Abstract
Effective therapies are limited for patients with parenteral nutrition-dependent short bowel syndrome. We previously showed that intestinal expression of the transcriptional coregulator tetradecanoyl phorbol acetate-induced sequence 7 (tis7) is markedly increased during the adaptive response following massive small bowel resection and tis7 plays a role in normal gut lipid metabolism. Here, we further explore the functional implications of tis7 deletion in intestinal lipid metabolism and the adaptive response following small bowel resection. Intestinal tis7 transgenic (tis7tg), tis7−/−, and wild-type (WT) littermates were subjected to 50% small bowel resection. Mice were fed a control or a high-saturated-fat (42% energy) diet for 21 days. Survival, body weight recovery, lipid absorption, mucosal lipid analysis, and the morphometric adaptive response were analyzed. Quantitative real-time PCR was performed to identify tis7 downstream gene targets. Postresection survival was markedly reduced in high-fat, but not control, diet-fed tis7−/− mice. Decreased survival was associated with anastomotic inflammation and intestinal obstruction postresection. High-fat, but not control, diet-fed tis7−/− mice had increased intestinal IL-6 expression. Intestinal lipid trafficking was altered in tis7−/− compared with WT mice postresection. In contrast, high-fat diet-fed tis7tg mice had improved survival postresection compared with WT littermates. High-fat diet feeding in the setting of tis7 deletion resulted in postresection anastomotic inflammation and small bowel obstruction. Tolerance of a calorie-rich, high-fat diet postresection may require tis7 and its target genes. The presence of luminal fat in the setting of tis7 deletion promotes an intestinal inflammatory response postresection.
Keywords: intestinal adaptation, lipid metabolism, gut inflammation, short bowel syndrome
a dynamic morphological and functional gut adaptive response occurs in rodents after loss of small bowel surface area due to surgical resection. The morphological response in the remnant intestine is characterized by increased villus height and crypt depth and increased crypt cell production rate, associated with an early surge in stem cell numbers and crypt fission (6, 10, 11). The functional adaptive response is characterized by increased nutrient, fluid, and electrolyte absorption (10). Morphological and metabolic adaptation also occurs in humans following loss of functional small bowel surface area, although the process is more variable and depends on the patient's underlying disease(s) and overall morbidity (11). Elucidation of the molecular basis of this response, which is still poorly understood, has important potential therapeutic implications.
We have identified the immediate early gene tis7 (tetradecanoyl phorbol acetate-induced sequence 7) as a putative regulator of the gut adaptive response (15, 19, 23, 26). Tis7 is a transcriptional coregulator (21) that translocates into the nucleus, where it interacts with the SIN 3 protein complex that has histone deacetylase activity (21, 22). This complex binds to DNA-binding proteins that interact with specific promoter-binding sites to regulate gene transcription (21, 22). We have shown that tis7 mRNA expression increases up to eightfold in remnant mouse ileum after 50% midjejunoileal resection (15). This increase in tis7 mRNA expression occurs in adapting enterocytes, the principal absorptive cell of the gut, and in crypt cells (15).
To elucidate the function of tis7 in the normal gut, we generated transgenic mice in which promoter sequences from the rat liver fatty acid-binding protein gene were utilized to preferentially direct overexpression of tis7 in crypt and villus epithelium (23). These mice exhibited increased adiposity, despite reduced total body mass and weight, and increased rates of intestinal triglyceride (TG) absorption (23). In contrast, tis7−/− mice have normal weight and body fat but when fed a high-fat diet are protected from weight gain and have reduced adiposity compared with high-fat diet-fed wild-type (WT) mice (26). High-fat diet-fed tis7−/− mice had evidence of altered lipid metabolism, with reduced and delayed lipid absorption in the jejunum, with shunting of free fatty acids and TG to the ileum/distal gut. However, net lipid absorption as measured by fecal fat quantification methods was unchanged. The role of tis7 in gut adaptation was also studied using tis7−/− mice. After resection, tis7−/− mice had a blunted early (by 72 h) adaptive morphometric response, with reduced villus height and reduced crypt cell proliferative index, which resolved by 10 days postresection.
To further explore the functional implications of tis7 deletion on the metabolic gut adaptive response postresection, we performed 50% intestinal resections and fed mice a high-fat diet for 3 wk postresection. We examined survival, weight gain, and the adaptive response postresection. We show that tis7 deletion in high-fat, but not control, diet-fed mice results in increased mortality postresection, associated with intestinal obstruction, inflammation, and altered intestinal mucosal lipid concentrations, despite similar rates of body weight recovery postresection. In contrast, intestinal tis7 overexpression is protective in high-fat diet-fed mice postresection, with an improved survival rate compared with WT littermates and a small, but significant, increase in lipid absorption. We have generated a novel model of anastomotic inflammation and intestinal obstruction postresection. Small bowel obstruction following intestinal resection is a common clinical problem for which there are no preventive or effective medical therapies; this model may be used to understand how specific diets may contribute to the underlying pathogenesis and to the search for potential preventive and therapeutic strategies.
MATERIALS AND METHODS
Mice.
The Washington University School of Medicine Animal Studies Committee reviewed and approved all animal experimentation. The tis7−/− mice were generated as described elsewhere (20). The mice are congenic on a C57BL/6 background; their respective WT C57BL/6 littermates were used as controls for all tis7−/− experiments. The tis7 transgenic (tis7tg) mice were generated as described elsewhere (23) on a FVB/N background. WT FVB/N littermates were used as controls for all experiments with tis7tg mice. The liver fatty acid-binding promoter was used to overexpress tis7 specifically in villus and crypt enterocytes of the small bowel and in colonocytes of the proximal colon. All mice were bred and housed together in the same room of the Washington University School of Medicine mouse facility. The mice were fed a standard rodent chow diet (Picolab 20, Ralston Purina), except as described below, and were kept on a strict 12:12-h light-dark cycle.
Intestinal resection surgery.
At 2 days prior to surgery, the animals were fed ad libitum a control liquid diet, which is equivalent to standard rodent chow (see below). On the day of surgery, mice were anesthetized with ketamine (87 mg/kg)-xylazine HCl (13 mg/kg) and isoflurane inhalation. As previously described (15), a 50% small bowel resection, starting 3 cm from the ligament of Treitz and ending 8 cm from the ileocecal junction, was performed. A primary end-to-end anastomosis was made between the remaining jejunum and ileum, and the abdominal cavity was closed in two layers. Sham surgeries were also performed as control: a single incision was made 9 cm from the ileocecal junction, the intestine was resutured at this site, and the abdominal cavity was closed as described above. Mice received 0.2 mg of gentamicin in 0.5 ml of saline, buprenorphine (0.03 mg/kg ip) for analgesia, and 0.9% sodium chloride (2.5 ml ip) for fluid resuscitation. Mice recovered in an Air Shields isolette incubator at 31°C overnight and were euthanized on postresection day 21. Remnant duodenum-jejunal, ileal, and anastomotic segments were harvested and frozen in liquid nitrogen or fixed in formalin. Mice that stopped feeding, as assessed by monitoring daily food intake, or were not moving freely and appeared distressed, as evidenced by a hunched posture or excessive weight loss, were euthanized.
