Abstract
Partial bladder outlet obstruction (pBOO)-induced remodeling of bladder detrusor smooth muscle (DSM) is associated with the modulation of cell signals regulating contraction. We analyzed the DSM from obstructed murine urinary bladders for the temporal regulation of RhoA GTPase and Rho-activated kinase (ROCK), which are linked to Ca2+ sensitization. In addition, the effects of equibiaxial cell stretch, a condition thought to be associated with pBOO-induced bladder wall smooth muscle hypertrophy and voiding frequency, on the expression of RhoA, ROCK, and C-kinase-activated protein phosphatase I inhibitor (CPI-17) were investigated. DSM from 1-, 3-, 7-, and 14-day obstructed male mice bladders and benign prostatic hyperplasia (BPH)-induced obstructed human bladders revealed overexpression of RhoA and ROCK-β at the mRNA and protein levels compared with control. Primary human bladder myocytes seeded onto type I collagen-coated elastic silicone membranes were subjected to cyclic equibiaxial stretch, mimicking the cellular mechanical stretch in the bladder in vivo, and analyzed for the expression of RhoA, ROCK-β, and CPI-17. Stretch caused a significant increase of RhoA, ROCKβ, and CPI-17 expression. The stretch-induced increase in CPI-17 expression occurs at the transcriptional level and is associated with CPI-17 promoter binding by GATA-6 and NF-κB, the transcription factors responsible for CPI-17 gene transcription. Cell stretch caused by bladder overdistension in pBOO is the likely mechanism for initiating overexpression of the signaling proteins regulating DSM tone.
Keywords: partial bladder outlet obstruction, cell stretch, human bladder, benign prostatic hyperplasia, calcium sensitization
detrusor smooth muscle (DSM) hypertrophy is associated with alteration of the signaling pathways that initiate and maintain force in the bladder wall smooth muscle during partial bladder outlet obstruction (pBOO). These alterations have been observed in animal models and men with benign prostatic hyperplasia (BPH)-induced obstruction (7, 9, 14, 19). In both animal models and humans, removal of the obstruction causes regression of smooth muscle hypertrophy, and the bladder function returns relatively to normal in most instances. However, in some cases, bladder function does not return to normal, and the reason for this is unclear. It is most likely due to the failure of altered signaling mechanisms to reverse back to normal, as seen in incomplete reversal of myosin isoform expression after 2-wk pBOO in rabbits (14).
In general, activation of smooth muscle requires phosphorylation of the myosin regulatory light chain (MLC20) by Ca2+/calmodulin-dependent myosin light chain kinase (MLCK) (2, 45). Under certain physiological conditions, smooth muscles have been shown to produce tone without an increase in cytosolic Ca2+ (43). This is achieved by lowering myosin light chain phosphatase (MLCP) activity through phosphorylation of the MLCP regulatory subunit (MYPT1) by Rho-associated protein kinase (ROCK). The low MLCP activity reduces MLC20 dephosphorylation, resulting in the maintenance of phosphorylated myosin. This causes the muscle to develop and maintain force at low Ca2+ concentrations. This mechanism, termed calcium sensitization, is believed to play an important role in maintaining the basal smooth muscle tone, especially in visceral smooth muscles (30, 44). In addition, Thr38 phosphorylation of C-kinase-activated protein phosphatase I inhibitor (CPI-17) by protein kinase C (PKC) in response to agonists inhibits MLCP activity (16), altering the levels of myosin light chain phosphorylation and Ca2+ sensitivity in smooth muscle.
We previously reported differential, PKC-mediated DSM contraction with corresponding alterations of PKC expression, activity, and CPI-17 phosphorylation in obstructed bladders, depending on whether the bladder function is compensated or decompensated (11). CPI-17 expression was decreased upon silencing of the GATA-6 transcription factor in cultured bladder smooth muscle (BSM) cells and in BSM from NF-κB knockout (KO) mice (9). Moreover, force maintenance by BSM strips from KO mice was decreased compared with wild-type mice. GATA-6 and NF-κB overexpression was associated with CPI-17 overexpression in BSM from men with BPH-induced bladder hypertrophy and the pBOO mouse model (9). These results indicated that transcription factors NF-κB and GATA-6 are involved in the activation of CPI-17 gene expression; however, the initial stimulus for this transcriptional activation remains to be established.
The GATA family of zinc finger transcription factors plays an essential role in cardiac- and smooth muscle-specific gene transcription (10, 22, 26, 50). The vertebrate GATA transcription factors share a highly conserved domain composed of two zinc fingers, and this domain is responsible for specific binding to a consensus motif 5′-GATA-3′ in target gene promoters (25, 34, 39). GATA-6 directly controls the expression of various contractile and signaling proteins in smooth muscles (9, 22, 42). GATA-6 protein was shown to be associated with pathological cardiac and smooth muscle hypertrophy (8, 46). NF-κB subunit c-Rel stimulates cardiac hypertrophy and fibrosis (17). In smooth muscle, NF-κB and GATA-6 directly control the expression of proteins involved in calcium sensitization, and aberrant expression of these transcription factors deregulates these proteins, thereby inhibiting smooth muscle contraction during smooth muscle remodeling and hypertrophy associated with pathological conditions (9).
Urodynamic overload appears to be an underlying stimulus for structural and functional changes in obstructed bladders. Clinical pressure-flow studies show that high-pressure voiding is consistently observed in patients with BPH-induced pBOO (32). Studies from the pBOO rat model showed significantly increased micturition pressure in obstructed bladders compared with controls (27). An increase in bladder wall tension and mechanical stretch is considered to be the trigger that induces both the hypertrophic structural response and functional alterations observed following bladder obstruction (51). During filling and emptying, the bladder is subjected to cyclic physical deformation of the detrusor myocytes. The in vitro model for the urodynamic load increases observed in detrusor myocytes under pBOO conditions is repetitive stretching and relaxation applied to cultured BSM cells (4, 52).
