Abstract
Renal medullary function is characterized by corticopapillary concentration gradients of various molecules. One example is the generally decreasing axial gradient in oxygen tension (Po2). Another example, found in animals in the antidiuretic state, is a generally increasing axial solute gradient, consisting mostly of NaCl and urea. This osmolality gradient, which plays a principal role in the urine concentrating mechanism, is generally considered to involve countercurrent multiplication and countercurrent exchange, although the underlying mechanism is not fully understood. Radial oxygen and solute gradients in the transverse dimension of the medullary parenchyma have been hypothesized to occur, although strong experimental evidence in support of these gradients remains lacking. This review considers anatomic features of the renal medulla that may impact the formation and maintenance of oxygen and solute gradients. A better understanding of medullary architecture is essential for more clearly defining the compartment-to-compartment flows taken by fluid and molecules that are important in producing axial and radial gradients. Preferential interactions between nephron and vascular segments provide clues as to how tubular and interstitial oxygen flows contribute to safeguarding active transport pathways in renal function in health and disease.
Keywords: epithelial transport, hypoxia, mathematical modeling, microcirculation, urine concentrating mechanism
Architecture of Medullary Microcirculatory Pathways
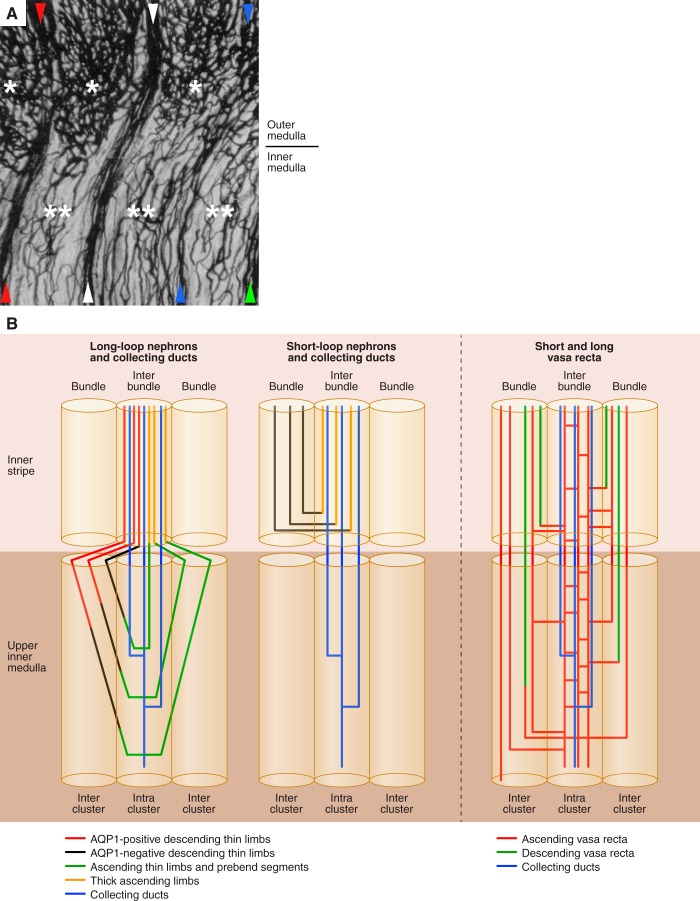
pioneering studies by moffat and Fourman (42) and Rollhauser et al. (59) clearly illustrated that the renal medulla is perfused primarily by 1) descending and ascending vasa recta positioned within vascular bundle regions that carry plasma in a papillary or cortical direction, respectively, and by 2) venous capillary networks that carry plasma chiefly in a cortical direction (Fig. 1). Their studies showed that venous capillary networks of the inner stripe of the outer medulla are positioned within interbundle regions, spatially separate from the vascular bundles, and that these networks are continuous, to some degree, with inner medullary capillary networks. Outer medullary capillary networks are associated with thick ascending limbs, descending limbs of long loops, and collecting ducts, whereas inner medullary capillary networks are associated primarily with collecting ducts and thin ascending limbs. Our understanding of vascular anatomy has been further advanced by others (3, 4, 36, 37, 63).
Fig. 1.
Regional distribution of nephrons and blood vessels of the rat inner stripe of the outer medulla and inner medulla. A: blood vessels at outer medullary-inner medullary border of India ink-injected kidney, formalin-fixed, embedded in celloidin, sectioned, cleared, and mounted; modified with permission from Ref. 42. Vascular bundles are denoted by arrowheads (see text for further details). Capillary networks of the outer medullary interbundle regions (*) and inner medullary intracluster regions (**) are shown. B: schematic diagram of nephrons and blood vessels in medullary regions; modified with permission from Ref. 52. Outer medullary interbundle regions are occupied by collecting ducts (CDs), thick ascending limbs, descending thin limbs of long-loop nephrons, and networks of fenestrated interconnecting capillaries (ascending vasa recta) (2, 27). The inner medullary intracluster regions are occupied by collecting ducts, networks of fenestrated interconnecting capillaries (ascending vasa recta), ascending thin limbs, and to a lesser extent aquaporin-1-null descending thin limbs (19, 55, 74). Unbranched ascending vasa recta residing in the medullary interbundle and intracluster regions (27) are not shown in B.
