Abstract
CD73 metabolizes extracellular 5′-AMP to adenosine; yet recent experiments in brain tissue suggest that CD73 is not required for the metabolism of 5′-AMP to adenosine because of tissue nonspecific alkaline phosphatase (TNAP), which like CD73 is a GPI-anchored ecto-enyzme with 5′-nucleotidase activity. Because adenosine importantly regulates renovascular function, we investigated whether both TNAP and CD73 are involved in the renovascular metabolism of 5′-AMP. To test this, we examined in isolated, perfused mouse kidneys the metabolism of 5′-AMP (applied to the lumen of the renal vasculature via intrarenal artery administration) to adenosine by measuring renal venous levels of 5′-AMP, adenosine, and inosine (adenosine metabolite) by mass spectrometry. In one study, we compared 5′-AMP metabolism in naive CD73+/+ (wild-type, n = 16) vs. CD73−/− (knockout, n = 16) kidneys; and in a second study, we compared 5′-AMP metabolism in CD73+/+ (n = 9) vs. CD73−/− (n = 8) kidneys pretreated with levamisole (1 mmol/l; TNAP inhibitor). In naive kidneys, 5′-AMP increased renal venous 5′-AMP, adenosine, and inosine, and these responses were similar in CD73+/+ vs. CD73−/− kidneys. Levamisole per se did not inhibit renovascular 5′-AMP metabolism; however, in the presence of levamisole, 5′-AMP increased renal venous 5′-AMP threefold more in CD73−/− vs. CD73+/+ kidneys and knockout of CD73 inhibited 5′-induced adenosine and inosine by 81 and 86%, respectively. TNAP mRNA, protein, and activity were similar in CD73+/+ vs. CD73−/− kidneys. In conclusion, CD73 and TNAP play interactive roles to metabolize luminally applied 5′-AMP in the renal vasculature such that inhibition of both is required to inhibit the production of adenosine.
Keywords: CD73, tissue nonspecific alkaline phosphatase, adenosine, inosine, 5′-AMP
cd73 is a glycosylphosphatidylinositol (GPI)-anchored ecto-5′-nucleotidase that resides on the cell surface and in most organ systems is critically involved in the conversion of extracellular 5′-AMP to adenosine (4). With regard to the kidney, CD73 participates in the production of adenosine that mediates tubuloglomerular feedback (3), provides renoprotective adenosine that renders the kidneys more able to tolerate acute ischemia-reperfusion (5), and produces adenosine in response to chronic exposure to angiotensin II leading to A2B receptor-induced chronic renal disease and hypertension (24). Surprisingly, however, preliminary experiments suggest that when exogenous 5′-AMP is delivered into the renal artery, the resulting increase in renal venous adenosine is normal in CD73−/− kidneys (9). Since adenosine output measured in the renal vein after administration of 5′-AMP into the renal artery likely reflects largely renovascular metabolism of adenosine, these findings suggest that alternative pathways of adenosine production may participate in the metabolism of 5′-AMP to adenosine in the luminal aspect of the renal vasculature. If true, this is an important concept because of the powerful renovascular effects of adenosine (21).
Like CD73, tissue nonspecific alkaline phosphatase (TNAP) is a GPI-anchored ecto-nucleotidase that can catalyze the conversion of 5′-AMP to adenosine and is the main isoform of alkaline phosphatase expressed in the kidney (25). Important work by Zhang and colleagues (23) demonstrates that TNAP mediates 5′-AMP conversion to adenosine in brains of CD73−/−, but not CD73+/+, mice and that TNAP can compensate for CD73 deficiency in the mouse brain. These facts motivate us to hypothesize that both CD73 and TNAP are coinvolved in the metabolism of 5′-AMP to adenosine in the renal vasculature and that inhibition or deletion of just one of either enzyme is insufficient to reduce renal vascular 5′-AMP metabolism because the alternative enzyme is present and provides an alternative metabolic route. Our objective here is to test this concept.
METHODS
Animals.
Male and female CD73−/− mice (3) (C57BL/6 × J129 background) and wild-type litter mates (CD73+/+ mice) were bred and genotyped (3) at the University of Pittsburgh. Mice used for experiments were 10 to 12 wk of age with similar numbers of male and female mice. Animals were housed at the University of Pittsburgh Animal Facility and fed Pro Lab RHM 3000 rodent diet (PMI Feeds, St. Louis, MO). The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Drugs.
Levamisole and 5′-AMP were obtained from Sigma (St. Louis, MO).
Isolated, perfused mouse kidney.
Mouse kidneys were isolated and perfused at a constant rate (1.5 ml/min) with Tyrode's solution as previously described by us (17). Kidneys were allowed to stabilize for 1 h before the protocols were initiated.
