Abstract
The goal of the present study was to determine if supraspinal pathways are necessary for inhibition of bladder reflex activity induced by activation of somatic afferents in the pudendal or tibial nerve. Cats anesthetized with α-chloralose were studied after acute spinal cord transection at the thoracic T9/T10 level. Dilute (0.25%) acetic acid was used to irritate the bladder, activate nociceptive afferent C-fibers, and trigger spinal reflex bladder contractions (amplitude: 19.3 ± 2.9 cmH2O). Hexamethonium (a ganglionic blocker, intravenously) significantly (P < 0.01) reduced the amplitude of the reflex bladder contractions to 8.5 ± 1.9 cmH2O. Injection of lidocaine (2%, 1–2 ml) into the sacral spinal cord or transection of the sacral spinal roots and spinal cord further reduced the contraction amplitude to 4.2 ± 1.3 cmH2O. Pudendal nerve stimulation (PNS) at frequencies of 0.5–5 Hz and 40 Hz but not at 10–20 Hz inhibited reflex bladder contractions, whereas tibial nerve stimulation (TNS) failed to inhibit bladder contractions at all tested frequencies (0.5–40 Hz). These results indicate that PNS inhibition of nociceptive afferent C-fiber-mediated spinal reflex bladder contractions can occur at the spinal level in the absence of supraspinal pathways, but TNS inhibition requires supraspinal pathways. In addition, this study shows, for the first time, that after acute spinal cord transection reflex bladder contractions can be triggered by activating nociceptive bladder afferent C-fibers using acetic acid irritation. Understanding the sites of action for PNS or TNS inhibition is important for the clinical application of pudendal or tibial neuromodulation to treat bladder dysfunctions.
Keywords: urinary bladder, neuromodulation, pudendal, tibial, cat
it is known that bladder reflexes are initiated by two types of afferent nerve fibers: Aδ-fibers and C-fibers (11). Under physiological conditions, bladder distention activates non-nociceptive afferent Aδ-fibers, which trigger a spinobulbospinal micturition reflex (6), whereas nociceptive afferent C-fibers are silent (13). Under pathological conditions, such as bladder irritation/infection, silent C-fibers are activated, triggering a spinal reflex in addition to a spinobulbospinal reflex and also cause urinary urgency, frequency, or incontinence. The supraspinal bladder reflex has been previously demonstrated in animal studies by large micturition contractions induced by saline distention of the bladder. After acute spinal cord transection rostral to the sacral spinal cord, the supraspinal bladder reflex is eliminated and reflex bladder contractions induced by saline distention disappear. Several weeks after spinal cord transection, spinal reflex bladder contractions can appear during saline distention due to reorganization of the spinal cord and/or plasticity of bladder afferent pathways (6, 10, 21). Although the spinal C-fiber reflex can be demonstrated after acute spinal cord transection by electrical stimulation of bladder afferents and recording of efferent activity on the pelvic nerve (4, 16), it has never been shown whether spinal reflex bladder contractions can be induced in acute spinal cord-transected cats by activating these afferent C-fibers using bladder irritants.
Overactive bladder syndrome (OAB) is characterized by urinary urgency typically accompanied by frequency and nocturia with or without urgency incontinence (1). Due to side effects and low efficacy, drug therapy for OAB has a poor compliance (2, 12, 17). Therefore, neuromodulation becomes an attractive option for OAB treatment (24). Pudendal (18, 19) and tibial (20) neuromodulation have been shown to be effective in the treatment of OAB. However, the central sites of action for pudendal or tibial neuromodulation are currently still unknown. Although previous animal studies (14, 22) have shown that both pudendal nerve stimulation (PNS) and tibial nerve stimulation (TNS) are effective in inhibiting bladder reflex contractions and increasing bladder capacity, whether the inhibition occurs in the spinal cord or supraspinally has not been determined.
The goals of the present study in cats were to determine 1) whether acetic acid (AA) can irritate the bladder, activate nociceptive afferent C-fibers, and induce bladder reflex activity after acute spinal cord transection at the thoracic level and 2) whether PNS or TNS inhibition of bladder reflex activity can still occur after the removal of supraspinal micturition reflex pathways. The results from this study indicate a prominent difference in the spinal mechanisms underlying PNS or TNS inhibition of bladder reflex activity.
MATERIALS AND METHODS
The protocol and animal use in this study were approved by the Animal Care and Use Committee of the University of Pittsburgh.
Surgical procedures.