The blood vessels that perfuse the rat renal medulla arise from efferent arterioles of midcortical and juxtamedullary glomeruli. After entering the outer stripe, each efferent arteriole continues as a descending vas rectum, with each vas rectum breaking up into as many as 30 descending vasa recta (DVR) (22). The deeper the originating glomerulus lies below the cortical surface, the deeper its descendant DVR extend into the medulla (14). As the DVR descend through the outer stripe and into the inner stripe of the outer medulla, a number of these DVR assemble to form vascular bundles that include AVR arising from deeper levels. These tightly organized bundles exist in the inner stripe of the outer medulla and the initial third of the inner medulla. At deeper inner medullary levels, these vessels fan out from each other, becoming diffusely distributed throughout the papillary interstitium (58, 74). Vessels within vascular bundles of rat and mouse undergo little or no branching (58, 74).
Within the outer medullary bundles and alongside the DVR and AVR lie descending limbs of short-loop nephrons, positioned either at the bundle periphery (rat) or positioned more centrally within the bundle (mouse; Psammomys) (2). The outer medullary vascular bundles are arranged with the shortest DVR (those breaking up into capillaries in the outer medulla) positioned at the periphery of the bundle (Fig. 1). Long DVR that terminate in the inner medulla lie near to the central core of the bundle (2). This separation of short and long DVR within each bundle indicates that preferential radial flows potentially occur even within vascular bundles; consequently, vascular shunts may lead to targeted delivery of plasma and red blood cells, which could significantly impact medullary oxygenation (see below) (11).
The longest DVR remain within the bundle at the outer medullary-inner medullary border. These long DVR continue to descend through the upper inner medulla within vascular bundles that become more loosely organized with increasing depth. The DVR and adjacent aquaporin-1 (AQP1)-positive descending thin limbs within the bundles are positioned in a way that would facilitate entry of reabsorbed water into the unbranched AVR and at the same time engage in countercurrent exchange of urea (see below). Short terminal portions of inner medullary DVR with continuous endothelia or that express the urea transporter UTB commonly become fenestrated and continue to descend before the vessel terminates in the venous capillary network, or before the vessel begins to ascend, as shown for the rat by ultrastructural studies (22, 63), functional studies (78), and by coexpression of UTB and plasmalemmal vesicle protein 1 (PV1) (55, 74). The overlap of UTB and PV-1 expression suggests that the fenestrations in the terminal portion of the DVR within the upper inner medulla may be insufficient for urea transport across the capillary wall without the presence of UTB (55).
As the DVR descend, they terminate at all levels of the outer and inner medulla, leading to a declining total DVR population at progressively deeper levels below the corticomedullary border. Each DVR typically terminates as a single junction with one fenestrated capillary (or less commonly, as a bilateral junction with several fenestrated capillaries) that continues in an ascending direction and undergoes extensive branching (23, 55, 74), so that DVR, collectively, feed into a complex network of capillaries (Fig. 1) (42, 59). Outer medullary capillary networks arise chiefly from DVR terminating in the outer medulla, and inner medullary capillary networks arise from DVR terminating in the inner medulla. The capillary network of the inner stripe is denser than the capillary networks of the outer stripe and inner medulla (Fig. 1).
In general, inner medullary ascending (venous) capillaries of inner medullary networks ascend some distance, then coalesce at regular intervals along the inner medullary axis to form AVR; however, capillaries of inner medullary intracluster networks lying near the outer medullary-inner medullary border ascend directly into the inner stripe, where they merge with the capillary network of the interbundle region. Consequently, only after passing from the inner medullary intracluster region directly into the outer medullary interbundle region do these capillaries coalesce to form AVR (Fig. 1) (23, 28, 42, 59). These capillaries may represent deeper extensions of interbundle capillary networks previously reported to lie in the inner part of the inner stripe and that link to AVR within the vascular bundles of the innermost outer medulla (27, 72). Capillary networks of the inner part of the inner stripe are thought to be spatially distinct from the capillary networks of the outer part of the inner stripe and the outer stripe, the latter ascending directly among the tubules in the interbundle region and linking to arcuate or interlobular veins (27, 72). The two capillary drainage patterns in the outer medulla suggest two defined regional blood flow patterns may exist (Fig. 1) (27, 72).
Specialized Compartments in the Inner Medulla
In the upper inner medulla of rodent kidneys such as rat and kangaroo rat, about three to five interconnecting fenestrated capillaries abut each collecting duct along its length, forming interstitial channels along the collecting duct axis. Ascending thin limbs combine with these segments to form well-defined interstitial compartments within the intracluster region, the so-called “interstitial nodal spaces” (21, 55, 56). Based on the preferential arrangements of collecting ducts (which reabsorb urea and water) and ascending thin limbs and prebend segments (which reabsorb NaCl but not water), we hypothesized that interstitial nodal spaces (and the intracluster region more generally) might be specialized sites for NaCl and urea mixing in the antidiuretic rat (30, 32, 53, 56).
