Abstract
Controlling meal-related glucose excursions continues to be a therapeutic challenge in diabetes mellitus. Mechanistic reasons for this need to be understood better to develop appropriate therapies. To investigate delayed gastric emptying effects on postprandial glucose turnover, insulin sensitivity, and β-cell responsivity and function, as a feasibility study prior to studying patients with type 1 diabetes, we used the triple tracer technique C-peptide and oral minimal model approach in healthy subjects. A single dose of 30 μg of pramlintide administered at the start of a mixed meal was used to delay gastric emptying rates. With delayed gastric emptying rates, peak rate of meal glucose appearance was delayed, and rate of endogenous glucose production (EGP) was lower. C-peptide and oral minimal models enabled the assessments of β-cell function, insulin sensitivity, and β-cell responsivity simultaneously. Delayed gastric emptying induced by pramlintide improved total insulin sensitivity and decreased total β-cell responsivity. However, β-cell function as measured by total disposition index did not change. The improved whole body insulin sensitivity coupled with lower rate of appearance of EGP with delayed gastric emptying provides experimental proof of the importance of evaluating pramlintide in artificial endocrine pancreas approaches to reduce postprandial blood glucose variability in patients with type 1 diabetes.
Keywords: gastric emptying, insulin action, pramlintide
glucose metabolism after a mixed meal is influenced by alterations in meal factors such as meal content and composition (14), humoral factors such as insulin/glucagon secretion and action (hepatic and peripheral) (2, 13), and gastrointestinal motility factors such as rate of gastric emptying (17, 32, 43). To understand the contribution of key factors controlling postprandial glucose metabolism, studies need to be performed with manipulation of determinants such as gastrointestinal motility while sophisticated techniques to precisely quantify glucose turnover and insulin action are used. Prior studies in humans with and without diabetes (20, 21, 26, 31, 37, 41, 42) have examined the role of delayed gastric emptying (GE) induced by pramlintide, an analog of islet amyloid polypeptide that is known to delay GE rate, to suppress postprandial glucagon concentration and increase satiety (7, 16, 22). These studies have demonstrated pramlintide-induced changes in postprandial glucose excursions (22, 41, 42) due to alterations in glucose turnover estimated with the dual-isotope technique (9, 15, 23, 24, 29, 41, 42).
However, the dual-isotope technique utilized for these studies (41, 42) underestimates rate of meal glucose appearance (MRa) and rate of glucose disappearance (Rd) while providing inaccurate estimates of rate of endogenous glucose production (EGP) due to non-steady-state errors (34). The triple-tracer approach minimizes these errors, enabling accurate estimation of the components of glucose turnover after a meal (3, 34). It does so by permitting simultaneous assessment of MRa and EGP by infusing [6-3H]glucose to mimic systemic appearance of [1-13C]glucose contained in the meal and infusing [6,6-2H2]glucose in a pattern mimicking anticipated changes in EGP after a mixed meal (3). This approach was subsequently shown to be better compared with the dual-tracer approach (34). Furthermore, to our knowledge, no prior study has examined the effect of delayed gastric emptying on insulin sensitivity (SI), β-cell responsivity, and disposition index (DI) by applying state-of-the-art C-peptide and oral minimal models.
To better understand the role of delayed GE on postprandial glucose metabolism, insulin action, and β-cell responsivity and function after a mixed meal, we applied the triple-tracer technique with the above models in healthy individuals, using pramlintide as a probe.
RESEARCH DESIGN AND METHODS
After approval by the Mayo Institutional Review Board, 16 healthy subjects were screened for the study after providing informed consent. Inclusion criteria included age 18–60 yr, BMI <40kg/m2, Hb A1c ≤6.0% (42.1 mmol/mol), serum creatinine ≤1.5 mg/dl, normal fasting and 2-h post-75-g oral glucose tolerance test (OGTT), and normal GE for solids and liquids measured with scintigraphy (4). Exclusion criteria included family history of diabetes in first-degree relatives, significant gastrointestinal symptoms by Bowel Symptom Questionnaire (33), disorders of the gastrointestinal tract, medications known to affect gastric emptying (e.g., erythromycin), or glucose metabolism (e.g., corticosteroids), pregnancy, breast feeding, and any active systemic disease.
Screen Visit 1
After an overnight fast, subjects reported to Clinical Research Unit (CRU) of the Mayo Clinic Center for Clinical and Translational Science in the morning. To confirm eligibility, a medical history, physical examination, and blood tests, including a pregnancy test in female subjects with child-bearing capacity, were evaluated; dietician consultation ensured subjects were on a weight-maintaining diet that met American Diabetes Association standards of macronutrient and calorie composition. A 75-g OGTT was performed to ensure normal glucose tolerance, and body composition was measured by dual-energy X-ray absorptiometry (1).
Screen Visit 2
GE of solids and liquids was measured by established and validated scintigraphic techniques (4) in subjects that were eligible to participate after the first screening visit. Data were summarized as the half-time (T1/2) separately for liquids and solids (4). Subjects with normal GE were scheduled for inpatient studies.
Inpatient Study Visits
Glucose metabolism was evaluated by the triple-tracer technique on two occasions, during which subjects were treated with pramlintide or no pramlintide in randomized order.
Day 1.
Subjects reported to the CRU at 4 pm. A DexCom 7 plus continuous glucose monitor (CGM) and a modular signal recorder (MSR) accelerometer (MSR Electronics, Seuzach, Switzerland) were placed and maintained for the rest of the inpatient study period as part of separate analyses. A mixed meal (10 kcal/kg, 55% carbohydrates, 15% protein, and 30% fat) was consumed at ∼5 PM. No other food was provided until the next morning. An intravenous cannula was inserted into a forearm vein at ∼8 PM for glucose tracer infusions.
Day 2.
A triple-tracer approach was performed as reported previously (1, 3, 18). At ∼4 AM, a primed continuous infusion of [6, 6-2H2]glucose was started. At ∼6 AM, an 18-gauge cannula was inserted in a retrograde fashion into a hand vein of the contralateral arm, and the hand was placed in a heated box, where the temperature was maintained at ∼55°C, for drawing of arterialized venous blood periodically throughout the study for measurements of glucose, glucose tracers, insulin, C-peptide, and glucagon concentrations. At ∼7 AM (T0), 30 μg of pramlintide was administrated subcutaneously, with the first bite of the mixed meal containing 75 g of dextrose labeled with [1-13C]glucose during the pramlintide study day. No pramlintide was provided on the other occasion. With the first bite of the meal, the [6, 6-2H2]glucose infusion rate was varied to mimic the anticipated changes in rate of EGP for the next 6 h. Additionally, an infusion of [6-3H]glucose was started and the rate varied to mimic the anticipated systemic rate of MRa. At ∼1 PM, all infusions were discontinued, the cannulae were removed, and the subject was provided lunch.
