Abstract
A compromise in the internal anal sphincter (IAS) tone and fibroelastic properties (FEP) plays an important role in rectoanal incontinence. Herein, we examined the effects of heme oxygenase (HO)-1 upregulation on these IAS characteristics in young rats. We determined the effect of HO-1 upregulator hemin on HO-1 mRNA and protein expressions and on basal IAS tone and its FEP before and after HO-1 inhibitor tin protoporphyrin IX. For FEP, we determined the kinetics of the IAS smooth muscle responses, by the velocities of relaxation, and recovery of the IAS tone following 0 Ca2+ and electrical field stimulation. To characterize the underlying signal transduction for these changes, we determined the effects of hemin on RhoA-associated kinase (RhoA)/Rho kinase (ROCK) II, myosin-binding subunit of myosin light chain phosphatase 1, fibronectin, and elastin expression levels. Hemin increased HO-1 mRNA and protein similar to the increases in the basal tone, and in the FEP of the IAS. Underlying mechanisms in the IAS characteristics are associated with increases in the genetic and translational expressions of RhoA/ROCKII, and elastin. Fibronectin expression levels on the other hand were found to be decreased following HO-1 upregulation. The results of our study show that the hemin/HO-1 system regulates the tone and FEP of IAS. The hemin/HO-1 system thus provides a potential target for the development of new interventions aimed at treatment of gastrointestinal motility disorders, specifically the age-related IAS dysfunction.
Keywords: rectoanal incontinence, smooth muscle tone
heme oxygenase (HO) is the rate-limiting enzyme in the catabolism of heme (1). HO degrades heme, producing carbon monoxide (CO), biliverdin, and Fe2+ (36). Of the two HO isoforms, HO-1 is an inducible enzyme, whereas HO-2 is constitutively expressed. In the basal state, HO-1 is weakly expressed in all mammalian tissues except liver and spleen where it is strongly expressed (10). However, several substances such as heme, metals, xenobiotics, and synthetic metalloporphyrins increase HO-1 expression and activity. Among them, hemin is considered to be the most powerful inducer of HO-1 (26). Recently, HO-1 has been successfully induced in humans by hemin (7).
Several studies have indicated that HO-1 protein itself and products of its activity are involved in the maintenance of cell metabolism and homeostasis in addition to being implicated in several pathological processes. For example, CO exerts vasorelaxant, antiapoptotic, and anti-inflammatory effects; biliverdin and bilirubin are antioxidant and anti-inflammatory. HO-1 is also a heat-shock protein (5). The physiological role of HO-1 has been proven by observations in HO-1 knockout mice (29). The first genetic deficiency of HO-1 in humans was reported in a young boy in 1999 who later died at age six (38). In addition, it has been shown that failure to upregulate HO-1 expression in response to oxidative stress leads to loss of interstitial cells of Cajal and delayed gastric emptying in nonobese diabetic mice (13).
Development of spontaneous tone and preservation of fibroelastic properties (FEP) of the internal anal sphincter (IAS) are of critical importance to maintenance of rectoanal continence (8, 24, 31, 35). Aging and other rectoanal motility disorders are associated with a loss of tone and derangement in the FEP of IAS, which leads to devastating clinical consequences, such as rectoanal or fecal incontinence (RI). It is well established that the RhoA-associated kinase (RhoA)/Rho kinase (ROCK) pathway is the major molecular determinant of basal tone in the IAS (32). The antihypertensive effect of hemin via regulating the RhoA/ROCK pathway in blood vessels has been demonstrated in previous studies (2). In addition, the extracellular matrix (ECM) proteins, such as collagen, elastin, and fibronectin, govern the FEP of IAS. In that regard, previously published studies have demonstrated that HO-1 deficiency is associated with excess ECM deposition during renal and peritoneal fibrosis (4, 21). However, the role of the hemin/HO-1 system in the regulation of tone and FEP of IAS has not been investigated.
Previous studies have identified the role of HO (predominantly HO-2 isoform) in neurally mediated relaxation of IAS (11). It was shown that CO, one of the products of HO, causes smooth muscle (SM) relaxation along with hyperpolarization of the SM cells (17). However, presently there are no studies that have addressed the nonneuronal roles of HO-1 in the IAS.
We therefore conducted the present studies to identify the role of the hemin/HO-1 system in the regulation of tone and FEP of IAS.
MATERIALS AND METHODS
Animals and tissue preparation.
The experiments were performed in 20- to 22-wk-old male Sprague-Dawley rats (300–350 g). The rats were randomly divided into the following three groups: control groups were injected with PBS, hemin group received intraperitoneal injection of hemin (15 mg·kg−1·day−1) for four consecutive days, and hemin + tin protoporphyrin (SnPP) IX group received SnPPIX (10 mg/kg ip) 30 min before hemin (25). Following this, rats in these groups were killed by decapitation, and IAS SM strips were prepared as described before (15, 16). Briefly, with the use of sharp dissection, the IAS strips exclusively from the circular SM layer of the muscularis propria (∼1 mm × 7 mm) were prepared in the oxygenated and 0.22 μm filtered Krebs physiological solution (KPS) at 40C. The composition of KPS was as follows (in mM): 118.07 NaCl, 4.69 KCl, 2.52 CaCl2, 1.16 MgSO4, 1.01 NaH2PO4, 25 NaHCO3, and 11.10 glucose. Identification of the IAS was established by the development of spontaneous basal tone of the SM strips and their relaxation with the electrical field stimulation (EFS) as described before (32).