Analysis of purines.
5′-AMP, adenosine, and inosine were quantified using ultraperformance liquid chromatography-tandem mass spectrometry (LC-MS/MS) as previously described by us (17).
Real-time PCR for TNAP mRNA.
Total RNA was isolated from kidneys obtained from both CD73+/+ and CD73−/− mice using TRIzol (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. With the use of gene-specific primers for mouse TNAP (Qiagen Quantitect Primer Assay catalog number QT00157717, Gaithersburg, MD), and GAPDH (Qiagen, catalog number QT01658692), semiquantitative real-time PCR was performed using an Applied Biosystems 7900HT Real Time PCR System (Carlsbad, CA). There were four samples (all from separate mice) per genotype and each sample was run in duplicate for each primer pair tested. The duplicates were averaged and the TNAP mRNA expression was normalized for GAPDH levels. Comparisons between genotypes were performed using the 2−ΔΔCT method (12).
Western blotting.
Western blotting was performed as previously described (22) using a rabbit anti-TNAP antibody from Novus Biologicals (Littleton, CO; catalog number, NBP1–95392). β-Actin was used as a loading control using a rabbit anti-β-actin antibody from Cell Signaling Technology (Danvers, MA; catalog number 4970).
TNAP activity.
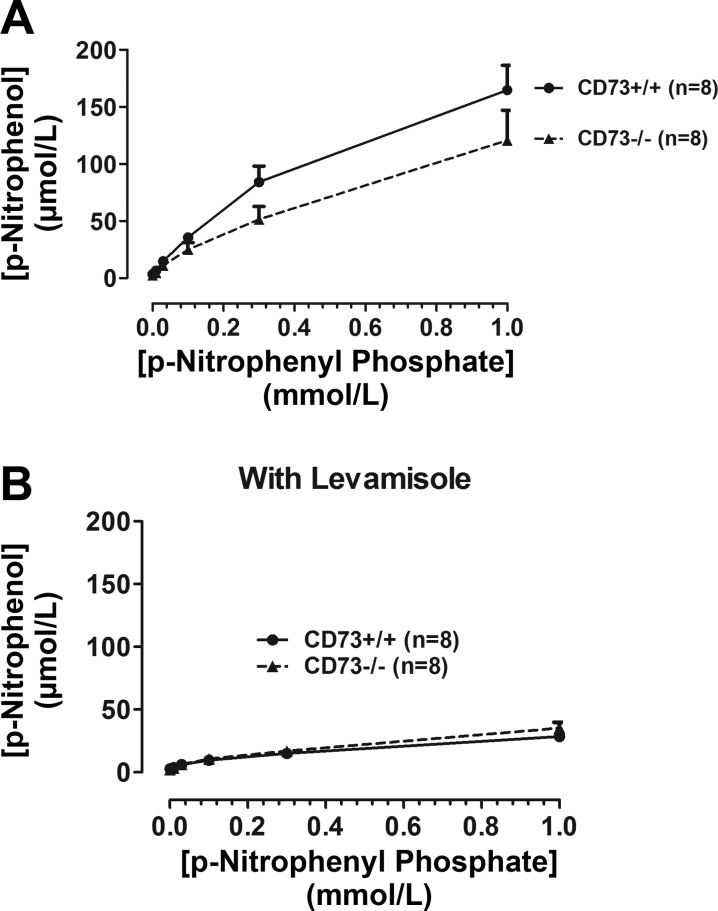
P-nitrophenyl phosphate was infused into CD73+/+ and CD73−/− kidneys to provide final concentrations in the renal artery of 0.01, 0.03, 0.1, 0.3, and 1 mmol/l (5 min at each concentration); and 1-min samples of renal venous perfusate were collected from 4 to 5 min into each infusion. After a 30-min washout period, this protocol was repeated in the presence of levamisole (1 mmol/l). TNAP converts p-nitrophenyl phosphate (colorless) to p-nitrophenol (yellow), which gives a strong absorbance signal at 405 nm (18). Therefore, absorbance at 405 nm was measured in the perfusate samples, and the levels of p-nitrophenol were calculated from a standard curve.
Protocol 1.
Kidneys from 16 CD73+/+ and 16 CD73−/− (n = 16) mice were isolated and perfused with Tyrode's solution. After 15 min, a basal 1-min sample of venous perfusate was collected and heat-inactivated for 90 s in boiling water to denature any enzymes present in the perfusate. Next, 5′-AMP was infused into the renal artery to provide a final concentration in the arterial perfuse of 10 μmol/l. Five minutes into the infusion of 5′-AMP, another 1-min sample of renal venous perfusate was obtained and heat-inactivated. Samples were stored at −80°C until analyzed by LC-MS/MS.