For the rest of the study day, the subject ambulated periodically on a treadmill at a pace (1.9 km/h) that mimicked activities of daily living as part of a separate study. Dinner was provided at ∼7 PM. The following morning, the CGM and accelerometers were removed; subjects were provided breakfast and subsequently dismissed.
GE Test With Pramlintide
Within 1 wk after completion of the second inpatient study visit, subjects underwent a repeat GE test as described in screen visit 2 above. However, during this visit, 30 μg of pramlintide was administered subcutaneously at the start of the test to determine the effects of pramlintide on liquid and solid GE rates.
Analytical Methods
Insulin was measured with a two-site electrochemiluminescence immunoenzymatic assay by DxI automated system (Beckman Instruments, Chaska, MN). C-peptide was measured by the Cobas e411 (Roche Diagnostics, Indianapolis, IN) using a two-site electrochemiluminescence immunometric assay. Glucagon was measured with a direct double-antibody radioimmunoassay (Linco Research, St. Charles, MO).
Plasma samples were placed on ice, centrifuged, and then separated and stored at −80°C until assay. Plasma glucose concentration was measured using a glucose oxidase method (YSI, Yellow Springs, OH). Plasma [6-3H]glucose-specific activity (SA) was measured by liquid scintillation counting as described (1). Plasma enrichment of [1-13C]glucose and [6, 6-2H2]glucose were measured using gas chromatography-mass spectrometry (Thermoquest, San Jose, CA) as described (3).
Calculations
Glucose kinetics.
Fasting and postprandial rates of glucose turnover were calculated as described (3). Briefly, the systemically infused [6-3H]glucose was used to trace the systemic rate of appearance of [1-13C]glucose contained in the meal, and [6, 6-2H2]glucose was used to trace the rate of appearance of endogenously derived glucose.
The ratio of plasma concentration of [6-3H]glucose to [1-13C]glucose [SA(t)] was used to calculate the rate of appearance of ingested [1-13C]glucose:
| (1) |
where INF3H is the infusion rate of [6-3H]glucose, G13C is the plasma concentration of [1-13C]glucose V is the volume of distribution and p is the pool fraction fixed to 200 and 0.65 ml/kg, respectively.
The total rate of appearance can be thus calculated as
| (2) |
where TTRmeal is the ratio of [1-13C]glucose and unlabeled glucose in the meal.
Similarly, the ratio of plasma concentration of [6,6-2H2]glucose to endogenously produced glucose [tracer-to-tracee ratio (TTR)] was used to calculate EGP:
| (3) |
where INF2H2 is the infusion rate of [6,6-2H2]glucose, Gend is the plasma concentration of endogenous glucose {calculated by subtracting the concentration of exogenously derived (ingested) glucose (i.e., plasma [1-13C]glucose concentration multiplied by TTR meal [1-13C]glucose enrichment)} from total plasma glucose concentration, and V is the volume of distribution and p is the pool fraction, fixed to 200 and 0.65 ml/kg, respectively.
Glucose rate of disappearance can then be calculated as
| (4) |
Meal Indices
The oral glucose minimal model (8, 11, 12) was used to interpret plasma glucose and insulin concentrations measured during the meal test. The meal was a mixed meal (10 kcal/kg) with 75 g of dextrose flavored with Jell-O, as described previously (3). Meal macronutrient contents are provided in Table 2. The model assumes that insulin action on glucose production and disposal emanates from a compartment remote from plasma, which is usually identified with the interstitium. The most important parameter of the model estimated from data is net insulin sensitivity (SI), which measures the overall effect of insulin to stimulate whole body (liver and periphery) glucose disposal and inhibit glucose production; details and calculations have been published previously (30). The oral C-peptide minimal model (35) was used together with the oral insulin minimal model (6) to interpret the interaction of plasma glucose with C-peptide and insulin, respectively. The oral C-peptide minimal model provides index of total β-cell responsivity (Φ) (10). In addition, we calculated total DI, a composite measurement of insulin secretion appropriate to the prevailing degree of insulin sensitivity (8), thus representing a marker of β-cell function.
Table 2.
Meal content
| Variables | No Pramlintide | Pramlintide |
|---|---|---|
| Energy, kcal | 582.7 ± 110.5 | 574.9 ± 94.4 |
| Carbohydrates | ||
| g | 76.2 ± 0.5 | 76.2 ± 0.4 |
| % | 53.5 ± 8.4 | 53.9 ± 7.7 |
| Protein | ||
| g | 25.4 ± 9.6 | 24.7 ± 8.2 |
| % | 16.9 ± 3.0 | 16.8 ± 2.7 |
| Fat | ||
| g | 19.8 ± 7.5 | 19.3 ± 6.6 |
| % | 29.6 ± 5.9 | 29.3 ± 5.5 |
| Fibers | ||
| g | 0 | 0 |
| % | 0 | 0 |
Data are means ± SD; n = 12.
Statistical Analyses
The sample size for this study was estimated on the basis of the determination that a sample size of 12 was the minimum sample size required to achieve stable variance estimates for estimation purposes (19, 36). Results were expressed as means ± SD with 95% confidence interval (CI) for the mean, unless otherwise stated. Given the fact that the study was designed as a crossover study, the considerations for carryover (treatment contamination from the first study visit carried forward to the second study visit) and period (or order) effects required attention. The study made an assumption that a carryover effect was not biologically plausible given the short half-life of pramlintide (∼3 h). A decision to not make a statistical adjustment for the period effect was determined based on the small sample size and the potentially advantageous larger residual error estimation (i.e., the variation in change in response attributable to the order effect would remain in the error term). As with the carryover effect, there was no a priori evidence to anticipate a period effect, as the study involved eating a labeled meal followed by standard experimental procedures. Thus, comparisons between visits with and without pramlintide were analyzed using a paired t-test with the assumption of no period or carryover effect. This analytical strategy was used for all outcome measurements presented in Table 3. For incremental area under the curve (iAUC), time 0 was used for baseline for all measurements. Differences were considered as statistically significant with P < 0.05 (2-sided). Analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Table 3.
