mask of interconnecting lines measuring 10 x 12 mm was placed over the image and the type of material (mineralised bone, soft tissue or implant material) at the intersection of each line was determined (total 225 intercept points per image). Assessments were used to evaluate the proportion of mineralised bone formed, soft tissue and implant material within each defect and at each of the 8 regions. For bone-implant contact, the type of tissue at the interface was determined at the points where lines intersected with the CaP biomaterial surface. Quantification of bone in pores > 20 μm were measured. Bone in pores < 20 μm were not measured as these sized pores occurred predominantly within the struts of the scaffold. Data was quantified and compared between groups.
FIGURE 1.
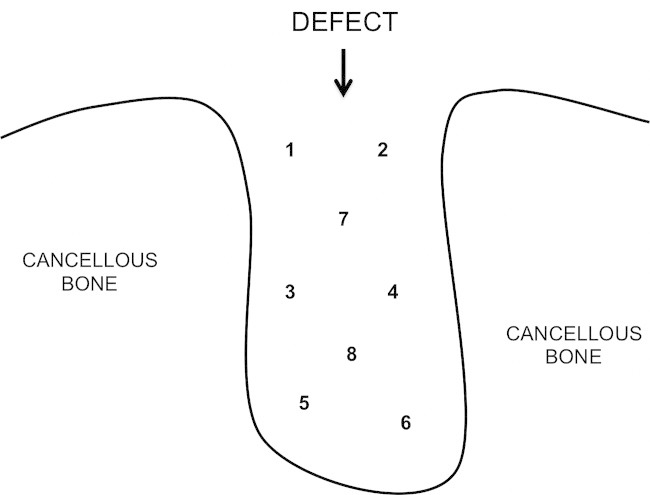
A diagram of a defect showing how the regions were divided for analysis.
Scanning electron microscopy
Thin sections were sputter-coated with a layer of gold palladium and viewed using backscattered Scanning Electron Microscopy (JSM-35C; Jeol, Welwyn Garden City, United Kingdom).
Statistics
All data collected from the study was input into a statistical software package (version 10.1; SPSS, Chicago, IL) for analysis. A Kolmogorov-Smirnov test showed that all data was nonparametric and a Mann Whitney U test was used to compare results obtained in the different experimental groups; p values < 0.05 were considered significant. Mean values are presented with standard error of mean.
RESULTS (4 WEEKS)
New bone formation
At 4 weeks results showed new bone formation within defects in all experimental groups. Highest bone values were measured in the TG2 group (13.32 ± 1.38%) when compared with TG3: 1.00 ± 0.16%; p = 0.021) and TG4: 3.28 ± 0.53%; p = 0.021). No significant difference was observed when TG2 data was compared with TG1 (7.64 ± 2.67%) [Figure 2(A)]. Significantly increased bone was measured in TG4 when compared with TG3 specimens (p = 0.021) and no significant difference was found when TG1 and TG3 were compared.
FIGURE 2.
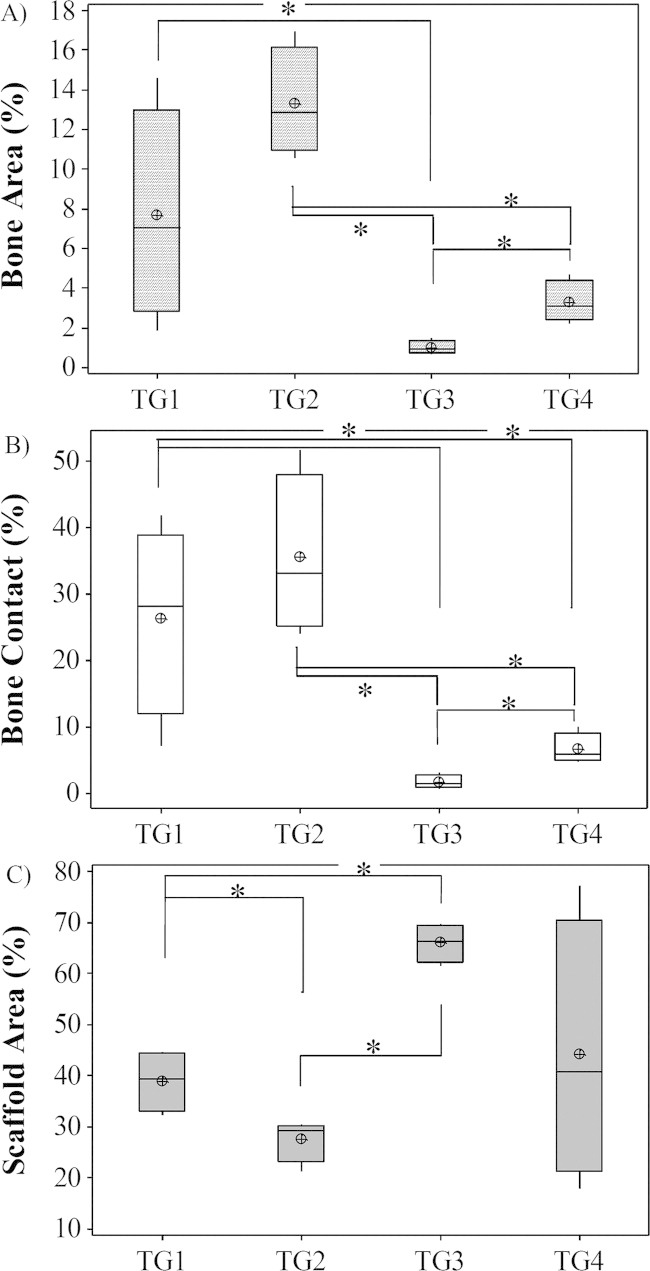
A Graph Comparing Bone area, contact and scaffold area in Defects at 4 Weeks post surgery: A) Bone area; B) Bone contact and C) Scaffold area (*p<0.05).
