Abstract
Nitric oxide (NO) regulates renal function. Luminal flow stimulates NO production in the thick ascending limb (TAL). Transient receptor potential vanilloid 4 (TRPV4) is a mechano-sensitive channel activated by luminal flow in different types of cells. We hypothesized that TRPV4 mediates flow-induced NO production in the rat TAL. We measured NO production in isolated, perfused rat TALs using the fluorescent dye DAF FM. Increasing luminal flow from 0 to 20 nl/min stimulated NO from 8 ± 3 to 45 ± 12 arbitrary units (AU)/min (n = 5; P < 0.05). The TRPV4 antagonists, ruthenium red (15 μmol/l) and RN 1734 (10 μmol/l), blocked flow-induced NO production. Also, luminal flow did not increase NO production in the absence of extracellular calcium. We also studied the effect of luminal flow on NO production in TALs transduced with a TRPV4shRNA. In nontransduced TALs luminal flow increased NO production by 47 ± 17 AU/min (P < 0.05; n = 5). Similar to nontransduced TALs, luminal flow increased NO production by 39 ± 11 AU/min (P < 0.03; n = 5) in TALs transduced with a control negative sequence-shRNA while in TRPV4shRNA-transduced TALs, luminal flow did not increase NO production (Δ10 ± 15 AU/min; n = 5). We then tested the effect of two different TRPV4 agonists on NO production in the absence of luminal flow. 4α-Phorbol 12,13-didecanoate (1 μmol/l) enhanced NO production by 60 ± 11 AU/min (P < 0.002; n = 7) and GSK1016790A (10 ηmol/l) increased NO production by 52 ± 15 AU/min (P < 0.03; n = 5). GSK1016790A (10 ηmol/l) did not stimulate NO production in TRPV4shRNA-transduced TALs. We conclude that activation of TRPV4 channels mediates flow-induced NO production in the rat TAL.
Keywords: mechano-transduction, TRP channels, nitric oxide synthase, shear stress, kidney, luminal flow
nitric oxide (NO) plays an important role in the regulation of vascular resistance and sodium and water excretion (16, 29, 32, 47, 56). As a result, it is a key player in the control of blood pressure. Inhibition of renal NO causes salt sensitivity of blood pressure (37, 70). Reductions in sodium excretion caused by diminishing renal NO can occur in the absence of changes in glomerular filtration rate or renal blood flow (33).
The medullary thick ascending limb is the nephron segment responsible for reabsorbing approximately one-third of the filtered sodium chloride creating the osmotic gradient necessary for water reabsorption in the collecting duct (7). Its normal function plays a critical role in extracellular fluid volume and blood pressure regulation. NO produced by NO synthase 3 acts as an autacoid to inhibit sodium chloride reabsorption in the thick ascending limb (45). We previously demonstrated that luminal flow stimulates NO production in this segment and that shear stress rather than pressure, cellular stretch, or ion delivery mediates flow-induced NO production (11).
As in other parts of the nephron, urinary flow through the thick ascending limb is a physiological parameter subjected to change (15, 25). Luminal flow can be acutely increased during volume overload or diuretic use, while chronically it can be increased in hypertension or glomerular hyperfiltration during the early stages of diabetic nephropathy (2, 14, 48). However, under physiological conditions, luminal flow also displays regular, rapid change mainly due to the tubuloglomerular feedback and peristalsis of the renal pelvis (15, 25).
The transient receptor potential vanilloid 4 (TRPV4) belongs to the superfamily of transient receptor potential (TRP) channels (46). This channel has been postulated as a sensor of mechanical stimulation in a variety of cells, including renal epithelial cells (41, 68). It has recently been shown that TRPV4 activation mediates flow-induced increases in intracellular calcium in the mouse cortical collecting duct (5). In the rat thick ascending limb, we found that activation of this channel mediates ATP release in response to hypotonicity-induced cellular stretch (54). TRPV4 also functions as a mechano-sensitive channel in endothelial cells where it mediates shear stress-induced NO production (40). Therefore, we hypothesized that TRPV4 activation mediates flow-induced NO production in the rat thick ascending limb.
MATERIALS AND METHODS
Animals.
We used male Sprague-Dawley rats weighing 100 to 150 g from Charles River Breeding Laboratories (Wilmington, MA). Rats were fed a diet containing 0.22% sodium and 1.1% potassium (Purina, Richmond, IN) for at least 5 days before the experiments. Protocols involving animals were previously reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University following the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Solutions and chemicals.
The physiological saline used to perfuse and bathe tubules was (in mmol/l) 130 NaCl, 4 KCl, 2.5 NaH2PO4, 1.2 MgSO4, 6 l-alanine, 0.1 l-arginine, 1 trisodium citrate, 5.5 glucose, 2 calcium dilactate, and 10 HEPES, pH 7.4 at 37°C. The calcium-free solution was (in mmol/l) 130 NaCl, 4 KCl, 1.2 MgSO4, 6 l-alanine, 0.1 l-arginine, 5.5 glucose, 0.1 EGTA, and 10 HEPES, pH 7.4 at 37°C. After being made, all solutions were adjusted, if necessary, to 290 ± 3 mosmol/kgH2O as measured by freezing-point depression.
4-Amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF FM-DA) was purchased from Invitrogen (Eugene, OR). Dimethyl sulfoxide from Sigma (St. Louis, MO) was used to prepare stock solutions of 10 mmol/l DAF FM-DA. Ruthenium red, 4α-phorbol 12,13-didecanoate (4αPDD), and GSK1016790A were purchased from Sigma. RN1734 was obtained from Tocris Bioscience (Bristol, UK).
Preparation of isolated and perfused medullary thick ascending limbs.