Outcome measurements of 2 study visits without and with pramlintide results
| Variable (Measurement) | No Pramlintide | Pramlintide | Difference | 95% CI for mean | P Value |
|---|---|---|---|---|---|
| Rate of gastric emptying | |||||
| Liquid, minutes to 50% clearance | 30 ± 9 | 110 ± 32 | 80 ± 33 | 59–101 | <0.001 |
| Solid, minutes to 50% clearance | 110 ± 29 | 173 ± 26 | 63 ± 26 | 46–79 | <0.001 |
| Glucose, mM | |||||
| iAUC 120 min | 324.0 ± 94.3 | 280.9 ± 101.7 | −43.2 ± 89.6 | −100.1 to 13.8 | 0.12 |
| iAUC 360 min | 365.8± 144.4 | 375.0 ± 122.1 | 9.2 ± 120.6 | −67.4 to 85.8 | 0.80 |
| Peak measurement | 9.7 ± 1.3 | 9.3 ± 1.1 | −0.4 ± 1.4 | −1.3 to 0.5 | 0.38 |
| Time to peak, min | 44 ± 12 | 86 ± 22 | 43 ± 26 | 26–59 | <0.001 |
| Insulin, pM | |||||
| iAUC 120 | 23,509.2 ± 7.769.4 | 14,641.8 ± 10,101 | −8,868.0 ± 7,565.4 | −1,3674.6 to −4,060.8) | 0.002 |
| iAUC 360 | 31,136.4 ± 10,012.8 | 2,7315.6 ± 13,848 | −3,819 ± 8,878.2 | (−9,460.2 to 1,821.6 | 0.16 |
| Peak measurement | 388.8 ± 163.8 | 281.4 ± 118.8 | −108.0 ± 135.6 | −193.8 to −21.6 | 0.019 |
| Time to peak, min | 51 ± 20 | 104 ± 23 | 53 ± 34 | (31, 74) | <0.001 |
| C-peptide, nM | |||||
| iAUC 120 min | 223.2 ± 78.4 | 127.9 ± 68.3 | −95.4 ± 68.9 | −139.1 to −51.6 | <0.001 |
| iAUC 360 min | 380.3 ± 146.4 | 326.0 ± 126.3 | −54.3 ± 79.7 | −104.9 to −3.6 | 0.038 |
| Peak measurement | 3.4 ± 1.0 | 3.0 ± 0.8 | −0.4 ± 0.8 | −1.0 to 0.1 | 0.084 |
| Time to peak, min | 81 ± 30 | 113 ± 20 | 31 ± 33 | 10–52 | 0.007 |
| Glucagon, pg/ml | |||||
| iAUC 120 min | −205.2 ± 1,574.4 | −1,345.8 ± 575.6 | −1,140.6 ± 1,664.9 | −2,198.5 to −82.8 | 0.037 |
| iAUC 360 min | 5,277.9 ± 4,907.3 | 2,132.3 ± 2,838.2 | −3,145.6 ± 5,105.9 | −6,389.8 to 98.5 | 0.056 |
| Peak measurement | 110.8 ± 34.4 | 112.3 ± 29.7 | 1.6 ± 24 | −13.7 to 16.8 | 0.82 |
| Time to peak, min | 215 ± 40 | 236 ± 35 | 21 ± 29 | 3–40 | 0.027 |
| Rate of EGP, μM·kg FFM−1·min | |||||
| iAUC 120 min | −1,052.1 ± 224.8 | −1,004.8 ± 267.1 | 47.3 ± 265.7 | −121.6 to 216.1 | 0.55 |
| iAUC 360 min | −2,331.6 ± 751.7 | −2,846.2 ± 946 | −514.6 ± 674.5 | −943.1 to −86.0 | 0.023 |
| Peak measurement | 15.5 ± 2.7 | 16.1 ± 2.1 | 0.7 ± 2.8 | −1.1–2.5 | 0.44 |
| Time to peak, min | 126 ± 156 | 67 ± 137 | −60 ± 191 | −181 to 62 | 0.30 |
| MRa,μM·kg FFM−1·min | |||||
| iAUC 120 min | 5,489.1 ± 967.6 | 4,049.0 ± 1,413.3 | −1,440.1 ± 999.7 | −2,075.3 to −804.9 | <0.001 |
| iAUC 360 min | 8,803.3 ± 1,523.9 | 9,497.0 ± 2,191.5 | 693.7 ± 1,164.2 | −46.0 to 1,433.4 | 0.063 |
| Peak measurement | 73.9 ± 13.1 | 63.2 ± 19.9 | −10.7 ± 16.6 | −21.2 to −0.1 | 0.048 |
| Time to peak, min | 46 ± 28 | 116 ± 39 | 70 ± 47 | 40–100 | <0.001 |
| Rd, μM·kg FFM−1·min | |||||
| iAUC 120 min | 4,359.3 ± 904.6 | 2,693.6 ± 1,420.5 | −1,665.6 ± 1,105.4 | −2,367.9 to −963.3 | <0.001 |
| iAUC 360 min | 6,767.4 ± 1,578.3 | 6,892.2 ± 1,922.8 | 124.7 ± 1,198 | −636.4 to 885.9 | 0.73 |
| Peak measurement | 76.7 ± 12.5 | 76.1 ± 22.2 | −0.6 ± 20.8 | −13.8 to 12.6 | 0.93 |
| Time to peak, min | 73 ± 26 | 131 ± 26 | 59 ± 30 | 39–78 | <0.001 |
| Indices of glucose action | |||||
| SI, 10−4 dl·kg−1·min−1 per μU/ml | 16.6 ± 8.3 | 21.5 ± 10.6 | 5.0 ± 6.6 | 0.8–9.2 | 0.024 |
| ϕ (β-cell responsivity, 10−9/min) | 15.6 ± 3.6 | 14.2 ± 3.2 | −1.4 ± 1.8 | −2.5 to −0.2 | 0.023 |
| DI, 10−14 dl·kg−1·min2 per pmol/l | 395.1 ± 103.2 | 465.1 ± 156.7 | 70 ± 137.1 | −17.1 to 157.1 | 0.10 |
Data are means ± SD; n = 12. CI, confidence interval; iAUC, incremental area under the curve; EGP, endogenous glucose production; FFM, fat-free mass; SI, insulin sensitivity; DI, disposition index.
