Abstract
Sestrin 2, initially identified as a p53 target protein, accumulates in cells exposed to stress and inhibits mammalian target of rapamycin (mTOR) signaling. In normal rat kidneys, sestrin 2 was selectively expressed in parietal epithelial cells (PECs), identified by the marker protein gene product 9.5. In adriamycin nephropathy, sestrin 2 expression decreased in PECs on day 14, together with increased expression of phosphorylated S6 ribosomal protein (P-S6RP), a downstream target of mTOR. Sestrin 2 expression was markedly decreased on day 42, coinciding with glomerulosclerosis and severe periglomerular fibrosis. In puromycin aminonucleoside nephropathy, decreased sestrin 2 expression, increased P-S6RP expression, and periglomerular fibrosis were observed on day 9, when massive proteinuria developed. These changes were transient and nearly normalized by day 28. In crescentic glomerulonephritis, sestrin 2 expression was not detected in cellular crescents, whereas P-S6RP increased. In conditionally immortalized cultured PECs, the forced downregulation of sestrin 2 by short hairpin RNA resulted in increased expression of P-S6RP and increased apoptosis. These data suggest that sestrin 2 is involved in PEC homeostasis by regulating the activity of mTOR. In addition, sestrin 2 could be a novel marker of PECs, and decreased expression of sestrin 2 might be a marker of PEC injury.
Keywords: sestrin 2, glomerular parietal epithelial cells, mammalian target of rapamycin
sestrin 2 is a stress-inducible protein, initially identified as a hypoxia-responsive gene product (7). Sestrin 2 accumulates within cells exposed to stress and plays an important role in suppressing the production of ROS, thus protecting cells from oxidative damage (6). In addition, sestrin 2 inhibits mammalian target of rapamycin (mTOR) signaling through a redox-independent mechanism, by activating AMP-activated protein kinase (AMPK) and phosphorylating tuberous sclerosis protein 2 (TSC2) (5). Although sestrin 2 knockout mice are reported to be fully viable and do not display any gross developmental abnormalities (30), a recent study (19) has shown that deletion of sestrin 2 exacerbates obesity-induced mTOR activation, glucose intolerance, insulin resistance, and hepatosteatosis.
Target of rapamycin (TOR) is a serine-threonine kinase that was originally identified in yeast mutants resistant to the effect of rapamycin (31). Subsequently, mTOR was cloned in mammalian cells (31). mTOR constitutes a part of two distinct multiprotein complexes, TOR complex 1 (TORC1), which is sensitive to rapamycin, and TORC2, which is not sensitive to rapamycin (4, 31). Activated TORC1 directly phosphorylates two proteins, p70 ribosomal protein S6 kinase (p70S6K) and 4E-binding protein 1 (4E-BP1), which stimulate ribosome biogenesis and translation to increase cell mass (14). In turn, phosphorylated (P-)p70S6K phosphorylates S6 ribosomal protein (S6RP), which also stimulates translation (31).
Persistent mTOR activation is associated with diverse diseases including cancer, allograft rejection, autoimmune disorders, cardiovascular diseases, and metabolic disorders (31). Studies in animals are underway to identify the roles of mTOR signaling in the pathogenesis of kidney diseases such as glomerular diseases, polycystic kidney, and renal cancer (14). Genetic deletion of TORC1 in mouse podocytes induces proteinuria and progressive glomerulosclerosis, whereas genetic reduction of podocyte-specific TORC1 in diabetic animals suppresses the development of diabetic nephropathy (11, 15).
Among resident cells in the glomeruli, glomerular parietal epithelial cells (PECs) remain poorly understood (23). A recent study (23) has shown that PECs are dynamic and constantly responsive to cues within the glomeruli. In juvenile mice, PECs have been shown to migrate to become podocytes (1). PECs also contribute to the development of the sclerotic lesions in focal segmental glomerulosclerosis (27). In addition, PECs have also been reported to function as a second barrier of the glomerular filtrate, with their tight junctions to prevent filtered protein from escaping into the extraglomerular space (22).
In the present study, we first demonstrated that sestrin 2 was predominantly expressed in normal rat PECs. We further evaluated the expression of sestrin 2 and mTOR signaling in normal and diseased kidneys and attempted to determine the role of sestrin 2 and the association between sestrin 2 and mTOR signaling using conditionally immortalized cultured PECs.
METHODS
Animals and experimental protocol.
All rats were purchased from Charles River Japan (Kanagawa, Japan) and fed a standard diet and given water ad libitum. Six-week-old male Wistar rats were used to investigate normal kidneys. For the induction of adriamycin (ADR) nephropathy, 6-wk-old male Wistar rats were administered a single injection of 7.5 mg/kg ADR (doxorubicin hydrochloride, Sigma-Aldrich, St. Louis, MO) via the tail vein; on days 0, 8, 14, and 42 after the injection, rats were euthanized by an injection of pentobarbital sodium (Kyoritsu Pharmaceutical, Tokyo, Japan), and kidney samples were harvested. For the induction of puromycin aminonucleoside (PAN) nephropathy, 6-wk-old male Wistar rats were administered a single injection of 100 mg/kg PAN (Sigma-Aldrich) via the tail vein, as previously described (29); on days 0, 9, and 28 after the injection, rats were euthanized. For the induction of crescentic glomerulonephritis, 7-wk-old male Wistar-Kyoto (WKY/NCrlCrlj) rats were administered a single injection of a nephritogenic monoclonal antibody at 80 μg/body (clone a84, Iwai Chemicals, Tokyo, Japan) via the tail vein; on day 10 after the induction, kidney samples were harvested.
All animal experiments were carried out in accordance with the Institute of Experimental Animal Research of Gunma University and were handled using protocols approved by the Animal Care Committee of Gunma University.
Measurement of urinary protein excretion.
Twenty-four-hour urine was collected under normal conditions and each week after the induction of nephritis using a metabolic cage. The urinary protein concentration was determined using a Bio-Rad protein assay kit (Nippon Bio-Rad Laboratories, Tokyo, Japan).
Primary antibodies.
The primary antibodies used in this study were as follows: rabbit polyclonal anti-sestrin 2 antibody (ProteinTech Group, Chicago, IL), mouse monoclonal anti-protein gene product (PGP)9.5 antibody (clone 13C4, Gene Tex, Irvine, CA), mouse monoclonal anti-β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit monoclonal anti-P-S6RP antibody (Ser235/236, Cell signaling Technology, Beverly, MA), mouse monoclonal anti-α-smooth muscle actin (α-SMA) antibody (clone 1A4, Sigma-Aldrich), rabbit anti-paired box 2 (PAX2) antibody (Invitrogen, Camarillo, CA), mouse monoclonal anti-CD44 antibody (clone OX-49, Acris Antibodies, San Diego, CA), rabbit monoclonal anti-P-p70S6K antibody (Thr389, Cell Signaling), and rabbit monoclonal anti-P-4E-BP1 antibody (Thr37/46, Cell Signaling).
Immunohistochemistry.