A mathematical model of the inner medullary urine concentrating mechanism has suggested that NaCl and urea could be sequestered within the intracluster region (30). However, others have argued that solute diffusion between the intracluster and intercluster tubules and vessels may be sufficiently large to prevent establishment of significant lateral NaCl and urea concentration gradients (69). Because the interstitial and luminal O2 concentrations are orders of magnitude lower than NaCl and urea, and because collecting ducts may have a high O2 consumption rate, a substantial lateral oxygen tension (Po2) gradient may be generated, such that Po2 is significantly higher in the intercluster regions than in the intracluster regions. By sequestering O2 within the intercluster regions, O2 delivery to the deep papilla may be preserved.
Countercurrent Exchange Pathways
Examples of countercurrent exchange can be found throughout the medulla: NaCl and urea are generally shunted from AVR to DVR in the process of countercurrent exchange, whereas O2 is shunted from DVR to AVR. These exchanges delay or prevent loss of solutes from the inner medulla and delay or reduce O2 delivery to the inner medulla. A number of spatial relationships have long been considered to underlie preferential urea countercurrent flows between tubular structures of the outer and inner medulla based on detailed descriptions in the following references (16, 22, 25, 31, 33, 37, 45, 49, 52, 56, 60, 73). NaCl and O2 gradients would also lead to spatially dependent NaCl and O2 countercurrent flows.
Throughout the outer medulla and most of the first half of the inner medulla, all DVR express detectable levels of the urea transporter protein UT-B as determined with immunohistochemistry; however, expression levels are significantly reduced at deeper levels of the inner medulla (23, 24, 54, 74). High transendothelial urea permeability in outer medullary DVR and low transendothelial urea permeability in the papillary DVR of the rat (47, 50, 51) correlate with these UTB expression patterns, providing support for UTB as the predominant pathway for urea flux in DVR. Alternate pathways for urea flux in DVR include the paracellular pathway and/or fenestrations, and these are also likely pathways for NaCl flux (47, 50, 51).
Transendothelial water flux in DVR occurs through the water channel AQP1, which is coexpressed with UT-B within most but not all of these segments (55). Water flux occurs through at least one additional pathway, which has not been identified (48). Water reabsorbed from the outer medullary DVR flows into AVR, thereby providing a shunt pathway for volume efflux, a process that potentially concentrates both plasma solutes and blood cells in DVR and reduces the fluid load that enters the inner medulla. All medullary AVR and capillaries are fenestrated and express PV-1, a protein associated with the fenestral diaphragm (38, 54, 64). Most vessels (both descending and ascending) in the deep papilla (∼2 mm above the papilla tip) are fenestrated (54, 57), with the fenestration fraction increasing with medullary depth (38), although vessels with continuous endothelium do exist in the terminal papilla (63). Fenestrations impart a very high transendothelial permeability to water and small solutes, but not to high-molecular-weight solutes and proteins. In particular in the deep papilla, fenestrations may facilitate the diffusion of oxygen from the DVR plasma into the interstitium.
Throughout the medulla, the DVR and AVR lying within the bundle region (in the outer medulla) or the intercluster region (in the inner medulla) appear to be arranged sufficiently close together to promote effective solute and O2 countercurrent exchange between them (Fig. 1). UTB-mediated countercurrent exchange of urea between descending and ascending vessels lying near to each other should be considered a feature primarily of the bundle zone in the outer medulla and the intercluster region through approximately the upper ∼50% of the inner medulla (7, 38, 55, 63, 74). Any significant degree of solute recycling between descending and ascending vessels within ∼2 mm above the papilla tip likely involves primarily fenestrated vessels. The disproportionately large fraction of fenestrated descending and ascending vessels in the deep papilla combined with unhindered Na and urea diffusion would account for the nearly equivalent Na and urea transendothelial permeabilities (studied with in vivo microinfusion), reported by Pallone et al. (49, 51) for DVR and AVR (vessels distinguished from each other by the direction of plasma flow) at the papillary tip of the adult female Munich-Wistar rat.
The proximity of AVR to the descending limbs of short-loop nephrons in the outer medullary vascular bundles suggests that countercurrent solute exchange could occur between these segments (58). A similar configuration is found in the upper inner medulla, where the vasa recta and the descending limbs are found in the intercluster region. Despite their proximity, water exchange may be limited: the descending segments of short-loop nephrons of rats, mice, and humans express little or no AQP1 (68, 76); similarly, in most of the terminal inner medullary descending limb segments of the rat and chinchilla, AQP1 protein expression is relatively low (Fig. 1) and transepithelial osmotic water permeability is nearly zero (12, 13, 45, 54).