The experimental protocols of the study were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University and were in accordance with the recommendations of the American Association for the Accreditation of Laboratory Animal Care.
In vivo administration of hemin.
Hemin (ferriprotoporphyrin IX chloride), an endogenous chloroporphyrin (Sigma Chemical, St. Louis, MO), was administered intraperitoneally at a dose of 15 mg·kg−1·day−1 for four consecutive days as described previously (23, 25). Hemin and SnPPIX (Porphyrin Products, Logan, UT) were dissolved in 0.1 M NaOH, titrated to pH 7.4 with 0.1 M HCl, and diluted 1:10 with phosphate buffer in darkness and protected from light. To determine whether the treatment of hemin increased the expression of HO-1, IAS tissues were analyzed every 24 h after treatment of hemin.
Measurement of HO-1 activity.
HO-1 activity was measured using a protocol described by Ryter et al. and Aziz et al. (3, 34), based on bilirubin detection. Briefly, Rat IAS SM lysates were made in buffer containing (in mM) 0.25 sucrose, 0.1 KCl, 1 Tris·HCl, and 0.4 phenylmethylsulfonyl fluoride, adjusted to pH 7.8. Lysates (200–400 μg) were incubated in 500 μl of reaction mixture containing heme (20 μM), rat liver cytosol (2 mg/ml), MgCl2 (2 mM), glucose-6-phosphate dehydrogenase (1 U/ml), glucose 6-phosphate (2 mM), β-NADPH (0.8 mM), and Tris·HCl (20 mM) and incubated at 370C for 1 h. The reaction was terminated by placing the tubes on ice and adding equal volumes of chloroform to it. To extract bilirubin, the tubes were vortexed for 30 s and then centrifuged for 10 min at 15,000 g. The optical density of the organic phase was read at 464 and 530 nm, and the concentration of bilirubin was determined using an extinction coefficient of 40 mM−1/cm−1. HO-1 activity was calculated as nanomoles bilirubin per milligram protein per hour and expressed as percentage increase from basal (normalized to be 100).
Measurement of isometric tension in IAS strips.
The SM strips were transferred to 2-ml muscle baths containing oxygenated KPS at 37°C. One end of the strip was anchored at the bottom of the muscle bath while the other end was connected to a force transducer (FORT10; WPI, Sarasota, FL). Isometric tension was measured by the PowerLab/8SP data acquisition system (AD Instruments, Colorado Springs, CO) and recorded with Chart 4.1.2 (AD Instruments). The SM strips were initially stretched to a tension of 1 g followed by 90 min of equilibration. During this period, the SM strips were replenished with fresh KPS every 20 min. The IAS SM strips were defined by their development of spontaneous tone and relaxation in response to EFS (20 V, 0.5-ms pulse duration, and 4 s of train duration with different frequencies ranging from 1 to 10 Hz).
Cell lysate preparation and Western blot analysis.
The SM strips were flash-frozen in liquid nitrogen and suspended in ice-cold homogenization buffer (10 mM Tris·HCl, pH 7.5, 5 mM MgCl2, 2 mM EDTA, 250 mM sucrose, and 1 mM dithiothreitol, 1% Triton X-100). The extract was centrifuged at 800 g for 10 min, and protein concentration in resultant supernatant was determined using a BCA Protein Assay Reagent Kit (Pierce). Protein (20 μg) in 20 μl of lysates were mixed with 2× Laemmli sample buffer (with final concentrations of 62.5 mM Tris, 1% SDS, 15% glycerol, 0.005% bromphenol blue, and 2% mercaptoethanol) and placed in a boiling water bath for 5 min. Proteins in the samples separated by SDS-polyacrylamide gel [7.5% gel for HO-1, HO-2, elastin, ROCKII, and myosin light chain phosphatase 1 (MYPT1); 15% gel for RhoA] were electrophoretically transferred onto a polyvinylidene difluoride membrane using an iBlot Dry Blotting System (Invitrogen, Carlsbad, CA) at room temperature (RT).
To block nonspecific antibody binding, the membranes were soaked for 1 h at RT in LI-COR buffer, following which they were incubated with the specific primary antibodies (1:1,000 for HO-1, HO-2, elastin, RhoA, ROCKII, MYPT1, and 1:20,000 for α-actin) diluted in LI-COR buffer containing 0.1% Tween 20 for 1 h at RT. After being washed with TBS with Tween 20 (TBS-T), three times for 10 min, the membranes were incubated with the IRdye680- and IRdye800-conjugated secondary antibody from LI-COR Biosciences in the dark (bovine anti-rabbit 1:10,000 for HO-1, HO-2, elastin, RhoA, ROCKII, and MYPT1). The membranes were washed three times for 10 min with TBS-T and finally kept in PBS buffer on shaker for 10 min at RT in the dark and scanned by a LI-COR Infrared scanner (LI-COR Biosciences). The relative densities were calculated by normalizing the integrated optical densities of each blot with that of α-actin.