RESULTS
Subject Characteristics
Of the 16 subjects screened for the study, one subject failed the first screen visit due to abnormal OGTT, one refused to consume eggs during the mixed meal, and one had IV access difficulty. Thirteen subjects entered the crossover phase of the study, but data from one subject were excluded because the subject could not ingest the entire meal. Demographic of subjects is shown in Table 1. Briefly, the mean (SD) age and body mass index were 36 (12) yr and 25.3 (4.0) kg/m2, respectively. Fasting plasma glucose concentrations, Hb A1C, and OGTT were normal, and subjects met inclusion criteria for normal GE prior to entry into the crossover phase of the study.
Table 1.
Baseline characteristics of the subjects completing the 2-meal study
| Variable | Means ± SD | Range |
|---|---|---|
| Age, yr | 36.0 ± 11.9 | 21.0–56.0 |
| Weight, kg | 72.4 ± 15.8 | 51.9–104.7 |
| Sex (M/F) | 9/3 | |
| Body mass index, kg/m2 | 25.3 ± 4.0 | 20.0–32.7 |
| Fat-free mass, kg | 48.5 ± 10.7 | 37.7–72.3 |
| Percent body fat, % | 32.4 ± 8.9 | 16.7–45.1 |
| Laboratory results | ||
| Fasting blood glucose, mM | 4.6 ± 0.4 | 4.3–5.5 |
| 2-h Postprandial glucose, mM | 5.3 ± 1.2 | 3.3–7.1 |
| Hb A1c | ||
| % | 5.1 ± 0.4 | 4.6–6.0 |
| mmol/mol | 32.0 ± 4.0 | 26.8–42.1 |
| Hemoglobin, g/dl | 13.4 ± 1.1 | 11.6–15.7 |
| Creatinine, mg/dl | 0.8 ± 0.2 | 0.6–1.2 |
| BUN | 14.1 ± 4.4 | 8.0–22.0 |
| TSH, IU/l | 2.0 ± 1.1 | 0.6–4.6 |
Data are means ± SD; n = 12 (9 males and 3 females). BUN, blood urea nitrogen.
Mixed-Meal Content
The mixed meal composition is provided in Table 2.
GE Percentages for Solids and Liquids
With pramlintide, the T1/2 (T50) for solid and liquid emptying was prolonged (Table 3). For liquids, an additional 80 min (95% CI: 59–101 min, P < 0.001) was required to reach 50% clearance during the GE study. Similarly, solids required an additional 63 min (95% CI: 46–79 min, P < 0.001). However, between-group difference in the prolongation of GE attributable to pramlintide was not statistically different for liquids and solids (mean: 18 min; 95% CI: −42 to 7 min; P = 0.14).
Plasma Glucose, Insulin, C-peptide, and Glucagon Concentrations
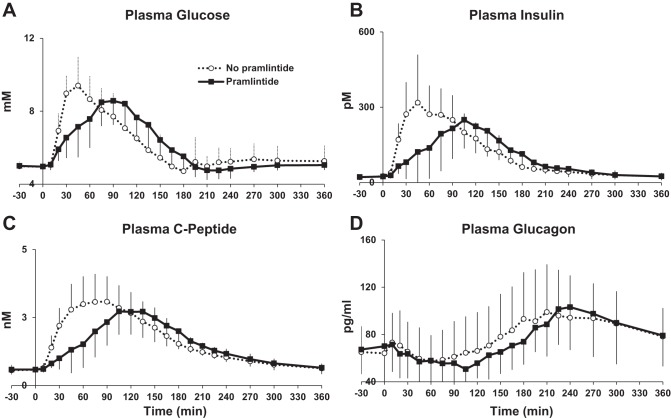
The peak postprandial glucose was delayed on average 43 min (95% CI: 26–59 min, P < 0.001) with the introduction of pramlintide (Fig. 1 and Table 3). This delay did not affect the total postprandial glucose excursion (difference: 9.2 mM over 6 h; 95% CI: −67.4 to 85.8 mM over 6 h; P = 0.80) or insulin concentration (difference: −3,819.0 pM; 95% CI: −9,460.2 to 1,821.6 pM over 6 h, P = 0.16) after the mixed meal, but the delay did have an impact on the measurements in the 2 h immediately following the meal (Table 3). Postprandial excursions of insulin (difference in iAUC: −8,868.0 pM over 2 h; 95% CI: −13,674.6 to −4,060.8 pM over 2 h; P = 0.002), C-peptide (difference in iAUC: −95.4 nM over 2 h; 95% CI: −139.1 to −51.6 nM over 2 h; P < 0.001), and glucagon (difference in iAUC: −1,140.6 pg/ml over 2 h; 95% CI: −2,198.5 to −82.8 pg/ml over 2 h; P = 0.037) were lower with pramlintide. Only the difference in C-peptide remained statistically significant over the entire 6-h period following the meal (P = 0.038; Table 3).
Fig. 1.
Glucose and islet hormone concentrations during triple-tracer meal studies without and with pramlintide in healthy subjects A: plasma glucose concentrations (time to peak delayed by pramlintide, P < 0.001). B: plasma insulin concentrations [incremental area under the curve (iAUC) 0–120 min, P = 0.002]. C: plasma C-peptide concentrations (iAUC 0–360 min, P = 0.038). D: plasma glucagon concentrations (iAUC 0–120 min, P = 0.037).
Ratio of [6-3H]glucose to [1-13C]glucose and Ratio of [6,6-2H2]glucose to Endogenous Glucose
As shown in Fig. 2A, the tracer/tracee ratio applied to calculate EGP was fairly constant apart from a change during the pramlintide study day between 150 and 240 min. As shown in Fig. 2B, the tracer/tracee ratio applied to calculate the Mra was also fairly constant for almost the entire duration (10–360 min) of the study apart from the initial perturbations (0–10 min) that are unavoidable when both the intravenously infused tracer and orally ingested tracee are entering the systemic circulation. Therefore, the triple-tracer approach applied here was successful in minimizing fluctuations in tracer/tracee ratios, enabling accurate measurements of postprandial glucose turnover.