Rats were anesthetized with ketamine (100 mg/kg body wt ip) and xylazine (20 mg/kg body wt ip). After anesthesia, the abdominal cavity was opened and the left kidney was bathed with 100 ml of ice-cold 150 mmol/l NaCl solution. Then, it was removed and placed in ice-cold physiological saline. With the use of a stereomicroscope at 4 to 10°C, tubules corresponding to medullary thick ascending limbs ranging from 0.7 to 1.1 mm were dissected free-hand, transferred to a temperature-regulated chamber mounted on an inverted microscope, and perfused using concentric glass pipettes at 37 ± 1°C as previously described (6, 8, 10, 11, 21). Luminal perfusion rates were 0 or 20 nl/min and basolateral flow rate was set at 0.6 ml/min.
Measurement of intracellular NO production.
Isolated medullary thick ascending limbs were bathed with 2–4 μmol/l DAF FM-DA in physiological saline for 15 min and then washed in dye-free physiological saline for 20 min. Some protocols were performed in an inverted microscope (TE-2000U; Nikon) where DAF FM was excited using a xenon arc lamp at 488 nm while emitted fluorescence was measured using a 515-nm long-pass dichroic mirror and a 535/50-nm barrier filter. Fluorescence was imaged with a ×40 immersion oil objective and a Coolsnap HQ digital camera (Photometrics, Tucson, AZ). Data were collected from regions of interest (ROI) and recorded using Metafluor version 7 imaging software (Universal Imaging, Downington, PA). Other protocols were performed using a different inverted microscope (TE-300; Nikon) where DAF FM was excited using a 491-nm argon laser at 55.6% intensity. Emitted fluorescence was measured using a 488-nm primary dichroic and a 500-nm long-pass barrier filter. Data were collected from ROIs and recorded using Voxcell imaging software (Visitech International). ROIs were set larger than the tubule to accommodate changes in shape.
NO production was measured in the absence of luminal flow for 5 min. Then, luminal flow was increased to 20 nl/min in the second period and NO was measured again for 5 min.
To study the effects of TRPV4 agonists, NO production was measured in the absence of luminal flow for 5 min. Then, 4α-phorbol 12,13-didecanoate or GSK1016790A was added to the basolateral bath in the second period and NO was measured again for 5 min. During this period, thick ascending limbs remained without luminal flow.
To test the contribution of extracellular calcium, a calcium-free solution was added to the luminal and basolateral side after the dye was washed with physiological saline. NO production was measured in the absence of luminal flow for 5 min. Then, luminal flow was increased to 20 nl/min in the second period and NO was measured again for 5 min.
In all protocols, fluorescence was measured at the beginning of each period once every 30 s. The change in emitted fluorescence over time was taken as NO production and expressed as arbitrary units (AU)/min.
In vivo adenoviral transduction of medullary thick ascending limbs.
Small hairpin TRPV4-RNA (shRNA) under the control of the H1 mouse RNA polymerase promoter or control negative sequence-shRNA was delivered to medullary thick ascending limbs using an adenovirus delivery system. As we previously described (39, 54), rats were anesthetized with ketamine (60 mg/kg body wt ip) and xylazine (20 mg/kg body wt ip). After anesthesia, a left-flanked incision was made to expose the left kidney. Then, the renal artery and vein were clamped. Immediately after the renal vessels were clamped, 4 infusions of 20 μl of adenovirus at a final concentration of 1.2 × 10−12 particles/ml were injected at the kidney cortico-medullary boundary. A 30-gauge needle was used to inject the virus at a flow rate of 20 μl/min. To avoid tissue bleeding and leakage of the virus, the needle was removed from the injection site 30 s after each infusion was completed. After a total time of 8 min, the vascular clamp was removed, the left kidney was returned to the abdominal cavity, and the wound was sutured. Animals were kept warm and under direct observation until they appeared alert and fully-recovered from anesthesia. We and others have found no irreversible histo-pathologic changes in the kidney after clamping the renal artery and vein for 8 min. This maneuver has been shown to increase transduction efficiency (12, 13, 28, 44, 61). Three days after the adenovirus injections, thick ascending limbs were carefully dissected from the vicinity of the injection site. We previously found that all cells around the injection site were effectively transduced (44).
TRPV4 protein expression measured by Western blotting.
Three days after the adenovirus injections were performed medullary thick ascending limb suspensions of the left (TRPV4shRNA-transduced) and the right (nontransduced) kidney from the same rat were obtained by collagenase digestion as we previously described (20, 54, 62). Suspensions were lysed in a buffer containing 20 mmol/l HEPES (pH 7.4), 2 mmol/l EDTA, 0.3 mmol/l sucrose, 1.0% NP-40, 0.1% sodium dodecyl sulfate, 5 μg/ml antipain, 10 μg/ml aprotinin, 5 μg/ml leupeptin, 4 mmol/l benzamidine, 5 μg/ml chymostatin, 5 μg/ml pepstatin A, and 0.105 mmol/l pf-block. Two and five μg protein were loaded into each lane of a polyacrylamide gel, separated by electrophoresis, and transferred to an Immobilon-P PVDF membrane (Bio Rad). Membranes were incubated in blocking buffer containing TBS-T plus 5% nonfat dried milk for 60 min and then with a primary in blocking buffer for 60 min at room temperature. Membranes were then washed in TBS-T and incubated with a 1:1,000 dilution of a secondary antibody against the appropriate IgG conjugated to horseradish peroxidase (GE Healthcare) in blocking buffer. The reaction was detected with a chemiluminescence kit (Thermo Scientific) and quantified by densitometry. Beta tubulin protein expression was used as a loading control to correct both TRPV4 and NOS 3 protein expression. Expression was calculated as the percentage change from control (nontransduced) thick ascending limbs from the same rat.