Bone-implant contact
Bone contact results showed that significantly increased contact values were found in TG2 (35.49 ± 6.03%) when compared with TG3 (1.75 ± 0.54%; p = 0.021) and TG4 (6.63 ± 1.15%; p = 0.021). No significant difference was found when this group was compared with TG1 (26.37 ± 7.17%). Significantly increased bone contact was seen in TG1 when compared with both TG3 (p = 0.021) and TG4 (p = 0.043). Significantly increased contact was measured in the TG4 group when compared with TG3 (p = 0.021) [Figure 2(B)].
Remaining implant area
Results showed significantly increased amounts of SiCaP present in TG3 defects (65.93 ± 1.88%) when compared with both TG2 (27.54 ± 2.07%; p = 0.021) and TG1 (38.89 ± 2.87%; p = 0.021). There was no significant difference in the amount of SiCaP present when TG3 and TG4 (44.12 ± 12.81%) were compared and when TG4 defects were compared with TG1 [Figure 2(C)].
RESULTS (8 WEEKS)
New bone formation
Results at 8 weeks showed that total bone area had significantly increased within defects in all groups investigated when compared with the 4-week time point (p = 0.021 in all cases). At 8 weeks, the highest increase in bone was measured in TG4 (42.78 ± 3.36%) and lowest in TG3 (30.36 ± 3.49%) (TG1: 34.95 ± 1.73%; TG2: 36.50 ± 2.30%). Results showed no significant differences between groups except when TG3 and TG4 specimens were compared (p = 0.043) [Figure 3(A)].
FIGURE 3.
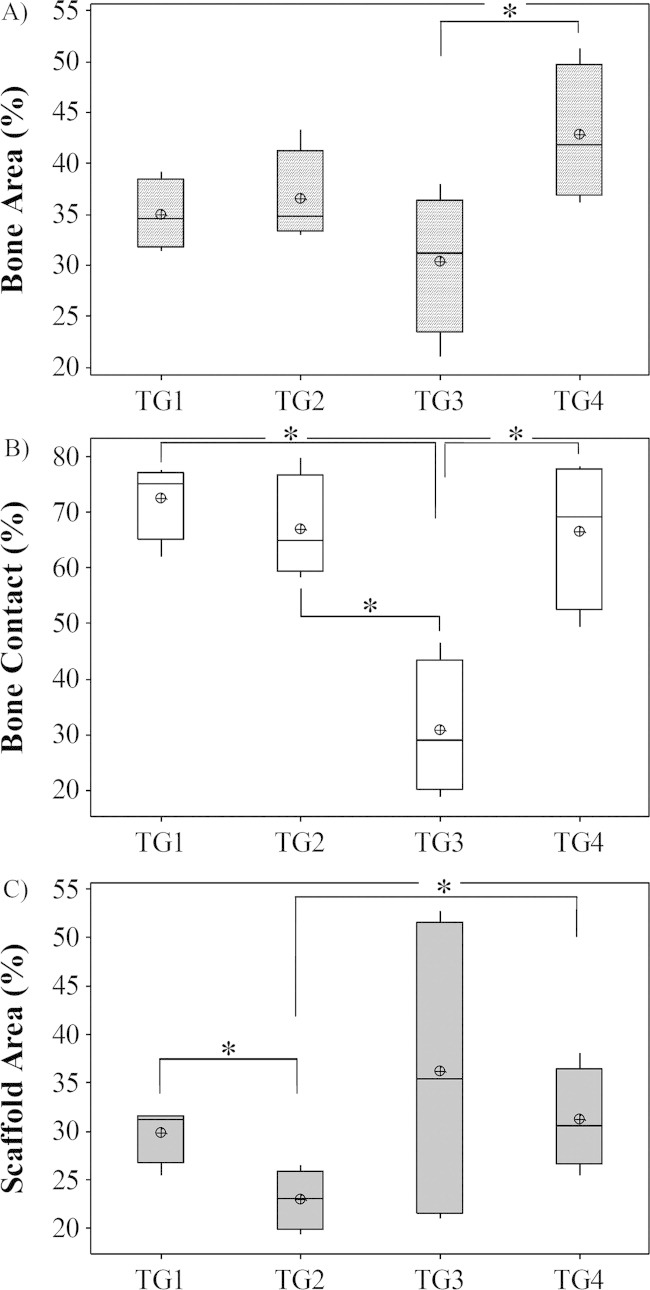
A Graph Comparing Bone area, contact and scaffold area in Defects at 8 weeks post surgery: A, Bone area; B, Bone contact; C, scaffold area (*p < 0.05).