Fig. 2.
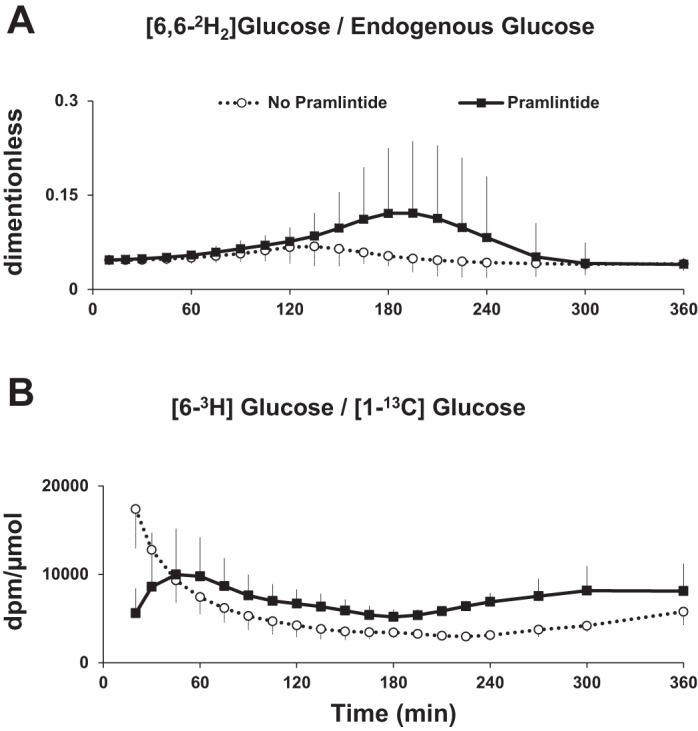
A: [6-3H]glucose/[1-13C]glucose concentrations during triple-tracer studies without and with pramlintide in healthy subjects. B: [6,6-2H2]glucose/endogenous glucose concentrations during triple-tracer studies without and with pramlintide in healthy subjects.
MRa, EGP, and Rd
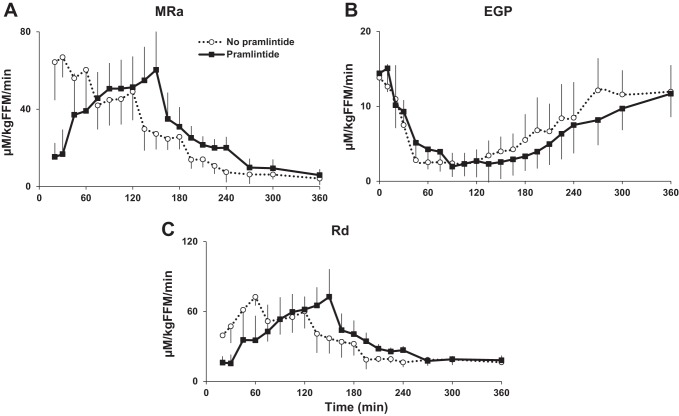
The iAUC of MRa was lower with pramlintide for 0–120 min after the meal [difference in iAUC: −1,440.1 μM·kg fat-free mass (FFM)−1·min over 2 h; 95% CI: −2,075.3 to −804.9 μM·kg FFM−1·min over 2 h; P < 0.001; Fig. 3 and Table 3]. However, the iAUC of MRa during the entire 0–360 min did not differ with or without pramlintide (difference in iAUC: 693.7 μM·kg FFM−1·min over 6 h; 95% CI: −46.0 to 1,433.4 μM·kg FFM−1·min over 6 h; P = 0.063). The time to peak of rate of MRa was delayed (difference: 70 min; 95% CI: 40–100 min; P < 0.001) with pramlintide.
Fig. 3.
A: rate of meal glucose appearance (MRa) during triple-tracer studies without and with pramlintide in healthy subjects (iAUC 0–120 min, P < 0.001). B: rates of endogenous glocose production (EGP) during the same studies without and with pramlintide (iAUC 0–360, P = 0.023). C: rates of whole body glucose disappearance (Rd) without and with pramlintide in healthy subjects (iAUC 0–120 min, P < 0.001).
In contrast, the iAUC of EGP did not differ with pramlintide for 0–120 min after the meal (difference in iAUC: 47.3 μM·kg FFM−1·min over 2 h; 95% CI: −121.6 to 216.1 μM·kg FFM−1·min over 2 h; P = 0.55). However, the iAUC of EGP during the entire 0–360 min was lower (difference in iAUC: −514.6 μM·kg FFM−1·min over 6 h; 95% CI: −943.1 to −86.0 μM·kg FFM−1·min over 6 h; P = 0.023) with pramlintide, whereas there were no differences in time to nadir of EGP (difference: −60 min; 95% CI: −181 to 62 min; P = 0.30) with or without pramlintide.
The iAUC of Rd was lower (difference in iAUC: −1,665.6 μM·kg FFM−1·min over 2 h; 95% CI: −2,367.9 to −963.3 μM·kg FFM−1·min over 2 h; P < 0.001) with pramlintide for 0–120 min after the meal. However, the iAUC of Rd during the entire 0–360 min did not differ with or without pramlintide (difference in iAUC: 124.7 μM·kg FFM−1·min over 6 h; 95% CI: −636.4 to 885.9 μM·kg FFM−1·min over 6 h; P = 0.73). The time to peak Rd was delayed (difference: 59 min; 95% CI: 39–78 min; P < 0.001) with pramlintide.