In rats and mice, the terminal inner stripe segment of short descending limbs, and the initial inner medullary segments of some long descending limbs, express the urea transporter UT-A2 (68). The traditional assumption is that some of the urea ascending from the inner medulla via the AVR is secreted into the UT-A2-positive segment of the short descending limb. If true, deletion of UT-A2 should induce a significant urine concentrating defect. However, that concentrating defect was not observed in UT-A2 knockout mice (67) nor in UT-A2/UT-B double knockout mice (35). What is particularly surprising is that, instead of further aggravating the urine concentrating mechanism, the abolition of UT-A2 permeability in the UT-A2/UT-B double knockout mice restored a nearly normal urine osmolality, which is severely impaired in the UT-B knockout mice (35). An alternative hypothesis is that urea is reabsorbed from, rather than secreted into the UT-A2 segment, a scenario that is likely if urea is actively secreted into the pars recta (73). If so, UT-A2 may play a role in the transient build-up of a urea and osmolality gradient in the inner medulla, rather than the generation of steady-state gradients. This hypothesis, in brief, is based on the observation that for the urea in the short descending limb to return to the inner medulla, the transit time required for the UT-A2-mediated reabsorption and the course through the DVR is shorter than the transit time through the thick ascending limb and collecting duct (for a more complete discussion, see the study by Layton and Bankir) (29).
Total vascular outflow from the rat inner medulla exceeds vascular inflow by ∼30%, the excess outflow representing fluid taken up from descending tubular segments (78). Despite the excess fluid, AVR flow is likely slower than DVR flow, because AVR exceed DVR by about twofold in the rat inner medulla, as determined by functional analyses (5, 39, 78) and by reconstruction of unbranched vessels lying in the bundle region (74). A slower AVR flow would provide a longer time for AVR to equilibrate with local interstitial fluid. As a result, in a concentrating kidney, AVR fluid lags local interstitial fluid osmolality by only a small amount, thereby minimizing any diluting effect and improving countercurrent exchanger efficiency (39). In contrast, there is an AVR/DVR ratio of about four for the anatomic ratio of all fenestrated and nonfenestrated vessels (determined at 2 mm above the papilla tip) in the Munich-Wistar rat (20, 74). The additional fenestrated vessels include interconnecting capillaries in addition to the AVR determined by functional studies. Interconnecting capillaries lie distant from DVR and are unlikely to directly participate in countercurrent exchange with DVR. However, the interconnecting capillaries, which run vertically and laterally, form the interstitial nodal spaces along with collecting ducts and ascending thin limbs and carry absorbate from these segments into the nonbranching AVR in bundle regions. The degree to which interconnecting capillaries join AVR and contribute to their flow has not been quantified for the rat medulla but has been shown to be minimal in the mouse (58).
Medullary Metabolic Activity and Oxygenation
In the mammalian kidney, despite high blood flow and oxygen delivery, Po2 is relatively low, especially in the renal medulla, which has been measured to be ∼20 and 10 mmHg in the outer and inner medulla, respectively (46). That marked discrepancy between blood supply and oxygenation can be attributed, in large part, to the kidney's high oxygen consumption per tissue weight. The Na-K-ATPase (the sodium pump), the protein complex that drives Na reabsorption, singularly consumes the greatest proportion of energy in the kidney. Na reabsorptive processes expend ∼90% of total renal oxygen; ∼30% of renal Na transport and 30% of renal oxygen consumption are consumed in the outer medulla (41). Renal ATP production occurs chiefly through aerobic metabolism; however, under anaerobic conditions such as has been predicted for the inner medulla, metabolism could consist substantially of anaerobic glycolysis (1, 34). Metabolic activity may be rate limiting for transepithelial solute transport, and this transport itself may, in turn, regulate metabolic activity. The processes by which oxygen is delivered to renal transport epithelia may increase oxygen demand, the direct implication being that renal metabolic autoregulation itself creates challenges with balancing hypoxia and hyperoxia. A host of control mechanisms and signaling pathways are involved in maintaining this balance (44). Due to the complexities involved, systems analyses of blood flow pathways and mathematical models of the multiple functional inputs to these processes are useful for understanding oxygen requirements and metabolism associated with fluid and solute transport in the renal medulla.
Because of the significant distance between the O2-carrying outer medullary DVR, which lie in the bundle regions, and the thick ascending limbs, which lie in interbundle regions and because of the thick limbs' high metabolic requirements, Po2 in the thick ascending limbs near the outer-inner medullary boundary is very low (<10 mmHg). This contrasts with Po2 measured in renal veins (50 mmHg), efferent arterioles (45 mmHg), and proximal and distal tubules (40 mmHg) (71). Hypoxia-induced necrosis of cells lining thick limbs and long-loop descending limbs (but not short-loop descending limbs) gave functional evidence for corticopapillary and transverse Po2 gradients in the rat outer medulla (6, 26, 62). Thick ascending limbs at the periphery of vascular bundles escape necrosis, and, at least in the mouse kidney, these are predominantly long-loop thick ascending limbs (58). Po2 gradients along the corticopapillary axis and projecting radially from the vascular bundles are predicted by a mathematical model of oxygen transport that was based on the complex structural organization of the outer medulla (10, 11). The low Po2 surrounding the thick limbs may be partially alleviated by the Bohr effect, which states that a lower pH reduces the binding affinity of hemoglobin for O2. Burke et al. (8) observed an increase in pH, from 7.20 to 7.31, after addition of furosemide, a finding that suggests that the interstitial fluid surrounding the thick ascending limbs may have a lower pH than that in the vascular bundles. The presumed greater acidity of blood in the interbundle region where the thick limbs are located, relative to that in the vascular bundles, may facilitate the release of O2 (9).