RT-PCR.
Total RNAs from the IAS were isolated and purified by the acid guanidine-phenol-chloroform method (14) and quantified by the measurement of absorbance at 260 nm on a spectrophotometer. Total RNA (2.0 μg) was subjected to first-strand cDNA synthesis using oligo(dT) primers (Promega, Madison, WI) and the Omniscript RT Kit (Qiagen, Germantown, MD) in a final volume of 20 μl at 42°C for 60 min. PCR was performed in Promega 2× Master Mix (Promega) in a final volume of 25 μl, using a Perkin-Elmer Thermal Cycler (PerkinElmer Life and Analytical Sciences). The PCR conditions consisted of 94°C for 2 min, followed by 35 cycles of 94°C for 30 s (denaturation), 59°C for 30 s (annealing), and 72°C for 1 min (extension). In the end, it was allowed a final extension at 72°C for 7 min. The PCR products were separated on 1.5% (wt/vol) agarose gel containing ethidium bromide and were visualized with ultraviolet light. The relative densities of HO-1, HO-2, elastin, RhoA, ROCKII, and MYPT1 were calculated by normalizing the densities of each blot with that of β-actin.
Chemicals and reagents.
α-Actin and β-actin antibodies were from Sigma. HO-1, HO-2, elastin, RhoA, ROCKII, and MYPT1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). IRdye680- and IRdye800-conjugated mouse, goat, and rabbit secondary antibodies were obtained from LI-COR Biosciences (Lincoln, NV). SnPPIX was purchased from Porphyrin Products.
Data analysis.
Results were expressed as means ± SE and graphed using GraphPad Prism 5.0 (Graph Pad Software, La Jolla, CA). Statistical significance between two different groups was tested using ANOVA and unpaired t-test. Linear regression analysis was used to identify the significance in difference between slopes of curves. A P value of <0.05 was considered to be statistically significant.
RESULTS
Effect of hemin pretreatment on HO-1 mRNA and protein expression.
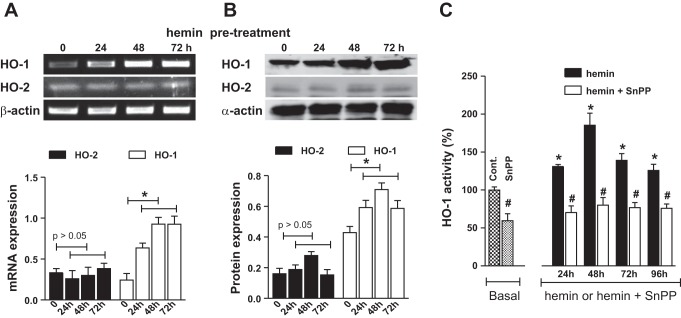
IAS from hemin-pretreated rats had a significant increase in the expression of mRNA and protein levels of HO-1 compared with control animals (P < 0.05; n = 4; Fig. 1). An increase in HO-1 (and not HO-2) expression was observed following 24 h of hemin pretreatment, and remained higher throughout the hemin treatment (data shown for 24 and 72 h; Fig. 1A, B), compared with controls. Likewise, hemin pretreatment caused a significant increase in the basal HO-1 activity following 24 h of pretreatment and was sustained throughout the treatment periods of 48, 72, and 96 h (P < 0.05; Fig. 1C). Additionally, HO-1 inhibitor SnPPIX attenuated both the basal and hemin-induced increase in the HO-1 activity (P < 0.05; n = 4).
Fig. 1.
A: RT-PCR analysis for heme oxygenase (HO)-1 and HO-2 mRNA expression. B: Western blot analysis for protein expression of HO-1 and HO-2 in the internal anal sphincter (IAS) of rats before (control; labeled as 0) and after 24, 48, and 72 h of consecutive hemin pretreatments. The corresponding quantitative data for the mRNAs and Western blot analyses are given as bar graphs. Data show significant increases in the expressions of both the mRNAs and protein following hemin pretreatment (*P < 0.05; n = 4). All data represent means ± SE. C: data show significant (8; P < 0.05; n = 4) increase in the basal activity of HO-1, 24, 48, 72, and 96 h following hemin pretreatment (*). Note that pretreatment of tin protoporphyrin (SnPP) IX (administered 30 min before hemin) causes a significant (#P < 0.05; n = 4) decrease in the basal HO-1 activity and in the hemin-induced increase.
Effect of HO-1 upregulation on basal IAS tone and on the characteristics of 0 Ca2+-induced relaxation of IAS.