Protocol 2.
This protocol was as described for protocol 1, with the exceptions that the Tyrode's solution contained 1 mmol/l of levamisole and utilized kidneys from nine CD73+/+ and eight CD73−/−. The concentration of levamisole used completely inhibits TNAP activity (1, 2).
Statistics.
Data were analyzed by paired or unpaired Student's t-test as appropriate. The criterion of significance was P < 0.05. All values in text and figures are means ± SE.
RESULTS
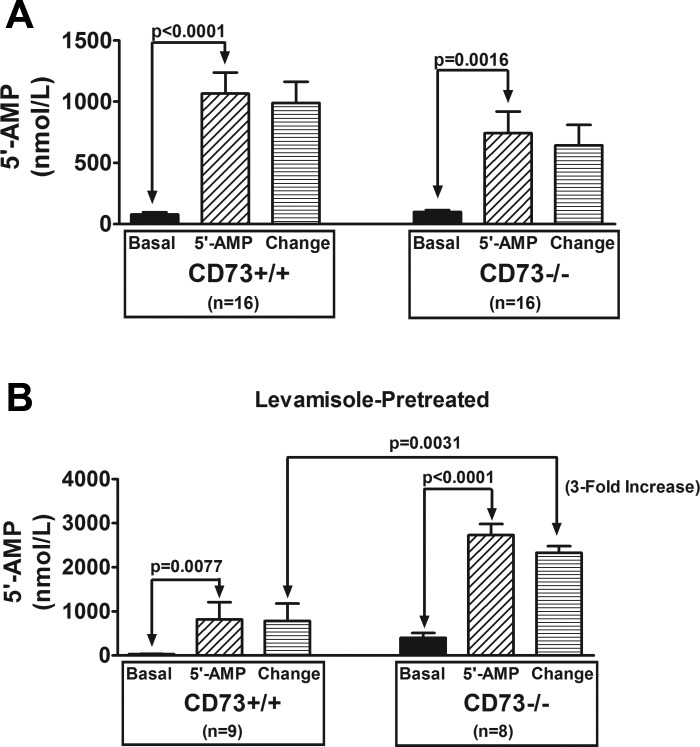
As shown in Fig. 1A, infusions of 5′-AMP into the renal artery of naive kidneys significantly increased renal venous perfusate levels of 5′-AMP in both CD73+/+ and CD73−/− kidneys. Since 5′-AMP was detected in the venous perfusate under basal conditions, we calculated the change in renal venous 5′-AMP concentrations by subtracting basal levels from levels measured during infusions of 5′-AMP. The change in renal venous 5′-AMP induced by administration of 5′-AMP into the renal artery was not significantly different between CD73+/+ vs. CD73−/− kidneys, even though the sample size was large (n = 16 per group). These data indicate that knocking out CD73 has little, if any, effect on the ability of the renal vasculature to extract 5′-AMP as the 5′-AMP passes from the renal artery to the renal vein.
Fig. 1.
Bar graphs show the levels of 5′-AMP in the renal venous perfusate in CD73+/+ and CD73−/− kidneys under basal conditions and during infusions of 5′-AMP into the renal artery (final concentration of 5′-AMP in arterial perfusate of 10 μmol/l). Also shown is the change in renal venous perfusate levels of 5′-AMP induced by 5′-AMP administration (i.e., 5′-AMP minus basal). A: kidneys were not pretreated with levamisole. B: kidneys were levamisole-pretreated (1 mmol/l; final concentration in the arterial perfusate). All values represent means ± SE.
In contrast to naive kidneys, in levamisole-pretreated kidneys the effect of knocking out CD73 on the renovascular extraction of 5′-AMP was striking (Fig. 1B). In this regard, although infusions of 5′-AMP into the renal artery significantly increased renal venous perfusate levels of 5′-AMP in both CD73+/+ and CD73−/− kidneys, the change induced by 5′-AMP administration was significantly greater (enhanced by threefold) in CD73−/− kidneys. These results show that when TNAP is inhibited, knocking out CD73 impairs the ability of the renal vasculature to extract 5′-AMP as the 5′-AMP passes from the renal artery to the renal vein.
