Abstract
Probiotics, including Lactobacilli, are commensal bacteria that have been used in clinical trials and experimental models for the prevention and treatment of diarrheal disorders. Our previous studies have shown that Lactobacillus acidophilus (LA) and its culture supernatant (CS) stimulated Cl−/HCO3− exchange activity, acutely via an increase in the surface levels of downregulated in adenoma (DRA, SLC26A3) and in long-term treatments via increasing its expression involving transcriptional mechanisms. However, the role of LA in modulating DRA activity under inflammatory conditions is not known. Current in vitro studies using human intestinal epithelial Caco-2 cells examined the efficacy of LA or its CS in counteracting the inhibitory effects of interferon-γ (IFN-γ) on Cl−/HCO3− exchange activity. Pretreatment of cells with LA or LA-CS for 1 h followed by coincubation with IFN-γ significantly alleviated the inhibitory effects of IFN-γ on Cl−/HCO3− exchange activity. In the in vivo model of dextran sulfate sodium-induced experimental colitis (3% in drinking water for 7 days) in C57BL/6J mice, administration of live LA (3 × 109 colony-forming units) via oral gavage attenuated colonic inflammation. LA administration also counteracted the colitis-induced decrease in DRA mRNA and protein levels. Efficacy of LA or its secreted soluble factors in alleviating inflammation and inflammation-associated dysregulation of DRA activity could justify their therapeutic potential in inflammatory diarrheal diseases.
Keywords: probiotics, Caco-2, diarrhea, interferon-γ, dextran sulfate sodium, downregulated in adenoma
the movement of water and ions (especially Na+ and Cl−) across the luminal membrane is an important function of colonic epithelial cells in physiological conditions. This movement is impaired in most forms of diarrheal diseases, including those associated with inflammatory bowel diseases. Two members of the SLC26 gene family, SLC26A3 or downregulated in adenoma (DRA) and SLC26A6 or putative anion transporter-1 (PAT-1), are known to play important roles in the apical Cl−/HCO3− exchange process in intestine. Decreased DRA expression, and its functional impairment have been shown to be associated with various diarrheal disorders (3, 16, 24, 38). Also, DRA mRNA expression has been shown to be significantly decreased by proinflammatory cytokines IL-1β, interferon-γ (IFN-γ), as well as in patients with ulcerative colitis (31, 38).
Probiotics are live microorganisms that when administered in adequate amounts confer health benefits to the host. Probiotics have been previously demonstrated to modulate cytokine production, strengthen mucosal barrier, and reduce inflammation in in vitro and experimental colitis models (20, 22, 30). All of these mechanisms may contribute to their therapeutic efficacy in colitis; however, the specificity of individual probiotic strains and their precise mechanisms of action are poorly understood. In this regard, earlier studies have shown probiotic Lactobacillus-mediated alleviation of inflammation-associated chloride secretion in response to cytokines or enteropathogen infection (30). We have previously shown that the probiotic L. plantarum or their secreted soluble factors counteract TNF-α-induced downregulation of expression and function of SMCT-1, a transporter for short-chain fatty acids, in rat intestinal IEC-6 cells (4). Also, we have previously demonstrated Lactobacillus acidophilus (LA) to stimulate Cl−/HCO3− exchange activity via an increase in the surface levels (6) and expression of the Cl−/HCO3− exchanger DRA in both in vitro and in vivo models (6, 28). However, there are no studies on the modulation of DRA activity by probiotics under inflammatory conditions.
In the current study, we have examined the role of LA in reversing the inflammatory cytokine-induced downregulation of DRA in human intestinal epithelial Caco-2 cells and in a dextran sulfate sodium (DSS) colitis model in mice. For this, we first measured the Cl−/HCO3− exchange activity in Caco-2 cells treated with IFN-γ (30 ng/ml) in the presence or absence of LA or culture supernatant (CS) for 24 h. Interestingly, pretreatment of LA or LA-CS significantly alleviated the inhibitory effects of IFN-γ on Cl−/HCO3− exchange activity. In a mouse model of DSS-induced experimental colitis, oral gavage of live LA showed alleviation of colitis-associated weight loss, diarrheal phenotype as well as downregulation of DRA mRNA and protein levels. In conclusion, our study shows the efficacy of LA and their secreted soluble factors in counteracting the proinflammatory effects of cytokines and justifies the potential therapeutic role of LA or LA-derived bioactive factors in inflammatory disorders of the intestine.
MATERIALS AND METHODS
Materials
Caco-2 cells were procured from ATCC. Radionucleotide 125I was obtained from PerkinElmer (specific activity 17.0 Ci/mg). RNeasy kits for RNA extraction were obtained from Qiagen (Frederick, MD), and the real-time qRT PCR kit was from Stratagene (La Jolla, CA). 4,4′-Diisothiocyanate-stilbene-2,2′-disulfonic acid (DIDS) was procured from Sigma-Aldrich (St. Louis, MO). Human recombinant IFN-γ was obtained from Sigma. Common reagents for SDS-PAGE such as ammonium per sulfate, acrylamide, and bis-acrylamide were from Fisher Scientific (Pittsburgh, PA).
Cell Culture
Caco-2 cells were grown in T-75 cm2 culture flasks at 37°C in a 5% CO2-95% air incubator in Minimum Essential Medium containing 20% FBS, 20 mM HEPES, 100 IU/ml penicillin, and 100 mg/ml streptomycin. Cells (2 × 104) seeded per well in 12-well Transwell inserts were used between passages 25 and 45. Fully differentiated confluent monolayers were used for the experiments (10–12 days postplating).
Bacterial Culture
The following Lactobacilli species, with ATCC strain numbers given in parentheses, were used in this study: LA (4357), L. rhamnosus (53103), L. plantarum (14917), and L. casei (393). These species were grown overnight in MRS broth (Difco, Detroit, MI) at 37°C without shaking. Bacteria were spun down by centrifuging at 3,000 rpm for 10 min. For in vitro studies, CS was separated from spun down bacteria, filtered through a 0.22-μm filter, and diluted in cell culture media (1:10) for further use. For treating the cell monolayers, the bacterial pellet was washed with DMEM-F-12 media (Invitrogen Life Technologies, Carlsbad, CA) containing 5 mg/l mannose and resuspended in the same media (6). For in vivo studies, 3 × 109 colony-forming units (CFU) of bacteria in 200 μl PBS, as reported earlier from our laboratory (28), were gavaged per animal for the first 2 days of DSS treatments in LA or LA + DSS groups.