Because ∼80% of the DVR do not pass into the inner medulla, ∼80% of the O2 supplied to the outer medulla does not perfuse the inner medulla. Mathematical models suggest that, owing to the separation of the DVR from the thick ascending limbs, most (>90%) of the O2 supplied to those deep-reaching DVR at the corticomedullary junction reaches the inner medulla (9, 77). The functional role of the vascular bundles of preserving oxygen delivery to the deep inner medulla is underscored in a recent modeling study (17). The authors conducted a simulation of a hypothetical configuration in which nephrons and vessels are more homogeneously distributed. In particular, the separation between the DVR and thick limbs are much reduced. That simulation predicted a drastic decrease in oxygen delivery to the inner medulla, with the terminal inner medulla almost deprived of oxygen (17).
The vascular structural organization of the upper inner medulla has an analogous impact on oxygenation of that region. While the thin limbs do not have significant active transport (40, 43), functional and modeling studies support a brisk active NaCl reabsorption in the inner medullary collecting duct, (18, 70). A radial oxygen gradient across the inner medullary interstitium has been hypothesized on the basis of permeability and flux values estimated for transport in the inner medullary collecting duct (69). A substantial radial oxygen gradient has been predicted in a mathematical model of medullary oxygen transport (17).
The ratio of Na transport and O2 consumption is relatively low in the medullary collecting duct compared with other nephron segments. Together with the low medullary Po2, anaerobic glycolysis may be important in the collecting duct, as suggested by findings in several studies. When aerobic ATP production is directly inhibited, outer medullary collecting duct cells in the mouse maintain ATP levels that are ∼80% of the control (i.e., the case when the cells are not inhibited) (66), and when Na-K-ATPase is directly inhibited in the rabbit, O2 consumption levels are maintained nearly equal to noninhibited levels of 30–40% (75). In the rat inner medullary collecting duct, cellular ATP levels have been found to reach 40% of controls when oxygen metabolism is inhibited with rotenone (65).
Conclusions and Future Directions
The architecture of the medullary circulatory system is commonly interpreted in terms of how it aids in preserving the corticopapillary solute gradient. This design conflicts with the ability of capillaries to provide oxygen to medullary structures at concentrations that equal those known to exist in the renal cortex. Improved understanding of medullary blood flow will aid in better understanding disorders associated with fluid and solute imbalances. Integrative studies on regional perfusion in vivo are essential for understanding inner medullary blood flow and its regulation, and the impact on various diseases and disorders.
We conclude with an example that illustrates the synergy between model simulations and experimental studies. A recent modeling study by Edwards et al. (15) considers the effect of Na-K-2Cl cotransporter (NKCC2) isoform regulation on NaCl transport in the thick ascending limb and macula densa. Their results suggest that modulation of differential splicing of NKCC2 by a low-salt diet, which induces a shift of NKCC2-A to the B isoform (61) primarily in the thick ascending limb and macula densa cells, significantly enhances NaCl reabsorption along the thick ascending limb. Simulation results also suggest that the isoform shift hyperpolarizes the macula densa basolateral cell membrane, which, taken in isolation, may inhibit the tubuloglomerular feedback signal. However, excessive early distal salt delivery and thus renal salt loss during a low-salt diet may be prevented by an asymmetric tubuloglomerular feedback response, which may be more sensitive to flow increases. This is but one example where mathematical models have provided insights into the mechanism behind disorders and generated ideas for improving outcomes of procedures. A multidisciplinary approach that combines experimental and modeling techniques may have the best chance at attaining a true understanding of renal function and metabolism under physiological and pathophysiological conditions (e.g., hypertension and diabetes).
GRANTS
Research in the authors' laboratories has been supported by the National Science Foundation, IOS-0952885 (T. Pannabecker) and DMS-0340654 (A. Layton), and by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-08333 (T. Pannabecker) and DK-89066 (A. Layton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.L.P. and A.T.L. prepared figures; T.L.P. and A.T.L. drafted manuscript; T.L.P. and A.T.L. edited and revised manuscript; T.L.P. and A.T.L. approved final version of manuscript.