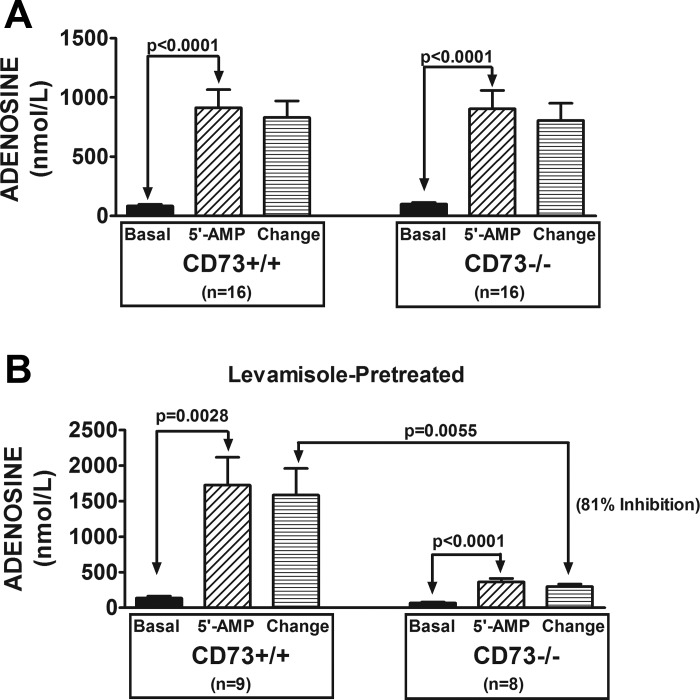
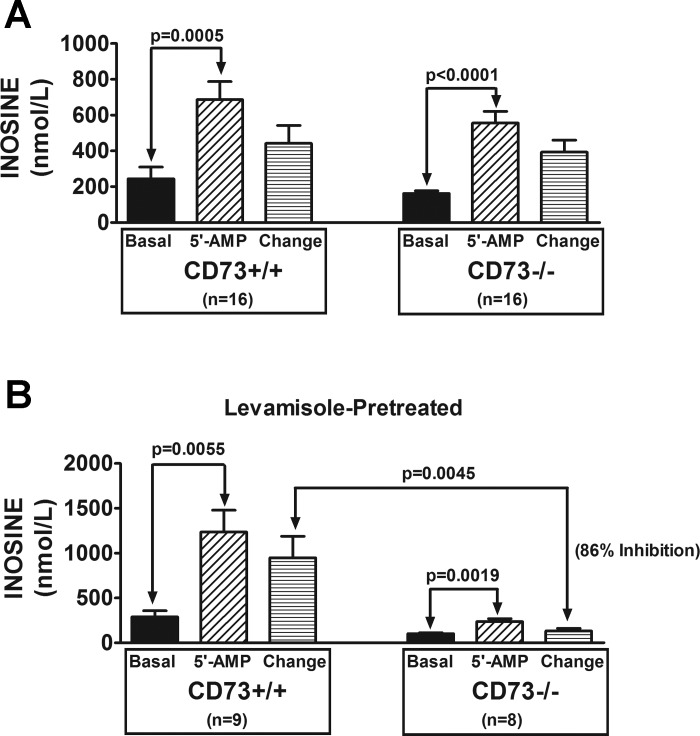
In naive kidneys, arterial infusions of 5′-AMP significantly increased renal venous perfusate levels of adenosine (Fig. 2A); however, this response was not affected by knocking out CD73. In contrast to naive kidneys, in levamisole-pretreated kidneys knocking down CD73 inhibited the 5′-AMP-induced renovascular production of adenosine by 81% (Fig. 2B). Similar results were observed for inosine, the primary downstream metabolite of adenosine. As shown in Fig. 3A, in naive kidneys, arterial infusions of 5′-AMP significantly increased renal venous perfusate levels of inosine; and this response was not affected by knocking out CD73. In contrast to naive kidneys, in levamisole-pretreated kidneys knocking down CD73 inhibited the 5′-AMP-induced renovascular production of inosine by 86% (Fig. 3B). These results show that deletion of CD73 has no effect on the renovascular metabolism of 5′-AMP to adenosine and inosine when TNAP is active; but when TNAP is blocked, knockdown of CD73 nearly abolishes adenosine/inosine production.
Fig. 2.
Bar graphs show the levels of adenosine in the renal venous perfusate in CD73+/+ and CD73−/− kidneys under basal conditions and during infusions of 5′-AMP into the renal artery (final concentration of 5′-AMP in arterial perfusate of 10 μmol/l). Also shown is the change in renal venous perfusate levels of adenosine induced by 5′-AMP administration (i.e., 5′-AMP minus basal). A: kidneys were not pretreated with levamisole. B: kidneys were levamisole-pretreated (1 mmol/l; final concentration in the arterial perfusate). All values represent means ± SE.
Fig. 3.