Diets and monitoring postresection surgery.
After resection, mice were housed in individual cages and fed ad libitum a liquid control or a liquid high-fat diet daily. The nutrient content of the control diet is equivalent to standard rodent chow and consists of 200 g of protein, 99 g of fat, and 47 g of fiber per kilogram of diet and water, providing 14.24 MJ/kg diet (4.5% of calories from fat) (AIN-93M Purified Liquid Diet-Control 7100080, Dyets, Bethlehem, PA). The high-saturated-fat (Western) diet contains 173 g of protein, 225 g of milk fat, and 1.5 g of cholesterol per kilogram of diet and water, providing 18.84 MJ/kg diet (modified, high-fat AIN-93M Purified Liquid Diet 710147, Dyets), with a total of 42% of energy derived from fat. This diet contains 0.47% ω-3, 2.56% ω-6, and 18% ω-9 (oleic acid) fatty acids. The amount of liquid diet consumed and the weight of each mouse were recorded daily.
Morphometric analysis.
All histological studies were performed in the Advanced Imaging Tissue Analysis Core of the Digestive Disease Research Core Center. Sections were stained with hematoxylin-eosin, and villus heights and crypt depths were measured in preoperative ileal segments (obtained from the ileum adjacent to the site of resection) and in postoperative remnant ileum (obtained on the day of euthanization). Digital images were acquired for morphometry using AxioVision (version 4.7) software (Zeiss). Measurements were taken from ≥25 well-oriented crypt-villus units. Sections from the anastomotic site were also stained with hematoxylin-eosin for inflammatory cell analysis and processed for immunohistochemical analysis (see below). On the day of euthanization, all mice were given 5-bromodeoxyuridine (5-BrdU, 16 g/l, 2.5 mg/kg ip). Crypt cell proliferation was measured by 5-BrdU incorporation detected by an anti-BrdU antibody (1:1,000 dilution; Accurate Chemical & Scientific). Crypt cell proliferation rate was measured as percentage of labeled crypt cells, which was calculated by counting the number of 5-BrdU-labeled cells per crypt and dividing by the total number of cells per crypt. At least 10 full-length crypts were evaluated in each sample.
Fecal fat analysis.
The mice were placed into individual metabolic cages with mesh cage bottoms to allow collection and prevent ingestion of feces during days 19–21 of the experiment. Fat analysis was performed by Folch's extraction, as described elsewhere (17). Briefly, feces were collected daily into separate sterile 15-ml conical tubes. One 24-h collection per mouse was used for fecal fat analysis. Feces were dried overnight in a 60°C oven, and 0.2 g of feces was removed. The dried feces were divided into two samples, each of which was added to a 2-ml microcentrifuge tube containing 0.2 ml of 1-mm glass beads, with 0.8 ml of deionized distilled water, incubated at room temperature for 2 h, and blended for 4 min. Then 0.5 ml of Folch reagent (2:1 chloroform-methanol) was added to each tube, and samples were vortexed. Samples were recombined, and an additional 4 ml of Folch reagent were added. Samples were vortexed, incubated for 30 min at room temperature, and centrifuged at 2,000 rpm for 5 min. The lower chloroform phase was transferred to a previously weighed 5-ml glass vial, and an additional 2 ml of Folch reagent were added to the remaining homogenate, which was vortexed and centrifuged, with collection of the chloroform phase into the same vials. The scintillation vials were dried under N2 gas for 30 min, vacuum-dried at 60°C for 30 min, cooled, and weighed. Percentage of fecal fat in the fecal sample was then calculated as [total lipid (mg)/amount of stool analyzed (mg)] × 100. Percentage of unabsorbed fat was calculated as total stool collected (g) × %fecal fat/dry food weight consumed (g) × 21.2%. Percentage of unabsorbed fat was subtracted from 100 to obtain the percentage of fat absorbed. Liquid food (control or high-fat diet) was used as a control for each measurement.
Immunohistochemical analyses.
For all immunohistochemical procedures, antigens were unmasked with citrate-based DIVA decloaker buffer (Biocare, Concord, CA). NF-κB p65/relA expression was examined postresection in anastomotic sites from high-fat diet-fed tis7−/− and WT mice by immunohistochemistry using a rabbit monoclonal antibody to human NF-κB p65/relA protein, which cross-reacts with murine NF-κB p65/relA (1:800 dilution; Cell Signaling, Danvers, MA). Antigen-antibody complexes were detected by horseradish peroxidase (HRP). T cells were detected with an anti-human CD3 antibody that cross-reacts with mouse (1:100 dilution; AbD Serotec, Raleigh, NC) and streptavidin-HRP. B cells were detected with an anti-human CD45R/B220 antibody that cross-reacts with mouse (1:200 dilution; eBioscience, San Diego, CA) and with HRP-polymer (Biocare). Macrophages were detected with anti-mouse F4/80 (1:200 dilution; Abcam, Cambridge, MA) and streptavidin-HRP.
Genotyping.
Tail genomic DNA was isolated using EZ High MW Mouse Tail DNA Isolation Kit (EZ BioResearch, St. Louis, MO). PCR was performed using KlenTaq LA polymerase (St. Louis, MO) with the forward tis7tg oligonucleotide primer 5′-CTG CAC CAG CTG GCG TTT GAC ACC TAC CAG-3′ and the reverse tis7tg primer 5′-TTT CTG TTG TGT TTC CTC CCT GTT GGA GGG-3′. FVB/N WT primers are 5′-TGG ACA GGA CTG GAC CTC TGC TTT CC-3′ and 5′-TAG AGC TTT GCC ACA TCA CAG GTC AT-3′. PCR genotyping for the tis7−/− mice included the forward primers 5′-TAG CCA AAG CAA CAT TAC ATC-3′ (RK60), 5′-GAT GTG CTG CAA GGC GAT TAA GTT-3′ (RK40), and 5′-GTA TCC TGT CCA GGT AAT CCT-3′ (RK38).
Quantitative real-time PCR.
Quantitative real-time PCR (qRT-PCR) was performed as previously described (18, 26). Primers for each gene were designed using Primer Express 2.0 (Applied Biosystems). Genes and primers are summarized in Table 1.
Table 1.