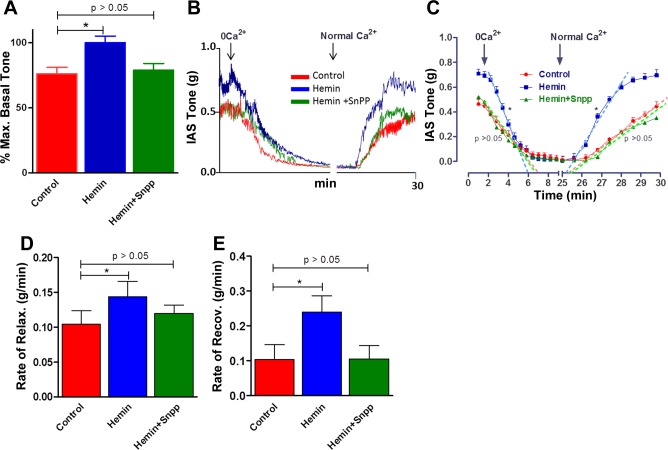
Quantitative data showing significant increase in the basal tone (P < 0.05; n = 4) with hemin pretreatment on the basal IAS tone are given in Fig. 2A. This increase in the basal IAS tone appears to be selective since it was significantly blocked by administration of HO-1 inhibitor SnPPIX so that the IAS tone values in the presence of hemin + SnPPIX were not significantly different from those in control (P > 0.05; n = 4). Typical tracings of the force measurements from the SM strips of IAS on the basal tone and changes in the relaxation characteristics following 0 Ca2+ before and after hemin pretreatment are given in Fig. 2B.
Fig. 2.
A: bar graphs show significant increase in the basal tone in hemin-pretreated rats compared with control animals. B: actual tracing showing decrease in the spontaneous basal tone at a faster rate in response to 0 Ca2+ in the IAS of hemin-pretreated vs. control rats. C: quantitative time course comparing the decreases in the IAS tone following 0 Ca2+ in control vs. hemin and hemin + SnPPIX. These data show significant increases in the IAS tone following hemin pretreatment (*P < 0.05; n = 4) that is blocked by pretreatment with SnPPIX. D and E: quantitative data showing significant increases in the rates of relaxation (with 0 Ca2+) and recovery (following normal Ca2+) (*P < 0.05; n = 4), respectively, in the hemin-pretreated group, which are blocked by the combination of hemin and SnPPIX. The regression lines (denoted by broken lines) were drawn by Prism Software.
Hemin pretreatment caused a significant increase in the IAS tone and in the velocities of relaxation with 0 Ca2+ and its recovery following normal KPS (P < 0.05; n = 4; Fig. 2C). These increases were attenuated by SnPPIX pretreatment to the levels not significantly different from control values (P > 0.05; n = 4; Fig. 2C).
Actual values for the velocities of relaxation and recovery following normal KPS are given in Fig. 2, D and E, respectively. (In these experiments, because of the variability of the protocols, it was not feasible to compare the total durations of relaxations following changes in Ca2+.) These data suggest an enhancement in the FEP of the IAS SM following hemin pretreatment. In these experiments the control animals received the vehicle for hemin and SnPPIX.
Effect of HO-1 upregulation on the characteristics of EFS-induced relaxation of IAS.
Figure 3A provides time course data for the absolute changes in basal IAS tone in response to the EFS (5 Hz). Data show significant increases in the basal IAS tone, decreases in the magnitude of relaxation, and increases in the velocities of relaxation and recovery of the IAS tone following EFS. Data in Fig. 3B further revealed that hemin pretreatment caused a significant decrease in the EFS-induced relaxation of the IAS in a frequency-dependent manner (P < 0.05; n = 4). Additionally, the data show that the attenuated IAS relaxation with EFS in the presence of hemin alone was significantly reversed by the prior treatment of HO inhibitor SnPPIX so that the values in the presence of the combination are not significantly different from controls (P > 0.05; n = 4; Fig. 3, A and B).
Fig. 3.
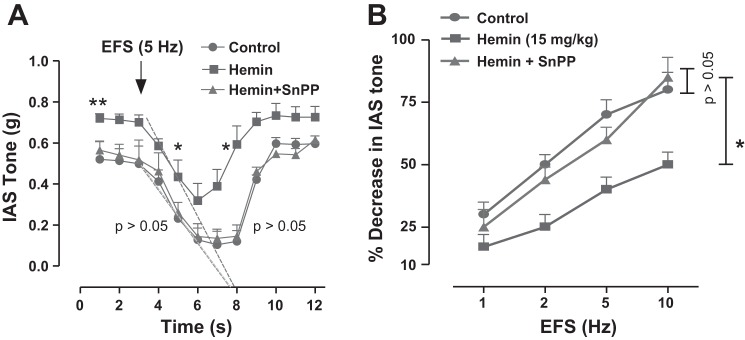
A: quantitative data show the time course of the IAS relaxation with electrical field stimulation (EFS, 5 Hz) in control that is significantly suppressed by hemin pretreatment (8; P < 0.05; n = 4). SnPPIX pretreatment in the hemin-pretreated IAS strips blocks the blunted EFS response so that the EFS responses in the presence of the combination are not significantly different from control (P > 0.05; n = 4). Data further show significant increase in the velocities of the relaxation and recovery of the IAS relaxation with EFS (*P < 0.05; n = 4) calculated by the extrapolated regression lines drawn (as broken lines) by the software. Note: for clarity, regression lines are drawn only for the relaxation phase and not for the recovery of relaxation phase. B: quantitative data show the frequency-dependent relaxation of the IAS that is significantly attenuated by hemin pretreatment (*P < 0.05; n = 4), reversible to the control levels by SnPPIX (P > 0.05).