Twelve cats (6 male cats and 6 female cats, 2.9–4.5 kg, Liberty Research, Waverly, NY) were anesthetized with isoflurane (2–3% in O2) during surgery and then with α-chloralose (65 mg/kg iv and supplemented as needed) during data collection. A tracheotomy was performed, and a tube was inserted to keep the airway patent. A catheter was inserted into right carotid artery to monitor systemic blood pressure. The right cephalic vein was catheterized for the intravenous injection of drugs and fluid. Heart rate and blood O2 were monitored by a pulse oximeter (9847V, NONIN Medical, Plymouth, MN) attached to the tongue.
Through an abdominal incision, the ureters were isolated, tied, and cut for external drainage of urine. A double lumen catheter was inserted into the bladder via a small cut in the proximal urethra and secured by a ligature around the urethra. One lumen was connected to a pump to slowly infuse (1–3 ml/min) saline or 0.25% AA into the bladder. The other lumen was attached to a pressure transducer to measure bladder pressure. The right pudendal nerve was dissected via a 3- to 4-cm incision in the sciatic notch lateral to the tail. A tripolar cuff electrode (NC223pt, MicroProbe, Gaithersburg, MD) was implanted around the pudendal nerve. A bipolar cuff electrode (NC223pt, MicroProbe) was also implanted on the left tibial nerve above the ankle. Stimulation electrodes were connected to a stimulator (S88, Grass Medical Instruments, Quincy, MA) via a constant-voltage stimulus isolator (SIU5, Grass Medical Instruments). A switch was inserted between the electrodes and stimulus isolator so that the pudendal or tibial nerve could be stimulated individually. A laminectomy was performed to expose the spinal cord at the thoracic (T9/T10) level for complete spinal cord transection during the experiment. Another laminectomy was performed at the lumbosacral (L7-S3) spinal cord level for the injection of lidocaine or complete transection of the spinal cord and S1-S3 spinal roots at the end of the experiments. The spinal cord was covered with saline-soaked cotton, and the skin and muscle layers were then closed by sutures.
Experimental protocol.
Uniphasic rectangular pulses (0.2-ms pulse width) of different frequencies (0.5, 1, 5, 10, 20, and 40 Hz) were used for PNS or TNS. The stimulation threshold (T), defined as the minimal intensity for inducing anal/toe twitch at 5 Hz, was determined at the beginning of the experiment. Initially, cystometrograms (CMGs) were performed by slowly infusing the bladder with saline to determine the bladder capacity, which was defined as the bladder volume threshold required to induce a micturition contraction of large amplitude (>30 cmH2O) and long duration (>20 s). Because the urethra was ligated to prevent bladder emptying, the bladder was emptied manually after each CMG by withdrawing saline through the catheter. Multiple CMGs were performed to ensure reproducibility of the saline control capacity. The spinal cord was then completely transected at the T9/T10 level. Thirty minutes after spinal cord transection, a saline CMG was performed to confirm the disappearance of the large-amplitude micturition contraction. The bladder infusion was then changed from saline to 0.25% AA to irritate the bladder, activate nociceptive bladder afferent C-fibers, and induce spinal reflex bladder activity. During the AA CMG, bladder capacity was determined by the volume threshold required to induce a bladder contraction of an amplitude of >10 cmH2O. To ensure reproducibility of the AA control capacity, multiple CMGs were performed by filling and manually emptying the bladder. With the bladder filled with AA solution to a volume above the micturition volume threshold and maintained under isovolumetric conditions, PNS (2T) or TNS (4T) of different frequencies (0.5, 1, 5, 10, 20, and 40 Hz) was then applied in a random order for ∼2-min duration at 3- to 5-min intervals to inhibit spinal reflex bladder activity. After the isovolumetric tests, the following four CMGs were performed with AA infusion: 1) control CMG without PNS, 2) CMG during 2T PNS, 3) CMG during 4T PNS, and 4) control CMG without PNS to determine any poststimulation effect. The four CMGs were then performed again with TNS instead of PNS.
At the end of experiments, when the bladder was filled with AA and maintained under isovolumetric conditions, hexamethonium, a ganglionic blocking agent (10 mg/kg, Sigma-Aldrich, St. Louis, MO), was administered intravenously to suppress spinal reflex bladder activity in seven cats. Ten minutes later, 1–2 ml lidocaine (2%, Hospira, Lake Forst, IL) was injected into the S1-S3 spinal cord in four cats to block any residual reflex bladder activity. In another cat, the spinal roots (S1-S3) were cut, and the spinal cord was completely transected between L7 and S1.
Data analysis.
The bladder capacity measured during each CMG was normalized to the initial saline control capacity in the same animal, which allowed for comparisons between animals. Bladder capacities were averaged for each condition and reported as means ± SE. Isovolumetric bladder contractions were measured by the area under the bladder pressure trace, maximal contraction amplitude, or contraction frequency during PNS/TNS and were normalized to isovolumetric bladder contractions during the same time period before the stimulation. A t-test or ANOVA followed by Dunnett multiple-comparisons test was used to determine the statistical significance (P < 0.05).