Primers used for quantitative RT-PCR
| Primer |
||
|---|---|---|
| Forward | Reverse | |
| IL-6 | 5′-AGA CAA AGC CAG AGT CCT TCA GAG A-3′ | 5′-GCC ACT CCT TCT GTG ACT CCA GC-3′ |
| KC | 5′-GCA CCC AAA CCG AAG TCA TAG CCA-3′ | 5′-GCG TTC ACC AGA CAG GTG CCA-3′ |
| IL-10 | 5′-GCA CCC ACT TCC CAG TCG GC-3′ | 5′-GGC TTG GCA ACC CAA GTA ACC CTT A-3′ |
| TNF-α | 5′-ATG AGC ACA GAA AGC ATG ATC-3′ | 5′-TAC AGG CTT GTC ACT CGA ATT-3′ |
| IL-1β | 5′-ACG GAC CCC AAA AGA TGA AG-3′ | 5′-TTC TCC ACA GCC ACA ATG AG-3′ |
| 18S | 5′-TCG AGG CCC TGT AAT TGG AA-3′ | 5′-CCC TCC AAT GGA TCC TCG TT-3′ |
| GAPDH | 5′-TGT GTC CGT CGT GGA TCT GA-3′ | 5′-CCT GCT TCA CCA CCT TCT TGA-3′ |
RNA was extracted from full-thickness intestinal tissue using TRIzol reagent (Invitrogen). RT-PCR was performed using an Applied Biosystems StepOnePlus RT-PCR system with default settings and a Fast SYBR Green Master Mix. GAPDH and 18S were used as internal controls. Relative gene expression was determined using the comparative cycle threshold number method, as described elsewhere (16).
Intestinal lipid extraction.
Intestinal lipids were extracted using methods described elsewhere (4, 24, 25). Briefly, two 0.5- to 0.8-cm-long frozen jejunal segments were homogenized twice in 1.0 ml of 1× PBS at 22,500 rpm for 5 s and incubated on ice for 5 min. Then 0.8 ml of homogenate was mixed with 5 ml of Folch reagent (2:1 chloroform-methanol) and 0.1 ml of 0.5% sulfuric acid, incubated at room temperature for 0.5 h, and then centrifuged at 2,000 rpm for 5 min. The organic phase containing lipids was isolated, and volume was recorded for calculation of total lipid. To prepare an aqueous lipid sample for the lipid concentration measurement, a mixture of 650 μl of the lipid extract and 100 μl of 0.5% Triton X-100-chloroform was blown dry with N2 gas at 45°C for 15 min; then 100 μl of deionized distilled water were added, and the solution was incubated at 37°C for 15 min. The remaining homogenate was centrifuged at 10,000 g for 2 min, and the supernatant containing soluble cytosolic proteins was saved. Lipid extracts, aqueous lipid samples, and cytosolic protein samples were stored at −20°C until use.
Intestinal lipid and protein concentration measurements.
The concentration of TG, total cholesterol, nonesterified fatty acids (NEFA), and free cholesterol in aqueous lipid samples was determined using the L-type TG M kit, Cholesterol E kit, HR Series NEFA-HR(2), and Free Cholesterol E kit (Wako Diagnostics, Richmond, VA), respectively, in a microplate reader (BioTek, Winooski, VT). For conversion of the measured TG and total cholesterol concentrations from mg/dl to mmol/l, the concentrations were multiplied by the coefficients 0.0113 and 0.0259, respectively, provided by the manufacturer. The cytosolic protein concentration was determined using the CB-X protein assay kit (G-Biosciences, St. Louis, MO) in a microplate reader. Total cytosolic protein concentrations were used to normalize the lipid content.
Assessment of neutrophil effector function.
Neutrophil effector function was measured by examination of the respiratory burst in blood from tis7−/− and WT mice in collaboration with M. Dinauer (Washington University). Neutrophil superoxide production was quantified by flow cytometry using dihydrorhodamine 123 (DHR 123) (13). DHR 123 is used as a peroxidase substrate for the respiratory burst in neutrophils. DHR is oxidized to rhodamine by hydrogen peroxide, and rhodamine fluorescence is detected and measured by flow cytometry in the neutrophil gate.
Statistical analysis.
Statistical analyses were performed using Prism 5.0 (GraphPad Software, La Jolla, CA). Survival analysis was performed utilizing the Gehan-Breslow-Wilcoxon test. Comparisons of daily body weight changes were analyzed by paired Student's t-test. Differences in ability to achieve and exceed preoperative body weight were evaluated by χ2 analysis. Changes in villus height, crypt depth, and gene expression levels were assessed by paired or unpaired Student's t-test or by two-way ANOVA and Bonferroni's post hoc analysis. Values are means ± SE. Differences were considered significant at P ≤ 0.05.
RESULTS
Increased mortality in tis7−/− mice fed a high-fat diet postresection.
To elucidate the role of tis7 in the gut's metabolic adaptive response following resection, tis7−/− mice and WT littermate controls were subjected to 50% small bowel resection and fed a high-saturated-fat liquid diet (42% of calories from fat) or an isocaloric control liquid diet (4.5% calories from fat, nutritionally equivalent to standard rodent chow). Mortality rates were not significantly different between tis7−/− and WT mice fed the control diet postresection (19% vs. 12% mortality, P = not significant; Fig. 1A). However, we observed 61% overall mortality in tis7−/− mice compared with 6% mortality in WT littermate control mice fed the high-fat diet (P = 0.001; Fig. 1B). These mortality rates excluded mice that died within 24 h of surgery due to anesthesia or surgical mishaps (e.g., anesthesia overdose or bleeding). To determine the etiology of the increased mortality in tis7−/− mice fed a high-fat diet, we performed postmortem analyses on a subset of these mice (n = 24 mice subjected to resection, with 18 early deaths; Table 2). We found intestinal obstruction at the anastomosis in 11 of 18 tis7−/− mice fed the high-fat diet postresection. Of the additional seven deaths, one was a surgical mishap, in which death occurred at <24 h, for four of the deaths there was no obvious cause, one death was due to dehiscence (complete opening) of the surgical anastomosis, and one was due to a leak at the anastomosis. Only 1 of 17 operated WT mice died; this death occurred on postoperative day 6, and the cause of death could not be identified (P = 0.0001, deaths in tis7−/− vs. WT mice, by χ2 analysis).
Fig. 1.

Increased mortality in high-fat diet-fed tis7−/− mice after 50% small bowel resection. A: tis7−/− mice and wild-type (WT) littermates fed a control low-fat liquid diet have similar postoperative mortality rates following 50% intestinal resection (19% and 12%, respectively, P = not significant, n = 16 WT and 16 tis7−/− mice). B: when fed a high-saturated-fat liquid diet, tis7−/− mice had significantly increased mortality compared with WT littermates (61% vs. 6%, n = 17 WT and 41 tis7−/− mice). *P = 0.001 (by Gehan-Breslow-Wilcoxon survival test).