Primary antibodies included the following: TRPV4: rabbit polyclonal anti-TRPV4 (Alomone Labs) 1:1,000 dilution; nitric oxide synthase (NOS) type 3: mouse anti-NOS III (BD Transduction Laboratories) 1:1,000 dilution; and beta tubulin: rabbit polyclonal anti-beta tubulin (Abcam) 1:5,000 dilution.
Statistical analysis.
Data are presented as means ± SE. A paired Student's t-test was used to calculate the difference between no flow and flow conditions or between no flow and no flow ± TRPV4 agonists conditions in each experimental group. A P value of <0.05 was considered significant.
RESULTS
We started our studies by testing the ability of different TRPV4 blockers to inhibit flow-induced NO production. First, we used the general TRPV antagonist ruthenium red. Increasing luminal flow from 0 to 20 nl/min raised NO production from 8 ± 3 to 45 ± 12 AU/min (n = 5; P < 0.05; Fig. 1). However, in the presence of the TRPV antagonist ruthenium red (15 μmol/l), increasing luminal flow did not increase NO production (from 18 ± 5 vs. 20 ± 5 AU/min; n = 5; Fig. 2). To show that the effect of ruthenium red was due to inhibition of TRPV4 and not some other protein, we used a chemically distinct pharmacological TRPV4 selective agonist RN 1734. When luminal flow was increased in the presence of RN 1734 (10 μmol/l), NO levels did not change significantly (11 ± 7 vs. 9 ± 2 AU/min; n = 5; Fig. 3).
Fig. 1.
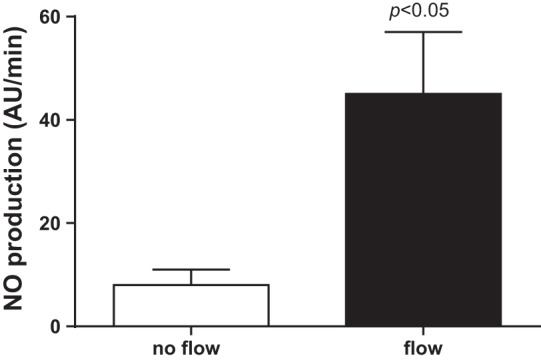
Effect of increasing luminal flow on nitric oxide (NO) production by isolated thick ascending limbs (P < 0.05 compared with no flow conditions; n = 5).
Fig. 2.
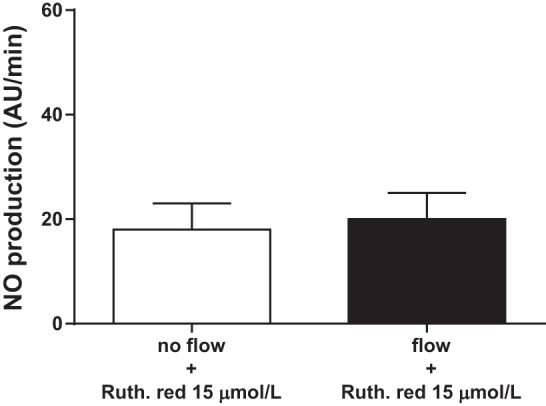
Effect of increasing luminal flow on NO production by isolated thick ascending limbs in the presence of the transient receptor potential vanilloid (TRPV) antagonist ruthenium red (n = 5).
Fig. 3.
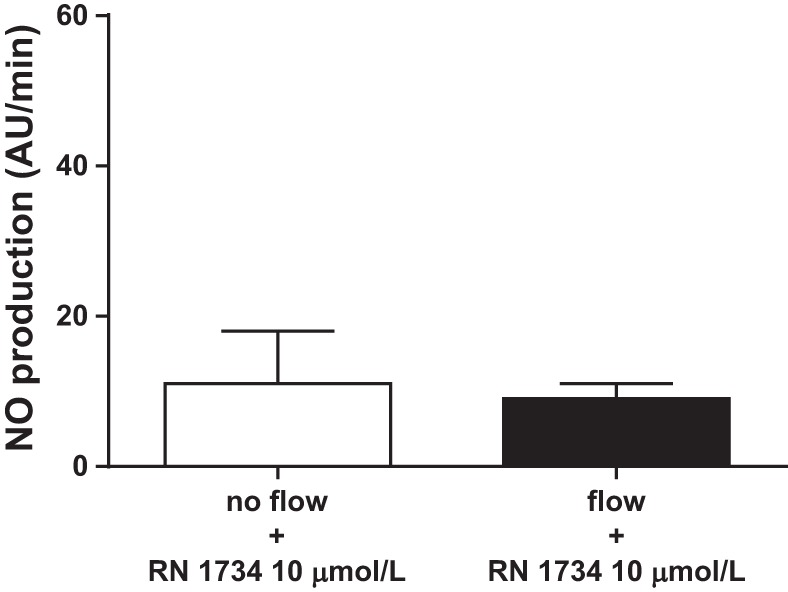
Effect of increasing luminal flow on NO production by isolated thick ascending limbs in the presence of the TRPV4 antagonist RN 1734 (n = 5).