Bar graphs show the levels of inosine in the renal venous perfusate in CD73+/+ and CD73−/− kidneys under basal conditions and during infusions of 5′-AMP into the renal artery (final concentration of 5′-AMP in arterial perfusate of 10 μmol/l). Also shown is the change in renal venous perfusate levels of inosine induced by 5′-AMP administration (i.e., 5′-AMP minus basal). A: kidneys were not pretreated with levamisole. B: kidneys were levamisole-pretreated (1 mmol/l; final concentration in the arterial perfusate). All values represent means ± SE.
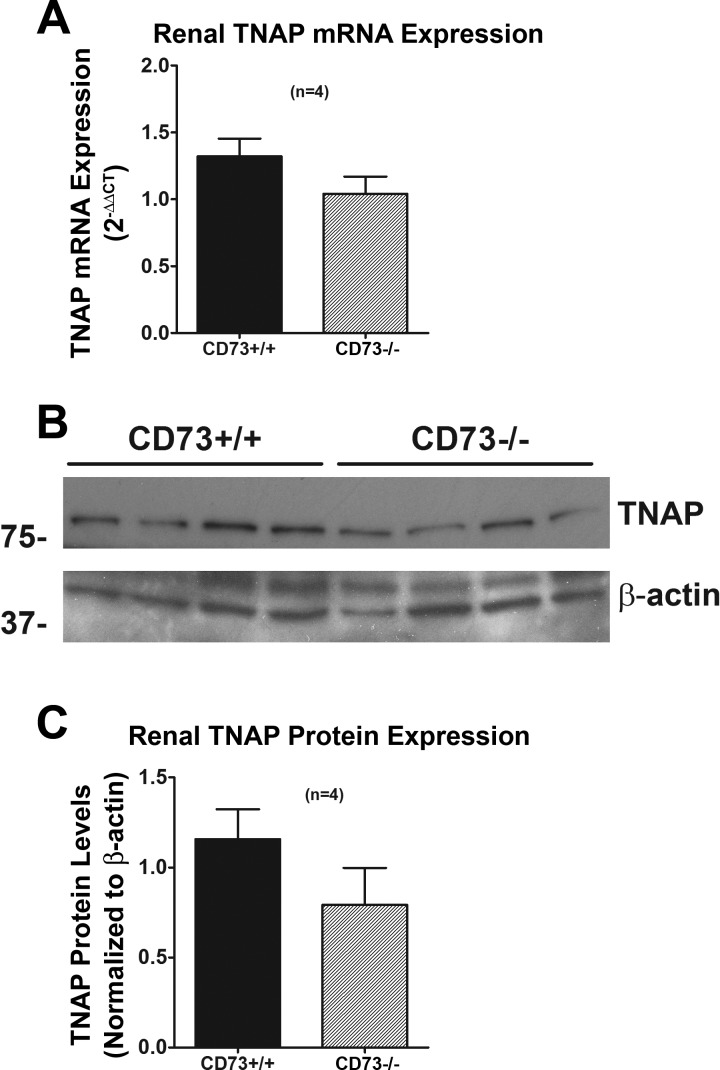
To determine whether lifelong deletion of CD73 affects the renal levels of TNAP, we measured TNAP mRNA and protein (Fig. 4). Neither TNAP mRNA nor protein expression was altered by knocking out CD73. Likewise, TNAP activity was similar in CD73+/+ vs. CD73−/− kidneys (Fig. 5). These results indicate that TNAP expression/activity is not upregulated by chronic deletion of CD73.
Fig. 4.
A: bar graph shows renal expression of tissue nonspecific alkaline phosphatase (TNAP) mRNA (by real-time PCR) in CD73+/+ vs. CD73−/− kidneys. B: Western blots show TNAP protein expression in CD73+/+ and CD73−/− kidneys. C: bar graph shows densitometry results from Western blots normalized to β-actin (loading control). All values represent means ± SE.
Fig. 5.
Bar graphs show the levels of p-nitrophenol in the renal venous perfusate during administration of p-nitrophenyl phosphate (TNAP substrate that is converted to p-nitrophenol) into the renal artery at 0.01, 0.03, 0.1, 0.3, and 1 mmol/l (5 min at each concentration). These experiments were performed in both CD73+/+ and CD73−/− kidneys both before (A) and after (B) administration of levamisole (1 mmol/l). All values represent means ± SE.
DISCUSSION
CD73 and TNAP have many characteristics in common. Both are ecto-enzymes, are GPI-anchored to cell membranes with the catalytic domains facing the extracellular space, contain metal ions (e.g., Zn2+), are glycosylated, have similar molecular weights, form homomeric dimers, are widely expressed in many tissues/cells, can be released as soluble forms, and can metabolize 5′-AMP to adenosine (25). Despite these similarities, investigators have focused nearly exclusively on CD73 as “the” adenosine-generating enzyme and have mostly ignored the possible contributions of TNAP in this regard.