Table 2.
Necropsy results for mice that died after resection prior to day 21 (end of experiment)
| Genotype | Diet | Cause of Death | Postoperative Day of Death |
|---|---|---|---|
| C57BL/6 | |||
| WT littermates of tis7−/− | High-fat | Unknown (n =1 of 17 operated) | Day 6 (n = 1) |
| Tis7−/− | High-fat | Obstruction (n = 11) | Obstruction (n = 11): day 3 (n = 4), day 4 (n = 4), day 5 (n = 1), day 15 (n = 2) |
| Dehiscence (n = 1) | Dehiscence: day 3 (n = 1) | ||
| Anastomotic leak (n = 1) | Leak: day 4 (n = 1) | ||
| No obvious cause (n = 4) | No obvious cause: day 3 (n = 2), day 4 (n = 1), day 7 (n = 1) | ||
| <24 h (n = 1) (total n = 18 with early death of 24 operated)* | |||
| FVB/N | |||
| WT littermates of tis7tg | High-fat | Small bowel obstruction with hemorrhage (n = 4), hemorrhage (n = 2) (total n = 6 early death of 12 operated) | Obstruction with hemorrhage (n = 4): day 2 (n = 1), day 4 (n = 3) |
| Hemorrhage (n = 2): day 2 (n = 2) | |||
| tis7tg | High-fat | Small bowel obstruction with hemorrhage (n = 2) | Obstruction with hemorrhage: day 3 (n = 1), day 4 (n= 1) |
| Hemorrhage (n = 1) (total n = 3 early death of 13 operated)† | Hemorrhage: day 5 (n = 1) |
n, Number of mice.
P = 0.0001 vs. WT. †P = 0.05 vs. WT.
To determine whether increased mortality was dependent on gut resection or might be due to the stress of surgery in the setting of a high-fat diet, sham-operated tis7−/− mice and WT littermates were fed a high-fat or a control diet for 21 days. There was no increase in mortality in either group compared with each other or with WT littermates fed the high-fat diet postresection (data not shown).
Increased anastomotic inflammation in tis7−/− mice.
To begin to elucidate the mechanisms regulating the increased mortality of high-fat diet-fed tis7−/− mice postresection predominantly from small bowel obstruction, we analyzed the histology of hematoxylin-eosin-stained sections of intestine at the anastomosis and at sites distant from the anastomosis (n = 4–5 mice per group). High-fat diet-fed WT mice had normal-appearing anastomoses with intact crypts and villi (mild distortion is attributable to altered orientation of anastomotic tissue in paraffin blocks; Fig. 2, A and B, and Fig. 3A). In contrast, in high-fat diet-fed tis7−/− mice, there was an increase in mucosal and submucosal lamina propria and serosal inflammatory cells at the anastomosis (Fig. 2, C–F, and Fig. 3, B–D). Increased inflammatory infiltrates were observed as early as postoperative day 4 (Fig. 2, C and D, and Fig. 3, B and C). In the tis7−/− mice that survived to day 21, a persistent, lymphocyte-predominant mucosal inflammatory cell infiltrate was seen at the anastomosis (Fig. 3D), with persistent serosal and submucosal inflammatory cell infiltrates (Fig. 2, E and F). Submucosal and serosal inflammatory cells were mainly composed of macrophages, as detected by F4/80 immunostaining (Fig. 4, A–C), and were increased in tis7−/− compared with WT intestines. Mucosal infiltrates were composed of a predominance of B lymphocytes (CD45R+) with scattered T lymphocytes (CD3+) (Fig. 4, D and E). There was no histological evidence of inflammation in the control diet-fed tis7−/− or WT mice (data not shown).
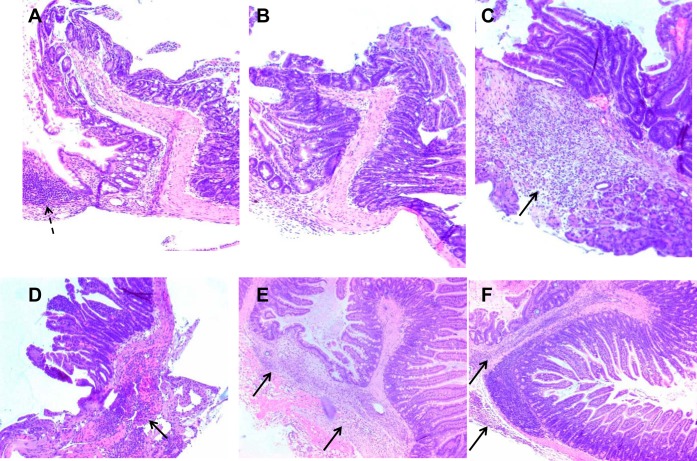
Fig. 2.
Increased anastomotic inflammation in tis7−/− mice fed a high-fat diet postresection. A–F: representative low-power views of histological sections of hematoxylin-eosin-stained surgical anastomotic sites (n = 4–5 mice per group). A and B: anastomoses harvested from high-fat diet-fed WT littermate mice that survived to postoperative day 21. Dashed arrow in A shows Peyer's patch. Crypts and villi are present at the anastomotic site, with some distortion resulting from altered tissue orientation in paraffin. C and D: anastomoses from tis7−/− mice that died before day 21. Arrows indicate submucosal and serosal inflammation. E and F: persistent anastomotic inflammation in high-fat diet-fed tis7−/− mice that survived to day 21. Arrows indicate submucosal and serosal inflammation. Magnification: ×100 (A–C) and ×50 (D–F).
Fig. 3.
Increased mucosal and submucosal inflammation in tis7−/− mice fed a high-fat diet postresection. A–D: representative high-power views of histological sections of hematoxylin-eosin-stained surgical anastomotic sites (n = 4–5 mice per group). A: high-fat diet-fed WT littermate. Crypt-villus architecture at the anastomotic site is well preserved. B: anastomosis from a high-fat diet-fed tis7−/− mouse that developed obstructive symptoms and expired on postoperative day 4. Note marked inflammatory infiltrate in the villus core (arrows). C: additional view of anastomosis from the high-fat diet-fed tis7−/− mouse in B showing serosal inflammation (arrows). D: anastomosis from a high-fat diet-fed tis7−/− mouse that survived until the end of the experiment (euthanized at postresection day 21). Note persistent inflammatory infiltrate in the villus core. Magnification: ×200.
Fig. 4.
Characterization of the anastomotic inflammatory infiltrate in tis7−/− mice fed a high-fat diet postresection. A: representative hematoxylin-eosin-stained anastomotic site from a high-fat diet-fed tis7−/− mouse that survived to postoperative day 21. Increased inflammatory cells are noted in the submucosa and serosa (arrows). B and C: section in A immunostained using an anti-F4/80 antibody to detect macrophages (arrows). D: B lymphocytes infiltrate the lamina propria of the villus core, detected using an anti-CD45R antibody. E: scattered increase in T lymphocytes in the villus core lamina propria. Magnification: ×100 (A and B) and ×200 (C–E).