In this study, we show that the temporal increase of CPI-17 expression in mice following surgical pBOO and men with BPH-induced obstruction (9) is associated with overexpression of the small GTPase RhoA and ROCK, which are also linked to Ca2+ sensitization. In addition, we determined the effect of cyclic equibiaxial stretch, a condition thought to be associated with pBOO-induced bladder distention, on the expression of these signaling proteins in bladder myocytes in culture. Stretch induced a significant increase of RhoA, ROCK-β, and CPI-17 expression in cultured human detrusor myocytes. The stretch-induced increase in CPI-17 expression is also associated with CPI-17 promoter binding by GATA-6 and the p50 and c-Rel subunits of NF-κB to chromatin. Our data suggest that cell stretch caused by bladder overdistension in pBOO causes CPI-17 gene activation and is the likely stimulus that initiates the overexpression of the signaling proteins regulating DSM tone.
MATERIALS AND METHODS
Surgical induction of pBOO in the mouse model.
All the procedures for creating pBOO were approved by the Institutional Animal Care and Use Committees (IACUC) at the Children's Hospital and the University of Pennsylvania. Adult male mice were subjected to partial surgical ligation of the urethra as described previously (3). Sham-operated animals underwent an identical procedure until the suture was tied down, and then the suture was removed and the abdomen closed. At the desired time points following pBOO, the mice were euthanized, the bladders harvested, and the DSM layers separated from the mucosa and serosa for biochemical and molecular biological studies.
Human bladder samples.
After obtaining institutional review board approval (University of Pennsylvania, IRB 803645), frozen bladder tissue samples were obtained from Dr. Gomes of the University of Sao Pãolo, Brazil (IRB protocol no. 811/04). The tissue samples were identified only by age and disease. Bladder tissue biopsy specimens were collected from patients aged 62 to 78 yr, who underwent suprapubic prostatectomy to treat BPH. All patients had severe lower urinary tract symptoms and were preoperatively characterized as having overt bladder outlet obstruction. Bladder outlet obstruction was assessed via multichannel urodynamics using the bladder outlet obstruction index. Patients were characterized as having an obstruction when the bladder outlet obstruction index was >40. All definitions conform to the standardized terminology of the International Continence Society. Control BSM samples were acquired from patients undergoing ureteral reimplantation and from nondiseased bladder tissue from patients with bladder cancer undergoing cystectomy. The reasons for ureteral reimplantation in the control group were distal ureteral stenosis and vesicoureteral reflux in one patient each. No patients in the control group exhibited voiding symptoms, had an American Urological Association symptom score higher than 8, or had clinical symptoms of BPH. Age-matched control individuals were used for comparison with the BPH group.
RNA extraction and RT-PCR.
RNA was extracted from bladder smooth muscle tissue devoid of mucosa and serosa and from cultured human BSM cells using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The cDNA synthesis was carried out using standard procedures as described previously (9).
Protein extraction and immunoblot analysis.
Murine BSM tissue devoid of mucosa and serosa and human BSM samples were pulverized into a fine powder. Protein was extracted from tissues and analyzed by Western blot using standard protocols (36). Briefly, the total protein from each sample was separated by SDS-PAGE and transferred to PVDF membranes (Millipore, Bedford, MA). The membranes were incubated with a rabbit polyclonal anti-CPI-17 (US Biological, Swampscott, MA), RhoA, and ROCK-β (Sigma-Aldrich, St Louis, MO). Immunoreactive proteins were visualized with an anti-rabbit, peroxidase-conjugated, secondary antibody using Super Signal Reagent (Pierce, Rockford, IL). Equal loading between lanes was confirmed by probing the membranes after stripping with an anti-GAPDH antibody (Abcam, San Francisco, CA).
Mechanical cell stretching.
Primary human bladder detrusor myocytes were obtained from Lonza (Walkersville, MD) and grown in smooth muscle growth-2 medium at a cell density of 1 × 105 cells/cm2. The cells were grown on a type I collagen-coated silicon membrane (Specialty Manufacturing, Saginaw, MI). Mechanical deformation of the cells was performed using a Tensorcell III, a device designed to apply precise and reproducible biaxial strains (supplied by CT Group, Crested Butte, CO) to the membrane on which the cells were grown (18). It has previously been shown that the cells experience essentially the same deformations as the substrate on which they are grown (5). Design, calibration, and description of the equibiaxial strain system for cultured cells have been previously reported (20). The cells were seeded (1 × 105 cells/well) and incubated for 24 and 48 h in nutrient medium. For experimental wells, biaxial strain was applied by stretching the elastic membrane cyclically for 24 and 48 h to provide 2.5% strain. Details of the strain level estimation, the design of the Tensor stretch instrument, and computer program are based on published data (18, 48). The strain level of 2.5% was used in this study because this condition was found to be sufficient to produce molecular changes without inducing apparent cell injury (12). For controls, cells were cultured in the apparatus wells under the same conditions but without subjecting them to mechanical strain. After completion of the stretch regimen, control and stretched cells were scraped off and processed for RNA and protein analyses. Cells from at least six individual wells were pooled to extract sufficient quantities of RNA for analysis. Stretch and control experiments were processed simultaneously and analyzed identically.
Immunohistochemistry and confocal microscopy.