RESULTS
Spinal reflex bladder activity induced by AA irritation.
During saline CMGs, micturition contractions of large amplitude (>30 cmH2O) and long duration (>20 s) could be induced when the volume reached the bladder capacity (9.5 ± 0.9 ml, n = 12 cats; Fig. 1A). After complete spinal cord transection at the T9/T10 level, this large micturition reflex disappeared during saline CMGs even after the baseline bladder pressure reached more than 40 cmH2O at a much larger bladder volume (161.0 ± 16.1% of the pretransection saline capacity; Fig. 1A). Infusion of AA into the bladder induced spinal reflex bladder contractions of small amplitude (<30 cmH2O) and short duration (<20 s) at a significantly (P < 0.01) smaller bladder volume that was 60.8 ± 6.4% of the saline capacity before spinal cord transection (Fig. 1, A and B).
Fig. 1.
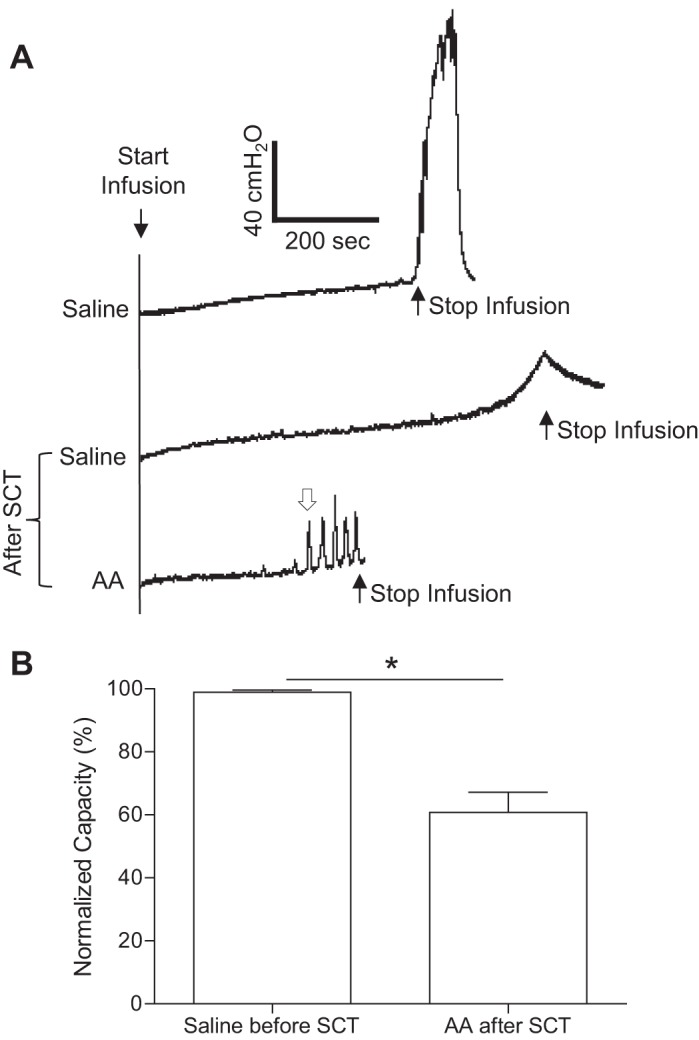
Effect of acute spinal cord transection (SCT) at the T9/T10 level on reflex bladder activity. A: cystometrograms (CMGs) during intravesical saline or 0.25% acetic acid (AA) infusion before and after SCT. The infusion rate was 1 ml/min. B: summarized results from 12 cats. The open arrow in A marks the contraction that was used to determine bladder capacity after SCT. The bladder capacity was normalized to the measurement during saline infusion before SCT. The infusion rate was 1–3 ml/min. *Significant difference (P < 0.01 by a paired Student's t-test).
Spinal reflex bladder contractions were not only induced during AA infusion (Fig. 1A) but also during isovolumetric conditions after the AA infusion was stopped at bladder volumes ∼20–30% more than the AA capacity (see the first trace before hexamethonium treatment in Fig. 2A). Under these conditions, hexamethonium (10 mg/kg iv) significantly (P < 0.01) reduced the amplitude of spinal reflex bladder contractions from 19.3 ± 2.9 to 8.5 ± 1.9 cmH2O (Fig. 2B). The contraction amplitude was further significantly (P < 0.01) reduced to 4.2 ± 1.3 cmH2O (Fig. 2B) by the injection of lidocaine (2%, 1–2 ml) into the S1–S3 spinal cord (n = 4 cats). In another hexamethonium-treated cat without lidocaine injection, complete spinal transection between the L7 and S1 spinal cord and cutting the S1–S3 spinal roots reduced the contraction amplitude from 6.8 to 3.1 cmH2O. The frequency of bladder contractions (1.9 ± 0.3/min) was not changed by either hexamethonium or lidocaine treatment (Fig. 2C).