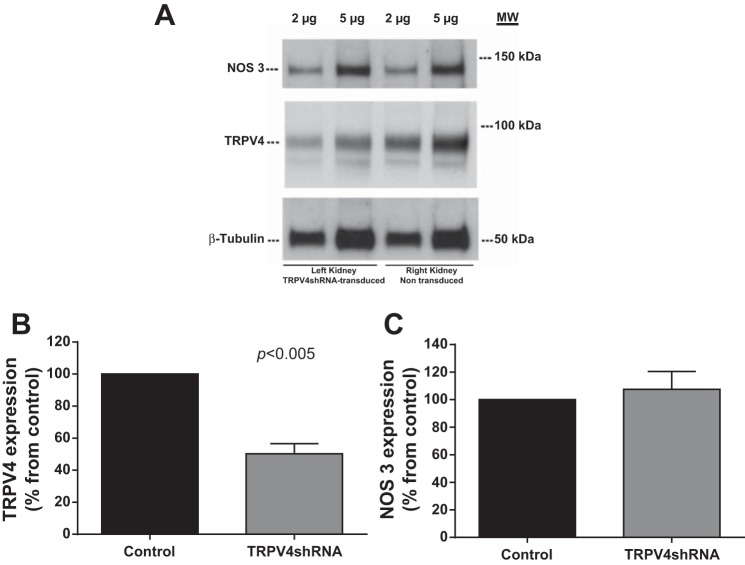
To specifically target TRPV4 expression, we transduced medullary thick ascending limbs with an adenovirus that expressed TRPV4shRNA. Transducing thick ascending limbs with this virus reduced TRPV4 protein expression by 50.3 ± 6.3% (n = 4; P < 0.005) in the left kidney (TRPV4shRNA-transduced) compared with the right kidney (nontransduced; Fig. 4B). However, TRPV4shRNA had no effect on NOS 3 protein expression (change: −7.5 ± 13%; Fig. 4C).
Fig. 4.
A: representative Western blots from the left TRPV4shRNA-transduced kidney and the right nontransduced kidney. Two and five micrograms of protein were loaded and TRPV4, nitric oxide synathase (NOS) 3, and β-tubulin expressions were measured. B: TRPV4 protein expression decreased by 50.3 ± 6.3% in the left kidney (TRPV4shRNA-transduced) compared with the right kidney (nontransduced; P < 0.005; n = 4). C: NOS 3 protein expression levels did not change in the left kidney (TRPV4shRNA-transduced) compared with the right kidney (nontransduced).
In a separate set of experiments we studied the effect of luminal flow on NO production after 3 days of transducing thick ascending limbs with a TRPV4shRNA. In control (nontransduced) thick ascending limbs luminal flow increased NO production by 47 ± 17 AU/min (n = 5; P < 0.05). However, in TRPV4shRNA-transduced thick ascending limbs luminal flow did not stimulate NO production (Δ10 ± 15 AU/min; n = 5; Fig. 5). To show that this phenomenon was not due to any undesired effects of the adenoviral transduction technique, we repeated these experiments in control negative sequence-shRNA-transduced thick ascending limbs. Similar to nontransduced thick ascending limbs, luminal flow increased NO production by 39 ± 11 AU/min (P < 0.03; n = 5) in thick ascending limbs transduced with a control negative sequence-shRNA. Together, these data show that TRPV4 inhibition blocks flow-induced NO production in the rat medullary thick ascending limb.
Fig. 5.
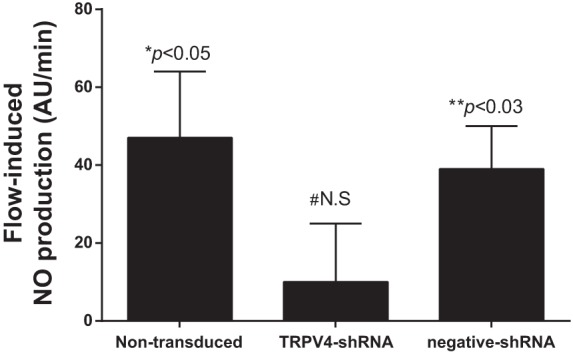
Effect of increasing luminal flow on NO production by isolated thick ascending limbs from control nontransduced thick ascending limbs; TRPV4shRNA-transduced thick ascending limbs and control negative sequence shRNA-transduced thick ascending limbs (*P < 0.05 compared with no flow conditions in the same thick ascending limb; n = 5). #N.S., compared with no flow conditions in the same thick ascending limb; n = 5. N.S., nonsignificant. **P < 0.03 compared with no flow conditions in the same thick ascending limb; n = 5.
Then, we tested whether the activation of TRPV4 was sufficient to augment NO production, mimicking the effect of mechanical stimulation. To study this possibility, we tested the effect of two different TRPV4 agonists on NO production in the absence of mechanical stimulation exerted by luminal flow. In the absence of luminal flow, 4αPDD (1 μmol/l) increased NO production by 60 ± 11 AU/min (n = 7; P < 0.002) while the selective TRPV4 agoinst GSK1016790A (10 ηmol/l) stimulated NO production by 52 ± 15 AU/min (n = 5; P < 0.03). GSK1016790A (10 ηmol/l) did not increase NO production in TRPV4shRNA-transduced thick ascending limbs (Δ8 ± 4 AU/min; n = 5; Fig. 6). These results indicate that the effects generated by luminal flow on NO production in the thick ascending limb can be reproduced by directly activating TRPV4 using exogenous compounds.
Fig. 6.