Bone-implant contact
Percentage bone contact also significantly increased at this time point when compared with the 4 week data in all groups (p = 0.021 in all cases). At 8 weeks, highest values were measured in TG1 (72.63 ± 3.51%) and lowest in TG3 specimens (30.87 ± 6.15%) (TG2: 67.09 ± 4.66%; TG4: 66.54 ± 6.74%). Significantly increased bone contact was seen in all groups when compared with TG3 (p = 0.021 in all cases) [Figure 3(B)]. No other significant differences between groups were identified.
Remaining implant area
In all groups the amount of SiCaP scaffold present within the defect had decreased when compared with data obtained 4 weeks post surgery, however a significant decrease was seen only within TG1 (29.89 ± 1.48%) (p = 0.021) and TG3 (36.15 ± 8.27%) defects (p = 0.021). Results showed that significantly more scaffold was present in TG1 when compared with TG2 (23.00 ± 1.55%) (p = 0.043) and significantly increased SiCaP was present in TG4 (31.22 ± 2.62%) when compared with TG2 defects (p = 0.043) [Figure 3(C)]. No other significant differences were found at this time point. The largest implant resorption rate was measured in the TG3 group with a loss of 45.17% of scaffold over the 4-week period. The amount of SiCaP present in TG1 had decreased by 23.14%, TG2 by 16.49%, and TG4 by 29.24%.
Light and backscattered electron microscopy
At 4 weeks post surgery and in all experimental groups, a trend was seen where areas within the proximal aspect of the defect and regions close to the periphery showed increased new bone formation when compared with regions located within the centre of the defect. In areas close to the periphery, some granules were surrounded by bone however few areas of new bone formation were observed within the centre of the defect. In all groups bone formation appeared to occur by direct intra-membranous ossification without the formation of chondroid tissue. Osteoblasts were seen actively depositing bone (Figure 4). In all groups, bone appeared to be “conducted” along the surface of the implant and in many places new bone had bridged SiCaP granules together. Areas of vascular invasion were observed within soft tissue and throughout the defect in all groups. In TG2, SiCaP granules were notably smaller when compared with TG1 and notably larger when compared with TG3 and TG4 (Figures 5 and 6). Due to the smaller size of the granules in the TG3 and TG4 groups, graft within the defects appeared more evenly distributed and more densely packed. In addition and in these two groups, light microscopy showed thick basinophylic collagen fibres branching between the SiCaP granules forming a network onto which intramembraneous bone formed (Figure 7). At 8 weeks and in all groups, the majority of granules throughout each defect were in direct contact with and surrounded by mature lamellar bone (Figure 8). Bone formation was seen both within the macro and strut porosity. As particle size decreased, the amount of particulate ceramic debris increased and these areas were encapsulated by soft tissue. Macrophages were observed phagocytosing particulate debris however, their presence did not seem to have an adverse effect on bone growth and in many instances, osteoblasts could be seen actively depositing osteoid immediately adjacent to particulate SiCaP.
FIGURE 4.
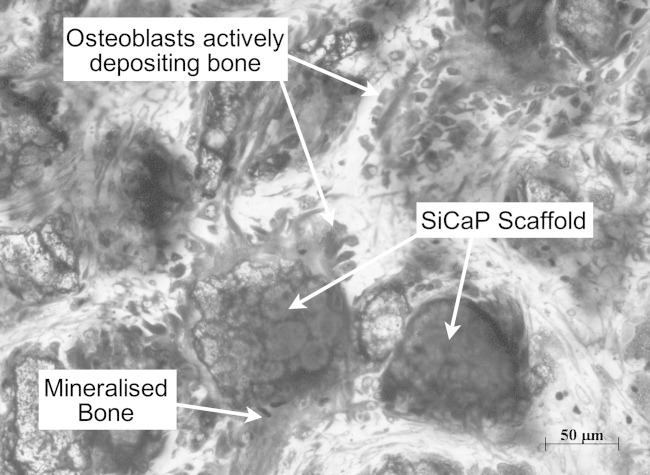
Osteogenesis at the surface of SiCaP granules in TG3 located at the centre of the defect 4 weeks post-implantation (Mag. 20×).
FIGURE 5.