Fig. 2.
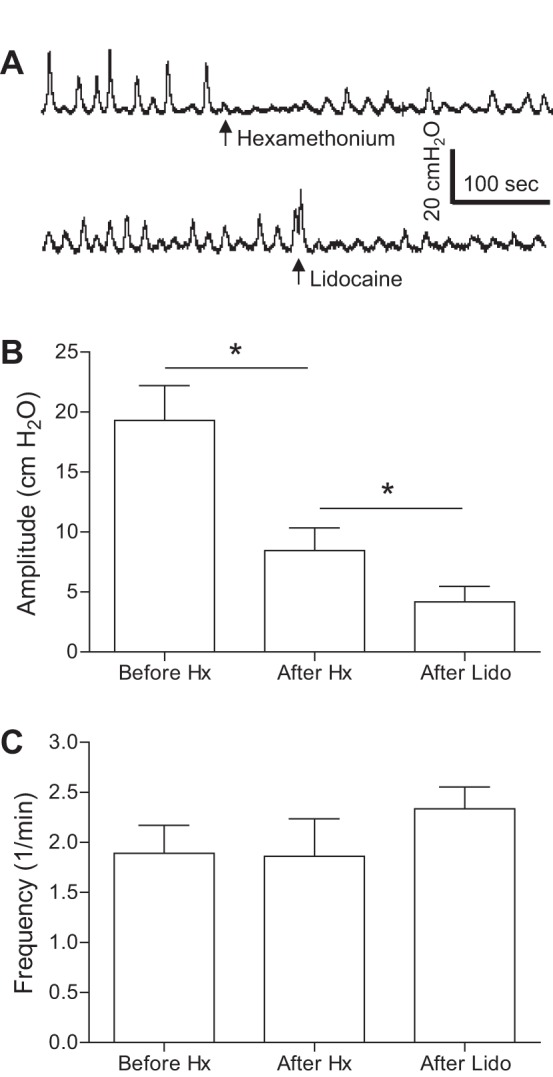
Spinal reflex bladder activity induced by bladder irritation using 0.25% AA after acute SCT at the T9/T10 level. A: under isovolumetric conditions, the maximal amplitude of rhythmic bladder contractions was significantly reduced by intravenous injection of 10 mg/kg hexamethonium (Hx). The contraction amplitude was further reduced by injection of 0.5 ml of 2% lidocaine (Lido) into the sacral S1 spinal cord. B: maximal contraction amplitude. C: contraction frequency. In total, seven cats were tested with Hx followed by Lido injection in four cats. *Significant difference (P < 0.01 by a paired Student's t-test).
Effect of PNS and TNS on spinal reflex bladder activity.
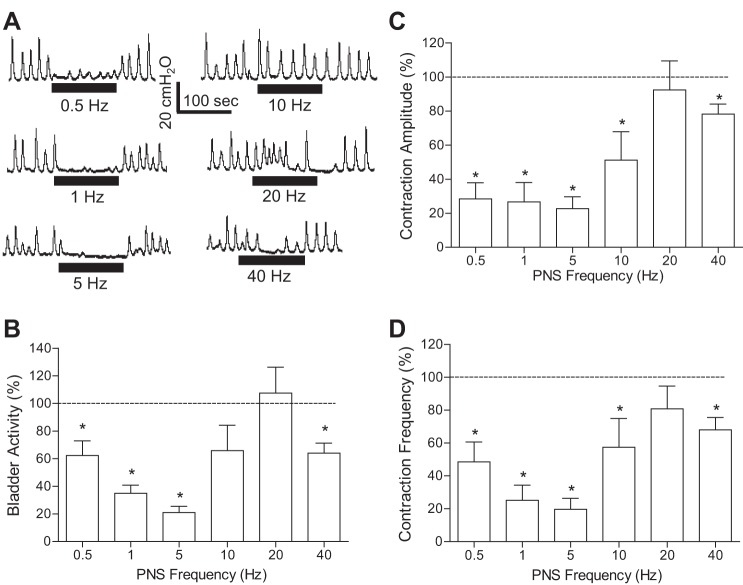
The effect of PNS on spinal reflex bladder activity induced by AA irritation was dependent on the stimulation frequency (Fig. 3). Under isovolumetric AA conditions, PNS at 2T intensity significantly (P < 0.05) suppressed spinal reflex bladder contractions at frequencies of 0.5–5 and 40 Hz but had no effect at 20 Hz (Fig. 3, B and C). At 10 Hz, it only reduced the maximal contraction amplitude (Fig. 3B) and contraction frequency (Fig. 3D). The PNS inhibition was quickly reversible after stimulation (Fig. 3A). During repeated AA CMGs, 5-Hz PNS at 2T or 4T intensity significantly (P < 0.01) increased bladder capacity from 65.2 ± 9.4% to 92.5 ± 12.0% and 107.6 ± 14.8% of the saline control capacity, respectively (Fig. 4).