Treatment of Caco-2 Cells
Bacterial suspensions in DMEM-F-12 media were diluted to OD600 nm = 0.2 in the same media and applied to the apical surface of cell monolayers at a multiplicity of infection of 50 for indicated time periods (6). For Cl−/HCO3− (OH−) exchange activity, cells were pretreated for 1 h from the apical side with LA bacterial suspension described above, or with the bacteria-free CS diluted in a ratio of 1:10 in serum-reduced medium (1% FBS) and then coincubated with IFN-γ from the basolatral side (30 ng/ml) for an additional 24 h. Similarly for promoter studies, 24 h posttransfection, cells were pretreated with LA-CS for 1 h followed by coincubation of IFN-γ (30 ng/ml) for an additional 24 h or incubated with IFN-γ (30 ng/ml) or LA-CS for 24 h alone in serum-reduced medium (1% FBS) (34).
Cl−/HCO3− Exchange Activity
Cl−/HCO3− exchange activity was assessed as 125I− uptake (in place of 36Cl− uptake) carried out in well-differentiated polarized Caco-2 cells as described previously (16, 32). Briefly, after treatment, the cell monolayers were incubated in base medium containing 20 mM HEPES, pH 8.5, at room temperature. After 30 min, base medium was removed, and the cells were rapidly washed with 1 ml tracer-free mannitol uptake buffer containing 260 mM mannitol, 20 mM Tris/2-(N-morpholino)ethanesulfonic acid, pH 7.0. This was followed by incubation with or without 600 μM DIDS in uptake buffer for 5 min, since this time period is within the linear range of 125I− uptake in cells. The radioactive 125I− in sodium iodide (3 mM) was added to the uptake buffer at a concentration of 1.0 μCi/ml. Uptake was stopped by removing the radioactivity-containing buffer and washing the cells rapidly two times with ice-cold PBS, pH 7.2. Furthermore, the cells were solubilized by 0.5 N NaOH for 4 h, and protein concentration was measured by the Bradford method (8). Radioactivity was measured by a Packard tri-Carb 1600 TR Liquid Scintillation Analyzer (Packard Instruments; PerkinElmer, Waltham, MA). The Cl−/HCO3− (OH−) exchange activity was then calculated as DIDS-sensitive 125I− uptake, and the specific activity is expressed as nanomoles per milligram protein per 5 min.
Transfections
Caco-2 cells were transfected with DRA promoter (p-1,183/+114), cloned upstream of the luciferase reporter gene in pGL2-Basic and β-galatosidase expression vector by electroporation using an Amaxa Nucleofactor System as described previously (34). Activities of Firefly Luciferase and β-galatosidase were measured according to the manufacturer's instructions (Promega, Madison, WI). DRA promoter activity was expressed in terms of relative luciferase activity normalized to β-galatosidase activity.
In Vivo Studies
All of the animal studies were approved by the Animal Care Committee of the University of Illinois at Chicago and Jesse Brown Veteran Affairs Medical Center. C57BL/6J mice (male, 8 wk old) were obtained from Jackson Laboratories (Bar Harbor, ME). Four groups of mice (8 animals/group) were used in this study for DSS induction of mild colitis as described previously (21, 33). In two of the groups 3% (wt/vol) DSS (mol mass 36–50 kDa; MP Biomedicals, Solon, OH) was given orally in drinking water for 7 days, whereas the control groups received drinking water only. In one of the two DSS groups, LA suspended in sterile PBS was administered by oral gavage (3 × 109 CFUs) along with DSS one time per day for the first 2 days (LA + DSS), whereas the other group received sterile PBS with no bacteria. Mice were daily monitored for weight change. On day 8, mice were euthanized, the entire colon was removed, and the length and weight were recorded. Mucosa was scraped from the distal colon for RNA and protein extraction.
Myeloperoxidase Activity
Myeloperoxidase (MPO) activity in the distal colon was assessed using the method of Krawisz et al. with minor modifications (18). MPO activity was normalized to the amount of protein in supernatant as measured by Bradford's method (8) and calculated as units per gram protein and expressed relative to control (considered as 100).
Real-Time PCR
RNA was extracted from mouse colonic mucosal samples using Qiagen RNeasy kits. RNA was reverse transcribed and amplified using a Brilliant SYBR Green qRT-PCR Master Mix kit (Stratagene). Mouse DRA was amplified with gene-specific primers. Mouse histone or GAPDH was amplified as an internal control (28). Relative levels of DRA mRNA were expressed as percent of control normalized to histone or GAPDH.
Western Blotting
Tissue lysates were prepared from the scraped colonic mucosa using cell lysis buffer (Cell Signaling, Danvers, MA). Lysates were run on an 8% gel and then transferred onto nitrocellulose membrane. Immunoblotting was carried out with anti-DRA affinity-purified antibody as previously described (6). Bands were visualized with enhanced chemiluminescence detection reagents.
Immunofluorescence Staining in Mouse Colonic Tissues
Sections of colonic tissues from different mice groups were snap-frozen in optimal cutting temperature embedding medium. For immunostaining, 5-μm frozen sections were fixed with 1% paraformaldehyde in PBS for 10 min at room temperature. Fixed sections were washed in PBS, permeabilized with 5% Nonidet P-40 for 5 min, and blocked with 5% normal goat serum (NGS) for 30 min. Tissues were incubated with DRA antibody (1:100) in PBS with 1% NGS for 90 min at room temperature. After being washed, sections were incubated with Alexa Fluor 594-conjugated goat anti-rabbit IgG and Alexa Fluor 488-conjugated phalloidin (5 U/ml; Invitrogen) for 60 min. Sections were then washed and mounted under cover slips using Slowfade Gold antifade with DAPI reagent (Invitrogen). Sections were imaged using a Carl Zeiss LSM 510 laser-scanning confocal microscope equipped with ×20 water immersion objective.
Statistical Analysis
Data are presented as means ± SE of 3–8 independent experiments. Differences between control vs. treated were analyzed using one-way ANOVA with Tukey's test. Differences were considered significant at P ≤ 0.05.
RESULTS
Long-Term Treatment with LA-CS Stimulates Cl−/HCO3− Exchange Activity in Caco-2 Cells
Our earlier studies showed that short-term treatments with LA and L. rhamnosus were effective in enhancing Cl−/HCO3− (OH−) exchange activity (5). In the current studies, the effects of long-term treatments with various Lactobacillus species on Cl−/HCO3− (OH−) exchange activity were evaluated. Postconfluent Caco-2 monolayers were treated with the CS (diluted 1:10 in DMEM) from different species of Lactobacillus for 24 h, and Cl−/HCO3− exchange activity was measured as DIDS-sensitive 125I uptake. As shown in Fig. 1, LA-CS significantly enhanced Cl−/HCO3− exchange activity (∼2-fold) in Caco-2 cells after 24 h. The CS of L. rhamnosus, L. plantarum, and L. casei showed no significant effect. Therefore, all further in vitro or in vivo studies were carried out only with LA.