The frozen BSM tissue samples from normal and pBOO mice and from healthy and BPH-induced human bladder were used to prepare 5-μm-thick frozen sections and mounted on a glass slide. Immunohistochemistry of the bladder sections was carried out as described (53). Antibodies against smooth muscle-specific markers SM22 and myosin heavy chain were used to determine the smooth muscle-specific localization of these proteins in murine and human bladders, respectively. The rabbit polyclonal antibodies RhoA and ROCK-β (Sigma-Aldrich) were used as the primary antibodies for the immunofluorescence analysis. The secondary antibodies used were anti-rabbit Cy3-conjugated and anti-mouse and anti-goat Alexa Fluor 488-conjugated antibodies (Invitrogen), anti-mouse smooth muscle myosin heavy chain (Sigma-Aldrich), and anti-goat SM22 (Abcam). Negative-control sections were prepared using antibody preabsorbed with the antigen used to raise the antibody (data not shown). Laser-scanning confocal immunofluorescence microscopy was performed at room temperature using a Confocal microscope (Fluoview FV 1000; Olympus) and a ×60 oil immersion, numerical aperture 1.45 lens to determine the localization of RhoA and ROCK-β in murine and human BSM tissues. Images were captured as single acquisitions or as a series of time-lapse Z-stacks using Fluoview FV10-ASW software (Olympus) and imported into Image J (National Institutes of Health) using LOCI Bio-Formats for Image J. The Cy3 fluorescence emission was imaged using the red channel. The FITC fluorescence emission of SM22 (a smooth muscle-specific protein) was detected using the green channel. The nuclei were stained with DAPI imaged using the blue filter.
Preparation of nuclear extracts.
Nuclear extracts from stretched and nonstretched BSM were prepared essentially, as previously described (40). Briefly, cells were washed with phosphate-buffered saline and resuspended in ice-cold buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EGTA, 0.1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride). The cells were allowed to swell on ice for 15 min, lysed with 1% (vol/vol) Nonidet P-40, and centrifuged. The nuclear pellet was resuspended in a buffer containing 20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride and rocked on a mixer at 4°C for 15 min. The nuclear extract was centrifuged, and the supernatant was frozen in aliquots and stored at −70°C.
Transcription factor ELISA.
Transcription factor (TF) ELISAs are DNA-binding ELISAs that quantify the specific transcription factor-DNA interactions in mammalian tissue and cell extracts (6, 28, 38). In this assay, activated NF-κB p50 or GATA-6 protein from the nuclear extracts binds to its consensus binding site on biotinylated oligonucleotides. These oligonucleotides are then immobilized on a streptavidin-coated 96-well plate. The NF-κB p50 or GATA-6 bound to the oligonucleotides is probed with an antibody directed against NF-κB p50 or GATA-6 followed by horseradish peroxidase (HRP)-conjugated secondary antibody. HRP reacts with chromogenic substrate to provide a sensitive colorimetric readout, which is quantified by microplate reader (spectrophotometer) at 450 nm. In this experiment, nuclear extracts (5 μg) were analyzed by an Affymetrix Transcription Factor ELISA assay to determine the NF-κB p50 or GATA-6 DNA binding activity, as described by the manufacturer (Affymetrix, Santa Clara, CA). Briefly, nuclear extracts isolated from stretched and nonstretched cells were incubated with biotinylated NF-κB or GATA-6 consensus DNA sequences in a transcription assay buffer to allow the formation of protein/DNA complexes. In parallel, oligonucleotide competition experiment was performed using the indicated fold excess of unlabeled wild-type and mutant oligonucleotides to determine the specificity of DNA binding to NF-κB and GATA-6. The reaction mixture was preincubated with competitor DNA before the addition of the biotin-labeled oligonucleotide probe. NF-κB or GATA-6 protein in the nuclear extracts bound to biotinylated consensus sequences was immobilized to the streptavidin-coated plate, and any unbound material was washed away. NF-κB or GATA-6 protein was probed with primary antibody, followed by incubation with HRP-conjugated secondary antibody. The protein-DNA complex was detected by chromogenic substrate. Absorbance of the samples was measured at 450 nm using microplate reader (Bio-Tek I Spectrophotometer Instruments, Winooski, VT).
Chromatin immunoprecipitation assay.
Chromatin immunoprecipitation (ChIP) assay is used to identify the interaction of the transcription factors with a specific DNA sequence under certain conditions in vivo. In cultured cells, proteins that interact with the DNA are crosslinked by formaldehyde, and the antibody is used to capture a specific DNA-interacting protein. The isolated DNA can be quantified by PCR. We made an attempt to identify the transcription factors that are recruited to CPI-17 promoter in BSM cells in stretch and nonstretched condition. ChIP assays were performed on cultured BSM cells from stretched and control as described previously (33). Chromatin samples (200 to 600 bp) were immunoprecipitated overnight at 4°C with antibodies specific for NF-κB p50, c-Rel, or GATA-6 (Abcam) or normal rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) using protein G magnetic beads. The beads were collected, and the DNA was eluted as described previously (33). An aliquot of sheared chromatin was purified using the QIAquick PCR purification kit (Qiagen, Valencia, CA) and used as an input control. All samples were treated with proteinase K (1 mg/ml) for 1 h and subjected to PCR. The PCR products were separated by agarose gel electrophoresis, DNA band intensities were quantified, and the data analyzed by normalization to the corresponding input values.
Transient transfection and promoter activity assays.
Promoter activity was measured using the reporter gene assay. The firefly luciferase gene that served as a reporter in this assay was cloned downstream of the CPI-17 promoter (9) in a promoterless DNA vector. The luciferase enzyme is synthesized after transient transfection of the CPI-17 promoter plasmid in cells only if the upstream DNA promoter sequences drive the transcription of the luciferase gene. The ability of the cloned CPI-17 promoter sequence to be activated by mechanical stretch is proportional to the amount of light produced by the oxidation of luciferin by luciferase in the presence of ATP in an in vitro reaction performed using cell extracts. Cotransfection with Renilla luciferase control reporter, expressed from a constitutive promoter, permits normalization for experimental variations such as cell number. We have used this promoter reporter assay to identify the cis-acting elements necessary for transcriptional induction of the CPI-17 promoter in response to mechanical stretch in BSM cells. We generated CPI-17 promoter mutant constructs, and these constructs were fused to firefly luciferase and used for transfection. The dual luciferase assay was used to quantify the firefly and Renilla luciferase enzyme activities in transfected cells.