Although we did not observe an increase in neutrophils in the inflammatory infiltrate, tis7 deletion has been shown to reduce neutrophil effector function, resulting in altered inflammatory responses in the lung (7). We examined neutrophil function in tis7−/− vs. WT mice by measuring superoxide production using DHR 123, as described elsewhere (13; see materials and methods). No differences in neutrophil respiratory burst function were observed in WT compared with tis7−/− mice (data not shown).
Because tis7 regulates NF-κB activation in muscle cells (12) and silencing tis7 in myoblasts induces acetylation and nuclear import of p65 (12), we examined intestinal NF-κB activation by detecting nuclear NF-κB p65/RelA expression, which is specifically increased during NF-κB activation (5). Intestinal anastomotic sites were analyzed by immunohistochemical analysis for nuclear p65 expression postresection in high-fat diet-fed WT and tis7−/− mice (n = 4 in each group). Representative sections are shown in Fig. 5. Patchy areas of increased nuclear expression were noted in lamina propria and submucosal lymphocytes in tis7−/− ileum (Fig. 5, B and D); many fewer immune cells were present in WT ileum, and rare cells showed nuclear p65 expression (Fig. 5, A and C). However, quantification of p65 expression by immunoblot revealed no significant differences between tis7−/− and WT postoperative ileal or jejunal nuclear expression (data not shown). Although this may reflect the patchiness of nuclear p65 expression, the increase in nuclear p65 in tis7−/− immune cells only suggests that this may be a nonspecific response secondary to the general increase in inflammation.
Fig. 5.
NF-κB p65/relA expression in intestinal anastomosis of high-fat diet-fed tis7−/− mice vs. WT littermates. Immunohistochemical analysis of NF-κB p65 expression was performed using an anti-p65/relA antibody on histological sections of anastomoses from high-fat diet-fed WT (A and C) and tis7−/− (B and D) mice postresection. Immunohistochemistry was performed on intestines from 4 mice per group; representative sections are shown. A and C: scattered immune cells stain brown, indicating nuclear p65 expression in immune cells along villus (A) and submucosa (C) of WT mice (arrows). B and D: nuclear p65 expression in tis7−/− immune cells. Increased numbers of nuclear p65+ immune cells are observed (arrows). Magnification: ×400.
Histological analysis of remnant intestine beyond the anastomosis showed evidence of mucosal and submucosal inflammation in tis7−/− mice that did not survive until postoperative day 21 (Fig. 6A). In contrast, tis7−/− mice that survived to day 21 had generally normal-appearing mucosa beyond the anastomosis (Fig. 6B). Analysis of tis7−/− mice that survived with partial intestinal obstruction revealed a marked increase in submucosal edema, with an increase in submucosal thickness compared with WT mice (Fig. 6, C and D, arrows). Trichrome stain to assess collagen expression and determine whether fibrosis was increased in tis7−/− mice revealed no significant increase in collagen (Fig. 6, C and D).
Fig. 6.
Histological features of high-fat diet-fed tis7−/− mice distant from the anastomosis and collagen expression at the anastomosis. A: hematoxylin-eosin-stained section of intestine distant from the anastomosis from a tis7−/− mouse that died before day 21. Arrows depict mucosal and serosal inflammation. B: section of intestine distant from the anastomosis from a tis7−/− mouse that survived to day 21. C and D: trichrome stain to detect collagen (blue) in tis7−/− and WT mice that survived to postoperative day 21. There was no increase in collagen, but an increase in submucosal edema was observed in the tis7−/− anastomosis (C, arrows) compared with WT in D. Magnification: ×100.
The adaptive response is preserved in tis7−/− mice that survive resection.
The morphometric adaptive response in the remnant small bowel following massive resection is characterized by increased crypt depths, villus heights, and crypt cell proliferation. In the tis7−/− mice that survived to postoperative day 21, the adaptive response was preserved, with the expected increase in ileal villus heights and crypt depths postresection, whether the mice were fed a control or a high-fat diet (Fig. 7, A–D). This was similar to WT mice. Also, there were no differences in crypt cell proliferation rates in WT compared with tis7−/− ileum postresection (Fig. 7E), as quantified by 5-BrdU incorporation.
Fig. 7.
Morphological adaptive response is preserved in high-fat diet-fed tis7−/− mice postresection. WT littermates or tis7−/− mice fed the control diet (A and C) or the high-fat diet (B and D) postresection show a preserved adaptive response with increased villus heights (A and B) and crypt depths (C and D). E: no differences in postoperative crypt cell proliferation rates between WT and tis7−/− mice fed the control or the high-fat diet (n = 5–10 mice per group). 5-BrdU, 5-bromodeoxyuridine. *P < 0.05.
Intestinal lipid metabolism is altered in tis7−/− mice postresection.
We previously showed that, compared with WT littermates, (unoperated) tis7−/− mice do not gain weight when chronically fed a high-fat diet (26) and tis7 deletion results in delayed lipid absorption and altered intestinal and hepatic lipid trafficking, with reduced intestinal TG, cholesterol, and free fatty acid mucosal levels in the jejunum but increased mucosal free fatty acid concentration in the ileum. We therefore examined mucosal lipid concentrations in the jejunum and ileum at postresection day 21 (Fig. 8). Similar to our previously published findings in unoperated mice (26), we observed reduced mucosal cholesterol and free fatty acid concentrations in post-op tis7−/− vs. WT jejunum (Fig. 8A) and a trend toward decreased TG concentration. Examination of tis7−/− vs. WT postoperative ileal lipid mucosal concentrations revealed no significant difference in mucosal cholesterol or TG concentrations, as observed in unoperated mice (Fig. 8B). However, in contrast to our previous observation that mucosal free fatty acid concentrations were increased in tis7−/− vs. WT unoperated ileum (21), we observed a significant decrease in mucosal free fatty acid concentration in tis7−/− vs. WT postoperative distal ileum (P = 0.04). Finally, total lipid absorption was measured by calculating fecal fat content and the percentage of ingested fat absorbed. As previously observed in unoperated mice, there were no significant differences in the percentage of lipid absorbed in high-fat diet-fed tis7−/− mice vs. WT mice postresection; however, there was a trend toward a decrease in fat absorption in tis7−/− mice (96.8% vs. 92% of ingested fat absorbed, P = 0.08). Thus mucosal lipid concentration is reduced in tis7−/− jejunum and ileum postoperatively following massive resection; the compensatory increase in mucosal free fatty acid concentration in unoperated tis7−/− ileum was not preserved in the shortened postoperative ileum.