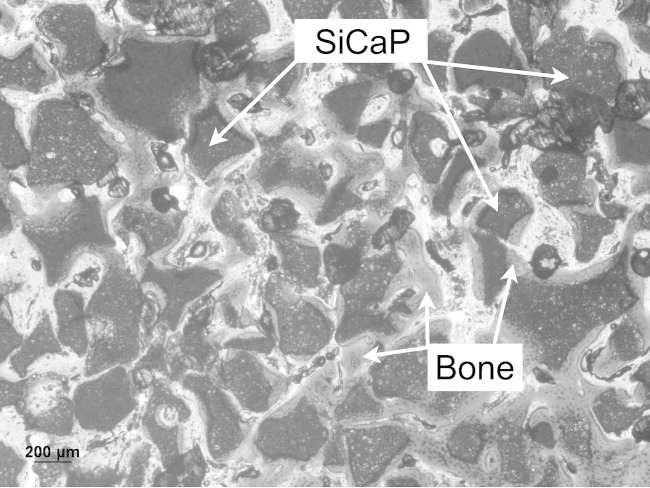
New bone growth at 4 weeks post surgery within TG1 and at region close to the periphery of the defect (Mag. 5×).
FIGURE 6.
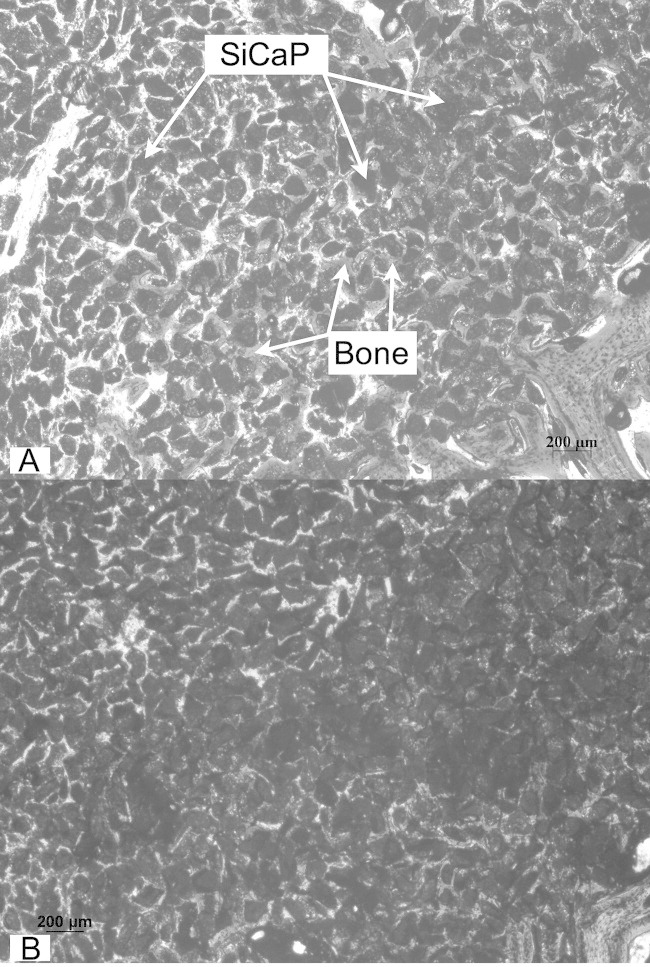
(a,b) A low-powered image of densely packed TG4 granules at a region close to the bottom of the defect showing intergranular bone formation despite tight packing at 4 weeks (Mag. fig a: 5×; fig b: 2.5×).
FIGURE 7.
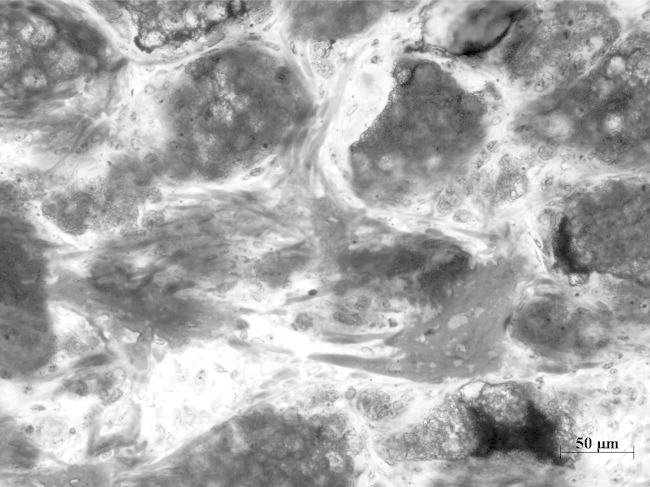
At 4 weeks collagen fibres branch between packed TG4 granules onto which bone is directly deposited. (Mag. 2.5×).
FIGURE 8.
A: A montage of SEM images through the defect of a TG2 specimen in the anterior/posterior direction at 4 weeks. B: A montage of SEM images through the defect of a TG3 specimen in the anterior/posterior direction at 4 weeks. In comparison to (A), the particle size is notably smaller. C: A montage of SEM images through the defect of a TG2 specimen in the anterior/posterior direction at 8 weeks.