Insulin Action (SI), β-Cell Responsivity (Φ), and DI
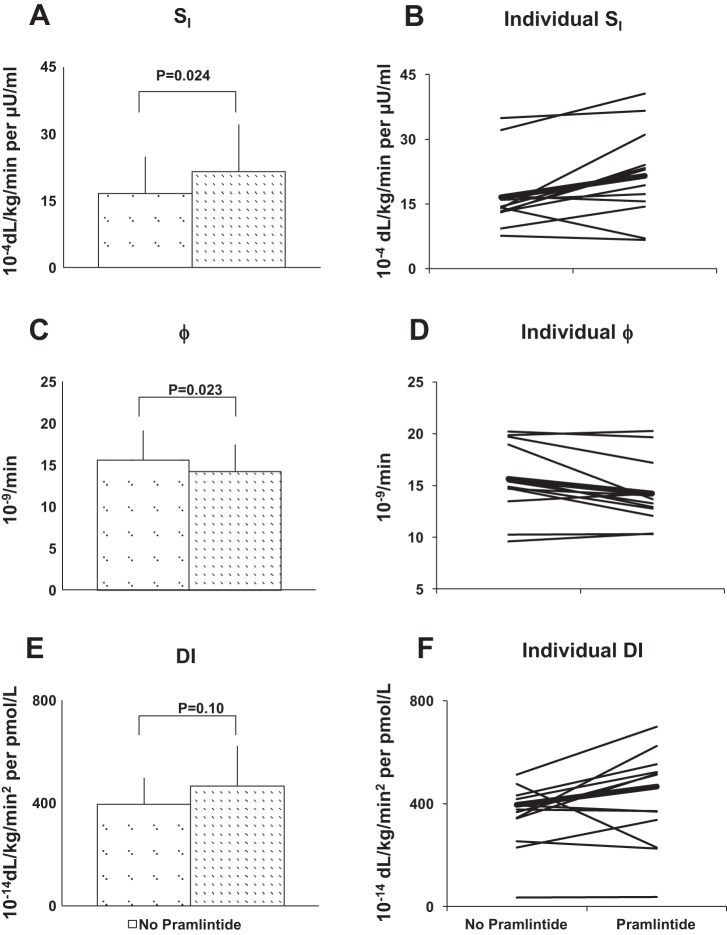
Whereas whole body insulin sensitivity was higher (difference: 5.0 × 10−4 dl·kg−1·min−1 per μU/ml; 95% CI: 0.8 to 9.2 × 10−4 dl·kg−1·min−1 per μU/ml; P = 0.024), Φ was lower (difference: −1.4 × 10−9/min; 95% CI: −2.5 to −0.2 ×10−9/min; P = 0.023) with pramlintide (Table 3 and Fig. 4). This resulted in an unchanged DI (difference: 70.0 ×10−14 dl·kg−1·min2 per pmol/l; 95% CI: −17.1 to 157.1 × 10−14 dl·kg−1·min2 per pmol/l; P = 0.10).
Fig. 4.
A: insulin sensitivity (SI) obtained during triple-tracer studies without and with pramlintide in healthy subjects (P = 0.024). B: model-based SI for all subjects shown individually. The thicker line shows mean estimates of SI for visits without and with pramlintide. C: Φ was lower with pramlintide (P = 0.023). D: Φ shown individually for each subject. E: disposition index (DI) was unchanged with pramlintide use but increased numerically, indicating modest effect size (P = 0.10). F: DI shown individually for each subject.
DISCUSSION
Applying state-of-the-art methods to estimate postprandial glucose turnover and β-cell function, this study demonstrates that delay in gastric emptying obtained with the administration of 30 μg of pramlintide administered at the start of a mixed meal in healthy subjects delays peak rate of meal glucose appearance. Furthermore, delayed gastric emptying lowered rate of EGP and improved insulin sensitivity while reducing β-cell responsivity, resulting in a net unchanged disposition index.
Pramlintide was used as a probe to delay gastric emptying, confirming prior studies in animals and in subjects with and without diabetes (17, 31, 41–43). This effect is probably mediated by vagal inhibition (24).
Delayed gastric emptying of liquids probably explains the marked delay in the time to peak meal glucose appearance rate and hence, time to peak postprandial glucose concentration. It is noteworthy that the carbohydrate (dextrose) component of the mixed meal was ingested in the semisolid form induced by mixing with Jell-O, which liquefies at body temperature in the stomach. The consequent slower rise in postprandial plasma glucose concentrations caused a delay in rise in insulin concentration, likely resulting in a delayed peak in the rate of Rd. However, despite the lower insulin concentrations during the first 2 h after the meal, the iAUC of EGP during the entire 0–360 min was lower with pramlintide. Furthermore, iAUC of C-peptide was lower with pramlintide, implying that portal insulin concentrations were also lower with than without pramlintide, further strengthening the conclusion that hepatic insulin action improved with pramlintide use.
It is also noteworthy that although delayed gastric emptying delayed postprandial rise in glucose and insulin concentrations and time to peak in rates of MRa and Rd, the integrated postprandial glucose and insulin concentrations and those of MRa and Rd over the entire period of 6 h were not affected. The higher rate of appearance of meal glucose resulting in higher glucose and insulin concentrations during the later postprandial period and, at the end of the study, plasma glucose, plasma hormone concentrations, and glucose turnovers all returning to baseline levels suggest that effects of a single dose of pramlintide last for a short period of time, as expected, and that it does not alter net systemic appearance of meal glucose and, therefore, splanchnic glucose uptake in healthy subjects. It is noteworthy that integrated response of EGP for the 6 h after the meal was lower with delayed gastric emptying, which was likely influenced by lower postprandial glucagon concentrations.
It is intriguing that delayed gastric emptying improved postprandial insulin sensitivity significantly, as measured by the oral minimal model. Whereas the insulin sensitivity change is impressive, the β-cell response is likely compensatory. The glucagon response over 6 h was not different, but the iAUC EGP was different. The improved insulin sensitivity coupled with lower rate of EGP with delayed gastric emptying suggests that delayed gastric emptying improved whole body insulin sensitivity by likely improving hepatic insulin action. In addition, iAUC of C-peptide was lower with pramlintide, implying that portal insulin concentrations were also lower with than without pramlintide. The observation that iAUC of EGP was lower with pramlintide despite a lower projected portal insulin concentrations further strengthens our conclusion that hepatic insulin action was improved with pramlintide use.
Furthermore, although delayed gastric emptying reduced direct β-cell responsivity to glucose, the net effect on DI, a measure of β-cell function, i.e., β-cell response appropriate to the degree of insulin resistance, remained unaltered. It is apparent from the unchanged DI that the true underlying change is the improvement in insulin sensitivity, and the observed changes in β-cell response are compensatory.
The reasons for the change in insulin sensitivity need to be investigated in more detail. Since glucagon concentrations were not different, the changes of glucagon made by pramlintide only cannot explain this effect. It has been well known that some peptides and nutrients, such as free fatty acids, modify hepatic insulin sensitivity (5, 28, 38, 39), and the improved whole body insulin sensitivity could be mediated by changes in other peptides or changes in other nutrients, such as free fatty acids.