Fig. 1.
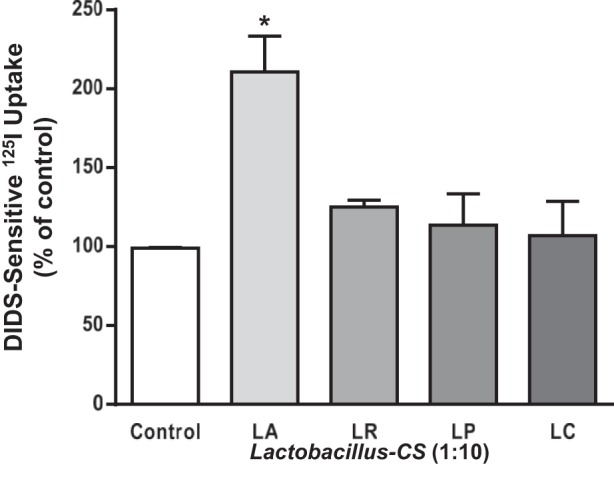
Long-term treatment with Lactobacillus acidophilus (LA)-culture supernatant (CS) stimulates Cl−/HCO3− exchange activity in Caco-2 cells. Overnight serum-starved postconfluent Caco-2 cells were treated with 1:10 dilution of CS of LA, L. rhamnosus (LR), L. plantarum (LP), and L. casei (LC) for 24 h, and apical Cl−/HCO3− exchange activity [4,4′-diisothiocyanate-stilbene-2, 2′-disulfonic acid (DIDS)-sensitive 125I− uptake] was measured. Values are means ± SE; n = 3 mice. *P < 0.05 compared with control.
Live LA or CS blocks IFN-γ-Induced Inhibition of Cl−/HCO3− Exchange Activity in Caco-2 Cells
We next examined whether LA can counteract the inhibitory effects of IFN-γ on DRA function. Postconfluent Caco-2 monolayers were pretreated with live bacteria or CS (LA-CS) from the apical surface for 1 h, which was continued for another 24 h with and without IFN-γ (30 ng/ml) added basolaterally. The concentrations of IFN-γ used in this study are based upon our previous studies showing inhibition of NHE3 (2) and DRA (34) gene expression by IFN-γ in Caco-2 cells as well as other studies (15). Live LA or LA-CS (1:10) significantly stimulated activity, whereas IFN-γ treatment significantly (P < 0.05 vs. control) inhibited the Cl−/HCO3− exchange activity in Caco-2 cells. Furthermore, both live LA (Fig. 2A) and LA-CS (Fig. 2B) completely blocked the IFN-γ-induced inhibition of Cl−/HCO3− exchange. These results show that either live bacteria or their secreted products in CS are enough to block the inhibition of Cl−/HCO3− exchange activity in response to IFN-γ treatment.
Fig. 2.
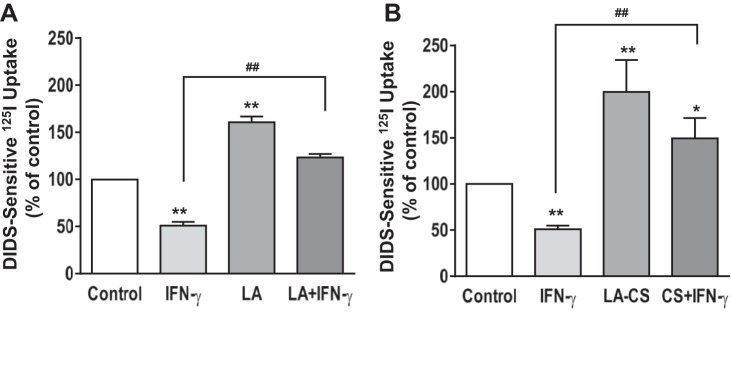
LA attenuates interferon-γ (IFN-γ)-mediated inhibition of apical Cl−/HCO3− exchange activity. Postconfluent Caco-2 monolayers were pretreated with live LA (A) or with LA-CS (B) from the apical surface for 1 h, which was continued for an additional 24 h, with and without IFN-γ (30 ng/ml) added basolaterally. DIDS-sensitive I125 uptake was then measured for 5 min. Values are means ± SE; n = 3. **P < 0.01 and *P < 0.05 compared with untreated control. ##P < 0.05 compared with IFN-γ.
LA-CS Increases DRA mRNA Levels and Blocks IFN-γ-Induced Decrease in DRA
We next examined whether LA counteracts the IFN-γ-mediated decrease in Cl−/HCO3− exchange activity via alterations in DRA mRNA levels. For these studies, cells were pretreated with LA-CS (1:10) from the apical surface for 1 h, which was continued for an additional 24 h, with and without IFN-γ (30 ng/ml) added basolaterally in serum-reduced medium (1% FBS). As reported previously by us (28) DRA mRNA levels were significantly increased (∼2.2-fold) in response to LA-CS, whereas IFN-γ treatment exhibited a 50% decrease in the mRNA levels. Consistent with the functional studies, the inhibitory effects of IFN-γ on DRA mRNA levels were blocked in cells treated with LA-CS (P < 0.01 vs. IFN-γ) as shown in Fig. 3.
Fig. 3.