The construction and mutagenesis of murine CPI-17 promoter was described in our earlier publication (9). GATA-6, NF-κB p50, and c-Rel cDNA were obtained from OriGene Technologies (Rockville, MD). Human primary BSM cells were obtained from Lonza. Plasmids were transfected into human primary BSM cells by electroporation using Amaxa Nucleofector II (Lonza Walkersville) according to the manufacturer's instructions. RhoA expression cDNA construct that encodes for the RhoA protein was transfected into human primary BSM cells by electroporation. The total proteins isolated from RhoA-transfected human BSM cells and purified CPI-17 recombinant protein were used as a positive control in immunoblot analysis. Firefly and Renilla luciferase activities were determined using a dual-luciferase reporter assay system (Promega, Madison, WI). Cleared extracts (100 μl per well) were prepared using the passive lysis buffer provided, and luciferase activity was measured according to the manufacturer's instructions. Initially, firefly luciferase activity was measured followed by the Renilla luciferase luminescence. The data are represented as the ratio of firefly to Renilla luciferase activity (relative Luciferase activity).
Statistical analysis.
Where appropriate, comparisons between control and experimental groups were performed using the Student's t-test. A P value of <0.05 was considered statistically significant. We used one-way ANOVA from GraphPad Prism software for multiple sample comparisons. A P value of <0.05 was considered statistically significant.
RESULTS
Temporal changes in RhoA expression following pBOO in mouse DSM.
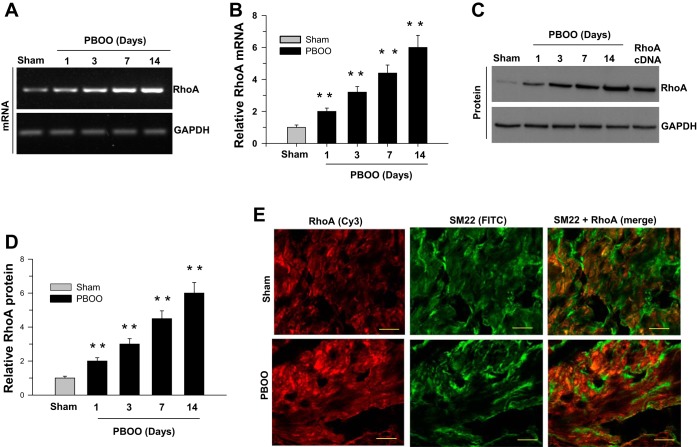
The temporal changes in RhoA expression following pBOO were analyzed at the mRNA and protein level (Fig. 1). RhoA expression increases postobstruction, both at the mRNA (Fig. 1, A and B) and protein levels (Fig. 1, C and D). At day 14, there is a fivefold increase in the RhoA expression when normalized to GAPDH. The RhoA protein is localized in smooth muscle cells, as determined by colocalization with SM22 (Fig. 1E). The immunoreactivity for RhoA in the DSM is higher in pBOO tissues compared with sham-operated mice.
Fig. 1.
Overexpression of RhoA in the partial bladder outlet obstruction (pBOO) murine model. RT-PCR (A and B) and immunoblot (C and D) analysis of RhoA mRNA and protein expression in sham-operated and pBOO mouse detrusor smooth muscle (DSM) tissues. GAPDH served as a loading control. B and D: quantification of RT-PCR (A) and immunoblot (C) data presented as the means ± SD (n = 10; 10 sham-operated and 10 pBOO mice DSM were used in this experiment). **P < 0.01 compared with the results for sham-operated control mice. E: murine bladder sections prepared from sham-operated and pBOO mice were stained with anti-RhoA (Cy3, red) and anti-SM22 (FITC, green) antibodies. Scale bars = 20 μm.
Temporal changes in ROCK expression following pBOO in mouse DSM.
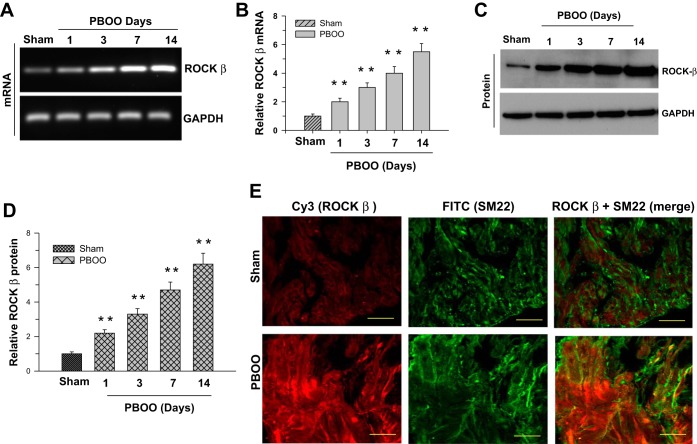
Figure 2 depicts the ROCK-β expression at the mRNA (Fig. 2, A and B) and protein levels (Fig. 2, C and D). ROCK-β expression continues to rise 1 day following obstruction. The expression difference between the 7-day and 14-day obstruction is reduced compared with the change between the 1-day and 7-day obstruction, indicating that the increased expression is leveling off after 14 days. The increased ROCK-β expression is localized in the smooth muscle cells (Fig. 2E). The number of ROCK-β-positive smooth muscle cells in obstructed mice bladder walls is higher than in the controls as shown in the immunofluorescence images.
Fig. 2.