In all groups at 4 weeks, backscattered scanning electron microscopy showed new bone formation within the defects with most bone growing preferentially along the surface of SiCaP granules and in some cases, especially in regions close to the periphery, bridging granules together. Large proportions of the central defect in all groups were devoid of bone at 4 weeks. SEM also showed the difference in granule size between groups (Figure 9). In places where bone formation had occurred, bone made contact with the outer surface of granules and also at times, within the pore structure. In all groups at 8 weeks, scanning electron microscopy showed mature lamellar bone surrounding SiCaP granules with extensive bone growth throughout the defects. In many instances and in all groups, Haversian systems lay adjacent the graft material. In two dimensions it was evident that bone was able to use the SiCaP as a scaffold to bridge bone within the defect. In both TG1 and TG2 packed defects isolated SiCaP granules were not seen and all were in contact through a bone bridge with others. However in both TG3 and TG4, isolated granules (some of which were surrounded by bone) were evident. Bone was in contact with the outer SiCaP surface and within the microporosity of the structure and also occurred in pores as small as 20 μm. In TG3 and TG4 at 8 weeks, bone formation was less uniform than in the other groups and although bone had bridged the entire defect, bone formation was greater towards the walls adjacent to the host bone.
FIGURE 9.
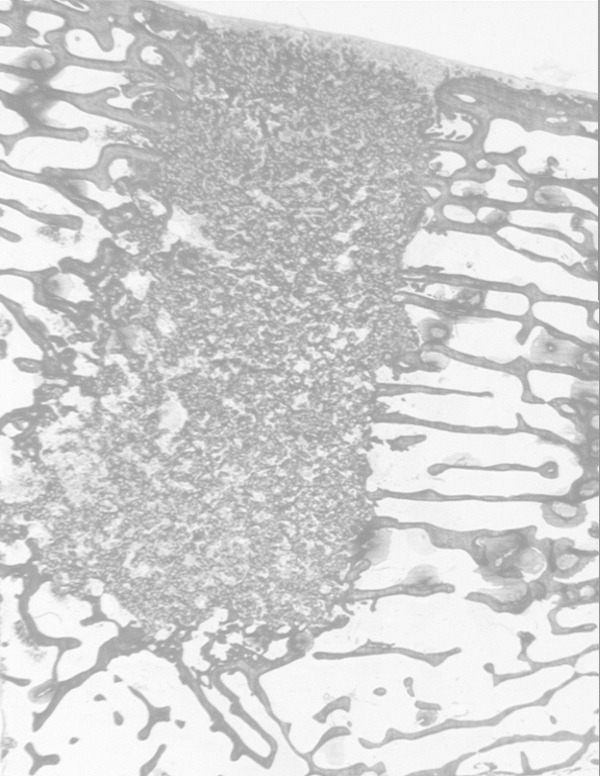
TG3 implant at 8 weeks duration showing soft tissue (S) enclosing defect site without any evidence of inflammation. The defect site is a mixture of bone and SiCaP granules distributed evenly throughout the site.
DISCUSSION
The aim of this study was to investigate the effect of granule size on the osteointegration of injectable silicate-substituted calcium phosphate in an ovine critical-sized femoral condyle defect model. The repair of critical-sized bone defects is challenging in the fields of maxillofacial and orthopaedic surgery and current therapies such as autografts and allografts are associated with various limitations.18–22 The repair rate of a bony defect is mainly dependent on defect size and osteogenesis is associated with migration of osteoblasts. Even in larger stable defects the faster migrating fibroblasts induce more fibrous tissue which becomes dominant in the bone defect.23 The critical-sized bone defect is defined as the smallest size intraosseous wound that will not spontaneously heal completely with bone tissue.23 In the ovine model this size is 8 × 15 mm2 and these defects are well suited for the placement of materials for the evaluation of the bone healing response to bone substitutes.17
In our study, four experimental groups containing silicate-substituted calcium phosphate of different granule size were investigated. Qualitative analysis using light microscopy identified new bone formation in all groups at both the 4- and 8-week time-points. In addition, no poloxamer carrier was identified in any of the defects at either time point. Light microscopy also showed good vascularisation in all groups with blood vessels evident both adjacent to granules and within the macro pores of the scaffold. No obvious differences were seen in the amount of vascularisation within any of the groups. Many areas of osteogenesis were seen adjacent to the granules with osteoblasts observed actively laying down bone. Light microscopy also identified collagen fibres in between granules onto which osteoid was deposited and subsequently mineralised. At both the 4 and 8-week time point, osteogenesis occurred both within the macropores and adjacent to the scaffold surface with no evidence of cartilage matrix deposition in any of the groups. Even with small granules bone formation occurred even though the number of macropores was reduced. This implies that macropore structure is not necessary for bone formation. A number of articles have indicated that porous bone graft substitute materials form bone preferentially on the concave surface of the pore.24–26 However, in our study due to the small size of the granules, the porous structure was reduced but bone formation still occurred indicating that macropores are not necessary provided an appropriate packing density between granules that allows bone formation.