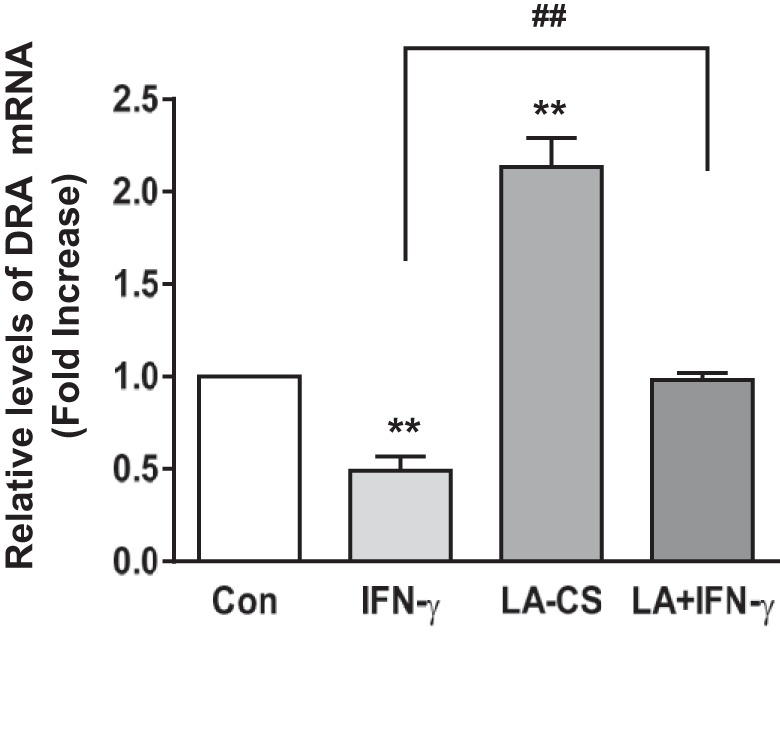
LA-CS increases downregulated in adenoma (DRA) mRNA levels and blocks IFN-γ-induced decrease in DRA mRNA. Cells were pretreated with LA-CS (1:10) from the apical surface for 1 h, which was continued for additional 24 h, with and without IFN-γ (30 ng/ml) added basolaterally in serum-reduced medium (1% FBS). The relative abundance of DRA mRNA was measured using gene-specific primers in Caco-2 RNA from different groups and was normalized to GAPDH mRNA as the internal control. Values are means ± SE; n = 4 independent experiments. **P < 0.01 compared with untreated control. ##P < 0.01, CS + IFN-γ compared with IFN-γ alone.
LA-CS Attenuates IFN-γ-Induced Inhibition of DRA Promoter Activity in Caco-2 Cells
We have previously shown that the activity of DRA full-length promoter construct p-1183/+114 exhibited (∼50%) inhibition in response to IFN-γ treatment compared with untreated controls (1, 34). In an attempt to examine whether LA-CS can reverse the effects of IFN-γ on DRA promoter, Caco-2 cells were transfected with DRA full-length promoter construct p-1183/+114. Twenty-four hours posttransfection, cells were treated with LA-CS (1:10 dilution) apically or IFN-γ basolaterally or coincubated with LA-CS and IFN-γ for 24 h, and DRA promoter activity was assessed. DRA promoter activity was markedly increased (∼1.5-fold) in response to LA-CS; IFN-γ treatment exhibited a 50% decrease in the activity, whereas the inhibitory effects of IFN-γ on DRA promoter activity were blocked in cells pre- and cotreated with LA-CS (P < 0.05 vs. IFN-γ) as shown in Fig. 4.
Fig. 4.
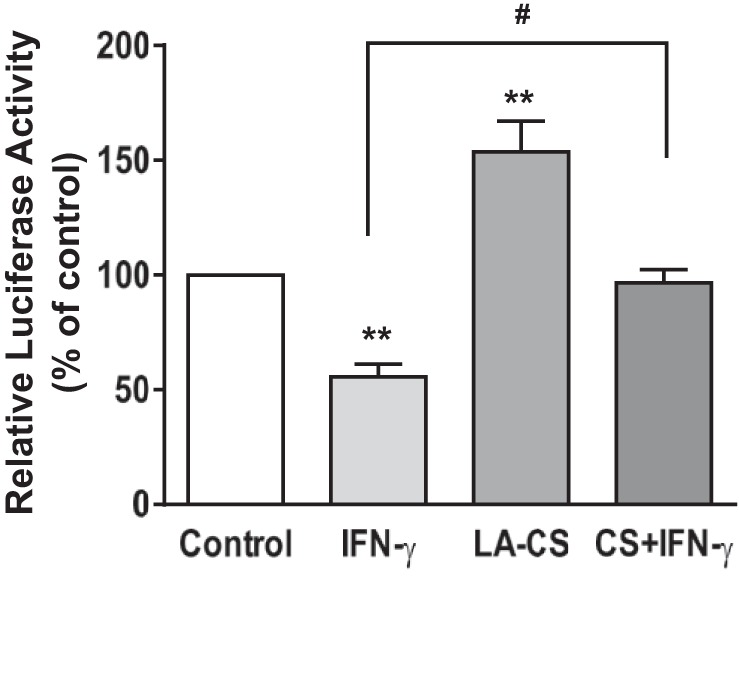
LA-CS stimulates DRA promoter activity and attenuates IFN-γ inhibition of DRA promoter activity. Caco-2 cells were transiently transfected with a 1.3-kb fragment of the promoter region of DRA gene cloned into pGL2 basic vector. At 24 h posttransfection cells were pretreated with LA-CS (1:10) from the apical surface for 1 h, which was continued for an additional 24 h with and without IFN-γ (30 ng/ml) added basolaterally in serum-reduced medium (1% FBS). Cells were then harvested 48 h posttransfection, and the promoter activity was measured by luciferase assay and normalized with β-gal activities. Values are means ± SE; n = 4 independent experiments. **P < 0.01 compared with untreated control. #P < 0.05, CS + IFN-γ compared with IFN-γ alone.
In Vivo Effects of LA on DSS-Induced Colitis in Mice
Our in vitro studies in Caco-2 cells clearly demonstrated that out of four Lactobacillus species tested, long-term treatment with LA showed maximum increase in Cl−/HCO3− exchange activity by increasing DRA expression. Also LA-CS was able to block the inhibition of Cl−/HCO3− exchange activity and repression of DRA promoter caused by IFN-γ in Caco-2 cells. Therefore, to get a comprehensive idea of the effect of this probiotic strain in a complex physiological setting, we next investigated the effects of LA on DRA expression in an in vivo mouse model of DSS-induced colitis.
LA did not attenuate DSS-induced loss of body weight.
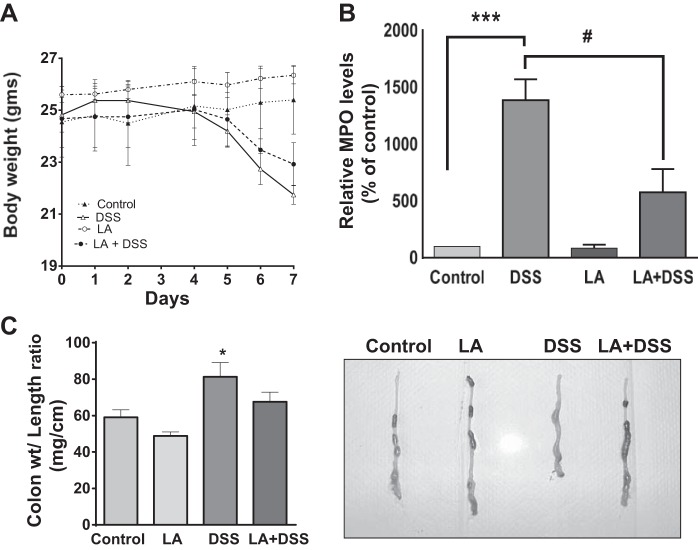
The DSS-induced colitis murine model is commonly used as a chemical injury model to address the pathogenesis of inflammatory bowel disease (14, 37). To test the efficacy of LA in ameliorating colitis, C57BL/6 mice were given 3% (wt/vol) DSS in drinking water for 7 days. As shown in Fig. 5A, significant weight loss was evident in the DSS-treated group compared with the control or LA-treated group. Whereas animals in the control group gained weight over a 1-wk period of observation, the mice in the DSS group showed significant reduction in weight over the same time, which was evident starting after the 5th day of DSS treatment and persisted until the 6th and 7th day compared with control. However, this weight loss was not attenuated in the LA + DSS mice group (Fig. 5A).