REFERENCES
- 1.Bagnasco S, Good D, Balaban R, Burg M. Lactate production in isolated segments of the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 248: F522–F526, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Bankir L, De Rouffignac C. Urinary concentrating ability: insights from comparative anatomy. Am J Physiol Regul Integr Comp Physiol 249: R643–R666, 1985 [DOI] [PubMed] [Google Scholar]
- 3.Bankir L, Kaissling B, Rouffignac CD, Kriz W. Vascular organization of the kidney of Psammomys obesus. Anat Embryol (Berl) 155: 149–160, 1979 [DOI] [PubMed] [Google Scholar]
- 4.Beeuwkes R, III, Bonventre JV. Tubular organization and vascular-tubular relations in the dog kidney. Am J Physiol 229: 695–713, 1975 [DOI] [PubMed] [Google Scholar]
- 5.Bottcher W, Steinhausen M. Microcirculation of the renal papilla of rats under control conditions and after temporary ischemia. Kidney Int 10: S74–S80, 1976 [PubMed] [Google Scholar]
- 6.Brezis M, Rosen S, Spokes K, Silva P, Epstein FH. Transport-dependent anoxic cell injury in the isolated perfused rat kidney. Am J Pathol 116: 327–341, 1984 [PMC free article] [PubMed] [Google Scholar]
- 7.Bulger RE, Trump BF. Fine structure of the rat renal papilla. Am J Anat 118: 685–722, 1966 [DOI] [PubMed] [Google Scholar]
- 8.Burke TJ, Malhotra D, Shapiro JI. Factors maintaining a pH gradient within the kidney: role of the vasculature architecture. Kidney Int 56: 1826–1837, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Edwards A, Layton AT. Effects of pH and medullary blood flow on oxygen transport and sodium reabsorption in the rat outer medulla. Am J Physiol Renal Physiol 298: F1369–F1383, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Edwards A, Layton AT. A mathematical model of O2 transport in the rat outer medulla. II. Impact of outer medullary architecture. Am J Physiol Renal Physiol 297: F537–F548, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Layton AT, Edwards A. A mathematical model of oxygen transport in the rat outer medulla, I. Model formulation and baseline results. Am J Physiol Renal Physiol 297: F537–F548, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou CL, Knepper MA. In vitro perfusion of chinchilla thin limb segments: segmentation and osmotic water permeability. Am J Physiol Renal Fluid Electrolyte Physiol 263: F417–F426, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Chou CL, Nielsen S, Knepper MA. Structural-functional correlation in chinchilla long loop of Henle thin limbs: a novel papillary subsegment. Am J Physiol Renal Fluid Electrolyte Physiol 265: F863–F874, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Christensen EI, Grann B, Kristoffersen IB, Skriver E, Thomsen JS, Andreasen A. Three-dimensional reconstruction of the rat nephron. Am J Physiol Renal Physiol 306: F664–F671, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Edwards A, Castrop H, Laghmani K, Vallon V, Layton AT. Effects of NKCC2 isoform regulation on NaCl transport in thick ascending limb and macula densa: a modeling study. Am J Physiol Renal Physiol 307: F137–F146, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87: 1083–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Fry BC, Edwards A, Sgouralis I, Layton AT. Impact of renal medullary three-dimensional architecture on oxygen transport. Am J Physiol Renal Physiol 307: F263–F272, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg LC, Mackie S, Tisher CC. Effect of low potassium-diet on Na-K-ATPase in rat nephron segments. Pflügers Arch 394: 113–117, 1982 [DOI] [PubMed] [Google Scholar]
- 19.Gilbert RL, Pannabecker TL. Architecture of interstitial nodal spaces in the rodent renal inner medulla. Am J Physiol Renal Physiol 305: F745–F752, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holliger C, Lemley K, Schmitt C, Thomas F, Robertson C, Jamison RL. Direct determination of vasa recta blood flow in the rat renal papilla. Circ Res 53: 401–413, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Issaian T, Urity VB, Dantzler WH, Pannabecker TL. Architecture of vasa recta in the renal inner medulla of the desert rodent Dipodomys merriami: potential impact on the urine concentrating mechanism. Am J Physiol Regul Integr Comp Physiol 302: R748–R756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamison RL, Kriz W. Urinary Concentrating Mechanism. New York: Oxford University Press, 1982 [Google Scholar]
- 23.Kim J, Pannabecker TL. Two-compartment model of inner medullary vasculature supports dual modes of vasopressin-regulated inner medullary blood flow. Am J Physiol Renal Physiol 299: F273–F279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YH, Kim DU, Han KH, Jung JY, Sands JM, Knepper MA, Madsen KM, Kim J. Expression of urea transporters in the developing rat kidney. Am J Physiol Renal Physiol 282: F530–F540, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Knepper MA, Roch-Ramel F. Pathways of urea transport in the mammalian kidney. Kidney Int 31: 629–633, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Kopolovic J, Brezis M, Spokes K, Silva P, Epstein F, Rosen S. The vulnerability of the thin descending limbs of Henle's loop in the isolated perfused rat kidney. Am J Kidney Dis 14: 25–30, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Kriz W. Structural organization of the renal medulla: comparative and functional aspects. Am J Physiol Regul Integr Comp Physiol 241: R3–R16, 1981 [DOI] [PubMed] [Google Scholar]