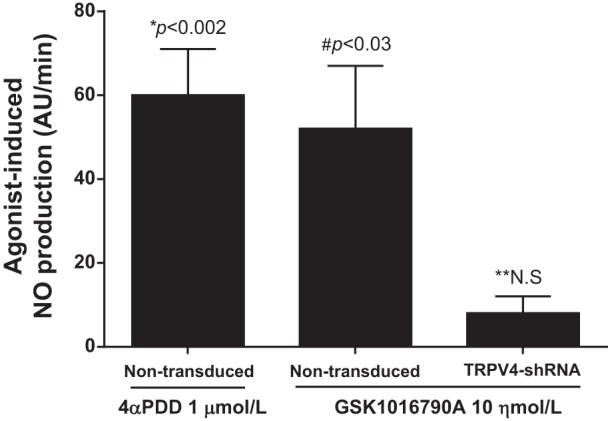
Effect of the TRPV4 agonist 4αPDD and GSK1016790A on NO production by isolated thick ascending limbs in the absence of luminal flow (*P < 0.002 compared with no flow conditions in the same nontransduced thick ascending limb; n = 7). #P < 0.03 compared with no flow conditions in the same nontransduced thick ascending limb; n = 5. **N.S., compared with no flow conditions in the same TRPV4shRNA-transduced thick ascending limb; n = 5.
Since TRPV4 has been shown to be a calcium-permeable channel, we studied the contribution of extracellular calcium on flow-induced NO production. In the absence of luminal flow, NO production was 10 ± 4 AU/min. Increasing luminal flow in the absence of extracellular calcium did not generate an increase in NO production by thick ascending limbs (14 ± 6 AU/min; n = 5). These data suggest that flow-induced NO production depends on extracellular calcium (Fig. 7).
Fig. 7.
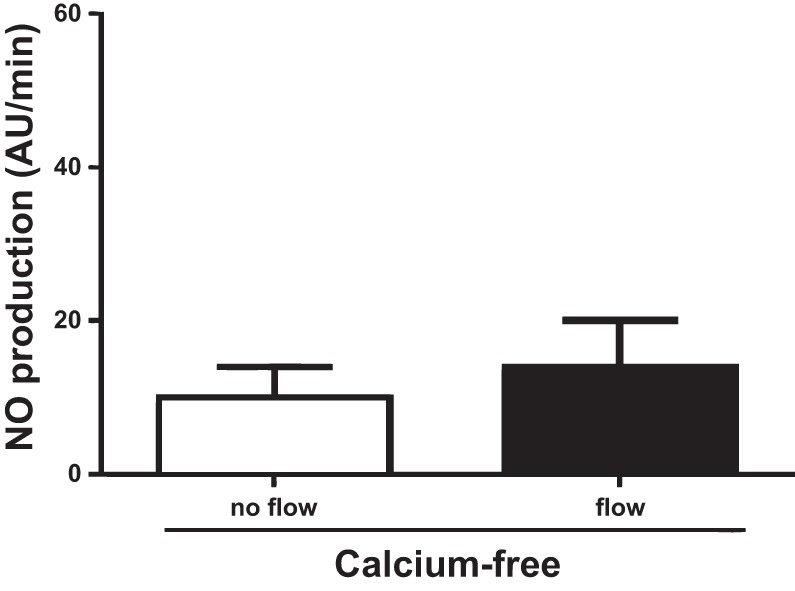
Effect of increasing luminal flow on NO production by isolated thick ascending limbs in the absence of extracellular calcium (n = 5).
DISCUSSION
Similar to our previous reports, we showed that luminal flow stimulates NO production in isolated, perfused thick ascending limbs (10, 11). Here, we first tested the effects of the general TRP antagonist ruthenium red and found that this compound was able to prevent flow-induced NO production. We also studied the effect of RN 1734, a selective TRPV4 antagonist (63). Our results show that RN 1734 also prevented flow-induced NO production. However, the expression of different TRP channels in the thick ascending limbs is not known in detail and RN 1734 has not been widely studied. Additionally, as any other pharmacological agent, the two TRPV4 antagonists used might lack specificity. To circumvent this dilemma, we specifically reduced TRPV4 expression by transducing thick ascending limb cells with a TRPV4shRNA. We found that this maneuver also blunted flow-induced NO production. Finally, we found that flow-induced NO production was prevented in the absence of extracellular calcium.
In a previous report, we found that flow-induced NO production was a NOS 3-dependent mechanism (11). Therefore, we also studied whether transducing thick ascending limbs with a TRPV4shRNA reduced NOS 3 expression. We found that TRPV4shRNA specifically reduced TRPV4 expression by 50% without affecting NOS 3 expression levels. These data necessarily suggest that the ability of a TRPV4shRNA to block flow-induced NO production was mediated by a reduction in TRPV4 expression levels rather than any change in NOS 3 expression.
Although we reduced TRPV4 expression by only 50%, this was sufficient to completely prevent flow-induced NO production. Why this did not result in only a 50% reduction in flow-induced NO is unclear. Possible explanations include: 1) since GSK1016790A failed to stimulate NO production in TRPV4shRNA-transduced thick ascending limbs, it is possible that the reduction of TRPV4 expression correlates with significant reductions in the membrane-targeted pool of the protein assuming this is the physiologically relevant pool; 2) since thick ascending limbs are isolated from the vicinity of the injection site, it is possible that TRPV4 expression in these tubules was reduced more than 50%, as seen before (44); 3) reductions in TRPV4 expression might alter the cytoskeleton structure and organization, which in turn may affect the capacity of epithelial cells to sense mechanical stimulation as shown before (42); and 4) our NO detection system is not sensitive enough to recognize smaller variations in NO production.
If TRPV4 mediates flow-induced NO production, then activation of the channel in the absence of flow should enhance NO synthesis. To test this, we used two separate activators of TRPV4, 4αPDD and GSK1016790A (40, 59, 64). In the absence of luminal flow, both activators stimulated NO production. These data show that activation of TRPV4 is not only necessary to obtain flow-induced NO production, but it is also sufficient.