Overexpression of Rho-activated kinase (ROCK)-β in the pBOO murine model. RT-PCR (A and B) and immunoblot (C and D) analysis of ROCK-β mRNA and protein expression in sham-operated and pBOO mouse DSM tissues. GAPDH served as a control. B and D: quantification of RT-PCR (A) and immunoblot (C) data presented as the means ± SD (n = 10; 10 sham-operated and 10 pBOO mice DSM were used in this experiment). **P < 0.01 compared with the results for sham-operated control mice. E: murine bladder sections prepared from sham-operated and pBOO mice were stained with anti-ROCK-β (Cy3, red) and anti-SM22 (FITC, green) antibodies. Scale bars = 20 μm.
RhoA and ROCK-β overexpression in the DSM from men with BPH-induced bladder outlet obstruction.
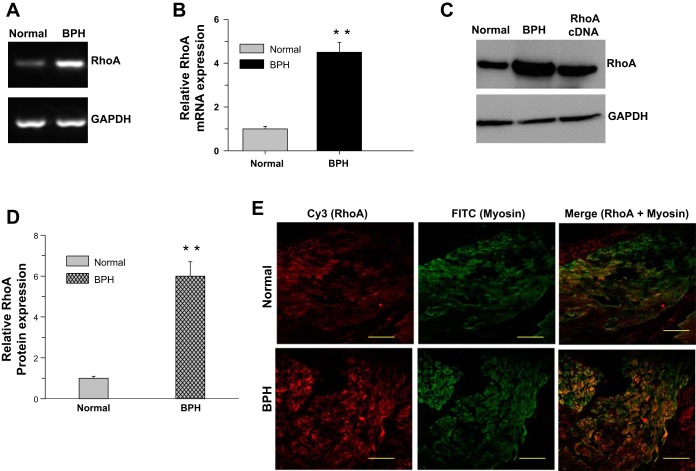
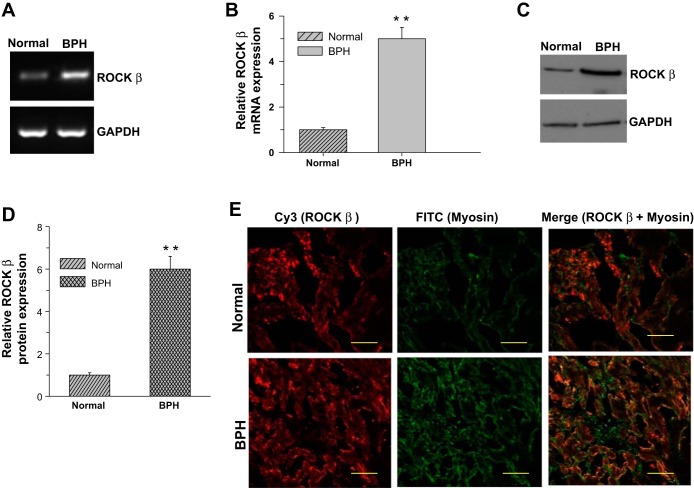
BPH induces DSM hypertrophy because of bladder outlet obstruction. To see whether the altered Ca2+ sensitization pathway protein expression in the pBOO mouse model is also present in the DSM from human bladders, bladder tissue samples were collected following open prostatectomy for the removal of enlarged (noncancerous) prostates. The expression of RhoA (Fig. 3, A and B) and ROCK-β (Fig. 4, A and B) mRNA is increased approximately fivefold. A similar increase in RhoA (Fig. 3, C and D) and ROCK-β (Fig. 4, C and D) protein expression was observed in the smooth muscle from obstructed bladders compared with controls. The expression of these proteins in the DSM was tested by immunostaining against smooth muscle-specific myosin and RhoA or ROCK-β. The immunostained DSM sections were examined using confocal microscopy. Both RhoA (Fig. 3E) and ROCK-β (Fig. 4E) are localized to myosin-positive cells, confirming that the immunostained cells are smooth muscle cells. The expression of these proteins is more prominent in the DSM from obstructed bladders (Figs. 3E, bottom, and Fig. 4E, bottom) compared with controls (Figs. 3E, top, and Fig. 4E, top).
Fig. 3.
RhoA overexpression in DSM from human bladders with benign prostatic hyperplasia (BPH)-induced bladder outlet obstruction. A–D: RNA and protein samples prepared from DSM of control and patients with BPH were subjected to RT-PCR and immunoblot analysis for RhoA. GAPDH was used as a loading control. B and D: quantification of RT-PCR (A) and immunoblot (C). Data are presented as the means ± SD (n = 6; 6 normal and 6 obstructed human bladders were used in this experiment). **P < 0.01 compared with the results for control (normal) human DSM. E: human bladder sections prepared from control and patients with BPH were stained with anti-RhoA (Cy3, red) and anti-smooth muscle myosin heavy chain (SMHC) (FITC, green) antibodies. Scale bars = 20 μm.
Fig. 4.
ROCK-β overexpression in DSM from human bladders with BPH-induced bladder outlet obstruction. A–D: RNA and protein samples prepared from DSM of control and patients with BPH were subjected to RT-PCR and immunoblot analysis for ROCK-β. GAPDH was used as a loading control. B and D: quantification of RT-PCR (A) and immunoblot (C). Data are presented as the means ± SD (n = 6; 6 normal and 6 obstructed human bladders were used in this experiment). **P < 0.01 compared with the results for control human DSM. E: human bladder sections prepared from control and patients with BPH were stained with anti-ROCK-β (Cy3, red) and anti-SMHC (FITC, green) antibodies. Scale bars = 20 μm.