- 28.Kriz W, Lever AF. Renal countercurrent mechanisms: structure and function. Am Heart J 78: 101–118, 1969 [DOI] [PubMed] [Google Scholar]
- 29.Layton AT, Bankir L. Impacts of active urea secretion into pars recta on urine concentration and urea excretion rate. Physiol Rep 1: e00034, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Layton AT, Gilbert RL, Pannabecker TL. Isolated interstitial nodal spaces may facilitate preferential solute and fluid mixing in the rat renal inner medulla. Am J Physiol Renal Physiol 302: F830–F839, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Layton AT, Layton HE. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol 289: F1346–F1366, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Layton AT, Layton HE, Dantzler WH, Pannabecker TL. The mammalian urine concentrating mechanism: hypotheses and uncertainties. Physiology 24: 250–256, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Functional implications of the three-dimensional architecture of the rat renal inner medulla. Am J Physiol Renal Physiol 298: F973–F987, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JB, Vance VK, Cahill GF., Jr Metabolism of C14-labeled substrates by rabbit kidney cortex and medulla. Am J Physiol 203: 27–36, 1962 [DOI] [PubMed] [Google Scholar]
- 35.Lei T, Zhou L, Layton AT, Zhou H, Zhao X, Bankir L, Yang B. Role of thin descending limb urea transport in renal urea handling and the urine concentrating mechanism. Am J Physiol Renal Physiol 301: F1251–F1259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemley KV, Kriz W. Anatomy of the renal interstitium. Kidney Int 39: 370–381, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Lemley KV, Kriz W. Cycles and separations: the histotopography of the urinary concentrating process. Kidney Int 31: 538–548, 1987 [DOI] [PubMed] [Google Scholar]
- 38.MacPhee PJ. Fluid uptake by the renal medullary vasa recta: An estimate based on a quantitative analysis of the distribution of fenestrae in the vasa recta of young Sprague-Dawley rats. Exp Physiol 83: 23–34, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Marsh DJ, Segel LA. Analysis of countercurrent diffusion exchange in blood vessels of the renal medulla. Am J Physiol 221: 817–828, 1971 [DOI] [PubMed] [Google Scholar]
- 40.Marsh DJ, Solomon S. Analysis of electrolyte movement in thin Henle's loops of hamster papilla. Am J Physiol 208: 1119–1128, 1965 [DOI] [PubMed] [Google Scholar]
- 41.McDonough AA, Thomson SC. Metabolic basis of solute transport. In: Brenner and Rector's The Kidney (9th ed.), edited by Taal MW, Chertow GM, Marsden PA, Skorecki KL, Yu ASL, Brenner BM. Philadelphia, PA: Elsevier, 2012, p. 138–157 [Google Scholar]
- 42.Moffat DB, Fourman J. The vascular pattern of the rat kidney. J Anat 97: 543–553, 1963 [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan T, Berliner RW. Permeability of the loop of Henle, vasa recta, and collecting duct to water, urea, and sodium. Am J Physiol 215: 108–115, 1968 [DOI] [PubMed] [Google Scholar]
- 44.Navar LG. Integrating multiple paracrine regulators of renal microvascular dynamics. Am J Physiol Renal Physiol 274: F433–F444, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Nawata CM, Evans KK, Dantzler WH, Pannabecker TL. Transepithelial water and urea permeabilities of isolated perfused Munich-Wistar rat inner medullary thin limbs of Henle's loop. Am J Physiol Renal Physiol 306: F123–F129, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neuhofer W, Beck FX. Cell survival in the hostile environment of the renal medulla. Annu Rev Physiol 67: 531–555, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Pallone TL. Characterization of the urea transporter in outer medullary descending vasa recta. Am J Physiol Regul Integr Comp Physiol 267: R260–R267, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Pallone TL, Edwards A, Ma T, Silldorff EP, Verkman AS. Requirement of aquaporin-1 for NaCl-driven water transport across descending vasa recta. J Clin Invest 105: 215–222, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pallone TL, Turner MR, Edwards A, Jamison RL. Countercurrent exchange in the renal medulla. Am J Physiol Regul Integr Comp Physiol 284: R1153–R1175, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Pallone TL, Nielsen S, Silldorff EP, Yang S. Diffusive transport of solute in the rat medullary microcirculation. Am J Physiol Renal Fluid Electrolyte Physiol 269: F55–F63, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Pallone TL, Work J, Myers RL, Jamison RL. Transport of sodium and urea in outer medullary descending vasa recta. J Clin Invest 93: 212–222, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pannabecker TL. Comparative physiology and architecture associated with the mammalian urine concentrating mechanism: role of inner medullary water and urea transport pathways in the rodent medulla. Am J Physiol Regul Integr Comp Physiol 304: R488–R503, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pannabecker TL. Structure and function of the thin limbs of the loops of Henle. In: Comprehensive Physiology, edited by Terjung RL. Bethesda, MD: Wiley, 2012, p. 2063–2086 [DOI] [PubMed] [Google Scholar]