This study is underpinned by state-of-the-art assessments of gastric emptying of solids and liquids and glucose metabolism with the triple-tracer approach in healthy subjects with normal glucose tolerance and gastric emptying. However, our study has its limitations. First, these assessments were limited to a single dose of pramlintide, resulting in an acute episode of delayed gastric emptying. Future studies should examine the effects of long-term alterations in gastric emptying caused by long-term pramlintide use on postprandial glucose tolerance and insulin action. Long-term assessments are germane because nausea related to pramlintide improves with time. It is unclear whether this improvement is related to tachyphylaxis of the pramlintide-induced deceleration of gastric emptying, similar to that reported for glucagon-like peptide-1 (27). Second, postprandial glucose turnover was measured after a mixed meal containing a monosaccharide (i.e., glucose). Future studies need to assess these parameters after complex carbohydrates (polysaccharide) containing mixed meals that mimic the real-world situation. Finally, the independent effects of altered glucagon secretion and delayed gastric emptying and the effects of pramlintide cannot be determined from this study. Additional studies using selective agents that separately manipulate gastric emptying and glucagon secretion are necessary in this context.
To conclude, we have demonstrated that delayed gastric emptying using pramlintide acutely improves insulin sensitivity in healthy subjects in the postprandial state with state-of-the-art techniques. Additional studies using selective agents that separately manipulate gastric emptying and glucagon secretion are necessary in the future. Recent studies have shown promising effects of pramlintide on postprandial glucose excursions during closed-loop control in individuals with type 1 diabetes (25, 40). Our study sets the stage for investigating the effects of delayed gastric emptying induced by pramlintide on postprandial glucose turnover and insulin action in closed-loop settings.
GRANTS
The work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-R01-085516 and DK-DP3-094331 and Grant No. UL1-TR-000135 from the National Center for Advancing Translational Science, a component of the National Institutes of Health (NIH). R. Basu was supported by DK-29953 and DK-90541. M. Schiavon, C. Dalla Man, and C. Cobelli were partially funded by Italian Ministero dell'Istruzione, dell'Università e della Ricerca (Progetto FIRB 2009).
DISCLOSURES
Dr. Yogish C. Kudva is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis. There are no conflicts of interest, financial or otherwise, to declare for any of the authors.
AUTHOR CONTRIBUTIONS
L.H., A.M., R.B., A.B., and Y.C.K. performed experiments; L.H., M.S., A.M., C.D.M., R.B., A.E.B., C.C., R.E.C., A.B., and Y.C.K. analyzed data; L.H., M.S., A.M., C.D.M., R.B., A.E.B., C.C., R.E.C., A.B., and Y.C.K. interpreted results of experiments; L.H., M.S., A.M., C.D.M., R.B., C.C., A.B., and Y.C.K. prepared figures; L.H., M.S., A.M., C.D.M., R.B., A.E.B., C.C., R.E.C., A.B., and Y.C.K. drafted manuscript; L.H., M.S., A.M., C.D.M., R.B., A.E.B., C.C., R.E.C., A.B., and Y.C.K. edited and revised manuscript; R.B., R.E.C., A.B., and Y.C.K. conception and design of research; Y.C.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We are deeply indebted to the research participants. Our sincere thanks to the staff of the Mayo Clinic Center for Translational Science Activities CRU, the GI Motility Core, the CRU Mass Spectroscopy Laboratory, and the CRU Immunochemical Core Laboratory, Pamela Reich (research assistant), Chad Clark (Laboratory Technician), Brent McConahey (research assistant), and Shelly McCrady Spitzer (research assistant). All persons mentioned above are at Endocrine Research Unit, Mayo Clinic, Rochester, MN. We thank Amylin Pharmaceuticals for providing some of the Pramlintide used in the study. The data for this study have been presented in part at the Advanced Technologies and Treatments for Diabetes Meeting in Paris, France, in February 2013 and in part at the Annual Scientific Meeting of the European Association for the Study of Diabetes in Barcelona, Spain, in September 2013.
REFERENCES
- 1.Basu A, Dalla Man C, Basu R, Toffolo G, Cobelli C, Rizza RA. Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care 32: 866–872, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, Cobelli C, Rizza RA. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 55: 2001–2014, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Basu R, DiCamillo B, Toffolo G, Basu A, Shah P, Vella A, Rizza RA, Cobelli C. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab 284: E55–E69, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bharucha AE, Camilleri M, Forstrom LA, Zinsmeister AR. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf) 70: 415–420, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burmeister MA, Ferre T, Ayala JE, King EM, Holt RM. Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Am J Physiol Endocrinol Metab 302: E334–E343, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Campioni M, Toffolo G, Basu R, Rizza RA, Cobelli C. Minimal model assessment of hepatic insulin extraction during an oral test from standard insulin kinetic parameters. Am J Physiol Endocrinol Metab 297: E941–E948, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman I, Parker B, Doran S, Feinle-Bisset C, Wishart J, Lush CW, Chen K, Lacerte C, Burns C, McKay R, Weyer C, Horowitz M. Low-dose pramlintide reduced food intake and meal duration in healthy, normal-weight subjects. Obesity (Silver Spring) 15: 1179–1186, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes 63: 1203–1213, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobelli CT, Ferrannini E. A model of glucose kinetics and their control by insulin, compartmental and non-compartmental approaches. Math Biosci 72: 291–315, 1984 [Google Scholar]