Fig. 5.
Oral administration of live LA in mice attenuates dextran sulfate sodium (DSS)-induced effects on body weight, myeloperoxidase (MPO) level, and the colon weight-to-length ratio. A: change in body weight during DSS treatment: mice were given 3% DSS in drinking water for 7 days. Control mice had only drinking water. LA (3 × 109 colony-forming units) were gavaged to control or DSS-treated mice for the first 2 days. Data were expressed as means ± SE of body weight in each animal (n = 4). B: MPO activity in distal colon during DSS treatment: measurement of colonic MPO activity, an index of neutrophil accumulation, was measured in the distal colonic mucosa of different groups, as described in materials and methods. Data calculated as MPO units/g protein and are expressed as %control (n = 4). ***P < 0.001 compared with untreated control. #P < 0.05 compared with DSS alone. C: changes in colon weight-to-length ratio in response to DSS treatment: C57BL/6 mice were administered 3% DSS in drinking water or LA as described above for 7 days. At the 8th day mice were killed, the entire colon was dissected out, and length and weight were recorded. Data were expressed as the means ± SE of the ratio of weight/length changes in each group (n = 7). *P < 0.05 compared with untreated control. Visual examination of colon from different groups suggests almost reversal of colon weight-to-length ratio in the LA + DSS group compared with DSS alone.
LA reduces MPO activity in acute DSS-treated mice colon.
Because the DSS-induced colitis mostly affects the distal part of the colon (26), a part (2 cm) of distal colon was used for determination of MPO activity. Colonic MPO level, an index of neutrophil accumulation, was significantly increased in DSS mice compared with control or LA mice (P < 0.01 vs. control or LA) (Fig. 5B). This increase in MPO levels was attenuated by LA treatment in the LA + DSS group (Fig. 5B). These results suggested that LA exerts its protective effects in the colon of mice with colitis by decreasing inflammation caused by DSS.
Changes in colon weight-to-length ratio.
Colon weight and length were recorded for each mouse immediately after death (8th day since the start of DSS treatment) (Fig. 5C). The length of the colon was significantly shortened (P < 0.05) in mice in the DSS group (Table 1). Although the colon length was greater in the LA group, compared with control, this was not statistically significant. Also, the DSS group showed a significant increase in the colon weight-to-length ratio compared with control (Fig. 5C), an indication of fluid accumulation that, however, was not significantly attenuated in the LA + DSS mice group. It should be noted that the pelleted stool content (Fig. 5C) as examined visually (qualitative measurement) appeared to decrease in DSS colon compared with control. In contrast, in LA + DSS mice this phenotype was less prominent. These results indicate that LA may have beneficial effects in colons of mice, including potential antidiarrheal effects.
Table 1.
Colonic weight and colon length of different treatment groups
| Control | LA | DSS | LA + DSS | |
|---|---|---|---|---|
| Length, cm | 6.48 ± 0.18 | 6.75 ± 0.40 | 5.04 ± 0.09* | 5.75 ± 0.24 |
| Weight, g | 0.38 ± 0.03 | 0.33 ± 0.02 | 0.41 ± 0.03 | 0.39 ± 0.03 |
Values are means ± SE. LA, Lactobacillus acidophilus; DSS, dextran sulfate sodium. Full colonic length and weight (excluding cecum) were measured immediately after death.
P < 0.05 vs. control.
LA Blocks DSS-Induced Decrease in DRA mRNA and Protein Expression in Distal Colon
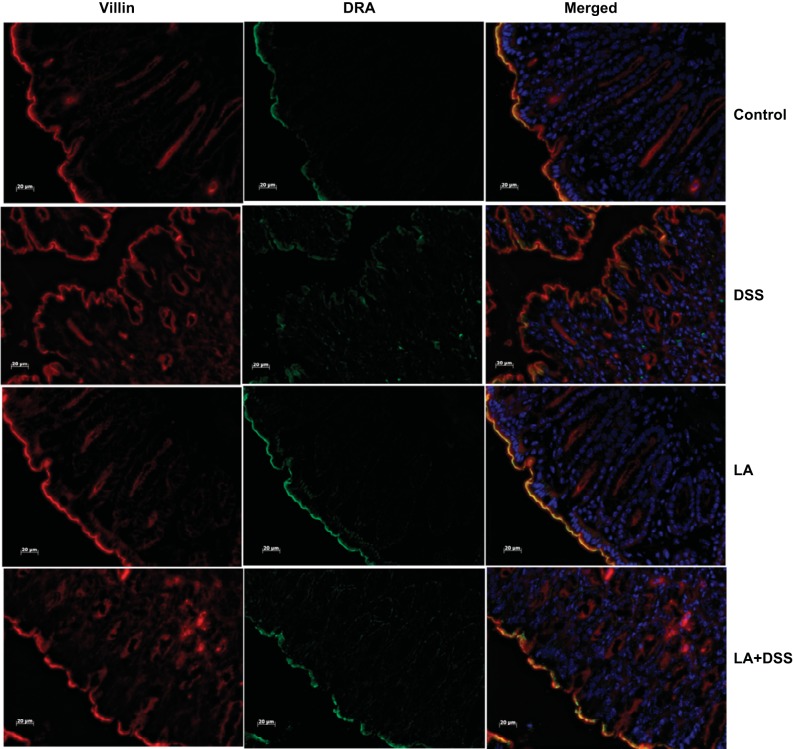
Our previous studies have already shown that DRA mRNA and protein expression in mice colonic tissue increases in response to live LA gavaged for 24 h (28). To examine whether LA could restore DSS-induced downregulation of DRA mRNA and protein expression, we examined DRA mRNA and protein levels in distal colonic mucosa of control, DSS, LA, and LA + DSS mice groups using real-time PCR and immunoblotting. As shown in Fig. 6, DRA mRNA levels in distal colon were significantly increased (∼1.5-fold) in response to LA administration. DSS caused a significant decrease in DRA mRNA and protein expression (∼50–60%) in distal colon. On the other hand, LA treatment to DSS mice significantly attenuated this decrease in the levels of DRA mRNA (Fig. 6) and protein (Fig. 7) in LA + DSS mice. LA-mediated reversal of DSS effects on DRA expression was also measured by immunofluorescence staining of colonic sections. As shown in Fig. 8, the apical membrane level of DRA was substantially reduced in response to DSS treatment. On the other hand, LA administration not only increased apical membrane DRA level compared with control but also almost completely blocked the inhibitory effects of DSS on DRA expression (Fig. 8). These results suggest that modulation of DRA protein expression by LA might be a major contributor to the observed antidiarrheal effects of LA in DSS colitis.