Kidneys were fixed in formalin and embedded in paraffin. Four-micrometer sections were stained with periodic acid-Schiff (PAS). For the immunohistochemical analysis, 4-μm sections were deparaffinized and rehydrated. Antigens were retrieved by microwaving at 500 W for 10 min in 10 mmol/l citric acid. Endogenous peroxidase activity was blocked with periodic acid (Nichirei, Tokyo, Japan) for 45 s. Sections were incubated with the primary antibodies at 4°C overnight followed by an incubation with the biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) for 30 min at room temperature and with horseradish peroxidase-avidin (Vector Laboratories) for 30 min at room temperature. Color development was performed using diaminobenzidine tetrahydrochloride solution (Nichirei). Sections were counterstained with methyl green or PAS.
Quantification by immunohistochemistry.
Glomerular damage was quantified by grading the severity of the glomerulosclerosis and mesangial expansion on PAS-stained sections on a scale of 0 to 4 (0 = no lesion, 1 = 0–25%, 2 = 25–50%, 3 = 50–75%, and 4 = 75–100%) as previously described (12). Fifty glomeruli from each rat were evaluated, and the average score was calculated. Sestrin 2 expression in PECs and periglomerular α-SMA expression were quantified on a scale of 0 to 4 (0 = no lesion, 1 = 0–25%, 2 = 25–50%, 3 = 50–75%, and 4 = 75–100% around Bowman's capsule) in each glomerulus. The average score of 50 glomeruli/section was obtained. The number of PAX2-positive cells attached to Bowman's capsule was counted in 50 glomeruli/section. The percent area of P-S6RP- or CD44-positive PECs per glomerulus was assessed using Photoshop CS6 (Adobe, San Jose, CA) as follows. First, the total number of pixels corresponding to the glomerulus was counted in the captured image of each glomerulus. After removal of the glomerular tuft, the number of pixels showing P-S6RP positivity (brown area) within Bowman's capsule was counted. The percent P-S6RP-positive area was calculated using the following formula: number of pixels showing P-S6RP positivity/total number of pixels corresponding to the glomerulus × 100 (in %). The average percentage of 50 glomeruli/section was calculated.
Western blot analysis.
Western blot analysis was performed as previously described elsewhere with some modifications (29). In brief, protein was extracted from cultured PECs using RIPA lysis buffer (1× Tris-buffered saline, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 0.004% sodium azide, Santa Cruz Biotechnology) containing PMSF, protease inhibitor cocktail, and sodium orthovanadate (Santa Cruz Biotechnology). Protein concentration was determined by the BCA protein assay (Pierce, Rockford, IL) in accordance with the manufacturer's directions. The protein extract (10 μg) was separated on 4–20% precast polyacrylamide gels (Nippon Bio-Rad) and transferred to a polyvinyl difluoride membrane (Immobilon-P, Millipore, Bedford, MA). After being blocked with 2% BSA to reduce nonspecific antibody binding, the membrane was incubated with primary antibodies overnight at 4°C, washed with Tris-buffered saline (20 mM Tris·HCl, 150 mM NaCl, and 0.1% Tween 20), and incubated with alkaline phosphatase-conjugated anti-rabbit IgG or anti-mouse IgG antibody (Promega, Madison, WI) at room temperature for 2 h. After a further wash, detection of the bound antibody was performed using chromogen 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (Sigma-Aldrich).
Cell culture.
Conditionally immortalized mouse PECs were generated as previously described (24). Cells were cultured on a 100-mm collagen type I-coated culture dish (Iwaki, Tokyo, Japan) in RPMI 1640 containing penicillin, streptomycin, amphotericin B, sodium pyruvate (1 mM), and 2% FBS. Cells were propagated at 33°C with 5 U/ml recombinant mouse interferon-γ (Millipore, Billerica, MA) and differentiated at 37°C in the absence of interferon-γ for 10 days. The AMPK activator aminoimidazole carboxamide ribonucleotide (AICAR) was purchased from Sigma-Aldrich.
Sestrin 2 knockdown with short hairpin RNA.
Sestrin 2 silencing was performed using short hairpin (sh)RNA by previously described methods (25). In brief, pLKO.1-puro lentiviral plasmids encoding shRNA for mouse sestrin 2 or nontarget control (NTC), together with a puromycin resistance gene, were purchased from Thermo Scientific Open Biosystems (Huntsville, AL). Human immunodeficiency virus-1-based lentiviral particles were generated in HEK293FT cells (American Type Culture Collection, Manassas, VA) by cotransfection of shRNA or NTC shRNA with pCMV R8.91 and pCMV-VSV-G using FuGENE6 reagent (Roche Diagnostics, Indianapolis, IN). Cultured PECs were infected with the lentiviral particles in the presence of 8 μg/ml polybrene for 5 h at 33°C. The effective viral titer was confirmed by observations of cell survival in the presence of 3 μg/ml puromycin (Invivogen, San Diego, CA). Five shRNA clones were tested for their ability to silence sestrin 2 mRNA, and the best performing clone was selected.
Measurements of apoptosis.
Apoptosis was measured by Hoechst 33342 (Dojindo Molecular Technologies, Tokyo, Japan) staining and by careful morphological analysis, as previously reported (13). Apoptotic cells were counted in six randomly selected fields and expressed as a percentage in triplicate. In addition, the degree of apoptosis was also assessed by measurements of caspase-3 activity using the APOPCYTO caspase colorimetric assay kit (Medical & Biological Laboratories, Nagoya, Japan) in accordance with the manufacturer's instructions.
Statistical analyses.
Data are expressed as means ± SE. Differences between two groups were compared using a two-tailed t-test. Comparisons of multiple groups were performed by ANOVA; if ANOVA revealed significance, Tukey's test was applied using IBM SPSS statistics 21 (IBM SPSS, Tokyo, Japan). P values of <0.05 were considered as indicative of statistically significance.
RESULTS
Expression of sestrin 2 in the normal rat kidney.
Immunohistochemical staining of the normal rat kidney revealed selective expression of sestrin 2 along Bowman's capsule (Fig. 1, A and B). The staining pattern of sestrin 2 was similar to that of PGP9.5 (Fig. 1C), a well-known PEC marker (26), indicating that sestrin 2 was predominantly expressed in PECs. Staining was absent elsewhere in the kidney and not detected when the primary antibody was removed as a negative control (data not shown).
Fig. 1.