- 10.Dalla Man C, Campioni M, Polonsky KS, Basu R, Rizza RA, Toffolo G, Cobelli C. Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes 54: 3265–3273, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 287: E637–E643, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Dalla Man C, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 49: 419–429, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Dalla Man C, Piccinini F, Basu R, Basu A, Rizza RA, Cobelli C. Modeling hepatic insulin sensitivity during a meal: validation against the euglycemic hyperinsulinemic clamp. Am J Physiol Endocrinol Metab 304: E819–E825, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elleri D, Allen JM, Harris J, Kumareswaran K, Nodale M, Leelarathna L, Acerini CL, Haidar A, Wilinska ME, Jackson N, Umpleby AM, Evans ML, Dunger DB, Hovorka R. Absorption patterns of meals containing complex carbohydrates in type 1 diabetes. Diabetologia 56: 1108–1117, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Ferrannini E, Simonson DC, Katz LD, Reichard G, Jr, Bevilacqua S, Barrett EJ, Olsson M, DeFronzo RA. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 37: 79–85, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Heptulla RA, Rodriguez LM, Bomgaars L, Haymond MW. The role of amylin and glucagon in the dampening of glycemic excursions in children with type 1 diabetes. Diabetes 54: 1100–1107, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Heptulla RA, Rodriguez LM, Mason KJ, Haymond MW. Gastric emptying and postprandial glucose excursions in adolescents with type 1 diabetes. Pediatr Diabetes 9: 561–566, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Hinshaw L, Dalla Man C, Nandy DK, Saad A, Bharucha AE, Levine JA, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, Basu A. Diurnal pattern of insulin action in type 1 diabetes: implications for a closed-loop system. Diabetes 62: 2223–2229, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Stat 4: 287–291, 2005 [Google Scholar]
- 20.Kong MF, King P, Macdonald IA, Stubbs TA, Perkins AC, Blackshaw PE, Moyses C, Tattersall RB. Infusion of pramlintide, a human amylin analogue, delays gastric emptying in men with IDDM. Diabetologia 40: 82–88, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Kong MF, Stubbs TA, King P, Macdonald IA, Lambourne JE, Blackshaw PE, Perkins AC, Tattersall RB. The effect of single doses of pramlintide on gastric emptying of two meals in men with IDDM. Diabetologia 41: 577–583, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Levetan C, Want LL, Weyer C, Strobel SA, Crean J, Wang Y, Maggs DG, Kolterman OG, Chandran M, Mudaliar SR, Henry RR. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care 26: 1–8, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Livesey G, Wilson PD, Dainty JR, Brown JC, Faulks RM, Roe MA, Newman TA, Eagles J, Mellon FA, Greenwood RH. Simultaneous time-varying systemic appearance of oral and hepatic glucose in adults monitored with stable isotopes. Am J Physiol Endocrinol Metab 275: E717–E728, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Mari A, Wahren J, DeFronzo RA, Ferrannini E. Glucose absorption and production following oral glucose: comparison of compartmental and arteriovenous-difference methods. Metabolism 43: 1419–1425, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Micheletto F, Dalla Man C, Kolterman O, Chiquette E, Herrmann K, Schirra J, Kovatchev B, Cobelli C. In silico design of optimal ratio for co-administration of pramlintide and insulin in type 1 diabetes. Diabetes Technol Ther 15: 802–809, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moyses C, Young A, Kolterman O. Modulation of gastric emptying as a therapeutic approach to glycaemic control. Diabet Med 13: S34–S38, 1996 [PubMed] [Google Scholar]
- 27.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 60: 1561–1565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira S, Park E, Mori Y, Haber CA, Han P, Uchida T, Stavar L, Oprescu AI, Koulajian K, Ivovic A, Yu Z, Li D, Bowman TA, Dewald J, El-Benna J, Brindley DN, Gutierrez-Juarez R, Lam TK, Najjar SM, McKay RA, Bhanot S, Fantus IG, Giacca A. FFA-induced hepatic insulin resistance in vivo is mediated by PKCδ, NADPH oxidase, and oxidative stress. Am J Physiol Endocrinol Metab 307: E34–E46, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radziuk J, Norwich KH, Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol Endocrinol Metab Gastrointest Physiol 234: E84–E93, 1978 [DOI] [PubMed] [Google Scholar]
- 30.Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, Basu A. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 61: 2691–2700, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samsom M, Szarka LA, Camilleri M, Vella A, Zinsmeister AR, Rizza RA. Pramlintide, an amylin analog, selectively delays gastric emptying: potential role of vagal inhibition. Am J Physiol Gastrointest Liver Physiol 278: G946–G951, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Samsom M, Vermeijden JR, Smout AJ, Van Doorn E, Roelofs J, Van Dam PS, Martens EP, Eelkman-Rooda SJ, Van Berge-Henegouwen GP. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care 26: 3116–3122, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med 111: 671–674, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Toffolo G, Basu R, Dalla Man C, Rizza R, Cobelli C. Assessment of postprandial glucose metabolism: conventional dual- vs. triple-tracer method. Am J Physiol Endocrinol Metab 291: E800–E806, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Toffolo G, Breda E, Cavaghan MK, Ehrmann DA, Polonsky KS, Cobelli C. Quantitative indexes of β-cell function during graded up&down glucose infusion from C-peptide minimal models. Am J Physiol Endocrinol Metab 280: E2–E10, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Van Belle G. Statistical Rules of Thumb (2nd Ed) New York: John Wiley & Sons, 2008 [Google Scholar]
- 37.Vella A, Lee JS, Camilleri M, Szarka LA, Burton DD, Zinsmeister AR, Rizza RA, Klein PD. Effects of pramlintide, an amylin analogue, on gastric emptying in type 1 and 2 diabetes mellitus. Neurogastroenterol Motil 14: 123–131, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Vestergaard ET, Djurhuus CB, Gjedsted J, Nielsen S, Møller N, Holst JJ, Jørgensen JO, Schmitz O. Acute effects of ghrelin administration on glucose and lipid metabolism. J Clin Endocrinol Metab 93: 438–444, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Vestergaard ET, Gormsen LC, Jessen N, Lund S, Hansen TK, Moller N, Jorgensen JO. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes 57: 3205–3210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinzimer SA, Sherr JL, Cengiz E, Kim G, Ruiz JL, Carria L, Voskanyan G, Roy A, Tamborlane WV. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care 35: 1994–1999, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woerle HJ, Albrecht M, Linke R, Zschau S, Neumann C, Nicolaus M, Gerich J, Goke B, Schirra J. Importance of changes in gastric emptying for postprandial plasma glucose fluxes in healthy humans. Am J Physiol Endocrinol Metab 294: E103–E109, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Woerle HJ, Albrecht M, Linke R, Zschau S, Neumann C, Nicolaus M, Gerich JE, Göke B, Schirra J. Impaired hyperglycemia-induced delay in gastric emptying in patients with type 1 diabetes deficient for islet amyloid polypeptide. Diabetes Care 31: 2325–2331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young AA, Gedulin B, Vine W, Percy A, Rink TJ. Gastric emptying is accelerated in diabetic BB rats and is slowed by subcutaneous injections of amylin. Diabetologia 38: 642–648, 1995 [DOI] [PubMed] [Google Scholar]