Quantitative data for the rates of the IAS relaxation and recovery, and total durations of the IAS relaxation following EFS (5 Hz) in control vs. hemin and hemin + SnPPIX are given in Fig. 4, A, B, and C, respectively. These data further revealed enhancement of FEP of the IAS with hemin via upregulation of HO-1.
Fig. 4.
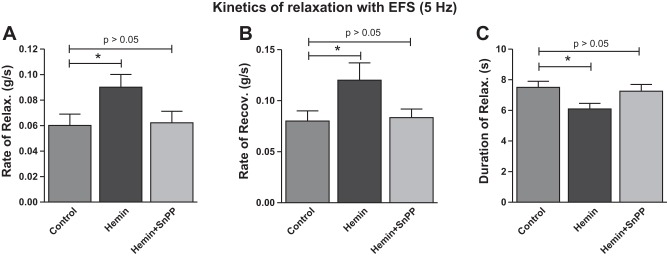
Kinetics of the IAS relaxation with EFS (5 Hz). A: rate of relaxation. B: rate of recovery. C: total duration of the IAS relaxation following EFS. Data show that hemin significantly accelerates the rates of relaxation and recovery and causes a significant decrease in the overall duration of relaxation with the EFS (*P < 0.05; n = 4), reversible by SnPPIX.
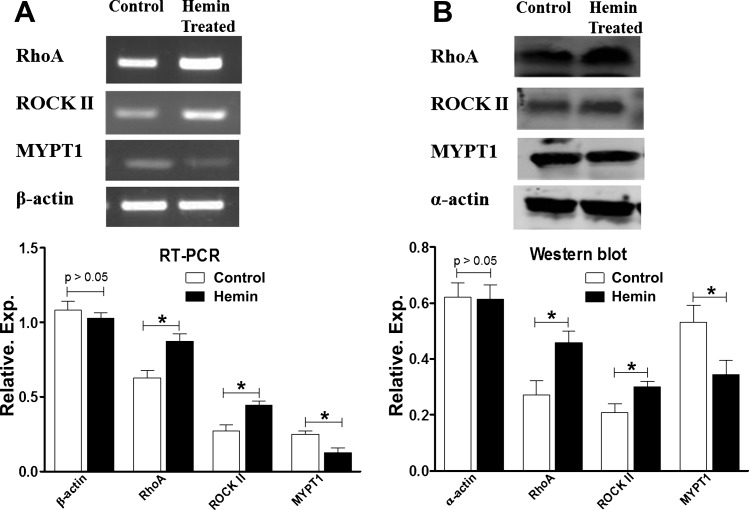
RT-PCR analysis for mRNA expression of RhoA/ROCKII in the IAS.
RT-PCR analysis revealed significantly higher gene expressions of RhoA and ROCKII in the IAS of the hemin-pretreated vs. control group (P < 0.05; n = 4; Fig. 5A). Conversely, expression of MYPT1 was higher in the control group than the hemin-pretreated group (P < 0.05; n = 4; Fig. 5A).
Fig. 5.
A: RT-PCR showing higher levels of gene expressions of RhoA-associated kinase (RhoA) and Rho kinase (ROCK) II, and lower levels of expressions of myosin light chain phosphatase 1 (MYPT1) in the IAS of hemin-pretreated rats compared with control rats (*P < 0.05; n = 4). B: Western blot showing higher levels of gene expressions of RhoA and ROCKII and lower levels of MYPT1 in the IAS of hemin-pretreated rats compared with control rats (*P < 0.05; n = 4).
Western blot analysis for protein expression of RhoA/ROCKII in the IAS.
The Western blot data in resemblance with the RT-PCR data showed that the IAS of the hemin-pretreated group had significantly higher expression levels of RhoA and ROCKII than the control group (P < 0.05; n = 4; Fig. 5B). However, in contrast to RhoA/ROCKII levels, MYPT1 levels in the IAS were significantly lower in the hemin-pretreated vs. the control group (P < 0.05; n = 4; Fig. 5B).