Fig. 6.
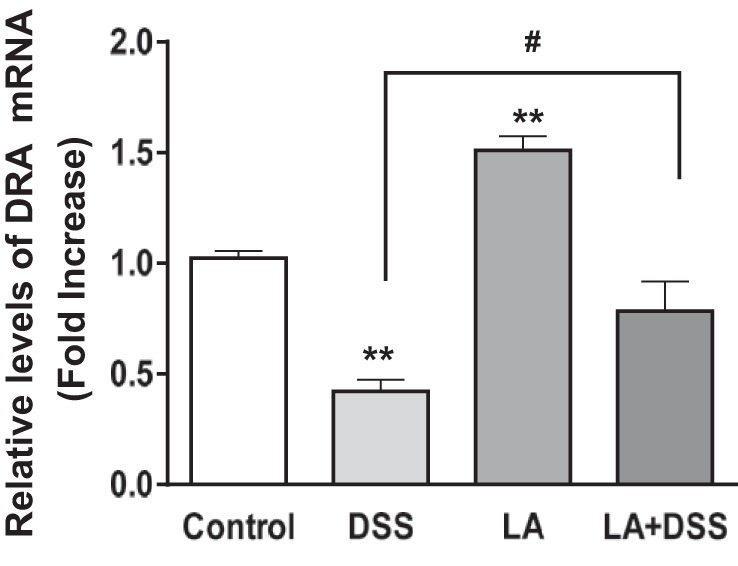
Oral administration of live LA attenuates DSS-induced decrease in DRA mRNA levels in mice colon: the relative abundance of DRA mRNA was measured, using gene-specific primers, in the distal colonic mucosa of different groups and was normalized to histone or GAPDH mRNA as the internal controls. Results represent means ± SE of 8 mice. **P < 0.01 compared with control. #P < 0.05, LA + DSS compared with DSS alone.
Fig. 7.
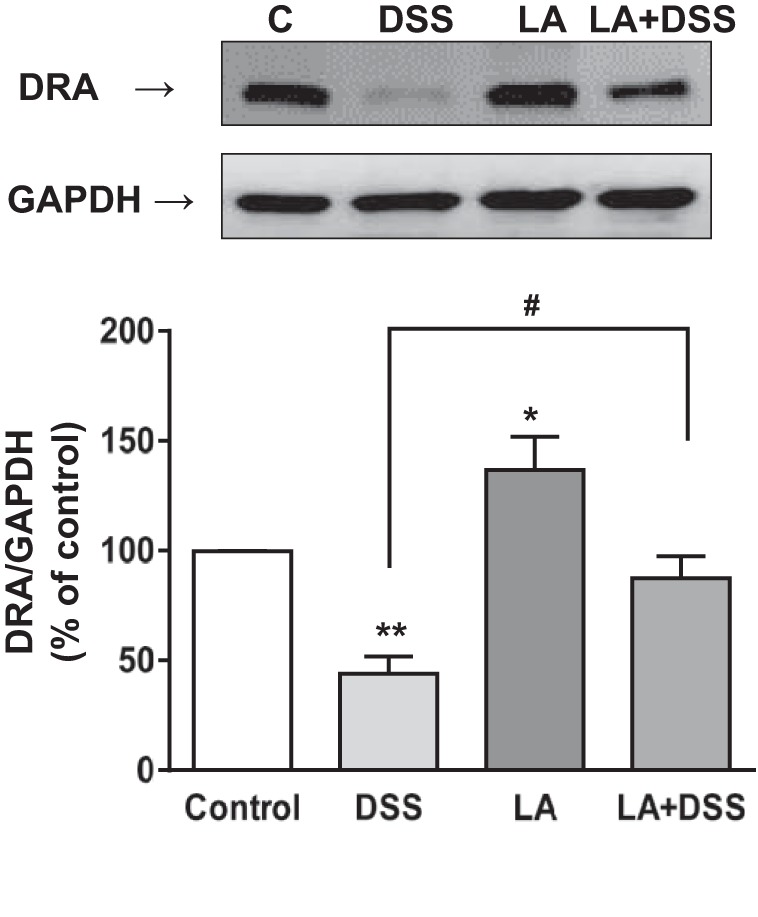
LA increases DRA protein levels and blocks DSS-induced decrease in DRA protein expression. DRA protein levels in distal colonic mucosal tissue lysates were measured by Western blot as described in materials and methods. A representative blot is shown. Bottom, densitometric analysis of relative band intensities. Results represent means ± SE of 8 mice. *P < 0.05 and **P < 0.01 compared with control. #P < 0.05, LA + DSS compared with DSS alone.
Fig. 8.
LA treatment blocks DSS-induced decrease in DRA immunostaining. Green, DRA; red, villin; blue, nuclei; scale bar = 20 μm.
DISCUSSION
Diarrhea associated with intestinal inflammatory diseases, including colitis, is the result of a complex interaction of multiple inflammatory mediators and their effects on the intestinal epithelium. Diarrhea can result partly in response to enhanced secretion of high levels of proinflammatory cytokines such as IFN-γ and TNF-α (7, 13, 17). Studies have shown that Caco-2 cells treated with proinflammatory cytokines (IFN-γ, IL-1β) have diminished expression of DRA, and the same is true for patients suffering from ulcerative colitis (34, 36). SLC26A3 or DRA is the main candidate gene for luminal human intestinal Cl−/HCO3− exchange (12). Its importance is further emphasized by DRA knockout mice, which exhibit diarrheal phenotype owing to loss of luminal membrane Cl−/HCO3− exchange activity (35). We have previously shown in in vitro and in vivo models that probiotics such as LA may have potential therapeutic value in treating diarrhea due to their efficacy in increasing Cl−/HCO3− exchange activity and DRA expression involving both short-term trafficking and long-term transcriptional changes, respectively (6, 28). Our group (4, 5) as well as others (30) have previously demonstrated that probiotics reverse the effects of inflammatory cytokines in human intestinal epithelial cells and inflammation in a mouse model (33). Experimental studies have implicated DRA repression as one of the potential events during intestinal inflammation (19, 38). However, the functional consequences of this repression have not been studied in detail. Also, nothing is known about the potential beneficial effects of probiotics on DRA (Cl−/HCO3− exchange) function and expression under inflammatory states such as colitis.