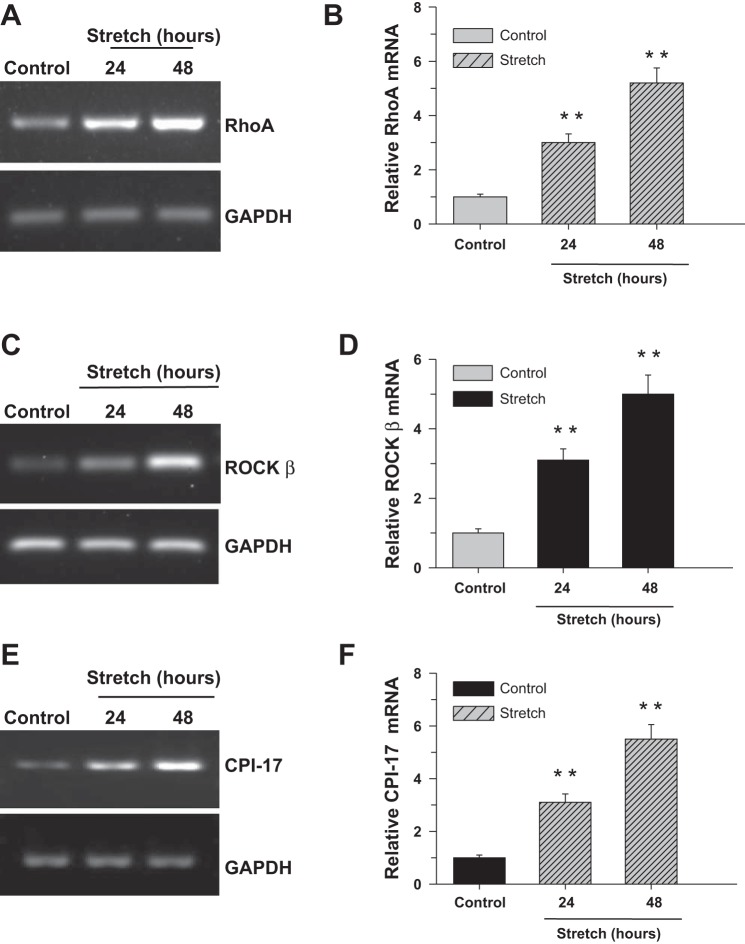
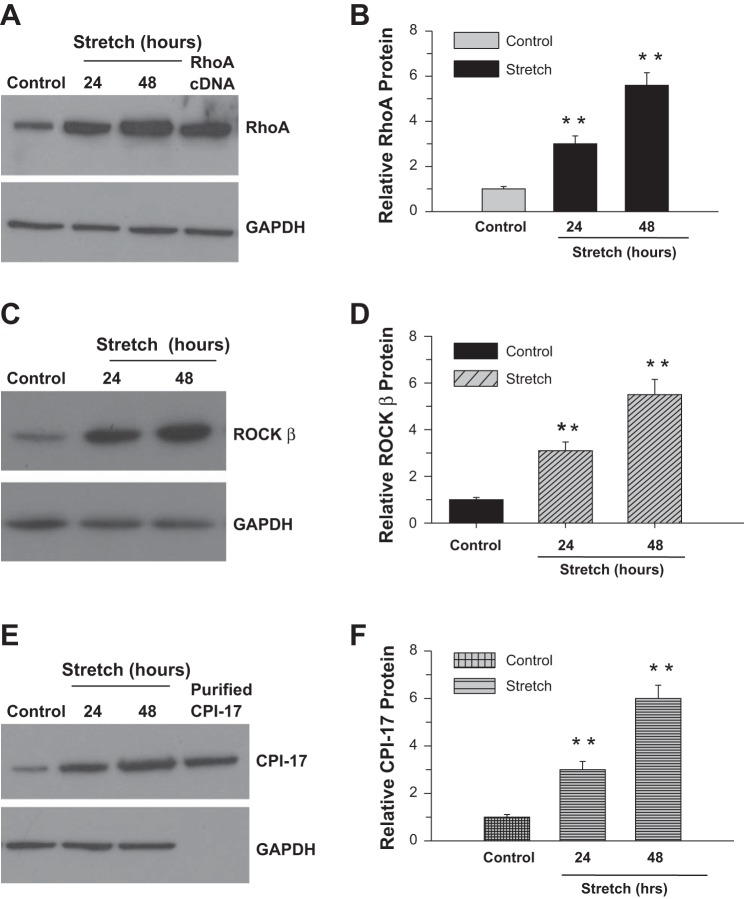
Stretch upregulates RhoA, ROCK-β, and CPI-17 expression.
In view of the cyclic stretch of DSM during normal bladder filling and contraction, the effect of cell stretch on Ca2+ sensitization pathway protein expression was determined at the mRNA (Fig. 5) and protein (Fig. 6) levels following application of equibiaxial stretching at 24 and 48 h. The mRNA (Fig. 5, A and B) and protein (Fig. 6, A and B) expression of RhoA is increased three- and sixfold following 24 and 48 h of stretching, respectively, compared with control cells. The effect of cell stretch on the expression of ROCK-β mRNA is shown in Fig. 5, C and D. There is a threefold increase in ROCK-β mRNA expression after 24 h. This is increased to fivefold after 48 h. Similar levels of increase in ROCK-β protein expression are shown in Fig. 6, C and D. Cell stretch also increased CPI-17 mRNA (Fig. 5, E and F) and protein (Fig. 6, E and F) expression compared with nonstretched cells.
Fig. 5.
Cyclic stretch induces RhoA, ROCK-β, and C-kinase-activated protein phosphatase I inhibitor (CPI-17) mRNA expression in bladder smooth muscle (BSM). Human bladder detrusor myocytes were subjected to cyclic stretch/relaxation for 24 or 48 h (A–F). Nonstretched cells were used as a control. RNA prepared from control and stretched cells were subjected to RT-PCR analysis for RhoA, ROCK-β, and CPI-17. GAPDH served as a loading control. B, D, and F: quantification of RT-PCR (A, C, and E). Data are presented as the means ± SD (n = 6; 6 of control and 6 of stretched cell samples from separate experiments were used in this experiment). **P < 0.01 compared with control cells.