Although the extant literature regarding the physiological role of TNAP in adenosine production is indeed sparse, there are several studies suggesting a contribution of TNAP to adenosine production. For example: 1) a report by Ohkubo and co-workers (13) suggests that the ecto-AMP phosphohydrolase activity of NG108–15 cells (cell line formed by fusing mouse neuroblastoma cells with rat glioma cells) is mediated by TNAP rather than CD73; 2) studies by Zhang and colleagues (23) demonstrate that 5′-AMP suppresses synaptic transmission similarly in brain slices from CD73+/+ vs. CD73−/− mice and that TNAP inhibition attenuates the effects of 5′-AMP only in CD73−/− brain slices; 3) Kuzhikandathil and co-workers (11) report that in the rat kidney 3′,5′-cAMP downregulates renal D1 receptor expression via a mechanism that is partially sensitive to the TNAP inhibitor levamisole; and 4) Pettengill et al. (14) report that both CD73 and TNAP are importantly involved in the generation of adenosine from 5′-AMP in human blood, particularly in neonatal blood. However, the role of CD73 vs. TNAP in the renal production of adenosine remains an open question.
Our interest in the role of TNAP in the renal production of adenosine originated from our prior work on the role of adenosine in renal sympathetic neurotransmission. In this regard, our previously published studies demonstrate that in isolated, perfused rat kidneys, pharmacological antagonism of A1 receptors attenuates renovascular responses to renal sympathetic nerve stimulation (10); and our subsequent experiments in isolated, perfused kidneys from A1 receptor null mice confirm the concept that renal sympathetic neurotransmission relies on A1 receptor signaling to provide for a full response to renal sympathetic activation (8). However, an examination of the effects of renal sympathetic nerve stimulation in CD73−/− vs. CD73+/+ kidneys reveals that CD73 is not required for normal renal sympathetic responses and apparently is not required for renal adenosine production (9). Taken together, our results suggest that renal adenosine production may be more complicated than just CD73 mediating the metabolism of 5′-AMP to adenosine.
The present study provides strong support for an important and interactive role for both CD73 and TNAP in the renovascular metabolism of 5′-AMP to adenosine. There are two main lines of evidence/reasoning for this conclusion. First, whether CD73 knockout alters the renovascular extraction of 5′-AMP depends on whether TNAP is inhibited. When TNAP is not inhibited, knockout of CD73 has no effect on the renovascular extraction of 5′-AMP, yet when TNAP is blocked, knockout of CD73 reduces renovascular extraction of 5′-AMP. Also, blockade of TNAP in CD73+/+ kidneys does not alter renovascular extraction of 5′-AMP, whereas TNAP inhibition does reduce renovascular extraction of 5′-AMP in CD73−/− kidneys. Second, whether CD73 knockout alters the renovascular production of adenosine and inosine from 5′-AMP depends on whether TNAP is inhibited. When TNAP is not inhibited, knockout of CD73 has no effect on adenosine/inosine production from 5′-AMP in the renal vasculature; yet when TNAP is blocked, knockout of CD73 reduces adenosine/inosine production. Moreover, in CD73+/+ kidneys, blockade of TNAP does not inhibit the renovascular production of adenosine/inosine from 5′-AMP, whereas TNAP inhibition does reduce renovascular production of adenosine/inosine in CD73−/− kidneys. Thus, the renovascular 5′-AMP → adenosine → inosine pathway is only inhibited when both CD73 and TNAP are blocked simultaneously. Together, these findings suggest that when the CD73 pathway is inhibited, the TNAP pathway compensates and vice versa.
The present work establishes that in the microenvironment of the luminal surface of the renal vasculature (likely mainly endothelial cells) neither CD73 nor TNAP per se is necessary for adenosine production from 5′-AMP. Apparently, in this situation lack of activity of one enzyme can be fully compensated for by activity of the other enzyme. However, we doubt that this is the case for all renal microenvironments. Specifically we hypothesize that in some renal microenvironments CD73 is primarily responsible for adenosine production from 5′-AMP and in other renal compartments TNAP primarily mediates the conversion of 5′-AMP to adenosine. And in yet other renal microenvironments although both CD73 and TNAP participate, loss of one activity is not fully compensated for by the activity of the other enzyme. This may be why Huang et al. (7) find that knockout of CD73 only attenuates, but does not abolish, the adenosine-dependent phenomenon of tubuloglomerular feedback.