Although luminal flow has been recognized as a parameter that alters several cellular processes, little is known about the mechanisms that transduce the extracellular mechanical stimuli into intracellular events such as NO production. For kidney epithelial cells, the most likely candidates are two members of the TRP family of cation channels, transient receptor potential polycystin 2 (TRPP2) and TRPV4 (17, 34, 36, 50, 60, 68). Our present results indicate that preventing TRPV4 activation blocks most of the NO production induced by luminal flow, suggesting that TRPV4 might be the only mechanosensitive channel involved in this effect. Nonetheless, it has been suggested that TRPP2, present in the primary cilia, can form heterodimers with TRPV4 to function as a mechanosensitive channel. In this regard, TRPV4 has been suggested to be an essential component of the TRPP2-TRPV4 heterodimer (31, 69). In fact, depletion of TRPV4 abolishes the ability of renal epithelial cells to sense luminal flow (31). Therefore, our results might represent different possibilities: 1) TRPV4 mediates flow-induced NO production by itself; 2) TRPV4-TRPP2 heterodimers mediate flow-induced NO production and TRPV4 blockade prevents the activation of the whole heterodimer as previously suggested; and 3) TRPV4 forms heterodimers with other TRP channels and its inhibition also prevents the activation of these heterodimers.
TRPV4 has been shown to be activated by different mechanical stimuli such as flow-induced shear stress and cellular stretch in several types of cells (54, 58, 68). In the kidney, this channel is expressed along the distal nephron (60). We previously showed that TRPV4 activation mediates hypotonicity-induced cellular stretch (54). In the collecting duct, it mediates flow-induced increases in intracellular calcium as well as flow-dependent potassium secretion (5, 58). Although luminal flow generates different mechanical stimuli such as shear stress, cellular stretch, and/or pressure, we previously demonstrated that shear stress rather than cellular stretch and/or pressure mediates flow-induced NO production in the thick ascending limb (11). In agreement with our results, TRPV4 activation has been also shown to mediate shear stress-induced NO production in endothelial cells (40).
NO plays a pivotal role in regulating blood pressure and kidney function (29, 32). Specifically in the thick ascending limb, NO inhibits sodium chloride reabsorption (43). In this segment, several other autacoids such as endothelin 1 (24), angiotensin II (22), and ATP (53) stimulate the production of NO. Here, we present data indicating that pharmacological stimulation of TRPV4 channels induces NO production in the absence of mechanical stimulation exerted by luminal flow. These results suggest that TRPV4 might be another important regulator of NO production in this segment.
Luminal flow is a physiological phenomenon present in tubular structures lined by a single layer of either epithelial or endothelial cells. Particularly in the nephron, the luminal flow of the forming urine is subjected to change under several circumstances. Similar to previous reports, we tested the effects of increasing luminal flow from 0 to 20 nl/min. These flow rates fall within the physiological range. Late proximal urinary flow rate was previously suggested to be between 7 and 27 nl/min. These flow rates can increase to 40–50 nl/min when animals are subjected to volume expansion treatments, diuretics use, or consumption of a high-salt diet which in turn causes pressure-natriuresis. Additionally, luminal flow might be decreased during dehydration states, ureteral obstruction, and/or during kidney hypoperfusion. Physiologically, urinary flow rate can even be halted or reversed due to peristaltic contractions of the renal pelvis (15, 26, 30, 52).
Mechanical stimulation induced by luminal flow regulates several physiological processes in the tubules of the nephron. In the proximal tubule, luminal flow increases epithelial sodium transport (65). In collecting duct cells, luminal flow increases sodium reabsorption by causing ENaC activation (51), stimulates potassium excretion (66, 67), and induces the release of endothelin 1 (35) and prostaglandin E2 (18), which in turn may further affect sodium transport. We previously showed that in addition to NO, luminal flow can affect other cellular mechanisms such as superoxide production (19, 27) and ATP release in the thick ascending limb (10). All of the molecules regulated by luminal flow participate in other cellular processes such as triggering of intracellular signaling cascades, activation of membrane-targeted receptors, and transport of ions across the epithelium. In this segment, luminal flow stimulates NO production, which in turn inhibits NaCl transport (9, 45). Additionally, luminal flow leads to increased superoxide generation, which in turn stimulates NaCl absorption (55). Furthermore, we recently reported that flow-induced NO reduces flow-induced superoxide production (23). Luminal flow also stimulates ATP release leading to further activation of thick ascending limb purinergic P2Y and P2X receptors (10). It has recently been shown that P2X activation decreases sodium reabsorption in the thick ascending limb (38).
Similar to other molecules regulated by luminal flow of the forming urine, NO plays a crucial role in renal physiology. The NO system is implicated in several mechanisms such as epithelial reabsorption of ions (57, 70), intracellular signaling cascades (3, 24), and oxygenation (1). This is best evidenced by the fact that defects in the NO system lead to hypertension, renal damage, and inflammation (4, 49). Therefore, uncovering how mechanical stimulation generated by luminal flow in nephron segments is transduced to an intracellular event such as NO production is crucial to our understanding of 1) pathophysiological states in which luminal flow is altered and 2) conditions that alter NO production.
GRANTS
This work was supported in part by grants to J. L. Garvin from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL 070985) and to P. D. Cabral from the American Heart Association (11POST7490010 and 13POST16280001).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.D.C. and J.L.G. conception and design of research; P.D.C. performed experiments; P.D.C. and J.L.G. analyzed data; P.D.C. and J.L.G. interpreted results of experiments; P.D.C. prepared figures; P.D.C. drafted manuscript; P.D.C. and J.L.G. edited and revised manuscript; P.D.C. and J.L.G. approved final version of manuscript.