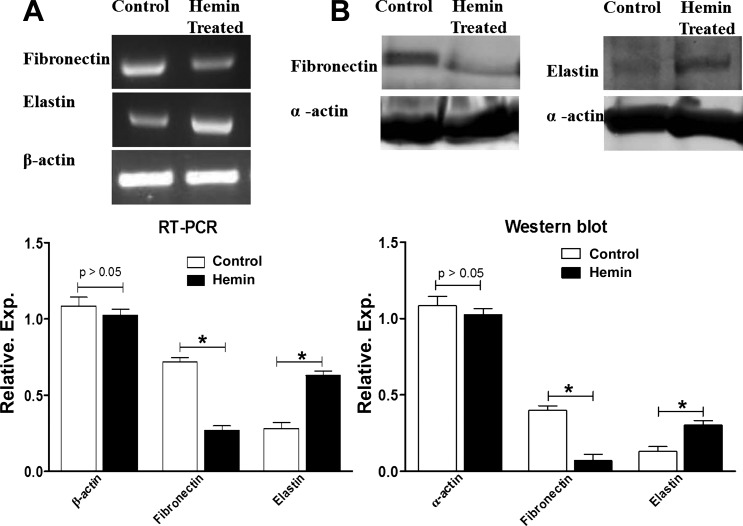
RT-PCR and western blot analyses for fibronectin and elastin expression in the IAS.
RT-PCR analysis revealed significantly higher mRNA expression of elastin in the IAS of hemin-pretreated vs. the control group (P < 0.05; n = 4; Fig. 6A). Conversely, expression of fibronectin was higher in the control group than the hemin-pretreated group (P < 0.05; n = 4; Fig. 6A).
Fig. 6.
A: RT-PCR showing higher levels of elastin gene expression and lower levels of fibronectin in the IAS of hemin-pretreated rats compared with control rats (*P < 0.05; n = 4). B: Western blot showing higher levels of elastin and lower levels of expressions in the IAS of hemin-pretreated rats compared with control rats (*P < 0.05; n = 4).
Western blot data in agreement with the RT-PCR data showed that the IAS of the hemin-pretreated group had significantly higher expression levels of elastin than the control group (P < 0.05; n = 4; Fig. 6B). By contrast, fibronectin levels in the IAS were significantly lower in the hemin-pretreated vs. the control group (P < 0.05). Collagen, fibronectin, and elastin are integral components of the ECM. A greater elastin-to-fibronectin ratio may be responsible for the improved FEP of the IAS of hemin-pretreated rats compared with controls. However, we did not find any significant difference in the mRNA and protein expression levels of collagen in the IAS of hemin-pretreated vs. control groups (data not shown).
DISCUSSION
The present studies have for the first time examined the nonneuronal roles of the hemin/HO-1 system in the IAS. In this study we have established that hemin treatment increases the tone and improves the FEP of IAS of rats. These changes seem to be, at least in part, associated with changes in the levels of RhoA/ROCKII, MYPT1, elastin, and fibronectin.
HO-1 upregulation by hemin increases the tone of IAS by upregulation of RhoA/ROCKII and by decreasing MYPT1 levels. The role of higher levels of RhoA/ROCK and lower levels of MYPT1 in mediating basal tone of the IAS has been previously demonstrated in rats as well as in humans (27, 28, 30, 32). The present data further suggest that upregulated RhoA/ROCKII and lower levels of MYPT1 may be responsible for the higher basal tone in the IAS of hemin-pretreated rats compared with controls. Interestingly, in vitro studies by Bortscher et al. have shown that hemin improves intestinal muscular contractility by inhibiting inducible nitric oxide (iNOS) (9). Similarly, Imai et al. (19) showed that vascular SM cell-directed overexpression of HO-1 suppresses the vasodilatory response to nitric oxide (NO), thereby leading to elevation of vascular tone and consequently arterial pressure. These findings, however, are in contrast to previous studies in the cardiovascular system where the hemin/HO-1 system has been shown to downregulate the RhoA/ROCK pathway leading to a decrease in vascular tone (2, 18). The observed differences between the previous studies in the cardiovascular system and the present ones may be explained on the basis of tissue-specific roles of the hemin/HO-1 system in the IAS. Additional studies, however, are necessary to identify the role of HO-1-mediated iNOS inhibition on IAS tone.
Studies by Karnik et al. (20) have shown that extracellular elastin is an important mediator of actin polymerization and contractile apparatus organization in vascular SM cells via the RhoA/ROCK signaling pathway. A similar role of elastin upregulation in the IAS of hemin-pretreated rats is one of the plausible mechanisms for the higher tone in the hemin-pretreated rats. However, the precise role of elastin in regulating the contractile phenotype of gastrointestinal SM needs to be determined.
In our study, the levels of ECM proteins, particularly fibronectin and elastin, appear to be regulated differently by the hemin/HO-1 system in the IAS. Stimulation of HO-1 by hemin upregulates elastin but downregulates fibronectin levels. Previous studies have reported that HO-1 reduces matrix metalloproteinase 9 levels and thereby prevents elastin degradation in the blood vessels (12, 37). However, it remains to be determined whether HO-1 produces upregulation of elastin at the gene level. In a recent study by Barikbin et al. (6) pharmacological upregulation of HO-1 by cobalt protoporphyrin IX was shown to prevent and reverse liver fibrosis by inhibiting hepatic stellate cell activation. Studies on the heart (22) have also shown that stimulation of HO-1 expression inhibited protein levels of procollagen type 1 but did not have any effect on fibronectin levels. By contrast, we found that HO-1 expression decreased fibronectin levels without producing any significant changes in collagen protein levels. The combined effect of elevated elastin levels and decreased fibronectin levels may lead to the observed improvement in the FEP of IAS in the hemin-pretreated group.