Fig. 6.
Cyclic stretch induces RhoA, ROCK-β, and CPI-17 protein expression in BSM. Human bladder detrusor myocytes were subjected to cyclic stretch/relaxation for 24 or 48 h (A–F). Equal amounts of whole cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibodies to RhoA, ROCK-β, and CPI-17. Nonstretched cells were used as a control. GAPDH served as a loading control. B, D, and F: quantification of proteins (A, C, and E). Data are presented as the means ± SD (n = 6; 6 of control and 6 of stretched cell samples from separate experiments were used in this experiment). **P < 0.01 compared with control cells.
Stretch upregulates GATA-6 and NF-κB DNA-binding activity.
Our previous study showed that CPI-17 transcription is activated by GATA-6 and NF-κB transcription factors that bind multiple GATA and κB motifs, respectively, on the CPI-17 promoter (9). To determine the activation of GATA-6 and NF-κB in human BSM cells in response to stretch, binding of nuclear NF-κB p50 or GATA-6 from stretched and nonstretched cells to their consensus sequence was measured using TF ELISA. Cells were exposed to cyclic stretch for 24 and 48 h, and GATA-6 and NF-κB p50 DNA-binding activity was assessed using a transcription factor ELISA. Figure 7A shows the binding of GATA-6 in control and the cells that were subjected to stretch to its consensus sequence. Figure 7B shows the binding of NF-κB p50 in control and the cells that were subjected to stretch to its consensus sequence. As shown in Fig. 7, A and B, GATA-6 (Fig. 7A) and NF-κB p50 (Fig. 7B) binding was increased in stretched cells compared with control. To verify the TF ELISA data, we carried out the oligonucleotide competition analysis. In competition analyses, an excess of wild-type (5×, 20×, and 50×), but not mutant (at GATA and GGACTCCC sequences), consensus oligonucleotides competed with the oligonucleotide sequence immobilized on the plate for binding to GATA-6 and NF-κB p50 in the TF ELISA, thereby confirming specificity of the interaction.
Fig. 7.
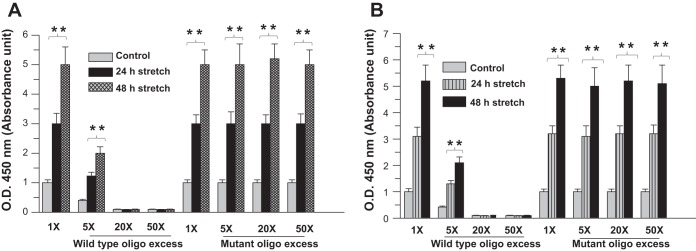
Stretch upregulates NF-κB and GATA-6 DNA binding activity. A and B: nuclear extracts from human BSM cells exposed to stretch or control were assayed using a transcription factor ELISA to determine DNA-binding activity of GATA-6 and NF-κB. The graph shows binding of GATA-6 (A) and NF-κB p50 (B) to their consensus oligonucleotides expressed as absorbance units at 450 nm. In oligonucleotide competition assay, increasing amounts of wild-type (WT) or mutant NF-κB-binding and WT or mutant GATA-6-binding consensus DNA sequence were added to the ELISA plate. Data are representative of at least 3 independent triplicate experiments. **P < 0.01 compared with control cells.
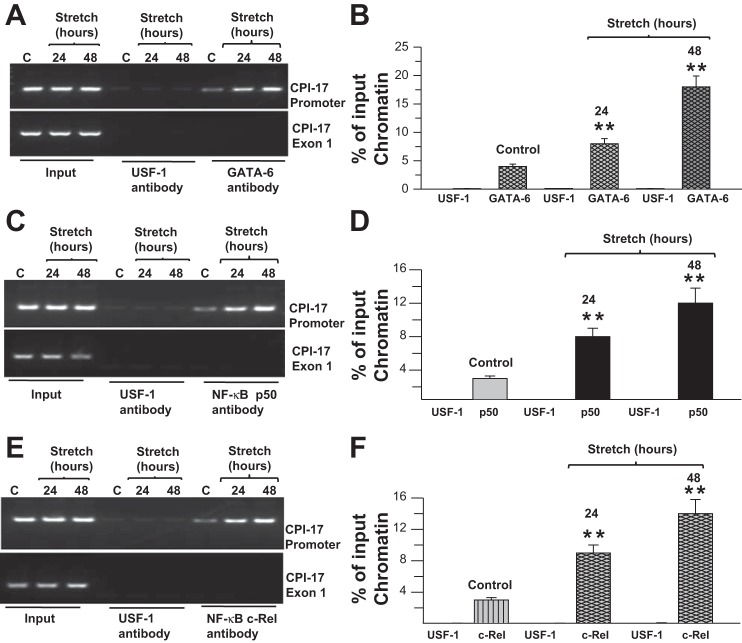
Stretch promotes the recruitment of GATA-6 and NF-κB to the CPI-17 promoter.