Fig. 3.
The inhibition of AA-induced spinal reflex bladder activity by pudendal nerve stimulation (PNS) is frequency dependent. A: under isovolumetric conditions, bladder rhythmic contractions were inhibited by PNS of different frequencies at an intensity of 2 times the threshold (T) for inducing anal twitching. The horizontal bars under the pressure trace indicate the duration of PNS (0.2 ms, T = 0.5 V). B–D: summarized results produced by 2T PNS. T = 1.0 ± 0.2 V (0.3–2.2 V). n = 12 cats. *Significantly different from 100% (P < 0.05 by a one-sample t-test). The bladder activity in B was measured as the area under the bladder pressure curve during PNS and then normalized to the measurement during the same time period before PNS. Similar normalization was also performed for the maximal contraction amplitude (C) and contraction frequency (D).
Fig. 4.
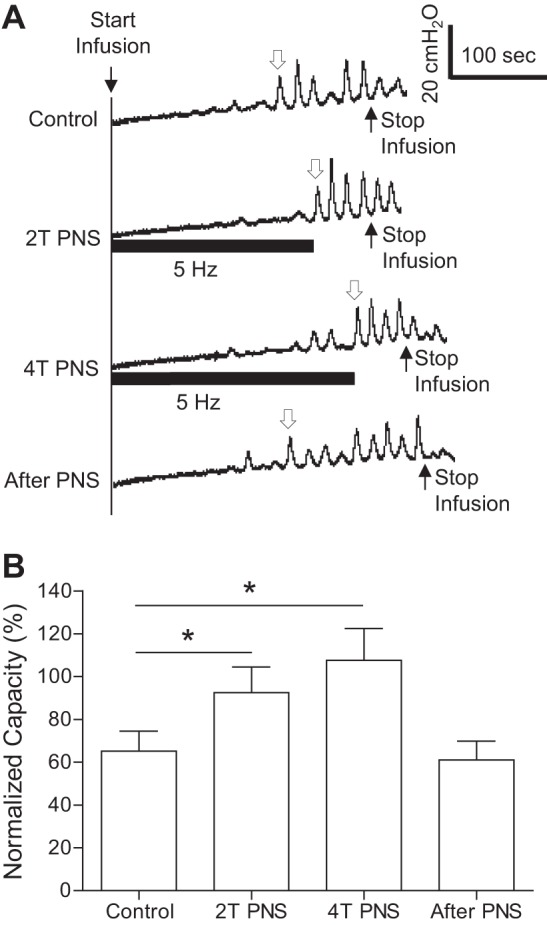
The inhibition of AA-induced spinal reflex bladder activity during a CMG by PNS at 5 Hz. A: examples of CMG traces with/without PNS at 2T or 4T intensity for inducing anal twitching. The horizontal bars under the pressure trace indicate the duration of PNS (0.2 ms, T = 2.2 V). The infusion rate was 2 ml/min. B: summarized results from 12 cats. The open arrow in A marks the contraction that was used to determine bladder capacity. Bladder capacity was normalized to the measurement during saline infusion before acute SCT. The infusion rate was 1–3 ml/min. T = 1.0 ± 0.2V (0.3–2.2V). *Significant difference (P < 0.05 by one-way ANOVA).
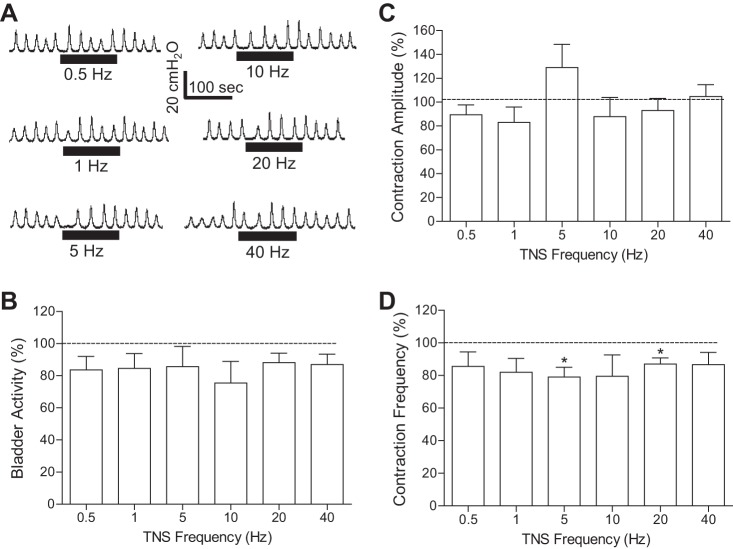
In contrast to PNS, TNS (4T) failed to inhibit spinal reflex bladder activity at all tested frequencies (0.5–40 Hz) after AA irritation under isovolumetric conditions (Fig. 5, A and B). More detailed analysis showed that TNS slightly reduced contraction frequency at 5 and 20 Hz (Fig. 5D) but did not change the maximal contraction amplitude (Fig. 5C). During repeated AA CMGs, 5-Hz TNS at both 2T and 4T intensity also failed to increase bladder capacity (Fig. 6).