We have also observed blunting of EFS-induced relaxation of IAS tissue strips in the hemin-pretreated group compared with controls. One of the plausible mechanisms could be inhibition of NOS by HO-1 expression, which has been shown in other tissues (9, 19). Further studies examining an interaction between NOS and HO-1 may unravel the possible mechanism for the observed suppressant effect of HO-1 upregulation on the EFS-induced IAS relaxation. Interestingly, according to our preliminary observations, rats in the hemin-pretreated group revealed a decrease in the number of fecal pellet output/24 h compared with controls. This could be because of the attenuation of the rectoanal inhibitory reflex coupled with a higher resting tone of IAS in the hemin-pretreated group. We speculate that, in the aging rats, the dysfunctional IAS (characterized by the lower IAS tone and compromised FEP, possibly accompanied with RI) may be rescued by hemin treatment rather than the observed decrease in the fecal pellet output in the normal rats.
Data demonstrating that SnPPIX reverses the effects of hemin pretreatment on basal tone and EFS-induced relaxation suggest the specificity of hemin effects to be via HO-1 activation. These effects combined with the changes in the mRNA and protein expression of RhoA/ROCK, MYPT1, elastin, and fibronectin suggest specific effects of HO-1 may occur at the gene level. However, short-term incubation (3 h) has also been shown to produce an effective (70%) inhibition of HO-1 activity in other systems (33). Such effects may be explained on the basis of differences in the pharmacokinetics of SnPPIX effects, and in the acute vs. long-term effects in different tissues.
In summary, the present data show that the hemin/HO-1 system regulates the tone and FEP of IAS. However, further work will be required to dissect the specific roles of heme catabolism products in mediating this effect. The hemin/HO-1 system thus provides a potential target for the development of new interventions aimed at treatment of gastrointestinal motility disorders.
GRANTS
The work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-035385 and an institutional grant from Thomas Jefferson University.
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: C.V.K., J.S., and S.K. performed experiments; C.V.K., J.S., and S.K. analyzed data; C.V.K., J.S., and S.K. prepared figures; C.V.K., S.K., and S.R. drafted manuscript; C.V.K., J.S., S.K., and S.R. approved final version of manuscript; J.S., S.K., and S.R. interpreted results of experiments; S.K. and S.R. edited and revised manuscript; S.R. conception and design of research.
REFERENCES
- 1.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60: 79–127, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Awede B, Lemaire MC, Hyvelin JM, Halimi JM, Bonnet P, Eder V. Hemin, a carbon monoxide donor, improves systemic vascular compliance by inhibiting the RhoA-Rhokinase pathway in spontaneous hypertensive rats. Eur J Pharmacol 626: 256–261, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Aziz MTA, Al-Asmar MF, Mostafa T, Atta H, Rashed L, Sabry D, Ashour S, Aziz ATA. Assessment of heme oxygenase-1 (HO-1) activity in the cavernous tissues of sildenafil citrate-treated rats. Asian J Androl 9: 377–381, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bang K, Jeong J, Shin JH, Kang JH, Kim CN, Yeom HJ, Yoon MO, Yang J, Ahn C, Hwang JI, Park MY, Kim JH, Lee KW. Heme oxygenase-1 attenuates epithelial-to-mesenchymal transition of human peritoneal mesothelial cells. Clin Exp Nephrol 17: 284–293, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Barbagallo I, Nicolosi A, Calabrese G, David S, Cimino S, Madonia M, Cappello F. The role of the heme oxygenase system in the metabolic syndrome. Curr Pharm Des In press [DOI] [PubMed] [Google Scholar]
- 6.Barikbin R, Neureiter D, Wirth J, Erhardt A, Schwinge D, Kluwe J, Schramm C, Tiegs G, Sass G. Heme oxygenase 1 prevents progression of liver fibrosis in Mdr2 knockout mice. Hepatology 55: 553–562, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Bharucha AE, Kulkarni A, Choi KM, Camilleri M, Lempke M, Brunn GJ, Gibbons SJ, Zinsmeister AR, Farrugia G. First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin Pharmacol Ther 87: 187–190, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharucha AE, Rao SSC. An update on anorectal disorders for gastroenterologists. Gastroenterology 146: 37–45, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bortscher S, Chang J, Vilz TO, Schafer N, Sommer N, Wehner S, Kalff JC, Overhaus M. Hemin induction of HO-1 protects against LPS-induced septic ileus. J Surg Res 178: 866–873, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Braggins PE, Trakshel GM, Kutty RK, Maines MD. Characterization of two heme oxygenase isoforms in rat spleen: comparison with the hematin-induced and constitutive isoforms of the liver. Biochem Biophys Res Commun 141: 528–533, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Chakder S, Cao GY, Lynn RB, Rattan S. Heme oxygenase activity in the internal anal sphincter: effects of nonadrenergic, noncholinergic nerve stimulation. Gastroenterology 118: 477–486, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Cheng C, Noordeloos AM, Joney V, Soares MP, Moll F, Pasterkamp G, Serruys PW, Duckers HJ. Heme oxygenase 1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation 119: 3017–3027, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Choi KM, Gibbons SJ, Nguyen TV, Stoltz GJ, Lurken MS, Ordog T, Szurszewski JH, Farrugia G. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology 135: 2055–2064, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 15.De Godoy MAF, De Oliveira AM, Rattan S. Angiotensin II-induced relaxation of anococcygeus smooth muscle via desensitization of AT1 receptor, and activation of AT2 receptor associated with nitric-oxide synthase pathway. J Pharmacol Exp Ther 311: 394–401, 2004 [DOI] [PubMed] [Google Scholar]