REFERENCES
- 1.Adler S, Huang H. Impaired regulation of renal oxygen consumption in spontaneously hypertensive rats. J Am Soc Nephrol 13: 1788–1794, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Arendshorst WJ, Beierwaltes WH. Renal tubular reabsorption in spontaneously hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 237: F38–F47, 1979 [DOI] [PubMed] [Google Scholar]
- 3.Bachmann S, Mundel P. Nitric oxide in the kidney: synthesis, localization, and function. Am J Kidney Dis 24: 112–129, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Bataineh A, Raij L. Angiotensin II, nitric oxide, and end-organ damage in hypertension. Kidney Int Suppl 68: S14–S19, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Berrout J, Jin M, Mamenko M, Zaika O, Pochynyuk O, O'Neil RG. Function of transient receptor potential cation channel subfamily V member 4 (TRPV4)as a mechanical transducer in flow- sensitive segments of the renal collecting duct system. J Biol Chem 287: 8782–8791, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burg M, Grantham J, Abramow M, Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol 210: 1293–1298, 1966 [DOI] [PubMed] [Google Scholar]
- 7.Burg MB. Thick ascending limb of Henle's loop. Kidney Int 22: 454–464, 1982 [DOI] [PubMed] [Google Scholar]
- 8.Burg MB, Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol 224: 659–668, 1973 [DOI] [PubMed] [Google Scholar]
- 9.Cabral PD, Garvin JL. Luminal flow regulates NO and O2− along the nephron. Am J Physiol Renal Physiol 300: F1047–F1053, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabral PD, Hong NJ, Garvin JL. ATP mediates flow-induced NO production in thick ascending limbs. Am J Physiol Renal Physiol 303: F194–F200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabral PD, Hong NJ, Garvin JL. Shear stress increases nitric oxide production in thick ascending limbs. Am J Physiol Renal Physiol 299: F1185–F1192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chetboul V, Klonjkowski B, Lefebvre HP, Desvaux D, Laroute V, Rosenberg D, Maurey C, Crespeau F, Adam M, Adnot S, Eloit M, Pouchelon JL. Short-term efficiency and safety of gene delivery into canine kidneys. Nephrol Dial Transplant 16: 608–614, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Defraigne JO, Pincemail J, Detry O, Franssen C, Meurisse M, Limet R. Preservation of cortical microcirculation after kidney ischemia-reperfusion: value of an iron chelator. Ann Vasc Surg 8: 457–467, 1994 [DOI] [PubMed] [Google Scholar]
- 14.DiBona GF, Rios LL. Mechanism of exaggerated diuresis in spontaneously hypertensive rats. Am J Physiol 235: 409–416, 1978 [DOI] [PubMed] [Google Scholar]
- 15.Dwyer TM, Schmidt-Nielsen B. The renal pelvis: machinery that concentrates urine in the papilla. News Physiol Sci 18: 1–6, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Eitle E, Hiranyachattada S, Wang H, Harris PJ. Inhibition of proximal tubular fluid absorption by nitric oxide and atrial natriuretic peptide in rat kidney. Am J Physiol Cell Physiol 274: C1075–C1080, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Flores D, Battini L, Gusella GL, Rohatgi R. Fluid shear stress induces renal epithelial gene expression through polycystin-2-dependent trafficking of extracellular regulated kinase. Nephron Physiol 117: p27–p36, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flores D, Liu Y, Liu W, Satlin LM, Rohatgi R. Flow-induced prostaglandin E2 release regulates Na and K transport in the collecting duct. Am J Physiol Renal Physiol 303: F632–F638, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garvin JL, Hong NJ. Cellular stretch increases superoxide production in the thick ascending limb. Hypertension 51: 488–493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Vicente A, Garvin JL. Angiotensin II-induced hypertension increases plasma membrane Na pump activity by enhancing Na entry in rat thick ascending limbs. Am J Physiol Renal Physiol 305: F1306–F1314, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good DW, Knepper MA, Burg MB. Ammonia and bicarbonate transport by thick ascending limb of rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 247: F35–F44, 1984 [DOI] [PubMed] [Google Scholar]
- 22.Herrera M, Garvin JL. Angiotensin II stimulates thick ascending limb NO production via AT2 receptors and Akt1-dependent nitric-oxide synthase 3 (NOS3) activation. J Biol Chem 285: 14932–14940, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrera M, Hong NJ, Garvin J. Flow-induced No suppresses flow-induced O2− in the thick ascending limb in vivo. Hypertension 56: e50–e166, 2010 [Google Scholar]
- 24.Herrera M, Hong NJ, Ortiz PA, Garvin JL. Endothelin-1 inhibits thick ascending limb transport via Akt-stimulated nitric oxide production. J Biol Chem 284: 1454–1460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holstein-Rathlou NH, Marsh DJ. A dynamic model of the tubuloglomerular feedback mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1448–F1459, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Holstein-Rathlou NH, Marsh DJ. Oscillations of tubular pressure, flow, and distal chloride concentration in rats. Am J Physiol Renal Fluid Electrolyte Physiol 256: F1007–F1014, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Hong NJ, Garvin JL. NADPH oxidase 4 mediates flow-induced superoxide production in thick ascending limbs. Am J Physiol Renal Physiol 303: F1151–F1156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Islam CF, Mathie RT, Dinneen MD, Kiely EA, Peters AM, Grace PA. Ischaemia-reperfusion injury in the rat kidney: the effect of preconditioning. Br J Urol 79: 842–847, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Ito S, Carretero OA, Abe K. Role of nitric oxide in the control of glomerular microcirculation. Clin Exp Pharmacol Physiol 24: 578–581, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Khuri RN, Strieder N, Wiederholt M, Giebisch G. Effects of graded solute diuresis on renal tubular sodium transport in the rat. Am J Physiol 228: 1262–1268, 1975 [DOI] [PubMed] [Google Scholar]