- 54.Pannabecker TL, Dantzler WH. Three-dimensional architecture of collecting ducts, loops of Henle, and blood vessels in the renal papilla. Am J Physiol Renal Physiol 293: F696–F704, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Pannabecker TL, Dantzler WH. Three-dimensional architecture of inner medullary vasa recta. Am J Physiol Renal Physiol 290: F1355–F1366, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Pannabecker TL, Dantzler WH, Layton HE, Layton AT. Role of three-dimensional architecture in the urine concentrating mechanism of the rat renal inner medulla. Am J Physiol Renal Physiol 295: F1271–F1285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pannabecker TL, Henderson C, Dantzler WH. Quantitative analysis of functional reconstructions reveals lateral and axial zonation in the renal inner medulla. Am J Physiol Renal Physiol 294: F1306–F1314, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Ren H, Gu L, Andreasen A, Thomsen JS, Cao L, Christensen EI, Zhai XY. Spatial organization of the vascular bundle and the interbundle region: three-dimensional reconstruction at the inner stripe of the outer medulla in the mouse kidney. Am J Physiol Renal Physiol 306: F321–F326, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Rollhauser H, Kriz W, Heinke W. Das gefass-system der rattenniere. Z Zellforsch Mikrosk Anat 64: 381–403, 1964 [PubMed] [Google Scholar]
- 60.Sands JM, Layton HE, Fenton RA. Urine concentration and dilution. In: Brenner and Rector's The Kidney (9th ed.), edited by Taal MW, Chertow GM, Marsden PA, Skorecki KL, Yu ASL, Brenner BM. Philadelphia, PA: Elsevier, 2012, p. 326–352 [Google Scholar]
- 61.Schiessl IM, Rosenauer A, Kattler V, Minuth WW, Oppermann M, Castrop H. Dietary salt intake modulates differential splicing of the Na-K-2Cl cotransporter NKCC2. Am J Physiol Renal Physiol 305: F1139–F1148, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Schurek HJ, Kriz W. Morphologic and functional evidence for oxygen deficiency in the isolated perfused rat kidney. Lab Invest 53: 145–155, 1985 [PubMed] [Google Scholar]
- 63.Schwartz MM, Karnovsky MJ, Venkatachalam M. Ultrastructural differences between rat inner medullary descending and ascending vasa recta. Lab Invest 35: 161–170, 1976 [PubMed] [Google Scholar]
- 64.Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci USA 96: 13203–13207, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stokes JB, Grupp C, Kinne RKH. Purification of rat papillary collecting duct cells: functional and metabolic assessment. Am J Physiol Renal Fluid Electrolyte Physiol 253: F251–F262, 1987 [DOI] [PubMed] [Google Scholar]
- 66.Uchida S, Endou H. Substrate specificity to maintain cellular ATP along the mouse nephron. Am J Physiol Renal Fluid Electrolyte Physiol 255: F977–F983, 1988 [DOI] [PubMed] [Google Scholar]
- 67.Uchida S, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki M. Impaired urea accumulation in the inner medulla of mice lacking the urea transporter UT-A2. Mol Cell Biol 25: 7357–7363, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wade JB, Lee AJ, Liu C, Ecelbarger C, Mitchell C, Bradford AD, Terris J, Kim GH, Knepper MA. UT-A2: a 55-kDa urea transporter in thin descending limb whose abundance is regulated by vasopressin. Am J Physiol Renal Physiol 278: F52–F62, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Weinstein AM. Identifying renal medullary neighborhoods—when do distances matter? Am J Physiol Renal Physiol 304: F1411–F1412, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weinstein AM. A mathematical model of the inner medullary collecting duct of the rat: pathways for Na and K transport. Am J Physiol Renal Physiol 274: F841–F855, 1998 [DOI] [PubMed] [Google Scholar]
- 71.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int 59: 230–237, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto K, Wilson DR, Baumal R. Blood supply and drainage of the outer medulla of the rat kidney: scanning electron microscopy of microvascular casts. Anat Rec 210: 273–277, 1984 [DOI] [PubMed] [Google Scholar]
- 73.Yang B, Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Renal Physiol 288: F881–F896, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Yuan J, Pannabecker TL. Architecture of inner medullary descending and ascending vasa recta: pathways for countercurrent exchange. Am J Physiol Renal Physiol 299: F265–F272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeidel ML, Silva P, Seifter JL. Intracellular pH regulation and proton transport by rabbit renal medullary collecting duct cells. Role of plasma membrane proton adenosine triphosphatase. J Clin Invest 77: 113–120, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhai XY, Fenton RA, Andreasen A, Thomsen JS, Christensen EI. Aquaporin-1 is not expressed in descending thin limbs of short-loop nephrons. J Am Soc Nephrol 18: 2937–2944, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Zhang W, Edwards A. Oxygen transport across vasa recta in the renal medulla. Am J Physiol Heart Circ Physiol 283: H1042–H1055, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Zimmerhackl B, Robertson CR, Jamison RL. Fluid uptake in the renal papilla by vasa recta estimated by two methods simultaneously. Am J Physiol Renal Fluid Electrolyte Physiol 248: F347–F353, 1985 [DOI] [PubMed] [Google Scholar]