It is conceivable that the situation in the kidney is even more complex than described above and involves an ensemble of enzymes that includes, but extends beyond, CD73 and TNAP. For example, Street and co-workers (20) demonstrate that in dorsal root ganglion neurons and spinal neurons knockout of CD73 decreases adenosine production by only 46% (at pH 7.4). However, simultaneous knockout of both CD73 and prostatic acid phosphatase (PAP) decreases adenosine production by 69%. Moreover, these investigators find that complete inhibition of adenosine biosynthesis can only be achieved by adding a TNAP inhibitor to neurons lacking both PAP and CD73 (19). It is noteworthy that in the present study, some adenosine was generated from 5′-AMP in levamisole-treated CD73−/− kidneys, suggesting the involvement of yet additional enzymes in the kidney.
An interesting difference between CD73 and TNAP is their interaction with ATP and ADP. In this regard, CD73 undergoes “feed-forward” inhibition by ATP and ADP (25). Consequently, when cells release ATP or ADP, CD73 is inhibited until ATP and ADP are mostly metabolized to 5′-AMP (for example, by CD39) (25). TNAP is known to metabolize ATP all the way to adenosine (ATP → ADP → 5′-AMP → adenosine) (25) and does not require the activity of CD39. Therefore, the ratio of importance of CD73 vs. TNAP in vivo may well depend on whether extracellular levels of ATP/ADP are high or low, with high ATP/ADP favoring the TNAP pathway and low ATP/ADP favoring the CD73 pathway. So the interactive roles of CD73 and TNAP may be temporally dynamic.
The ratio of importance of CD73 vs. TNAP may also depend on the presence or absence of hypoxia. As discussed by Poth and co-workers (16), hypoxia transcriptionally upregulates the expression of CD73 due to increased intracellular levels of hypoxia-inducible factor-1α. Whether this is true for TNAP is unknown. However, if hypoxia increases the expression of CD73, but not TNAP, the ratio of importance of CD73 vs. TNAP would also depend on the presence of hypoxia, which would favor the CD73 pathway.
What is the mechanism by which TNAP compensates for the lack of CD73 (and vice versa) in the renal vasculature? One possibility is that knockout of CD73 results in an increased expression of TNAP. However, this does not appear to be the case because the renal expressions of TNAP mRNA, protein, and activity do not significantly differ between CD73+/+ and CD73−/− kidneys. Another possibility is that when either CD73 is knocked out or TNAP is inhibited, 5′-AMP accumulates sufficiently behind the blocked pathway to accelerate the rate of adenosine production from the alternative pathway thus fully compensating for loss of one arm of the adenosine-producing mechanism. Full compensation would be expected as long as the alternative pathway is not saturated. The fact that TNAP can compensate for CD73 (and vice versa) when the 5′-AMP concentration is high (10 μmol/l) suggests that saturation of the alternative pathways with 5′-AMP is unlikely in vivo.
There are two important general implications of the current findings: 1) it is important to investigate nontraditional pathways of adenosine formation; and 2) in complex biological systems when a targeted pathway is obstructed genetically or pharmacologically, often alternate pathways are engaged. There are also several practical implications of our findings: 1) researchers should be aware that the lack of an effect of CD73 inhibition does not necessarily imply lack of a role for adenosine or adenosine receptors; 2) development of therapeutic strategies to manipulate adenosine production should include TNAP as a target; and 3) alkaline phosphatase per se could be used therapeutically to increase the local production of tissue-protective adenosine. Indeed, alkaline phosphatase is currently under investigation to protect against acute renal failure in patients with sepsis with encouraging preliminary findings (6, 15).
GRANTS
This work was supported by National Institutes of Health Grants DK091190, HL109002, HL069846, DK068575, and DK079307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.K.J. and P.M.K. conception and design of research; E.K.J. analyzed data; E.K.J. interpreted results of experiments; E.K.J. prepared figures; E.K.J. drafted manuscript; E.K.J. and P.M.K. edited and revised manuscript; E.K.J., D.C., J.D.V., K.J.-F., and P.M.K. approved final version of manuscript; D.C., J.D.V., and K.J.-F. performed experiments.
ACKNOWLEDGMENTS
We thank Dr. Jürgen B. Schnermann (National Institute of Diabetes and Digestive and Kidney Diseases) for contributing the breeding pairs of CD73 knockout mice that were used to establish a colony of these mice at the University of Pittsburgh.