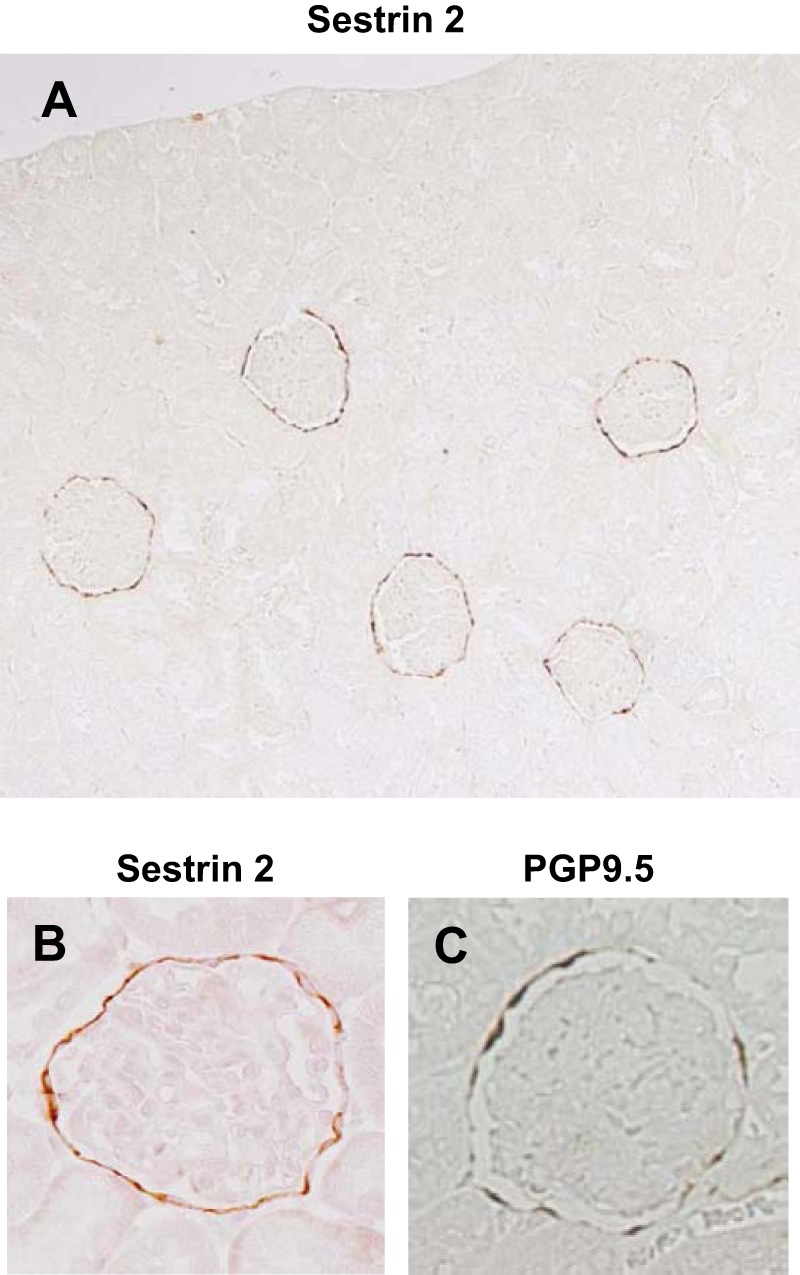
Immunohistochemical staining of sestrin 2 in the normal rat kidney. A and B: sestrin 2 was selectively expressed in parietal epithelial cells (PECs). C: protein gene product (PGP)9.5, a known marker of PECs, was also principally expressed in PECs. Original magnification: × 100 in A and ×400 in B and C.
Sestrin 2 staining decreases in ADR nephropathy.
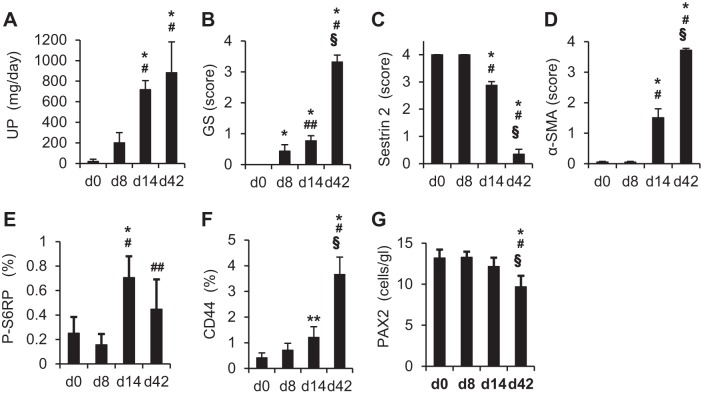
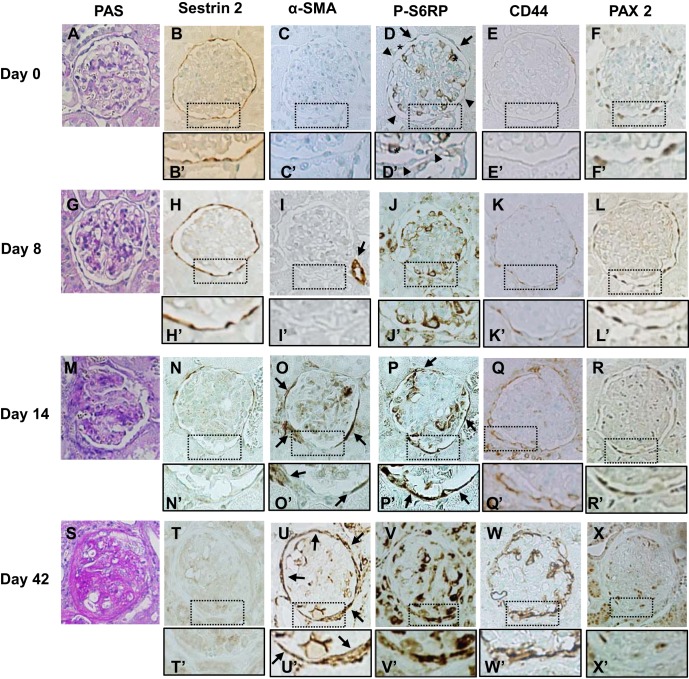
To determine sestrin 2 expression in PECs in experimental proteinuric diseases, the ADR model of focal segmental glomerulosclerosis was induced in rats. As shown in Fig. 2A, urinary protein excretion increased progressively after ADR injection. Severe glomerulosclerosis was observed in PAS-stained sections of the kidney on day 42 (Figs. 2B and 3, A, G, M, and S). Figures 2 and 3 show quantitation and representative pictures, respectively, of a variety of immunostains. In ADR nephropathy, sestrin 2 staining in PECs was unchanged until day 8 but decreased on day 14, with markedly decreased staining on day 42 (Figs. 2C and 3, B, H, N, and T). In contrast, α-SMA staining, which was observed only in the afferent or efferent arterioles on day 8 after ADR injection, was detected along Bowman's capsule on day 14, with a marked increase of its expression on day 42 (Figs. 2D and 3, C, I, O, and U). However, α-SMA-positive cells were not PECs, because they were located outside Bowman's capsule, as shown in Fig. 4, A and B. These data show that staining for sestrin 2 decreases in PECs in ADR nephropathy.
Fig. 2.
Changes of urinary protein excretion (UP), glomerulosclerosis (GS), and expression of each of the proteins in PECs in the rat model of adriamycin (ADR) nephropathy. Rats were injected with ADR, and urinary protein excretion and kidney sections were examined on days (d)0, 8, 14, and 42 (n = 6 at each time point). A: urinary protein excretion. B: glomerulosclerosis. C: sestrin 2. D: α-smooth muscle actin (α-SMA). E: phosphorylated S6 ribosomal protein (P-S6RP). F: CD44. G: paired box 2 (PAX2). The degree of GS (B) was estimated on periodic acid-Schiff (PAS)-stained kidney sections as described in methods. Degrees of sestrin 2 (C), α-SMA (D), P-S6RP (E), CD44 (F), and PAX2 (G) expression were estimated on immunohistochemically stained kidney sections using each specific antibody as described in methods. *P < 0.01 vs. day 0; #P < 0.01 vs. day 8; ##P < 0.05 vs. day 8; §P < 0.01 vs. day 14.
Fig. 3.