Fig. 5.
Tibial nerve stimulation (TNS) failed to inhibit the spinal reflex bladder activity induced by AA irritation. A: under isovolumetric conditions, rhythmic bladder contractions were not changed by TNS of different frequencies at an intensity of 4T for inducing toe twitching. The horizontal bars under the pressure trace indicate the duration of TNS (0.2 ms, T = 1.6 V). B–D: summarized results produced by 4T TNS. T = 1.8 ± 0.2V (1.2–3.5V). n = 12 cats. The bladder activity in B was measured as the area under the bladder pressure curve during TNS and then normalized to the measurement during the same time period before TNS. Similar normalization was also performed for the maximal contraction amplitude (C) and contraction frequency (D).
Fig. 6.
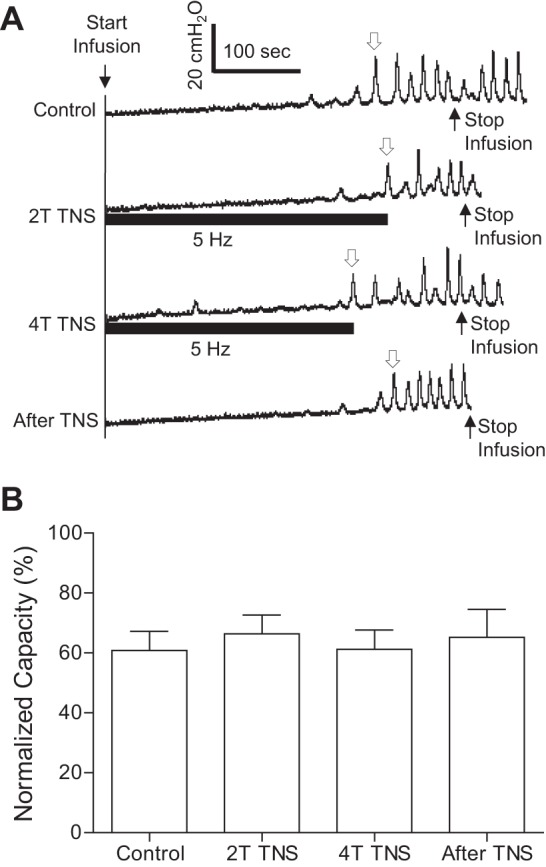
TNS at 5 Hz failed to increase bladder capacity during a CMG with AA infusion. A: examples of CMG traces with/without TNS at 2T or 4T intensity for inducing toe twitching. The horizontal bars under the pressure trace indicate the duration of TNS (0.2 ms, T =3.5 V). The infusion rate was 1 ml/min. B: summarized results from 12 cats. The open arrow in A marks the contraction that was used to determine bladder capacity. Bladder capacity was normalized to the measurement during saline infusion before acute spinal cord injury. The infusion rate was 1–3 ml/min. T = 1.8 ± 0.2V (1.2–3.5V).
DISCUSSION
This study demonstrated, for the first time in cats, that after acute spinal cord transection above the lumbosacral level, spinal reflex bladder contractions can be induced by activation of nociceptive afferent C-fibers using AA irritation (Figs. 1 and 2). Spinal reflex bladder contractions could be inhibited by PNS (Figs. 3 and 4) but not by TNS (Figs. 5 and 6), indicating a different mechanism of action for pudendal and tibial neuromodulation of bladder activity.