REFERENCES
- 1.Borgers M. The cytochemical application of new potent inhibitors of alkaline phosphatases. J Histochem Cytochem 21: 812–824, 1973 [DOI] [PubMed] [Google Scholar]
- 2.Borgers M, Thone F. The inhibition of alkaline phosphatase by l-p-bromotetramisole. Histochemistry 44: 277–280, 1975 [DOI] [PubMed] [Google Scholar]
- 3.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest 114: 634–642, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv Pharmacol 61: 301–332, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Kohle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol 18: 833–845, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Heemskerk S, Masereeuw R, Moesker O, Bouw MPWJM, van der Hoeven JG, Peters WHM, Russel FGM, Pickkers P, Group AS. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit Care Med 37: 417–423, e411, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Huang DY, Vallon V, Zimmermann H, Koszalka P, Schrader J, Osswald H. Ecto-5′-nucleotidase (cd73)-dependent and -independent generation of adenosine participates in the mediation of tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol 291: F282–F288, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Jackson EK, Cheng D, Mi Z, Verrier JD, Janesko-Feldman K, Kochanek PM. Role of A1 receptors in renal sympathetic neurotransmission in the mouse kidney. Am J Physiol Renal Physiol 303: F1000–F1005, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson EK, Cheng D, Mi Z, Verrier JD, Janesko-Feldman K, Kochanek PM. Role of CD73 in renal sympathetic neurotransmission in the mouse kidney. Physiol Rep 1: e00057, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson EK, Cheng D, Tofovic SP, Mi Z. Endogenous adenosine contributes to renal sympathetic neurotransmission via postjunctional A1 receptor-mediated coincident signaling. Am J Physiol Renal Physiol 302: F466–F476, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuzhikandathil EV, Clark L, Li Y. The extracellular cAMP-adenosine pathway regulates expression of renal D1 dopamine receptors in diabetic rats. J Biol Chem 286: 32454–32463, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods (Duluth) 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Ohkubo S, Kimura J, Matsuoka I. Ecto-alkaline phosphatase in NG108–15 cells: a key enzyme mediating P1 antagonist-sensitive ATP response. Br J Pharmacol 131: 1667–1672, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettengill M, Robson S, Tresenriter M, Millan JL, Usheva A, Bingham T, Belderbos M, Bergelson I, Burl S, Kampmann B, Gelinas L, Kollmann T, Bont L, Levy O. Soluble ecto-5′-nucleotidase (5′-NT), alkaline phosphatase, and adenosine deaminase (ADA1) activities in neonatal blood favor elevated extracellular adenosine. J Biol Chem 288: 27315–27326, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickkers P, Heemskerk S, Schouten J, Laterre PF, Vincent JL, Beishuizen A, Jorens PG, Spapen H, Bulitta M, Peters WHM, van der Hoeven JG. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care 16: R14, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poth JM, Brodsky K, Ehrentraut H, Grenz A, Eltzschig HK. Transcriptional control of adenosine signaling by hypoxia-inducible transcription factors during ischemic or inflammatory disease. J Mol Med 91: 183–193, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren J, Mi ZC, Jackson EK. Assessment of nerve stimulation-induced release of purines from mouse kidneys by tandem mass spectrometry. J Pharmacol Exp Ther 325: 920–926, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Sergienko EA, Millan JL. High-throughput screening of tissue-nonspecific alkaline phosphatase for identification of effectors with diverse modes of action. Nat Protoc 5: 1431–1439, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Street SE, Kramer NJ, Walsh PL, Taylor-Blake B, Yadav MC, King IF, Vihko P, Wightman RM, Millan JL, Zylka MJ. Tissue-nonspecific alkaline phosphatase acts redundantly with PAP and NT5E to generate adenosine in the dorsal spinal cord. J Neurosci 33: 11314–11322, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Street SE, Walsh PL, Sowa NA, Taylor-Blake B, Guillot TS, Vihko P, Wightman RM, Zylka MJ. PAP and NT5E inhibit nociceptive neurotransmission by rapidly hydrolyzing nucleotides to adenosine. Mol Pain 7: 80, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 86: 901–940, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Verrier JD, Jackson TC, Gillespie DG, Janesko-Feldman K, Bansal R, Goebbels S, Nave KA, Kochanek PM, Jackson EK. Role of CNPase in the oligodendrocytic extracellular 2′,3′-cAMP-adenosine pathway. Glia 61: 1595–1606, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D, Xiong W, Chu S, Sun C, Albensi BC, Parkinson FE. Inhibition of hippocampal synaptic activity by ATP, hypoxia or oxygen-glucose deprivation does not require CD73. PLos One 7: e39772, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R, Sun K, Glover L, Grenz A, Sun H, Tao L, Zhang W, Colgan SP, Blackburn MR, Eltzschig HK, Kellems RE, Xia Y. Elevated ecto-5′-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res 112: 1466–1478, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8: 437–502, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]