Immunohistochemical staining for each protein in PECs during ADR nephropathy. Rats were injected with ADR, and kidney sections were examined on day 0 (A–F), day 8 (G–L), day 14 (M–R), and day 42 (S–X). A, G, M, and S: PAS staining. Severe glomerulosclerosis was observed on day 42 (S). B, H, N, and T: sestrin 2. Sestrin 2 expression decreased on day 14 (N and N′) with an even more marked decrease observed on day 42 (T and T′). C, I, O, and U: α-SMA. α-SMA expression was not detected on day 0 (C and C′) or day 8 (I and I′) except in the small arteries (arrow in I). Expression of α-SMA was detected around the basement membrane of Bowman's capsule on day 14 (arrows in O and O′) and markedly increased on day 42 (arrows in U and U′). D, J, P, and V: P-S6RP. Along Bowman's capsule, weak P-S6RP expression was detected in some cells (arrows in D), but most cells were negative for P-S6RP expression on day 0 (arrowheads in D and D′). Within the glomerular tufts, some cells showed strong staining for P-S6RP (* in D and D′). These cells were identified as podocytes based on their localization. Strong expression of P-S6RP was observed in cells along Bowman's capsule on day 14 (arrows in P and P′) and day 42 (V and V′). E, K, Q, and W: CD44. CD44 was faintly expressed along Bowman's capsule on day 0 (E and E′) or day 8 (K and K′). CD44 expression increased on day 14 (Q and Q′) and markedly increased on day 42 (W and W′). F, L, R, and X: PAX2. PAX2 was detected in cells along Bowman's capsule in a nuclear localization. The number of PAX2-positive cells was mildly decreased on day 42 (X and X′). Original magnification: ×400.
Fig. 4.
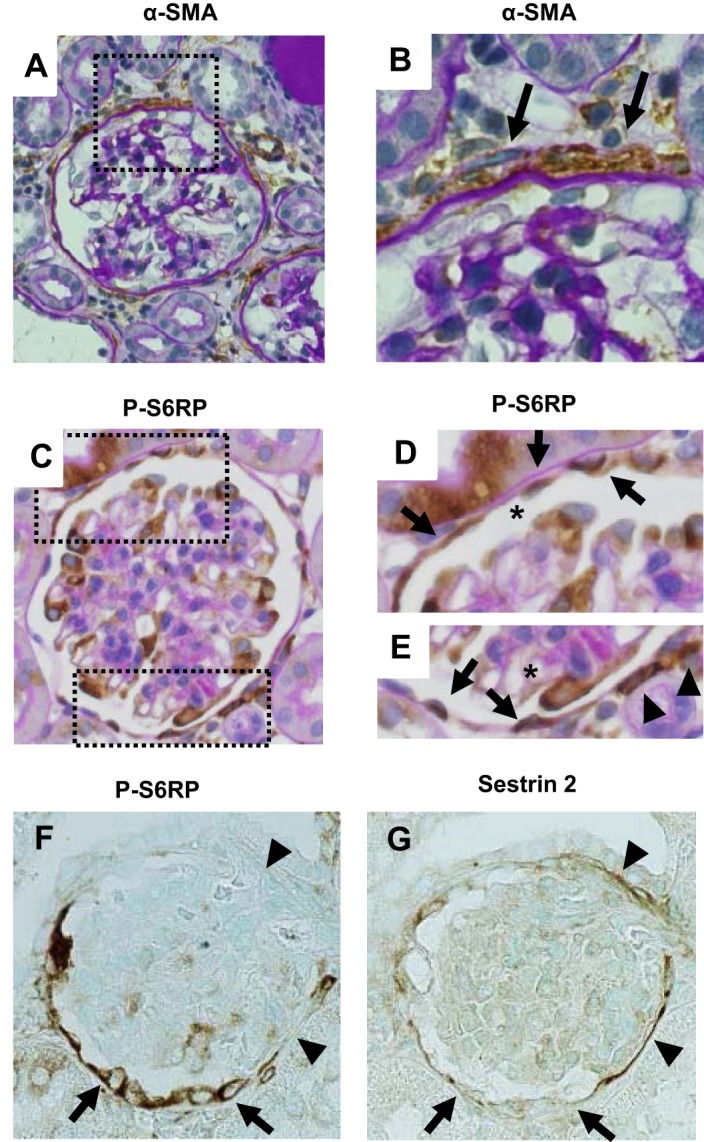
Immunohistochemical stainings for α-SMA, P-S6RP, and sestrin 2 in a rat model of ADR nephropathy. A and B: α-SMA immunostaining counterstained with PAS staining on day 42 in the model of ADR nephropathy. α-SMA-positive cells localized outside Bowman's capsule (arrow in B). C–E: P-S6RP immunostaining counterstained with PAS staining on day 14 of ADR nephropathy. P-S6RP-positive cells mainly localized within Bowman's capsule (arrows in D and E). A few P-R6RP-positive cells were also detected outside Bowman's capsule (arrowheads in E). F: P-S6RP. G: sestrin 2. Serial sections of P-S6RP (F) and sestrin 2 (G) on day 14 of ADR nephropathy showed a reciprocal relationship between sestrin 2 and P-S6RP expressions. PECs that showed strong P-S6RP expression (arrows in F) showed faint sestrin 2 expression (arrows in G). In contrast, PECs that showed no P-S6RP expression (arrowheads in F) showed sustained sestrin 2 expression (arrowheads in G). Original magnification: ×400.
S6RP staining and CD44 increase in ADR nephropathy.
Because sestrin 2 is considered a negative regulator of the mTOR pathway (5), the phosphorylation levels of S6RP, a direct phosphorylation target of mTOR, was examined using anti-P-S6RP antibody. P-S6RP staining in PECs was barely detected in control rats. In contrast, P-S6RP staining increased significantly by day 14 in ADR nephropathy (Figs. 2E and 3, D, J, P, and V). As shown in Fig. 4, C–E, P-S6RP-positive cells were detected almost exclusively along Bowman's capsule, in contrast to α-SMA-positive cells (Fig. 4, A and B). In addition, examination of serial sections of the kidney in ADR nephropathy revealed a reciprocal relationship between the expression of sestrin 2 and that of P-S6RP, i.e., PECs with increased P-S6RP expression had decreased sestrin 2 expression, whereas PECs with sustained sestrin 2 expression did not stain for P-S6RP (Fig. 4, F and G). Taken together, these data are consistent with the negative regulatory function of sestrin 2 on mTOR target P-S6RP.
CD44 has been described as an activation marker for PECs (10, 28). CD44 staining also increased significantly by day 14 in ADR nephropathy (Figs. 2F and 3, E, K, Q, and W), similar to P-S6RP staining. The number of PECs in ADR nephropathy was examined by PAX2 nuclear staining as we have previously reported. The number of PAX2-positive cells decreased slightly with the progression of ADR nephropathy, reaching statistical significance at day 42 (Figs. 2G and 3, F, L, R, and X). These data show that there was progressive activation of PECs in ADR nephropathy, despite a decrease in overall number.
Sestrin 2 expression in PAN nephropathy.