Fig. 8.

Reduced intestinal mucosal lipid concentrations in high-fat diet-fed tis7−/− mice postresection. Triglyceride (TG), nonesterified (free) fatty acid (NEFA), and total and free cholesterol mucosal concentrations were measured in WT and tis7−/− (KO) jejunum (A) and ileum (B) at postoperative day 21. *P < 0.05 (n = 3–4 mice per group).
To determine whether tis7−/− mice had reduced weight gain resulting from altered lipid metabolism, as previously observed in unoperated high-fat diet-fed tis7−/− mice, we measured daily food consumption and compared daily weights, which had been measured for each mouse for the entire experiment, up to postoperative day 21. Daily food consumption was the same in tis7−/− and WT mice that survived to day 21 (data not shown). There were no significant differences in overall weight gain expressed as percentage of body weight recovery between surviving tis7−/− and WT mice fed the control diet (Fig. 9A) or the high-fat diet (Fig. 9B). However, tis7−/− mice that died prior to day 21 showed reduced body weight recovery compared with surviving mice (n = 14 per group; Fig. 9C).
Fig. 9.
Body weight recovery in control or high-fat diet-diet fed tis7−/− and control WT littermate mice. The tis7−/− and control WT littermate mice were fed a control low-fat liquid diet equivalent to chow (A) or a high-saturated-fat liquid diet (B). No significant differences were noted in body weight recovery between control WT and tis7−/− mice fed either diet (n = 14–16 per group). C: body weight recovery was reduced in tis7−/− mice that died before postoperative day 21 compared with survivors. *P < 0.05 (n = 14 mice per group).
High-fat diet-fed tis7−/− mice have increased intestinal IL-6 expression.
To further explore the mechanism for the increase in anastomotic inflammation in tis7−/− mice fed a high-fat diet, we examined the pre- and postoperative ileal expression of inflammatory cytokines, including IL-6, KC (IL-8), TNF-α, and IL-10, by qRT-PCR. IL-6 expression did not change in high-fat diet-fed WT postoperative ileum compared with preoperative ileum (Fig. 10A). In contrast, tis7−/− mice that survived had increased ileal IL-6 mRNA expression compared with preoperative ileum (P < 0.03; Fig. 10A). Expression of KC, TNF-α, and IL-10 was unchanged in all groups (data not shown). Also when mice were fed the control diet, there were no differences in ileal IL-6 mRNA levels between postoperative WT and postoperative tis7−/− mice and between preoperative and postoperative mice within each genotype (Fig. 10B). Serum IL-6 levels were measured in tis7−/− and WT mice by ELISA and were unchanged; thus the inflammation appears to be localized to the intestine and is not systemic.
Fig. 10.

Ileal IL-6 mRNA expression is increased in high-fat diet-fed tis7−/− mice postoperatively. A: IL-6 mRNA expression is increased in the ileum in high-fat diet-fed tis7−/− mice postoperatively compared with preoperatively, but not in WT littermates postoperatively compared with preoperatively. *P = 0.016. B: IL-6 mRNA expression is unchanged in the ileum preoperatively vs. postoperatively in control diet-fed WT and control diet-fed tis7−/− mice (n = 5 mice per group).
Intestinal tis7 overexpression protects mice from mortality induced by a high-fat diet postresection.
We next evaluated the postoperative survival of high-fat diet-fed tis7tg mice and WT littermates. The tis7tg mice are on an FVB/N background, in contrast to the tis7−/− mice, which are congenic on a C57BL/6 background. To avoid confounding results due to strain differences, strain-matched WT littermates (also on a FVB/N background) were used as controls for all experiments with tis7tg mice. Interestingly, FVB/N WT mice tolerated the high-saturated-fat diet following 50% small bowel resection less well, as shown by higher postoperative mortality rates, than high-fat diet-fed C57BL/6 WT mice (Figs. 11A and 1B). However, a protective effect of intestinal tis7 overexpression on survival was observed in mice fed the high-fat diet, as littermate FVB/N tis7tg mice fed the high-fat diet postresection had 31% mortality compared with 56% mortality in WT mice (P = 0.044; Fig. 11A). Early deaths at <24 h were due to an increased incidence of hemorrhage in both groups; these mice were removed from the survival analysis. Necropsy of a subset of these mice revealed the cause of death in WT and tis7tg mice to be obstruction with hemorrhage or hemorrhage alone (Table 2), with a reduced incidence of both complications in tis7tg mice. Sham-resected tis7tg and WT littermates showed no difference in mortality, and both tolerated sham resection when fed a high-fat or a control diet.
Fig. 11.

Improved postresection survival and reduced IL-6 ileal expression in high-fat diet-fed tis transgenic (tis7tg) compared with WT littermate mice. A: survival of high-fat diet-fed tis7tg mice is increased compared with high-fat diet-fed WT littermates postresection (69% vs. 44%, n = 13 tis7tg and 18 WT). *P = 0.044 (by Gehan-Breslow-Wilcoxon survival test). B: increased IL-6 mRNA expression in the ileum of high-fat diet-fed WT mice postoperatively compared with preoperatively. *P = 0.003. In contrast, in high-fat diet-fed tis7tg (TG) mice, IL-6 mRNA in the ileum is not increased postoperatively compared with preoperatively. IL-6 mRNA expression is also significantly decreased in the ileum postoperatively in tis7tg mice compared with WT mice (n = 5–6 mice per group). *P = 0.009.
Histological analysis revealed evidence of an inflammatory response, as well as hemorrhage at the anastomosis, in high-fat diet-fed WT littermates that died early (Fig. 12, A and B). Similar findings were observed in tis7tg mice that died early, but the incidence of such findings was lower (Table 2). Both tis7tg and WT mice that survived to day 21 had normal-appearing anastomoses (Fig. 12, C and D).
Fig. 12.
Histological analysis of surgical anastomoses from high-fat diet-fed WT and tis7tg mice. Representative hematoxylin-eosin-stained sections of intestinal anastomoses were obtained from WT mice that died before day 21 (A and B) or survived until completion of the experiment on day 21 (D) and from tis7tg mice that survived until day 21 (C). In mice that died early, there was an increase in submucosal and serosal inflammatory cells and hemorrhage in the serosa at the anastomosis (A and B, arrows). Mice that survived had normal-appearing anastomoses (C and D).
We next used qRT-PCR to examine ileal IL-6 expression in tis7tg mice and WT littermates fed a high-fat diet postresection. Consistent with the higher mortality in high-fat diet-fed WT littermates postresection, IL-6 expression in WT tis7tg mice was increased postoperatively compared with preoperatively (P < 0.01; Fig. 11B). Conversely, IL-6 expression was not increased in high-fat diet-fed tis7tg mice postoperatively compared with preoperatively. Furthermore, postoperative ileal IL-6 mRNA levels were decreased in tis7tg mice compared with postoperative WT mice.