A study by Oreffo et al.27 showed that newly differentiated osteoprogenitor cells are more important and known to make a major contribution to bone defect filling and tissue repair when compared with pre-existing osteoblasts. A study by Weiβenbock et al.28 investigated the cellular response of human mesenchymal stem cells (hMSC's) on HA of different particle size in vitro and showed that hMSCs cultured on particles smaller than 100 μm showed highest cell viability and highest levels of alkaline phosphatase release. However, granule size had no significant effects on the up-regulation of osteocalcin and osteopontin in these cells. They concluded that substrate signals have a substantial effect on the phenotypic characteristics of a multipotent cell type, even in the presence of strong osteogenic inductive signals, and that the physical size of the biomaterial carrier may be crucial in the repair of tissues. However, studies have also shown that HA debris can cause inflammatory reactions and that small particles of HA (<20 μm) with an irregular geometry were reported to possess a cytotoxic potential by inducing strong inflammatory reactions compared with larger sized particles >20 μm.15 An in vitro study by Evans et al.29 measured an increase in the fibroblast mitotic rate in the presence of synthetic HA particles between 3.7 and 99 μm. An in vivo study by Oonishi et al.13 demonstrated that particles larger than 10 μm were required for successful bone augmentation. In our study and 8 weeks post surgery, increased bone was measured in defects adjacent to granules 90 – 125 μm in size however fragmentation of SiCaP (< 20 μm) was also evident in this smallest sized granule group (TG3 and 4). Less fragmentation was observed in the largest granule group (TG1). Where areas of fragmentation were seen, bone did not grow and particles were instead encapsulated within soft tissue. In localised regions particles were engulfed by macrophages, however SiCaP fragmentation did not impede new bone formation as osteoblasts were seen depositing bone immediately adjacent to particulate debris.
The bioactivity of calcium phosphate materials is believed to be associated with material dissolution and the release of calcium and phosphate ions from the implant surface followed by the precipitation of a biological apatite layer.30 Consequently it is thought that the surface rapidly absorbs more proteins and promotes the adhesion of osteoblasts in particular.31 Decreasing the size of the granules resulted in more space for granules and increased SiCaP surface area within the defect. This larger surface area may have provided an increase in dissolution of Ca and P ions leading to greater apatite deposition and more protein adsorption, osteoblast adhesion and increased bone growth.28 Resorption of the scaffold has a large influence on bone-bonding properties and the degradation characteristics of CaPs are governed by the chemical composition, crystal structure, crystal and grain size, microporosity, neck geometry, and neck dissolution rates of the material.13 In our study, the SiCaP investigated was identical in composition and manufacture and granule size was the only variable investigated. A study by Felipe et al.10 showed distinct dissolution rates for Bioglass of different size ranges. Results from the study reported a statistically significant difference favouring bone growth adjacent to granules 300–355 μm in size when compared with those of 90–710 μm in size. Bioglass particles of smaller size underwent faster resorption and substitution by new bone than did granules with a larger variation in size. A study by Shapoff et al.8 in monkey femurs, reported that granules of 100–300 μm resulted in significantly increased bone formation when compared with growth adjacent to 1000–2000 μm sized granules. Hall et al.32 compared Bioglass granules with small and large size ranges showing no significant difference in the amount of bone formed and in contact with the material surface after 4 months in the treatment of intrabony defects in the canine mandible. Results obtained by Hall et al. led Felipe et al. to speculate and conclude in their study that granule size may not affect bony formation during longer healing periods (>3 months) however, granules of a small size range leads to more rapid resorption and more rapid new bone formation at early time points following surgery. This is in contrast to results obtained in our study which showed that the smaller granules did not induce more rapid bone regeneration within the defect at 4 weeks when compared with the larger granules investigated. In addition, our study also demonstrated that the group showing highest amount of implant resorption over the 4–8 week time point [TG3 (45.17%)] also demonstrated least amounts of bone formation and bone-implant contact at 8 weeks when compared with all other groups.
A study by Fischer et al.33 investigated hydroxyapatite granules of varying size (212–300 μm and 500–706 μm) both in vitro and in vivo and reported abundant de novo bone formation adjacent to granule size 212–300 μm, whereas particles with a diameter of 500–706 μm showed no bone formation. This study concluded that not only is granule size important for bone formation to occur but also the three-dimensional architecture and microenvironment created by packing of the granules. Fischer et al. suggest that this 3D environment created by the packing of the granules provides a macroporosity and free space for invasion by body fluids, nutrients, host cell and blood vessels and that differences in bone formation in HA granules of different size may be linked to granule packing and that granules 500–706 μm in size created a macroporosity that was unfavorable for bone formation. Previous studies have demonstrated the significant effect that inter-granular space has on bone formation.6, 7 Our study did not quantify the packing spaces generated, but nevertheless osteogenesis was identified within the granular spaces in all groups. It is estimated that the inter-granular space was least in TG3 specimens (range ∼10–300 μm with an average of ∼50 μm between granules) as the smaller granules were packed together more tightly within the defect and largest in TG1 (range ∼100–300 μm with an average of ∼100 μm). It is also possible that the inter-granular space increased over time as the implant resorbed thereby influencing the degree and extent of bone formation within the different granules sizes. Qualitative observation showed a reduction in granule size however the shape of granules appeared to remain the same over the 8-week period. Further work is necessary to investigate the effect of inter-granular space. Nevertheless, results from our study showed that speed of bone growth was initially more rapid adjacent to larger particles however at a later stage, the smallest granules induced more bone formation. Clearly from our study there are a number of factors that affect bone formation and it appears that the size and packing density between granules is an important parameter.