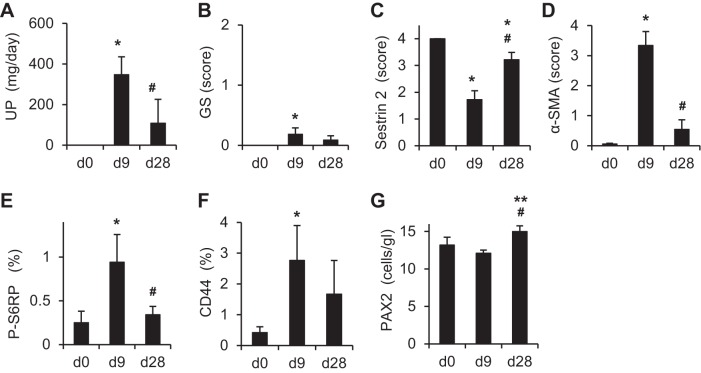
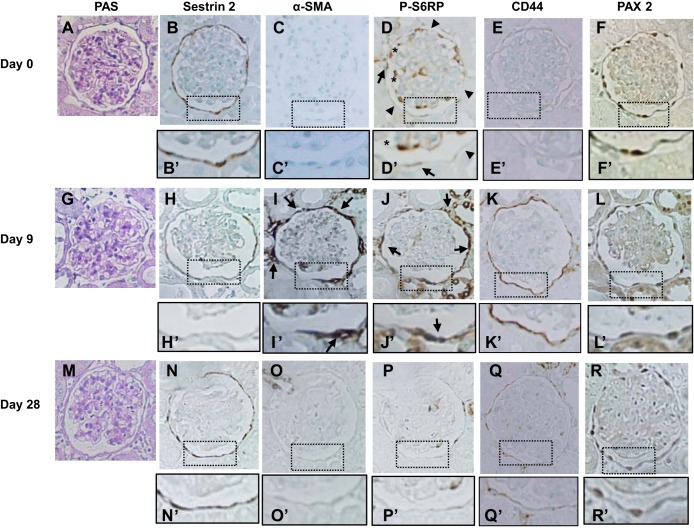
The expression of sestrin 2 was examined in PAN nephropathy, a model of minimal-change nephrotic syndrome. As shown in Fig. 5A, urinary protein excretion was markedly increased on day 9 of PAN nephropathy but almost normalized by day 28. As expected, glomerulosclerosis is very mild in PAN nephropathy (Figs. 5B and 6, A, G, and M). Sestrin 2 staining was moderately but significantly decreased on day 9; staining increased again on day 28 (Figs. 5C and 6, B, H, and N). In contrast to sestrin 2 expression, the number of α-SMA-positive cells outside Bowman's capsules was increased on day 9 but decreased again by day 28 (Figs. 5D and 6, C, I, and O). The reciprocal relationship between sestrin 2 and P-S6RP or CD44 was also observed in PAN nephropathy (Figs. 5, C, E, and F, and 6, D, E, J, K, P, and Q), where P-S6RP and CD44 increased on day 9, which coincided with reduced sestrin 2 staining. The number of PAX2-positive cells was slightly increased on day 28 (Figs. 5G and 6, F, L, and R).
Fig. 5.
Changes of urinary protein excretion, glomerulosclerosis, and expression of each of the proteins in PECs of the rat model of puromycin aminonucleoside (PAN) nephropathy. Rats were injected with PAN, and urinary protein excretion and kidney sections were examined on days 0, 9, and 28 (n = 6 at each time point). A: urinary protein excretion. B: glomerulosclerosis. C: sestrin 2. D: α-SMA. E: P-S6RP. F: CD44. G: PAX2. The degree of glomerulosclerosis (B) was estimated on PAS-stained kidney sections as described in methods. Degrees of sestrin 2 (C), α-SMA (D), P-S6RP (E), CD44 (F), and PAX2 (G) expression were estimated on immunohistochemically stained kidney sections using each specific antibody as described in methods. *P < 0.01 vs. day 0; **P < 0.05 vs. day 0; #P < 0.01 vs. day 9.
Fig. 6.
Immunohistochemical staining for each protein in PECs in a rat model of PAN nephropathy. Rats were injected with PAN, and kidney sections were examined on day 0 (A–F), day 9 (G–L), and day 28 (M–R). A, G, and M: PAS staining. B, H, and N: sestrin 2. Sestrin 2 expression decreased on day 9 (H and H′) but was restored on day 28 (N and N′). C, I, and O: α-SMA. Strong expression of α-SMA was detected around the basement membrane of Bowman's capsule on day 9 (arrows in I and I′) but disappeared on day 28 (O and O′). D, J, and P: P-S6RP. P-S6RP was detected weakly in some cells along Bowman's capsule (arrows in D and D′), but most cells were negative for P-S6RP expression on day 0 (arrowheads in D and D′). Strong expression of P-S6RP was observed in cells along Bowman's capsule on day 9 (arrows in J and J′), but the expression almost disappeared on day 28. Within the glomerular tufts, some cells showed strong staining for P-S6RP (* in D and D′). E, K, and Q: CD44. CD44 expression was increased on day 9 (K and K′) but returned to basal levels on day 28 (Q and Q′). F, L, and R: PAX2. PAX2 was detected in cells along Bowman's capsule in a nuclear localization. Original magnification: ×400.
Sestrin 2 expression in crescentic glomerulonephritis.
PECs proliferate to form crescents in crescentic glomerulonephritis (23). To determine sestrin 2 expression in crescents, Wistar-Kyoto rats were injected with anti-glomerular basement membrane (GBM) antibody. Massive proteinuria was observed on day 10 (4.0 ± 3.8 vs. 229.3 ± 27.5 mg/day, day 0 vs. day 10, P < 0.01). As shown in Fig. 7, sestrin 2 staining was not detected in the cellular crescents but was detected in cells lining Bowman's capsule not participating in the crescent. In contrast, P-S6RP and CD44 staining increased in a subpopulation of cells within the crescents.
Fig. 7.
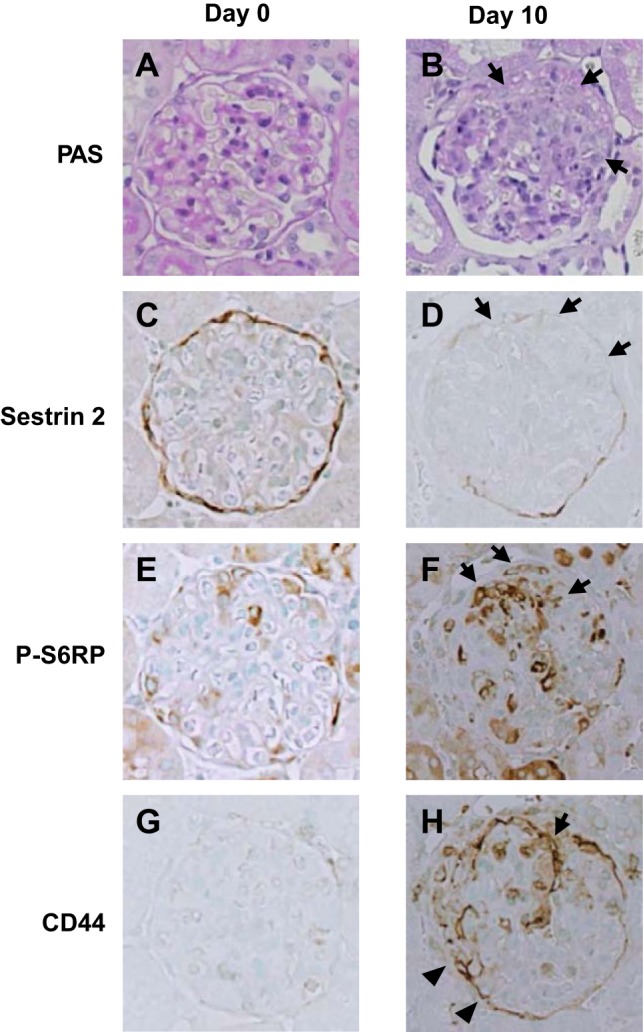
Immunohistochemical stainings for sestrin 2 and P-S6RP in anti- glomerular basement membrane (GBM) antibody-induced glomerulonephritis. Wistar-Kyotot rats were injected with anti-GBM antibody, and kidney sections were examined on day 0 (A, C, E, and G) and day 10 (B, D, F, and H). A and B: PAS staining. Cellular crescents were observed on day 10 (arrows in B). C and D: sestrin 2. Sestrin 2 expression was not detected in the area of the crescent formation (arrows in D). E and F: P-S6RP. Strong P-S6RP expression was detected within the crescents (arrows in F). G and H: CD44 expression was observed within the crescents (arrow in H) and PECs at the noncrescentic area (arrowheads in H). Original magnification: ×400.