Our previous studies showed that intestinal tis7 overexpression resulted in an increased rate of small bowel TG absorption (23). To determine whether the tis7-mediated increase in TG absorption also occurs in the adapting gut postresection, we assessed lipid absorption by quantifying the percentage of ingested fat that was absorbed. Feces were collected from high-fat diet-fed tis7tg mice and WT littermates on days 19–21 postresection. There was a small 0.8% increase in the percentage of fat absorbed in tis7tg mice fed the high-fat diet compared with WT littermates fed the high-fat diet (P = 0.04).
We next assessed morphological adaptation following resection in tis7tg vs. WT mice (Fig. 13). The villus and crypt adaptive response, characterized by an increase in villus height and crypt depth postresection, occurred as expected in preoperative vs. postoperative ileum from tis7tg vs. WT littermates fed the control or the high-fat diet (P < 0.05; Fig. 13, A–D). We also observed the expected postresection increase in crypt cell proliferation: >30% of ileal crypt cells were positive for 5-BrdU expression in each group postresection, with no significant differences between tis7tg mice and their respective WT littermates fed either diet (Fig. 13E).
Fig. 13.
Morphological adaptive response is preserved in high-fat diet-fed tis7tg mice postresection. WT littermates and tis7tg mice fed the control diet (A and C) or the high-fat diet (B and D) postresection show a preserved adaptive response, with increased villus heights (A and B) and crypt depths (C and D). E: no differences in postoperative crypt cell proliferation rates were noted between WT and tis7tg mice fed the control diet or the high-fat diet (n = 5–12 mice per group). *P < 0.05.
DISCUSSION
In the present study we have shown that tis7 plays a unique role in the intestinal response following loss of functional small bowel surface area. Compared with WT littermates, mice with tis7 deletion fed a high-fat diet but not a control diet following small bowel resection had a marked increase in mortality that was associated with increased gut inflammation and small bowel obstruction. The inflammatory changes were characterized by an influx of lymphocytes and macrophages, associated with submucosal edema and increased mucosal IL-6 expression. High-fat diet-fed tis7−/− mice postresection had altered jejunal and ileal lipid metabolism compared with WT mice, with reduced jejunal mucosal concentrations of free fatty acids and cholesterol and reduced ileal mucosal concentration of free fatty acids. These data suggest that tis7 deletion-induced changes in intestinal lipid metabolism and trafficking may result in increased susceptibility to an inflammatory response that predisposes to small bowel obstruction. In contrast, tis7tg mice had reduced mortality and reduced IL-6 expression compared with their WT littermates when fed a high-fat diet postresection. Increased intestinal tis7 expression also resulted in a small, but significant, increase in the percentage of lipid absorbed when mice were fed a high-fat diet postresection, again suggesting a possible link between changes in lipid metabolism induced by tis7, the inflammatory response to a high-fat diet, and postresection survival.
Our studies show that gut environmental factors (i.e., a high-fat diet) are required to induce postoperative intestinal inflammation in tis7−/− mice, since control diet-fed tis7−/− mice did not have postoperative anastomotic inflammation, increased IL-6 expression, or increased mortality (Fig. 5). Several studies have shown that a high-fat diet induces intestinal inflammation and barrier dysfunction. This occurred in association with alterations in gut microflora in obesity-prone rodents (1–3, 9) and in a colitis model (7), suggesting that high-fat diet-induced changes in microflora are at least part of the mechanism by which high-fat diets result in gut inflammation. We speculate that the altered intestinal trafficking of fatty acids and cholesterol induced by tis7 deletion may also generate proinflammatory gut microflora. Further examination of the effects of tis7 deletion and overexpression on the gut microflora in the setting of a high-fat diet represents an intriguing avenue to be explored.
We observed strain-specific differences in survival of high-fat diet-fed FVB/N compared with C57BL/6 WT mice postresection. Thus all experiments were performed only using WT littermates on the same genetic background as controls. The increased mortality of FVB/N WT compared with C57BL/6 mice occurs in part because of differences in their susceptibility to postoperative hemorrhage, in addition to inflammation induced by high-fat feeding. However, a specific role for tis7 in the inflammatory and obstructive process is further supported by our observation that high-fat feeding in tis7−/− mice induces a more robust inflammatory response, resulting in almost fivefold higher ileal IL-6 expression postresection than in high-fat diet-fed FVB/N WT mice postresection (Figs. 10A and 11B; note scale differences). Also, overexpression of tis7 in villus epithelial cells in tis7tg mice (FVB/N background) was partially protective, associated with a significant decrease in postoperative IL-6 expression, suggesting suppression of inflammation. Backcrossing of tis7tg mice into the C57BL/6 background shows that these mice retain their basal phenotype of increased adiposity (preliminary observations); future analyses of whether improved survival, adaptation, or body weight recovery following resection and high-fat feeding occurs in this background will be informative.
The tis7−/− mice have a global deletion of tis7 (20). Because tis7 is ubiquitously expressed, its deletion may result in increased postresection mortality due to other, unexamined extraintestinal effects. We were able to assign a cause of death to the majority of tis7−/− mice, but several mice had no obvious cause of death on necropsy (Table 2). Future experiments in mice with gut-specific deletion of tis7 are required to confirm that absence of intestinal tis7 is required for the increased mortality.
In addition to further defining the role of tis7 in the adapting gut, these studies have generated a novel murine model of inflammation and small bowel obstruction postresection. There is one previous report of a murine model of inflammation and fibrosis postresection (11) in which conventionally raised IL-10 null mice (with an intact enteric microflora) developed postsurgical inflammation and fibrosis after ileocecal resection (14). However, IL-10 null mice raised germ-free or WT conventionally raised mice did not develop inflammation or fibrosis. These mice are a widely used model of inflammatory bowel disease. Our postsurgical model of intestinal obstruction and inflammation is also dependent on the appropriate luminal environment and shares some features of inflammatory bowel disease, as intestinal inflammation is a hallmark of inflammatory bowel disease and intestinal obstruction is a common sequela of active inflammation in Crohn's disease. The role of gut microflora and interactions with the luminal nutritional environment, e.g., comparing high-fat, low-fat, high-protein, or high-carbohydrate diets, can be readily explored in the present model. In addition, the tis7−/− mouse may prove useful for elucidating the mechanisms that regulate postoperative small bowel obstruction following resection in humans and possibly help identify preventive therapies.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-46122, R01 DK-61216, and R01 DK-50466 (D. C. Rubin and M. S. Levin), Digestive Diseases Research Core Center Grant DK-52574, and Ruth L. Kirschstein National Research Service Award T32 DK-077653 (A. M. Garcia).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M.G., D.W., C.R., T.G., C.B., S.B., and E.A.S. performed the experiments; A.M.G., J.L., C.B., E.A.S., M.S.L., and D.C.R. analyzed the data; A.M.G., J.L., T.G., M.S.L., and D.C.R. interpreted the results of the experiments; A.M.G. drafted the manuscript; A.M.G., M.S.L., and D.C.R. edited and revised the manuscript; A.M.G., M.S.L., and D.C.R. approved the final version of the manuscript; B.W.W., M.S.L., and D.C.R. are responsible for conception and design of the research; D.C.R. prepared the figures.