Hexamethonium suppresses transmission in both parasympathetic and sympathetic ganglia by blocking neuronal nicotinic ACh receptors. The intravenous injection of hexamethonium significantly decreased the amplitude of isovolumetric bladder contractions (Fig. 2), indicating that these bladder contractions are dependent on the efferent output from the spinal cord. Since the sympathetic efferent output is inhibitory to the bladder (7), the reduction of contraction amplitude by hexamethonium indicates that these isovolumetric bladder contractions are mainly mediated by parasympathetic efferent output from the sacral spinal cord. However, the subsequent intrathecal injection of lidocaine (Fig. 2) or transection of S1–S3 spinal roots and the sacral spinal cord further reduced the contraction amplitude, which might be due to the fact that muscarinic as well as nicotinic mechanisms participate in transmission in bladder parasympathetic ganglia of the cat (8), and, therefore, hexamethonium-resistant ganglionic synapses may contribute to the propagation of efferent signals from the sacral spinal cord to the bladder. Another possibility is that hexamethonium produced an incomplete block of the nicotinic mechanism. However, this possibility is less likely since a very large dose of hexamethonium (10 mg/kg iv) was used in this study. After lidocaine injection or complete transection of the sacral cord and sacral roots to eliminate bladder afferent input, the residual small-amplitude (∼4 cmH2O) contractions are probably caused by intrinsic bladder muscle activity. The frequency of bladder contractions was similar before and after lidocaine or hexamethonium treatment (Fig. 2C), indicating that spinal reflex bladder contractions are very likely triggered by afferent activity induced by small intrinsic muscle contractions.
It is known that non-nociceptive bladder afferent Aδ-fibers can be activated by saline distension but that nociceptive bladder afferent C-fibers are silent during saline distension (13). After acute spinal cord transection, reflex bladder contractions could not be induced by saline distention at a bladder volume much larger than the bladder capacity (Fig. 1A), indicating that activation of non-nociceptive afferent Aδ-fibers by saline distention triggers the spinobulbospinal bladder reflex (6) but does not activate the spinal bladder reflex. Previous studies (4, 6, 16) in cats have also shown that the bladder efferent response evoked by electrical stimulation of bladder afferent Aδ-fibers is lost after acute spinal cord transection. However, after acute spinal cord transection, AA irritation induced reflex bladder contractions at a bladder volume much smaller than the saline capacity (Fig. 1). It is known that C-fibers, which are inactive during saline distention, become activated during bladder irritation (13). Therefore, it would be logical to conclude that AA irritation triggers a spinal bladder reflex by activating nociceptive bladder afferent C-fibers. This conclusion also agrees with previous observations (4, 16) in cats that after acute spinal cord transection, a long-latency reflex discharge can be evoked on bladder postganglionic nerves by electrical stimulation of bladder afferent C-fibers but not by afferent Aδ-fibers. However, this organization of the spinal micturition reflex might not be applicable to anesthetized rats because both C-fibers and Aδ-fibers can respond to saline distension of the bladder (11) and participate in the micturition reflex during saline distension (3).
It is also worth noting that a minimal AA volume is still required to induce spinal reflex bladder contractions (Fig. 1), indicating that the afferent limb of the spinal bladder reflex is mechanosensitive and that the reflex is dependent on both bladder distension and chemical stimulation of afferent nerves. This is consistent with the observation that silent C-fibers in cats activated by bladder irritation are also sensitive to bladder volume/distention (13). However, after acute spinal cord transection and irritation of the bladder with 0.25% AA, the volume threshold for eliciting reflex bladder contractions was still ∼60% of the saline capacity (Fig. 1B), whereas previous studies (14, 22) in cats have shown that an infusion of 0.25% AA can induce reflex bladder contractions at a much smaller volume (∼20% of the saline capacity) if the spinal cord is intact. The higher sensitivity to AA irritation in spinal cord-intact cats indicates either a facilitatory interaction between the Aδ-fiber-mediated supraspinal reflex and C-fiber-mediated spinal reflex or that AA also sensitizes afferent Aδ-fibers.
This study showed that PNS inhibition of reflex bladder activity can occur at the spinal cord level. There are two possible spinal mechanisms for PNS inhibition. First, pudendal afferent input can activate a group of inhibitory interneurons in the sacral spinal cord that have synaptic connections to excitatory neurons in the bladder reflex pathway. The suppression of PNS inhibition of reflex bladder activity by intrathecal administration of a GABAA receptor antagonist (picrotoxin) supports this mechanism (25). A second mechanism involves pudendal afferent activation of lumbar sympathetic efferent pathways in hypogastric nerves, which inhibits bladder activity via adrenergic inhibitory mechanisms in bladder smooth muscle and bladder ganglia (5, 7, 9, 15). The reduction of PNS inhibition of bladder activity in chronic spinal cats after transection of hypogastric nerves provides support for this mechanism (23). Thus, more studies are warranted to determine the possible contribution of the different spinal mechanisms to PNS inhibition of bladder reflexes.
PNS inhibition of spinal reflex bladder contractions is dependent on stimulation frequency (Fig. 3). Similar frequency-dependent PNS inhibition was also observed in spinal cord-intact and chronic spinal cord-injured cats (6, 21). This may indicate that similar mechanisms of action for PNS inhibition exist in different types of animal models (i.e., spinal cord-intact and acute and chronic spinal-transected cats).