In the current report, we have shown that pretreatment with LA or bacteria-free CS of LA counteracts the inhibitory effects of IFN-γ on DIDS-sensitive apical Cl−/HCO3− exchange activity. Parallel to function, IFN-γ-mediated inhibitory effects on DRA mRNA levels and promoter activity were abrogated by LA-CS. Consistent with our data, earlier reports have shown that pretreatment with live probiotics reversed the effects of TNF-α or IFN-γ on cystic fibrosis transmembrane conductance regulator (30). These results suggest that LA-derived soluble factors exert distinct effects to alleviate/block the cytokine effects on DRA expression and function. Our functional studies are limited by lack of DRA-specific inhibitor; however, given that LA-CS treatment does not affect PAT-1 expression (28) and our functional read out is parallel to changes in DRA mRNA and protein, it is highly likely that LA-mediated effects on apical Cl−/HCO3− exchange activity are mediated by DRA.
Our promoter studies have shown that LA-CS enhanced the activity of the full-length (p-1183/+114) DRA promoter. We have previously shown that IFN-γ inhibition of DRA promoter activity involved the JAK/STAT1 pathway (34). Hence, it can be speculated that LA-secreted soluble factors in the CS could reverse the effects of inflammatory cytokines either by blocking the phosphorylation of STAT-1 signaling pathways induced by IFN-γ or via blocking the binding of cytokine to its receptor. However, because live LA or LA-CS were added from the apical side of cell monolayers, whereas IFN-γ receptors are localized to basolateral membranes of polarized Caco-2 cells, it is unlikely that live LA or its supernatant-derived soluble factors counteracted IFN-γ effects via blocking its binding to the receptors. In addition, LA could also act via an independent pathway to counteract inhibition of promoter activity by IFN-γ. For example, involvement of p38, ERK1/2 MAPK, and phosphatidylinositol 3-kinase (PI 3-kinase) has been previously reported in LA-induced modulation of Cl− secretion in Caco-2 and HT29/cl.19a cells (30). We previously showed that acute stimulation in DRA activity by LA and increase in DRA levels on the plasma membrane involved PI 3-kinase (but not MEK1, MEK2, and p38 MAPK pathway)(6). It is possible that the PI 3-kinase-mediated pathway may be involved in observed effects of LA in counteracting the effects of cytokines. Future studies may focus on delineating exact signaling mechanisms underlying the effects of LA.
We extended our in vitro studies showing efficacy of LA in reversing the effects of inflammatory cytokine on DRA activity using an in vivo model of DSS colitis. Based on our previous study (33), we used 3% DSS for 7 days to induce mild colitis in mice, since DSS at high concentrations (4–5%) is known to cause ulcerations and erosion of the epithelium (23). Two doses of LA were chosen based on our previous study showing that, under similar conditions, LA attenuated inflammation and reversed the reduced expression of MDR-1 transporter in DSS-treated mice. Also, multiple studies suggest that probiotic bacteria remain colonized in the gut for at least 7 days to exert beneficial effects (27, 33). In DSS-induced colitis in mice, the most affected segment of the gastrointestinal tract is known to be the distal colon showing inflammation, shortening of length and loose fecal pellet, compared with control mice (29). Our studies also showed that mice administered DSS showed significant weight loss and shortening of the colon. However, these effects were not significantly reversed by administration of live LA. Furthermore, weight loss, MPO activity, and colon weight-to-length ratio (a potential readout for diarrheal phenotype) were significantly greater in the DSS group compared with all other groups. Occurrence of solid fecal pellet in LA + DSS mice indicated potential beneficial effects of LA on diarrheal phenotype and on colonic inflammation caused by DSS. Also, the inhibition of enhanced colonic MPO activity in DSS mice by LA reflects a potent anti-inflammatory effect of LA against tissue injury. The anti-inflammatory/immunomodulatory properties of various Lactobacillus species have previously been described by our group and others in experimental models of colitis (10, 25, 33). Studies have shown that fecal excretion of HCO3− is reduced in patients with active ulcerative colitis, which is caused by impairment of the colonic anion exchange process induced by inflammation (9). This is mainly due to a major decrease in the Cl−/HCO3− exchange activity in the luminal membrane, associated with a downregulation of the anion exchanger DRA (SLC26A3) in colitis (36). As expected, levels of DRA mRNA and protein expression were dramatically reduced in the distal colon of DSS-treated mice compared with control, whereas the other group where live LA was administrated along with DSS showed significantly less reduction in DRA mRNA and protein levels compared with the DSS-treated group alone. Our results are consistent with previous reports showing partial reversal of the effects of DSS in response to pretreatments with LA (11, 25). However, the mechanism of LA-mediated reduction in gut inflammation is not entirely clear. This could involve attenuation of effect of cytokines, beneficial effects on epithelial integrity, and improvement in barrier function (29).
Overall, our results demonstrated that the soluble factor(s) present in the CS of LA attenuate the IFN-γ-induced decrease in DRA function, mRNA and promoter activity. Also, live LA administration in vivo showed its effects by reversing the DSS-induced decrease in DRA mRNA and protein levels and appeared to have potential antidiarrheal effects in an experimental model of DSS colitis. These results are of critical importance in increasing our understanding of the molecular basis of the beneficial effects of LA and LA-derived molecules. Additionally, efficacy of LA supernatant to counteract inflammation-induced downregulation of DRA expression and function highlights the novel therapeutic potentials of LA-secreted soluble factors for diarrheal disorders associated with gut inflammation where live bacteria may be counterindicated for therapeutic purposes due to compromised barrier function of the intestine. In future studies, it will also be important to examine efficacy of LA as a therapeutic (after DSS treatment) rather than preventive agent in reversing inflammation and DRA expression.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-54016 (P. K. Dudeja), DK-81858 (P. K. Dudeja), DK-92441 (P. K. Dudeja), DK-71596 (W. A. Alrefai), DK-096258 (R. K. Gill), and DK 96254 (S. Saksena), the Bill & Melinda Gates Foundation Grant OPP1058288 (A. Borthakur), and by the Department of Veteran Affairs (P. K. Dudeja and W. A. Alrefai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: V.S., S.S., R.K.G., W.A.A., A.B., and P.K.D. conception and design of research; V.S., A.K., G.R., A.N.A., S.P., and M.N.J. performed experiments; V.S., A.K., G.R., A.N.A., S.P., M.N.J., and A.B. analyzed data; V.S., G.R., A.N.A., S.P., S.S., R.K.G., W.A.A., A.B., and P.K.D. interpreted results of experiments; V.S., A.K., and S.P. prepared figures; V.S., A.K., A.B., and P.K.D. drafted manuscript; S.S., R.K.G., W.A.A., A.B., and P.K.D. edited and revised manuscript; P.K.D. approved final version of manuscript.