- 16.De Godoy MAF, Dunn SR, Rattan S. Evidence for the role of angiotensin II biosynthesis in the rat internal anal sphincter tone. Gastroenterology 127: 127–138, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Farrugia G, Miller SM, Rich A, Liu X, Maines MD, Rae JL, Szurszewski JH. Distribution of heme oxygenase and effects of exogenous carbon monoxide in canine jejunum. Am J Physiol Gastrointest Liver Physiol 274: G350–G358, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Hyvelin JM, Maurel B, Uzbekov R, Motterlini R, Lermusiaux P. Hemin prevents in-stent stenosis in rat and rabbit models by inducing heme-oxygenase-1. J Vasc Surg 51: 417–428, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Imai T, Morita T, Shindo T, Nagai R, Yazaki Y, Kurihara H, Suematsu M, Katayama S. Vascular smooth muscle cell-directed overexpression of heme oxygenase-1 elevates blood pressure through attenuation of nitric oxide-induced vasodilation in mice. Circ Res 89: 55–62, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development 130: 411–423, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Kie JH, Kapturczak H, Traylor A, Agarwal A, Hill-Kapturczak N. Heme oxygenase-1 deficiency promotes epithelial-mesenchymal transition and renal fibrosis. J Am Soc Nephrol 19: 1681–1691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakkisto P, Siren JM, Kyto V, Forsten H, Laine M, Pulkki K, Tikkanen I. Heme oxygenase-1 induction protects the heart and modulates cellular and extracellular remodelling after myocardial infarction in rats. Exp Biol Med 236: 1437–1448, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Levere RD, Martasek P, Escalante B, Schwartzman ML, Abraham NG. Effect of heme arginate administration on blood pressure in spontaneously hypertensive rats. J Clin Invest 86: 213–219, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewicky-Gaupp C, Hamilton Q, Ashton-Miller J, Huebner M, DeLancey JO, Fenner DE. Anal sphincter structure and function relationships in aging and fecal incontinence. Am J Obstet Gynecol 200: 559–580, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martasek P, Schwartzman ML, Goodman AI, Solangi KB, Levere RD, Abraham NG. Hemin and l-arginine regulation of blood pressure in spontaneous hypertensive rats. J Am Soc Nephrol 2: 1078–1084, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 279: L1029–L1037, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Patel CA, Rattan S. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol 291: G830–G837, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Patel CA, Rattan S. Cellular regulation of basal tone in internal anal sphincter smooth muscle by RhoA/ROCK. Am J Physiol Gastrointest Liver Physiol 292: G1747–G1756, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA 94: 10919–10924, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rattan S, De Godoy MAF, Patel CA. Rho kinase as a novel molecular therapeutic target for hypertensive internal anal sphincter. Gastroenterology 131: 108–116, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Rattan S, Singh J. Basal internal anal sphincter tone, inhibitory neurotransmission, and other factors contributing to the maintenance of high pressures in the anal canal. Neurogastroenterol Motil 23: 3–7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rattan S, Singh J. RhoA/ROCK pathway is the major molecular determinant of basal tone in intact human internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 302: G664–G675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg DW, Drummond GS, Kappas A. The in vitro and in vivo inhibition of intestinal heme oxygenase by tin-protoporphyrin. Pharmacology 39: 224–229, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Ryter SW, Kvam E, Tyrrell RM. Heme oxygenase activity: current methods and applications. In: Stress Response, edited by Keyse SM. Totowa, NJ: Humana, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Speakman CTM, Hoyle CHV, Kamm MA, Swash M, Henry MM, Nicholls RJ, Chir M, Burnstock G. Abnormal internal anal sphincter fibrosis and elasticity in fecal incotninence. Dis Colon Rectum 38: 407–410, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Tenhunen R, Marver HS, Schmid. R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem 244: 6388–6394, 1969 [PubMed] [Google Scholar]
- 37.Wu ML, HOYC, Lin CY, Yet SF. Heme oxygenase-1 in inflammation and cardiovascular disease. Cardiovasc Dis 1: 150–158, 2011 [PMC free article] [PubMed] [Google Scholar]
- 38.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103: 129–135, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]