ACKNOWLEDGMENTS
We thank Kymberli Carter and Angela Felton (Morphology Core of the Digestive Diseases Research Core Center, Washington University School of Medicine) for excellent technical assistance, Mary Dinauer and Nancy Pech for help with neutrophil respiratory burst assays, and Lukas Huber and Ilja Vietor (Innsbruck Medical University, Austria) for providing tis7−/− mice.
Present address of A. M. Garcia: Department of Pediatrics, Oregon Health and Science University, Portland, OR.
REFERENCES
- 1.Barbier de La Serre C, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 299: G440–G448, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58: 1091–1103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem 26: 39–42, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Chandrakesan P, Ahmed I, Chinthalapally A, Singh P, Awasthi S, Anant S, Umar S. Distinct compartmentalization of NF-κB activity in crypt and crypt-denuded lamina propria precedes and accompanies hyperplasia and/or colitis following bacterial infection. Infect Immun 80: 753–767, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol 293: G1013–G1022, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487: 104–108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu Y, Harley IT, Henderson LB, Aronow BJ, Vietor I, Huber LA, Harley JB, Kilpatrick JR, Langefeld CD, Williams AH, Jegga AG, Chen J, Wills-Karp M, Arshad SH, Ewart SL, Thio CL, Flick LM, Filippi MD, Grimes HL, Drumm ML, Cutting GR, Knowles MR, Karp CL. Identification of IFRD1 as a modifier gene for cystic fibrosis lung disease. Nature 458: 1039–1042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laugerette F, Furet JP, Debard C, Daira P, Loizon E, Geloen A, Soulage CO, Simonet C, Lefils-Lacourtablaise J, Bernoud-Hubac N, Bodennec J, Peretti N, Vidal H, Michalski MC. Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am J Physiol Endocrinol Metab 302: E374–E386, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Levin MS, Rubin DC. (Editors). Intestinal Adaptation: The Biology of the Intestinal Response to Resection and Disease. Malden, MA: Blackwell, 2008, p. 45–54 [Google Scholar]
- 11.McDuffie LA, Bucher BT, Erwin CR, Wakeman D, White FV, Warner BW. Intestinal adaptation after small bowel resection in human infants. J Pediatr Surg 46: 1045–1051, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micheli L, Leonardi L, Conti F, Maresca G, Colazingari S, Mattei E, Lira SA, Farioli-Vecchioli S, Caruso M, Tirone F. PC4/Tis7/IFRD1 stimulates skeletal muscle regeneration and is involved in myoblast differentiation as a regulator of MyoD and NF-κB. J Biol Chem 286: 5691–5707, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson MP, Ayliffe MJ, Helbert M, Davies EG. A simple flow cytometry assay using dihydrorhodamine for the measurement of the neutrophil respiratory burst in whole blood: comparison with the quantitative nitrobluetetrazolium test. J Immunol Methods 219: 187–193, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Rigby RJ, Hunt MR, Scull BP, Simmons JG, Speck KE, Helmrath MA, Lund PK. A new animal model of postsurgical bowel inflammation and fibrosis: the effect of commensal microflora. Gut 58: 1104–1112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin DC, Swietlicki EA, Wang JL, Levin MS. Regulation of PC4/TIS7 expression in adapting remnant intestine after resection. Am J Physiol Gastrointest Liver Physiol 275: G506–G513, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Schwarz M, Lund EG, Setchell KD, Kayden HJ, Zerwekh JE, Bjorkhem I, Hertz J, Russell DW. Disruption of cholesterol 7α-hydroxylase gene in mice. II. Bile acid deficiency is overcome by induction of oxysterol 7α-hydroxylase. J Biol Chem 271: 18024–18031, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaker A, Swietlicki EA, Wang L, Jiang S, Onal B, Bala S, DeSchryver K, Newberry R, Levin MS, Rubin DC. Epimorphin deletion protects mice from inflammation-induced colon carcinogenesis and alters stem cell niche myofibroblast secretion. J Clin Invest 120: 2081–2093, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swietlicki E, Iordanov C, Fritsch H, Yi L, Levin MS, Rubin DC. Growth factor regulation of PC4/TIS7, an immediate early gene expressed during gut adaptation after resection. JPEN J Parenter Enteral Nutr 27: 123–131, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Vadivelu SK, Kurzbauer R, Dieplinger B, Zweyer M, Schafer R, Wernig A, Vietor I, Huber LA. Muscle regeneration and myogenic differentiation defects in mice lacking TIS7. Mol Cell Biol 24: 3514–3525, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vietor I, Huber LA. Role of TIS7 family of transcriptional regulators in differentiation and regeneration. Differentiation 75: 891–897, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Vietor I, Vadivelu SK, Wick N, Hoffman R, Cotten M, Seiser C, Fialka I, Wunderlich W, Haase A, Korinkova G, Brosch G, Huber LA. TIS7 interacts with the mammalian SIN3 histone deacetylase complex in epithelial cells. EMBO J 21: 4621–4631, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Iordanov H, Swietlicki EA, Wang L, Fritsch C, Coleman T, Semenkovich CF, Levin MS, Rubin DC. Targeted intestinal overexpression of the immediate early gene tis7 in transgenic mice increases triglyceride absorption and adiposity. J Biol Chem 280: 34764–34775, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Xie Y, Kennedy S, Sidhu R, Luo J, Ory DS, Davidson NO. Liver X receptor agonist modulation of cholesterol efflux in mice with intestine-specific deletion of microsomal triglyceride transfer protein. Arterioscler Thromb Vasc Biol 32: 1624–1631, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Y, Newberry EP, Young SG, Robine S, Hamilton RL, Wong JS, Luo J, Kennedy S, Davidson NO. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J Biol Chem 281: 4075–4086, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Yu C, Jiang S, Lu J, Coughlin CC, Wang Y, Swietlicki EA, Wang L, Vietor I, Huber LA, Cikes D, Coleman T, Xie Y, Semenkovich CF, Davidson NO, Levin MS, Rubin DC. Deletion of Tis7 protects mice from high-fat diet-induced weight gain and blunts the intestinal adaptive response postresection. J Nutr 140: 1907–1914, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]