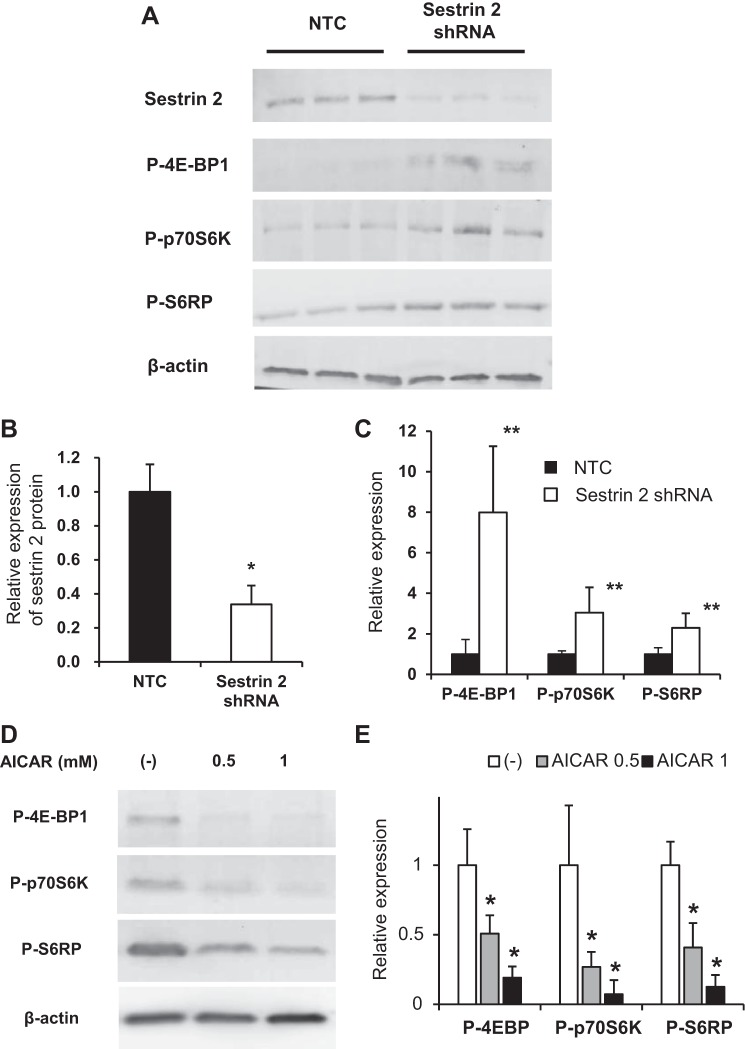
Downregulation of sestrin 2 by shRNA increased the activity of mTOR.
To examine the role of sestrin 2 in PECs, conditionally immortalized cultured mouse PECs were silenced for sestrin 2 using specific shRNA. The resultant decrease in sestrin 2 protein levels was confirmed by Western blot analysis compared with PECs transfected with NTC (Fig, 8, A and B). In sestrin 2-silenced PECs cultured under growth-restrictive conditions for 10 days, protein levels of P-4E-BP1, p70S6K, and S6RP (direct downstream targets of mTOR) increased significantly (Fig. 8, A and C). Because sestrin 2 inhibits the mTOR pathway via activation of AMPK and phosphorylation of TSC2 (5), we further examined the effect of the AMPK activator AICAR on sestrin 2 knocked down PECs. The increase in mTOR activation after forced knockdown of sestrin 2 was reversed by AICAR (Fig. 8, E and F).
Fig. 8.
Effect of sestrin 2 downregulation on the expression of mammalian target of rapamycin (mTOR) in conditionally immortalized cultured PECs. Conditionally immortalized cultured PECs were transfected with short hairpin (sh)RNA targeting sestrin 2 or nontarget control (NTC). Transfected PECs were then cultured under growth-restrictive conditions for 10 days, and proteins were extracted for Western blot analysis. A: Western blot analysis for sestrin 2 and the downstream targets of mTOR. Sestrin 2 expression was reduced in PECs transfected with shRNA targeting sestrin 2 compared with PECs transfected with NTC. Phosphorylation of downstream targets of mTOR [P-4E-binding protein 1 (P-4E-BP1), P-p70 ribosomal protein S6 kinase (P-p70S6K), and P-S6RP] was also increased in PECs transfected with shRNA targeting sestrin 2 compared with PECs transfected with NTC shRNA. B: relative expression of sestrin 2 protein detected by Western blot analysis. Compared with PECs transfected with NTC, a significant reduction of sestrin 2 protein expression was observed in PECs transfected with sestrin 2 shRNA. *P < 0.01. C: relative expression of the proteins downstream of P-mTOR. Expressions of P-4E-BP-1, P-p70S6K, and P-S6RP were significantly increased in PECs transfected with sestrin 2 shRNA compared with PECs transfected with NTC. **P < 0.05. D: Western blot analysis for the downstream targets of mTOR in PECs transfected with shRNA targeting sestrin 2 treated with the AMPK activator aminoimidazole carboxamide ribonucleotide (AICAR). Phosphorylation of the downstream targets of mTOR (P-4E-BP1, P-p70S6K, and P-S6RP) was reduced in sestrin 2 knocked down PECs. F: relative expression of the proteins downstream of P-mTOR. Expressions of P-4E-BP-1, P-p70S6K, and P-S6RP were significantly reduced in PECs transfected with sestrin 2 shRNA with AICAR treatment. *P < 0.01.