Previous studies in cats with an intact spinal cord have shown that TNS can inhibit AA-induced bladder reflex contractions (22). In the present study, after acute spinal cord transection at the T9/T10 level, TNS failed to inhibit AA-induced spinal reflex bladder contractions (Figs. 5 and 6). Therefore, it can be concluded that TNS requires supraspinal pathways to achieve the inhibition of afferent C-fiber-mediated bladder reflex. However, this does not mean that TNS inhibition cannot occur at the spinal cord level because tibial afferent input to supraspinal structures could, in turn, activate descending spinal pathways to inhibit the afferent C-fiber-mediated bladder reflex in the sacral spinal cord. Additional studies are clearly needed to further understand the neural pathways involved in tibial neuromodualtion.
In summary, this study demonstrated that nociceptive bladder afferent C-fibers can trigger reflex bladder contractions after acute spinal cord transection if they are activated by AA irritation. Furthermore, the experiments revealed that PNS- and TNS-induced neuromodulation of spinal reflex bladder activity can occur by very different mechanisms. Understanding the mechanisms underlying pudendal or tibial neuromodulation of the bladder reflex is important for treatment of OAB patients.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-094905, DK-090006, DK-102427, and DK-091253.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.X., M.J.R., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. conception and design of research; Z.X., M.J.R., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. performed experiments; Z.X., M.J.R., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. analyzed data; Z.X., M.J.R., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; Z.X., M.J.R., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. prepared figures; Z.X., M.J.R., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. drafted manuscript; Z.X., M.J.R., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; Z.X., M.J.R., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck PE, Victor A, Wein AJ. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21: 167–178, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE, Pehrson R. CNS involvement in overactive bladder: pathophysiology and opportunities for pharmacological intervention. Drugs 63: 2595–2611, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Chuang YC, Fraser MO, Yu Y, Beckel JM, Seki S, Nakanishi Y, Yokoyama H, Chancellor MB, Yoshimura N, de Groat WC. Analysis of the afferent limb of the vesicovascular reflex using neurotoxins, resiniferatoxin and capsaicin. Am J Physiol Regul Integr Comp Physiol 281: R1302–R1310, 2001 [DOI] [PubMed] [Google Scholar]
- 4.de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst Suppl 30: S71–S77, 1990 [DOI] [PubMed] [Google Scholar]
- 5.de Groat WC, Lalley PM. Reflex firing in the lumbar sympathetic outflow to activation of vesical afferent fibres. J Physiol 226: 289–309, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurons concerned with micturition in the cat. J Physiol 200: 87–108, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Groat WC, Saum WR. Sympathetic inhibition of the urinary bladder and of pelvic ganglionic transmission in the cat. J Physiol 220: 297–314, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groat WC, Saum WR. Synaptic transmission in parasympathetic ganglia in the urinary bladder of the cat. J Physiol 256: 137–158, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groat WC, Theobald RJ. Reflex activation of sympathetic pathways to vesical smooth muscle and parasympathetic ganglia by electrical stimulation of vesical afferents. J Physiol 259: 223–237, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol 235: 123–132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulur DM, Drake MJ. Management of overactive bladder. Nat Rev Urol 7: 572–582, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Häbler HJ, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425: 545–562, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Involvement of metabotropic glutamate receptor 5 in pudendal inhibition of nociceptive bladder activity in cats. J Physiol 589: 5833–5843, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindström S, Fall M, Carlsson CA, Erlandson BE. The neurophysiological basis of bladder inhibition in response to intravaginal electrical stimulation. J Urol 129: 405–410, 1983 [DOI] [PubMed] [Google Scholar]
- 16.Mazières L, Jiang C, Lindström S. The C fibre reflex of the cat urinary bladder. J Physiol 513: 531–541, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oefelein MG. Safety and tolerability profiles of anticholinergic agents used for the treatment of overactive bladder. Drug Saf 34: 733–754, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn 24: 643–647, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Peters KM, Killinger KA, Boguslawski BM, Boura JA. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol Urodyn 29: 1267–1271, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, Feagins BA. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 182: 1055–1061, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Tai C, Chen M, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC. Plasticity of urinary bladder reflexes evoked by stimulation of pudendal afferent nerves after chronic spinal cord injury in cats. Exp Neurol 228: 109–117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and non-nociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 302: F1090–F1097, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol 197: 225–234, 2006 [DOI] [PubMed] [Google Scholar]
- 24.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Lycklama á, Nijholt AA, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 178: 2029–2034, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Xiao Z, Reese J, Schwen Z, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of spinal GABAA receptors in pudendal inhibition of nociceptive and non-nociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 306: F781–F789, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]