CONCLUSIONS
The options for bone grafts used clinically are numerous and this study aimed to improve our knowledge of the biology and physiology that controls bone formation within SiCaP scaffolds. Although TG1 contained no polaxomer carrier, this group was included in this study as it represented graft material that is used clinically and therefore acted as control to our experimental groups. The results of this study showed that all grafts investigated are potentially suitable as a bone graft substitute material and at 8 weeks the smallest granule size encouraged increased amounts of bone within the defect. However, this study also showed that these small granules were also associated with higher amounts of SiCaP fragmentation and while they did not appear to have a negative influence on healing and bone formation, they may take up valuable space in which new bone could grow. Therefore, for more beneficial long-term results, particles sized 250–500 μm may be most advantageous to use. The SiCaP granules used in this study in combination with a poloxamer paste allows the injection of granules into defects which is able to induce bone formation rapidly and is an important option for the application of bone graft substitutes.
ApaTech Ltd supplied the graft materials and funded this study. Authors from the John Scales Centre were involved in the study design, data collection, analysis and interpretation. Authors who work for ApaTech Ltd have received payments or services either directly or indirectly (via ApaTech Ltd) in support of this work. No author has had any other relationships, or has engaged in any other activities that could be perceived to influence what is written in this work.
REFERENCES
- 1.Hing KA, Wilson LF, Buckland T. Comparative performance of three ceramic bone graft substitutes. Spine J. 2007;7:475–490. doi: 10.1016/j.spinee.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Vallet-Regi M, Arcos D. Silicon substituted hydroxyapatites: A method to upgrade calcium phosphate based implants. J Mater Chem. 2005;15:1509–1516. [Google Scholar]
- 3.Low KL, Tan SH, Sein SHS, Roether JA. Calcium phosphate-based composites as injectable bone substitute materials. J Biomed Mater Res Part B: Appl Biomater B. 2010;94:273–286. doi: 10.1002/jbm.b.31619. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Oh JH, Han I, Kim H-S, Chung SW. Grafting using injectable calcium sulphate in bone tumour surgery: Comparison with dimineralised bone matrix-based grafting. Clinics Orthop Surg. 2011;3:191–201. doi: 10.4055/cios.2011.3.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Dennison D, Ong JL. Protein adsorption and osteoblast precursor cell attachment to hydroxyapatite of different crystallinities. Int J Oral Maxillofac Implants. 2005;20:187–192. [PubMed] [Google Scholar]
- 6.Takeshita F, Ayukawa Y, Iyama S, Suetsugu T, Oishi M. Histological comparison of early wound healing following dense hydroxyapatite granule grafting and barrier placement in surgically created bone defects neighbouring implants. J Periodontol. 1997;68:924–932. doi: 10.1902/jop.1997.68.10.924. [DOI] [PubMed] [Google Scholar]
- 7.Habibovic P, Yuan H, van der Valk CM, Meijer G, van Blitterswijk CA, de Groot K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials. 2005;26:3565–3575. doi: 10.1016/j.biomaterials.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 8.Shapoff CA, Bowers GM, Levy B, Mellonig JT, Yukna RA. The effect of particle size on the osteogenic activity of composite grafts of allogeneic freeze-dried bone and autogenous marrow. J Peridontol. 1980;51:625–630. doi: 10.1902/jop.1980.51.11.625. [DOI] [PubMed] [Google Scholar]
- 9.Zaner DJ, Yukna RA. Particle size of peridontal bone grafting materials. J Peridontol. 1984;55:406–409. doi: 10.1902/jop.1984.55.7.406. [DOI] [PubMed] [Google Scholar]
- 10.Felipe ME, Andrade PF, Novaes AB, Jr, Grisi MFM, Souza SLS, Taba M Jr, Palioto DB. Potential of bioactive glass particles of different size ranges to affect bone formation in interproximal periodontal defects in dogs. J Periodontol. 2009;80:808–815. doi: 10.1902/jop.2009.080583. [DOI] [PubMed] [Google Scholar]
- 11.Yukna RA. Synthetic bone grafts in periodontics. Periodontol 2000. 1993;1:92–99. [PubMed] [Google Scholar]
- 12.Hirschorn JS, McBeath AA, Dustoor MR. Porous titanium surgical implant materials. J Biomed Mater Res Sympos. 1971;2:49. [Google Scholar]