- 31.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lahera V, Salom MG, Fiksen-Olsen MJ, Raij L, Romero JC. Effects of NG-monomethyl-l-arginine and l-arginine on acetylcholine renal response. Hypertension 15: 659–663, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Lahera V, Salom MG, Miranda-Guardiola F, Moncada S, Romero JC. Effects of NG-nitro-l-arginine methyl ester on renal function and blood pressure. Am J Physiol Renal Fluid Electrolyte Physiol 261: F1033–F1037, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Li Q, Montalbetti N, Wu Y, Ramos A, Raychowdhury MK, Chen XZ, Cantiello HF. Polycystin-2 cation channel function is under the control of microtubular structures in primary cilia of renal epithelial cells. J Biol Chem 281: 37566–37575, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Lyon-Roberts B, Strait KA, van Peursem E, Kittikulsuth W, Pollock JS, Pollock DM, Kohan DE. Flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol 300: F650–F656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mamenko M, Zaika OL, Boukelmoune N, Berrout J, O'Neil RG, Pochynyuk O. Discrete control of TRPV4 channel function in the distal nephron by protein kinases A and C. J Biol Chem 288: 20306–20314, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manning RD, Jr, Hu L, Tan DY, Meng S. Role of abnormal nitric oxide systems in salt-sensitive hypertension. Am J Hypertens 14: 68S–73S, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Marques RD, de Bruijn PI, Sorensen MV, Bleich M, Praetorius HA, Leipziger J. Basolateral P2X receptors mediate inhibition of NaCl transport in mouse medullary thick ascending limb (mTAL). Am J Physiol Renal Physiol 302: F487–F494, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Massey KJ, Hong NJ, Garvin JL. Angiotensin II stimulates superoxide production in the thick ascending limb by activating NOX4. Am J Physiol Cell Physiol 303: C781–C789, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 298: H466–H476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284: 21257–21264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. Am J Physiol Renal Physiol 287: F274–F280, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Ortiz PA, Hong NJ, Plato CF, Varela M, Garvin JL. An in vivo method for adenovirus-mediated transduction of thick ascending limbs. Kidney Int 63: 1141–1149, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Ortiz PA, Hong NJ, Wang D, Garvin JL. Gene transfer of eNOS to the thick ascending limb of eNOS-KO mice restores the effects of l-arginine on NaCl absorption. Hypertension 42: 674–679, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Plant TD, Strotmann R. TRPV4. Hand Exp Pharmacol 179: 189–205, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol Renal Physiol 276: F159–F163, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Pollock CA, Lawrence JR, Field MJ. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol Renal Fluid Electrolyte Physiol 260: F946–F952, 1991 [DOI] [PubMed] [Google Scholar]
- 49.Pollock JS, Pollock DM. Endothelin, nitric oxide, and reactive oxygen species in diabetic kidney disease. Contrib Nephrol 172: 149–159, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rohatgi R, Battini L, Kim P, Israeli S, Wilson PD, Gusella GL, Satlin LM. Mechanoregulation of intracellular Ca2+ in human autosomal recessive polycystic kidney disease cyst-lining renal epithelial cells. Am J Physiol Renal Physiol 294: F890–F899, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Schmidt-Nielsen B. The renal concentrating mechanism in insects and mammals: a new hypothesis involving hydrostatic pressures. Am J Physiol Regul Integr Comp Physiol 268: R1087–R1100, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Silva G, Beierwaltes WH, Garvin JL. Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension 47: 563–567, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Silva GB, Garvin JL. TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. Am J Physiol Renal Physiol 295: F1090–F1095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension 48: 467–472, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol 6: 89–94, 1995 [DOI] [PubMed] [Google Scholar]
- 57.Stoos BA, Garvin JL. Actions of nitric oxide on renal epithelial transport. Clin Exp Pharmacol Physiol 24: 591–594, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Taniguchi J, Tsuruoka S, Mizuno A, Sato JI, Fujimura A, Suzuki M. TRPV4 as a flow sensor in flow-dependent K+ secretion from the cortical collecting duct. Am J Physiol Renal Physiol 292: F667–F673, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1 -piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity. Part I. J Pharmacol Exp Ther 326: 432–442, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Tian W, Salanova M, Xu H, Lindsley JN, Oyama TT, Anderson S, Bachmann S, Cohen DM. Renal expression of osmotically responsive cation channel TRPV4 is restricted to water-impermeant nephron segments. Am J Physiol Renal Physiol 287: F17–F24, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Toosy N, McMorris EL, Grace PA, Mathie RT. Ischaemic preconditioning protects the rat kidney from reperfusion injury. BJU Int 84: 489–494, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Varela M, Herrera M, Garvin JL. Inhibition of Na/K ATPase in thick ascending limbs by NO depends on O2− and is diminished by high-salt diet. Am J Physiol Renal Physiol 287: F224–F230, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MA. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun 389: 490–494, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277: 13569–13577, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. Am J Physiol Renal Physiol 299: F1220–F1236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol 285: F629–F639, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Wu L, Gao X, Brown RC, Heller S, O'Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol 293: F1699–F1713, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Zhang ZR, Chu WF, Song B, Gooz M, Zhang JN, Yu CJ, Jiang S, Baldys A, Gooz P, Steele S, Owsianik G, Nilius B, Komlosi P, Bell PD. TRPP2 and TRPV4 form an EGF-activated calcium permeable channel at the apical membrane of renal collecting duct cells. PLos One 8: e73424, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou AP, Cowley AW., Jr Role of nitric oxide in the control of renal function and salt sensitivity. Curr Hypertens Rep 1: 178–186, 1999 [DOI] [PubMed] [Google Scholar]