REFERENCES
- 1.Alrefai WA, Wen X, Jiang W, Katz JP, Steinbrecher KA, Cohen MB, Williams IR, Dudeja PK, Wu GD. Molecular cloning and promoter analysis of downregulated in adenoma. Am J Physiol Gastrointest Liver Physiol 293: G923–G934, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Amin MR, Malakooti J, Sandoval R, Dudeja PK, Ramaswamy K. IFN-γ and TNF-α regulate human NHE3 gene expression by modulating the Sp family transcription factors in human intestinal epithelial cell line C2BBe1. Am J Physiol Cell Physiol 291: C887–C896, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Borenshtein D, Fry RC, Groff EB, Nambiar PR, Carey VJ, Fox JG, Schauer DB. Diarrhea as a cause of mortality in a mouse model of infectious colitis (Abstract). Genome Biol 9: R122, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borthakur A, Anbazhagan AN, Kumar A, Raheja G, Singh V, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus plantarum counteracts TNF-α-induced downregulation of SMCT1 expression and function. Am J Physiol Gastrointest Liver Physiol 299: G928–G934, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borthakur A, Bhattacharyya S, Kumar A, Anbazhagan AN, Tobacman JK, Dudeja PK. Lactobacillus acidophilus alleviates platelet-activating factor-induced inflammatory responses in human intestinal epithelial cells. PLoS One 8: e75664, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138: 1355–1359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 3: 521–533, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 9.Caprilli R, Frieri G, Latella G, Vernia P, Santoro ML. Faecal excretion of bicarbonate in ulcerative colitis. Digestion 35: 136–142, 1986 [DOI] [PubMed] [Google Scholar]
- 10.Chen CC, Louie S, Shi HN, Walker WA. Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr Res 58: 1185–1191, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chen LL, Wang XH, Cui Y, Lian GH, Zhang J, Ouyang CH, Lu FG. Therapeutic effects of four strains of probiotics on experimental colitis in mice. World J Gastroenterol 15: 321–327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chernova MN, Jiang L, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL. Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol 549: 3–19, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest 116: 2682–2694, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249, 1993 [PubMed] [Google Scholar]
- 15.Foley KF, Pantano C, Ciolino A, Mawe GM. IFN-γ and TNF-α decrease serotonin transporter function and expression in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 292: G779–G784, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428–437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indaram AV, Visvalingam V, Locke M, Bank S. Mucosal cytokine production in radiation-induced proctosigmoiditis compared with inflammatory bowel disease. Am J Gastroenterol 95: 1221–1225, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87: 1344–1350, 1984 [PubMed] [Google Scholar]
- 19.Lohi H, Makela S, Pulkkinen K, Hoglund P, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J. Upregulation of CFTR expression but not SLC26A3 and SLC9A3 in ulcerative colitis. Am J Physiol Gastrointest Liver Physiol 283: G567–G575, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 116: 1107–1114, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Marrero JA, Matkowskyj KA, Yung K, Hecht G, Benya RV. Dextran sulfate sodium-induced murine colitis activates NF-κB and increases galanin-1 receptor expression. Am J Physiol Gastrointest Liver Physiol 278: G797–G804, 2000 [DOI] [PubMed] [Google Scholar]
- 22.McCarthy J, O'Mahony L, O'Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, Fitzgibbon J, O'Sullivan GC, Kiely B, Collins JK, Shanahan F. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut 52: 975–980, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 296: G1140–G1149, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch 447: 710–721, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Nanda Kumar NS, Balamurugan R, Jayakanthan K, Pulimood A, Pugazhendhi S, Ramakrishna BS. Probiotic administration alters the gut flora and attenuates colitis in mice administered dextran sodium sulfate. J Gastroenterol Hepatol 23: 1834–1839, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98: 694–702, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Panigrahi P, Parida S, Pradhan L, Mohapatra SS, Misra PR, Johnson JA, Chaudhry R, Taylor S, Hansen NI, Gewolb IH. Long-term colonization of a Lactobacillus plantarum synbiotic preparation in the neonatal gut. J Pediatr Gastroenterol Nutr 47: 45–53, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298: G395–G401, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52: 988–997, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology 130: 731–746, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Saksena S, Dwivedi A, Singla A, Gill RK, Tyagi S, Borthakur A, Alrefai WA, Ramaswamy K, Dudeja PK. Characterization of the 5′-flanking region and regulation of expression of human anion exchanger SLC26A6. J Cell Biochem 105: 454–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saksena S, Gill RK, Tyagi S, Alrefai WA, Sarwar Z, Ramaswamy K, Dudeja PK. Involvement of c-Src and protein kinase C delta in the inhibition of Cl(-)/OH- exchange activity in Caco-2 cells by serotonin. J Biol Chem 280: 11859–11868, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Saksena S, Goyal S, Raheja G, Singh V, Akhtar M, Nazir TM, Alrefai WA, Gill RK, Dudeja PK. Upregulation of P-glycoprotein by probiotics in intestinal epithelial cells and in the dextran sulfate sodium model of colitis in mice. Am J Physiol Gastrointest Liver Physiol 300: G1115–G1123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saksena S, Singla A, Goyal S, Katyal S, Bansal N, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-γ. Am J Physiol Gastrointest Liver Physiol 298: G159–G166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Xiao F, Juric M, Li J, Riederer B, Yeruva S, Singh AK, Zheng L, Glage S, Kollias G, Dudeja P, Tian DA, Xu G, Zhu J, Bachmann O, Seidler U. Loss of downregulated in adenoma (DRA) impairs mucosal HCO3(-) secretion in murine ileocolonic inflammation. Inflamm Bowel Dis 18: 101–111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman SV, Merlin D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One 4: e6073, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H, Jiang W, Furth EE, Wen X, Katz JP, Sellon RK, Silberg DG, Antalis TM, Schweinfest CW, Wu GD. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am J Physiol Gastrointest Liver Physiol 275: G1445–G1453, 1998 [DOI] [PubMed] [Google Scholar]