- 13.Oonishi H, Hench LL, Wilson J. Comparative bone growth behaviour in granules of bioceramic materials of various sizes. J Biomed Mater Res. 1999;44:31–43. doi: 10.1002/(sici)1097-4636(199901)44:1<31::aid-jbm4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Kuroda T. Bone formation and mechanical properties of the cancellous bone defect site filled with hydroxyapatite granules. Nippon Seikeigeka Gakkai Zasshi. 1995;69:1037–1049. [PubMed] [Google Scholar]
- 15.Sun JS, Liu HC, Chang WH, Li J, Lin FH, Tai HC. Influence of hydroxyapatite particle size on bone cell activities: An in vitro study. J Biomed Mater Res. 1998;39:390–397. doi: 10.1002/(sici)1097-4636(19980305)39:3<390::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Gibson IR, Best SM, Bonfield W. Chemical characterization of silicon-substituted hydroxyapatite. J Biomed Mater Res. 1999;44:422–428. doi: 10.1002/(sici)1097-4636(19990315)44:4<422::aid-jbm8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Patel N, Brooks A, Clarke MT, Lee MT, Rushton N, Gibson IR, Best SM, Bonfield W. In vivo assessment of hydroxyapatite and silicate-substituted hydroxyapatite granules using an ovine defect model. J Mater Sci Mater Med. 2005;16:429–440. doi: 10.1007/s10856-005-6983-6. [DOI] [PubMed] [Google Scholar]
- 18.Tsiridis E, Spence G, Gamie Z, El Masry MA, Giannoudis PV. Grafting for periprosthetic femoral fractures: Strut, impaction or femoral replacement. Injury. 2007;38:688–697. doi: 10.1016/j.injury.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 19.Kwon B, Jenis LG. Carrier Materials for spinal fusion. Spine J. 2005;5:224S–230S. doi: 10.1016/j.spinee.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Fellah BH, Gauthier O, Weiss P, Chappard D, Layrolle P. Osteogenicity of biphasic calcium phosphate ceramics and bone autograft in a goat model. Biomaterials. 2008;29:1177–1188. doi: 10.1016/j.biomaterials.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Boyce T, Edwards J, Scarborough N. Allograft bone: the influence of processing on safety and performance. Bone Graft Bone Graft Sub. 1999;30:571–581. doi: 10.1016/s0030-5898(05)70110-3. [DOI] [PubMed] [Google Scholar]
- 22.Pelker RR, Friedlaender GE. Biomechanical aspects of bone autografts and allografts. Orthop Clin North Am. 1987;18:235–239. [PubMed] [Google Scholar]
- 23.Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299–308. [PubMed] [Google Scholar]
- 24.Ripamonti U. Molecular signals in geometrical cues sculpt bone morphology. S Afr J Sci. 2004;100:355–367. [Google Scholar]
- 25.Ripamonti U, Richter PW, Nilen RWN. Induction of bone formation by smart biphasic hydroxyapatite tricalcium phosphate biomimetic matrices in the non-human primate Papio ursinus. 33. J Cell Mol Med. 2008;12:2609–2621. doi: 10.1111/j.1582-4934.2008.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ripamontu U, Roden LC, Ferretti C, Klar RM. Biomimetic matrices self-initiating the induction of bone formation. 2011;22:1859–1870. doi: 10.1097/SCS.0b013e31822e83fe. [DOI] [PubMed] [Google Scholar]
- 27.Oreffo ROC, Driessens FC, Planell JA, Triffitt JT. Growth and differentiation of human bone marrow osteoprogenitors on novel calcium phosphate cements. Biomaterials. 1998;20:1845–1854. doi: 10.1016/s0142-9612(98)00084-2. [DOI] [PubMed] [Google Scholar]
- 28.Weißenbock M, Stein E, Undt G, Ewers R, Lauer G, Turhani D. Particle size of hydroxyapatite granules calcified from red algae affects the osteogenic potential of human mesenchymal stem cells in vitro. Cells Tissues Organs. 2006;182:79–88. doi: 10.1159/000093062. [DOI] [PubMed] [Google Scholar]
- 29.Evans EJ. Toxicity of hydroxyapatite in vitro: The effect of particle size. Biomaterials. 1991;12:574–576. doi: 10.1016/0142-9612(91)90054-e. [DOI] [PubMed] [Google Scholar]
- 30.Porter AE, Buckland T, Hing K, Best SM, Bonfield W. The structure of the bond between bone and porous silicon-substituted hydroxyapatite bioceramic implants. J Biomed Mater Res. 2006;78:25–33. doi: 10.1002/jbm.a.30690. [DOI] [PubMed] [Google Scholar]
- 31.Gregoire M, Orly I, Manankau J. The influence of calcium-phosphate biomaterials on human bone cell activates. An in vitro approach. J Biomed Mater Res. 1990;24:165–177. doi: 10.1002/jbm.820240204. [DOI] [PubMed] [Google Scholar]
- 32.Hall EE, Meffert RM, Hermann JS, Mellonig JT, Cochran DL. Comparison of bioactive glass to demineralised freeze-dried bone allograft in the treatment of intrabony defects around implants in the canine mandible. J Periodontol. 1999;70:526–535. doi: 10.1902/jop.1999.70.5.526. [DOI] [PubMed] [Google Scholar]
- 33.Fischer EM, Layrolle P, Van Blitterswijk CA, De Bruijn JD. Bone formation by mesenchymal progenitor cells cultured on dense and microporous hydroxyapatite particles. Tiss Eng. 2003;9:1179–1188. doi: 10.1089/10763270360728080. [DOI] [PubMed] [Google Scholar]