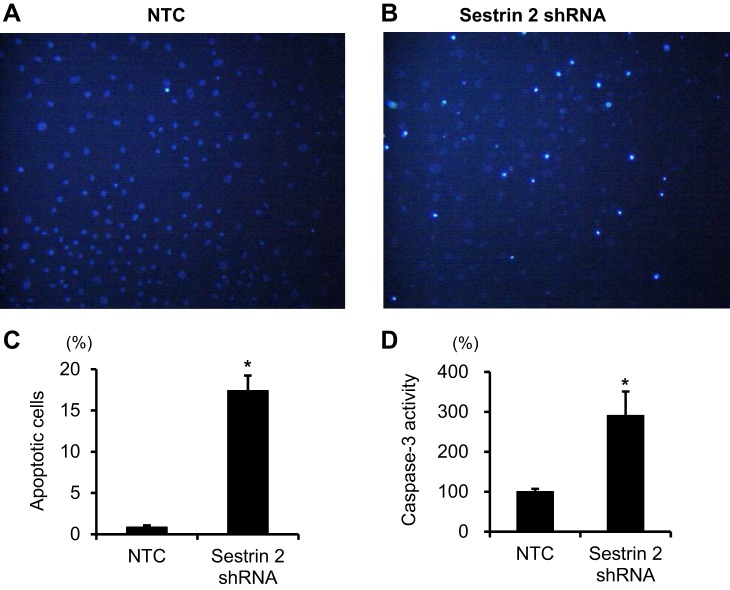
Finally, to determine the biological role of sestrin 2 in cultured PECs, apoptosis was measured. In transfected PECs with reduced levels of sestrin 2, apoptosis was significantly increased when measured by Hoechst 33342 staining and by caspace-3 activity (Fig. 9).
Fig. 9.
Effect of sestrin 2 downregulation on cellular apoptosis in conditionally immortalized cultured PECs. Conditionally immortalized cultured PECs were transfected with shRNA targeting sestrin 2 or NTC. Transfected PECs were then cultured under growth-restrictive conditions for 10 days, and the degree of apoptosis was determined. A–C: Hoechst 33342 staining in cultured PECs. A: PECs transfected with NTC showed faint apoptosis. B: increased apoptosis was observed in PECs transfected with sestrin 2 shRNA. C: apoptosis, as determined by Hoechst 33342 staining, was significantly increased in PECs transfected with sestrin 2 shRNA than in PECs transfected with NTC. *P < 0.01. D: the degree of apoptosis, as determined by caspase-3 staining, was also significantly increased in PECs transfected with sestrin 2 shRNA compared with cells transfected with NTC. *P < 0.01.
DISCUSSION
Recent studies have shown that many renal cell types express genes unique to that cell, which may serve not only as specific “markers” for identification but are also likely serve cell type-specific biological functions (23). Examples in podocytes include nephrin, podocin, NEPH1, glomerular epithelial protein 1, podocalyxin, and synaptopodin (21). In PECs, unique proteins include PAX2, PAX8, the tight junction proteins claudin-1 or occludin, ubiquitin-related protein PGP9.5, cell surface glycoprotein CD44, and the intermediate filament protein cytokeratin (2, 22, 24, 26). The first major finding of this study was that in glomeruli of normal rats, sestrin 2 was predominantly expressed in PECs. Sestrin 2 is a novel p53 target protein that accumulates in stressed cells (18). Just recently, increased expression of sestrin 2 was reported in renal proximal tubules in a model of renal ischemia-reperfusion injury (16). In addition, increased sestrin 2 expression was shown in cultured renal tubular cells (NRK-52E cells) exposed to oxidative stress (16). Taken together, the results of the present study show that in contrast to other cell types where sestrin 2 expression is related to stressors, sestrin 2 is constitutively expressed in normal adult PECs.
To better understand the biological role of constitutive sestrin 2 in PECs, three experimental models of nephrotic syndrome were studied. The second major finding was that sestrin 2 staining in PECs was closely linked with proteinuria. In both ADR and PAN models of podocyte injury characterized by proteinuria, sestrin 2 staining PECs was decreased, and this coincided with the presence of proteinuria. This was further highlighted by transient proteinuria in PAN being accompanied by a transient decline in sestrin 2 staining. Finally, in the anti-GBM model, reduced sestrin staining accompanied the proteinuria, but the decrease was selectively in proliferating cells of glomerular crescents. Taken together, although the models used were diseases of podocytes or the GBM, sestrin 2 staining was reduced in PECs.
Sestrin 2 inhibits mTOR signaling by activating AMPK and phosphorylation of TSC (5), and knockdown of sestrin 2 has been shown to activate mTOR pathway in other cell types (3, 5, 30). Therefore, we examined the downstream phosphorylation targets of mTOR, with an emphasis on P-S6RP. The third major finding of our study was decreased expression of sestrin 2 was accompanied by increased expression of P-S6RP during periods of heavy proteinuria in both ADR and PAN nephropathy models. This paradoxical expression suggests that sestrin 2 regulates the activity of mTOR in PECs, in both healthy and diseased conditions.
Interestingly, increased periglomerular fibrosis, as demonstrated by the accumulation of α-SMA-positive cells around Bowman's capsule, was only detected when PEC staining decreased for sestrin 2 and increased for P-S6RP. In addition, periglomerular fibrosis was reversible in the PAN nephropathy model when the proteinuria resolved, which coincided with the normalization of sestrin 2 staining in PECs. PECs have tight junctions and are considered to serve as a permeability barrier (22). Therefore, we hypothesize that heavy proteinuria induces PEC injury, which, in turn, results in a disruption of their tight junctions that allows leakage of protein into the extraglomerular space followed by the development of periglomerular fibrosis.
In contrast to the reversible changes of sestrin 2 expression and periglomerular fibrosis in the PAN nephropathy model, a model of transient nephrotic syndrome, a progressive decrease of sestrin 2 expression was observed in ADR nephropathy, a model of progressive nephrotic syndrome. In the ADR nephropathy model, the severe decrease of sestrin 2 expression in PECs was also associated with a decrease in the number of PECs, severe periglomerular fibrosis, and increased glomerular sclerosis, suggesting that prolonged PEC injury was closely linked to the glomerular and periglomerular injury. Although we could not detect any apoptotic cells within Bowman's space by TUNEL staining (data not shown) on day 42 of ADR nephropathy, we suppose that the reduction in the number of PEC was due to apoptosis and/or detachment of PECs after reduced sestrin 2 expression. Because apoptotic cells are likely detached and washed away in the urinary ultrafiltrate, there may only be a small window for the detection of apoptotic PECs in vivo (8).
Previous in vitro studies have shown that silencing of sestrin 2 increased apoptosis, including that of renal tubular cells (3, 6, 16, 20, 30). The fourth major finding of the present study was that silencing of sestrin 2 in cultured PECs increased phosphorylation of three downstream targets of TORC1 (4E-BP1, p70S6K, and S6RP). This correlated with increased apoptosis. Because immature PECs cultured under growth-permissive conditions stop proliferating and differentiate under growth-restrictive conditions, we supposed that the dysregulation of mTOR activity was associated with the apoptosis of PECs in vitro. The increased apoptosis by knockdown of sestrin 2 was reported in other cell types by different apoptotic stimuli (3, 5, 30). However, the precise mechanisms underlying the role of sestrin 2 in PEC survival need to be determined in the future.
The above data show that decreased expression of sestrin 2 was associated with a decrease in the number of PECs in the ADR nephropathy model and cultured PECs. Meanwhile, we observed that, in the crescentic glomerulonephritis model, in which PECs are considered to proliferate to form crescents, sestrin 2 expression was decreased in cells within the crescent, whereas P-S6RP expression was highly increased in these cells. Considering the role of mTOR in cell growth and proliferation (9, 31), the increased activity of mTOR in these cells seems explicable. Recently, Kurayama et al. (17) reported increased expression of P-S6RP in PECs before apparent crescent formation and a further increase in the expression in crescentic lesions in rat anti-GBM nephritis. In addition, they studied the effects of the mTOR inhibitor everolimus in this crescent model. Interestingly, early treatment with the mTOR inhibitor led to increased cellular necrosis of PECs, whereas later treatment reduced glomerular crescent formation. These findings demonstrate the complex role of the mTOR pathway in PECs.
Taken together, based on the present data, we propose the following model. In the normal rat kidney, sestrin 2 is predominantly expressed in PECs, in which minimal mTOR activity is detected. These conditions may be required for the maintenance of normal homeostasis of PECs under stress-free conditions. In contrast in injured PECs, we propose that the decreased expression of sestrin 2 leads to increased activity of mTOR. In PAN or ADR nephropathy models, injured PECs may lose their barrier function and the leakage of protein around Bowman's space causes periglomerular fibrosis. In addition, sustained injury of PECs in the ADR nephropathy model would lead to apoptosis and/or detachment of PECs, which, in turn, causes glomerulosclerosis. In contrast, in anti-GBM nephritis, decreased expression of sestrin 2 and increased activity of mTOR are associated with proliferation of PECs to form crescents. Although the reason why decreased sestrin 2 expression and increased mTOR activity are associated with different outcomes in PECs still remains unclear after this study, our data show that sestrin 2 could be a novel marker of PECs and that decreased expression of sestrin 2 might be a marker of PEC injury.
GRANTS
This work was funded by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan. S. J. Shankland was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-056799 and R21-DK-081835.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.H. and K.H. conception and design of research; H.H. and T.S. performed experiments; H.H., K.H., T.S., S.T., M.W., and A.M. analyzed data; H.H., K.H., T.S., S.T., M.W., A.M., T.O., and J.W.P. interpreted results of experiments; H.H. and K.H. prepared figures; H.H. and K.H. drafted manuscript; H.H., K.H., S.J.S., and Y.N. edited and revised manuscript; H.H., K.H., T.S., S.T., M.W., A.M., T.O., J.W.P., S.J.S., and Y.N. approved final version of manuscript.
REFERENCES
- 1.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bariety J, Mandet C, Hill GS, Bruneval P. Parietal podocytes in normal human glomeruli. J Am Soc Nephrol 17: 2770–2780, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Ben-Sahra I, Dirat B, Laurent K, Puissant A, Auberger P, Budanov A, Tanti JF, Bost F. Sestrin2 integrates Akt and mTOR signaling to protect cells against energetic stress-induced death. Cell Death Differ 20: 611–619, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budanov AV. Stress-responsive sestrins link p53 with redox regulation and mammalian target of rapamycin signaling. Antioxid Redox Signal 15: 1679–1690, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134: 451–460, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304: 596–600, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Budanov AV, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, Gorodin S, Fishman A, Chajut A, Einat P, Skaliter R, Gudkov AV, Chumakov PM, Feinstein E. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene 21: 6017–6031, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Chang AM, Ohse T, Krofft RD, Wu JS, Eddy AA, Pippin JW, Shankland SJ. Albumin-induced apoptosis of glomerular parietal epithelial cells is modulated by extracellular signal-regulated kinase 1/2. Nephrol Dial Transplant 27: 1330–1343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta 1804: 433–439, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Fatima H, Moeller MJ, Smeets B, Yang HC, D'Agati VD, Alpers CE, Fogo AB. Parietal epithelial cell activation marker in early recurrence of FSGS in the transplant. Clin J Am Soc Nephrol 7: 1852–1858, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121: 2197–2209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiramatsu N, Hiromura K, Shigehara T, Kuroiwa T, Ideura H, Sakurai N, Takeuchi S, Tomioka M, Ikeuchi H, Kaneko Y, Ueki K, Kopp JB, Nojima Y. Angiotensin II type 1 receptor blockade inhibits the development and progression of HIV-associated nephropathy in a mouse model. J Am Soc Nephrol 18: 515–527, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hiromura K, Pippin JW, Blonski MJ, Roberts JM, Shankland SJ. The subcellular localization of cyclin dependent kinase 2 determines the fate of mesangial cells: role in apoptosis and proliferation. Oncogene 21: 1750–1758, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Huber TB, Walz G, Kuehn EW. mTOR and rapamycin in the kidney: signaling and therapeutic implications beyond immunosuppression. Kidney Int 79: 502–511, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Ruegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121: 2181–2196, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishihara M, Urushido M, Hamada K, Matsumoto T, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Horino T, Fujieda M, Fujimoto S, Terada Y. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol 305: F495–F509, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Kurayama R, Ito N, Nishibori Y, Fukuhara D, Akimoto Y, Higashihara E, Ishigaki Y, Sai Y, Miyamoto K, Endou H, Kanai Y, Yan K. Role of amino acid transporter LAT2 in the activation of mTORC1 pathway and the pathogenesis of crescentic glomerulonephritis. Lab Invest 91: 992–1006, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 327: 1223–1228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JH, Budanov AV, Talukdar S, Park EJ, Park HL, Park HW, Bandyopadhyay G, Li N, Aghajan M, Jang I, Wolfe AM, Perkins GA, Ellisman MH, Bier E, Scadeng M, Foretz M, Viollet B, Olefsky J, Karin M. Maintenance of metabolic homeostasis by sestrin2 and sestrin3. Cell Metab 16: 311–321, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu SY, Lee YJ, Lee TC. Association of platelet-derived growth factor receptor beta accumulation with increased oxidative stress and cellular injury in sestrin 2 silenced human glioblastoma cells. FEBS Lett 585: 1853–1858, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Ohse T, Chang AM, Pippin JW, Jarad G, Hudkins KL, Alpers CE, Miner JH, Shankland SJ. A new function for parietal epithelial cells: a second glomerular barrier. Am J Physiol Renal Physiol 297: F1566–F1574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohse T, Pippin JW, Chang AM, Krofft RD, Miner JH, Vaughan MR, Shankland SJ. The enigmatic parietal epithelial cell is finally getting noticed: a review. Kidney Int 76: 1225–1238, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohse T, Pippin JW, Vaughan MR, Brinkkoetter PT, Krofft RD, Shankland SJ. Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture. J Am Soc Nephrol 19: 1879–1890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakairi T, Abe Y, Kopp JB. TGF-β1 reduces Wilms' tumor suppressor gene expression in podocytes. Nephrol Dial Transplant 26: 2746–2752, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirato I, Asanuma K, Takeda Y, Hayashi K, Tomino Y. Protein gene product 9.5 is selectively localized in parietal epithelial cells of Bowman's capsule in the rat kidney. J Am Soc Nephrol 11: 2381–2386, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Grone HJ, Floege J, Moeller MJ. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1262–1274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 20: 2604–2615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi S, Hiromura K, Tomioka M, Takahashi S, Sakairi T, Maeshima A, Kaneko Y, Kuroiwa T, Nojima Y. The immunosuppressive drug mizoribine directly prevents podocyte injury in puromycin aminonucleoside nephrosis. Nephron Exp Nephrol 116: e3–e10, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Wempe F, De-Zolt S, Koli K, Bangsow T, Parajuli N, Dumitrascu R, Sterner-Kock A, Weissmann N, Keski-Oja J, von Melchner H. Inactivation of sestrin 2 induces TGF-β signaling and partially rescues pulmonary emphysema in a mouse model of COPD. Dis Model Mech 3: 246–253, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471–484, 2006 [DOI] [PubMed] [Google Scholar]