We tested the binding of GATA-6 and NF-κB, the transcription factors that activate CPI-17 transcription to the CPI-17 promoter, using a ChIP assay. Samples immunoprecipitated with anti-GATA-6 antibody yielded prominent PCR products in stretch (Fig. 8A, lanes 8 and 9) compared with control cells (Fig. 8A, lane 7); however, samples immunoprecipitated with anti-upstream stimulatory factor (USF)-1 transcription factor antibody failed to yield PCR products (Fig. 8A, lanes 4, 5, and 6). Similarly, samples immunoprecipitated with anti-NF-κB p50 and anti-c-Rel antibodies yielded prominent PCR products in stretch (Fig. 8, C and E, lanes 8 and 9) compared with control (Fig. 8, C and E, lane 7) human BSM cells, whereas samples immunoprecipitated with anti-USF-1 transcription factor antibody failed to yield PCR products (Fig. 8, C and E, lanes 4, 5, and 6). Because the input is a certain percentage of chromatin without going through the immunoprecipitation process and indicative for the presence and amount of chromatin used in the ChIP reaction, the data derived from the input sample were used to normalize the amount of chromatin. The relative intensities of the bands shown in Fig. 8, B, D, and F, were determined as a percentage of input chromatin (Fig. 8, A, C, and E). GATA-6 binding to the CPI-17 promoter is increased fourfold (Fig. 8, A and B) in equibiaxial stretched cells compared with nonstretched cells. CPI-17 promoter binding to NF-κB p50 and c-Rel are also increased following cell stretch (Fig. 8, C–F). These data indicate that stretch promotes the binding of GATA-6 and NF-κB to the CPI-17 promoter.
Fig. 8.
Stretch upregulates NF-κB and GATA-6 DNA binding to CPI-17 promoter. A–F: chromatin samples prepared from control (marked as C) and stretched DSM cells were immunoprecipitated with anti-GATA-6, anti-NF-κB p50 anti-c-Rel, and anti-upstream stimulatory factor (USF)-1 antibodies. The anti-USF-1 antibody served as a negative control. Precipitated fragments were PCR amplified using primers specific for the GATA and κB motifs on the mouse CPI-17 promoter (top) or CPI-17 exon-1 (bottom). B, D and F: quantification of band intensities from agarose gels (A, C, and E) are presented as a percentage of input chromatin. Data are presented as the means ± SD (n = 6; 6 of control and 6 of stretched cell samples from separate experiments were used in this experiment). **P < 0.01 compared with control cells.
Cotransfection of human BSM cells with the 1.333-kb CPI-17 promoter reporter construct and either GATA-6 or NF-κB cDNA resulted in activation of the 1.333-kb promoter in stretched cells compared with nonstretched cells (Fig. 9A). As shown in Fig. 9A, cotransfection with GATA-6 and NF-κB significantly upregulated CPI-17 promoter activity compared with either GATA-6 or NF-κB alone in response to stretch, suggesting that these two transcription factors act additively to activate the CPI-17 gene. Transfection analysis of Mut1, Mut2, and Mut3 1.33-kb CPI-17 promoters in human BSM demonstrated that mutation of the GATA and κB motifs (separately or in combination) reduced promoter activity. Thus both GATA and κB motifs are required for GATA-6- and NF-κB-mediated transcriptional activation of the 1.33-kb CPI-17 promoter in response to stretch. These data indicate that stretch promotes the transcriptional activation of CPI-17 promoter activity through GATA-6 and NF-κB transcription factors.
Fig. 9.
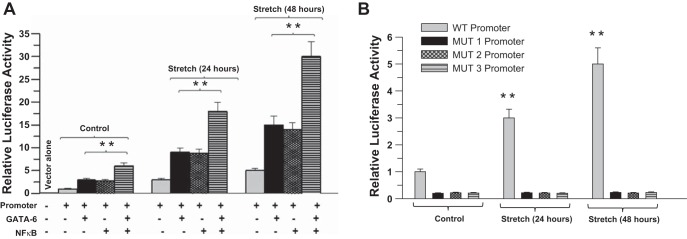
Stretch promotes the transcriptional activation of CPI-17 promoter by GATA-6 and NF-κB in primary BSM cells. A: human BSM cells were cotransfected with murine WT CPI-17 promoter luciferase constructs and GATA-6, NF-κB p50, and c-Rel cDNA. Luciferase activity was measured in each sample after 72 h and is presented relative to that of Renilla luciferase. Data are presented as means ± SD from 5 independent experiments. **P < 0.01 compared with stretched cells. B: human BSM cells were transfected with murine WT or mutant CPI-17 promoter luciferase constructs. The NF-κB binding site (κB motif) mutation in the 1.33-kb CPI-17 promoter is designated MUT1. The GATA-6 binding site (multiple GATA motifs) mutation is designated MUT2. The double mutant of both Mut1 and Mut2 mutations is designated MUT3. Luciferase activity was measured in each sample after 72 h and is presented relative to that of Renilla luciferase. Data are presented as means ± SD from 5 independent experiments. **P < 0.01 compared with stretched cells.
DISCUSSION
Bladder outlet obstruction leads to structural and functional changes in urinary bladders, presumably stimulated by urodynamic overload. Outlet obstruction results in bladder wall smooth muscle hypertrophy in both animal models and men with BPH. Patients with BPH-induced pBOO consistently produce high-pressure voiding (15) consistent with observations from the pBOO rat model (27). In patients with BPH, bladder outlet obstruction increases the mechanical stretch of the bladder wall during micturition (29). This BOO-derived mechanical stretch has been investigated as a possible trigger for both structural hypertrophy and functional alterations reported in the detrusor muscle after obstruction (51). In vitro models of mechanical stretch, where BSM cells are cultured on a deformable membrane, has been used to demonstrate that the mechanical stretch modifies gene expression and transcription (1, 37). In general, cyclic strain has been used as a model of the strain experienced by BSM cells during obstruction although clear evidence for the in vivo physiological existence of cyclic strain of BSM cells has not been presented.
We evaluated the changes in the expression levels of Ca2+ sensitization pathway proteins RhoA and ROCK. These proteins, along with CPI-17, help to maintain myosin phosphorylation without an increased cytosolic calcium concentration by decreasing phosphatase activity and inhibiting dephosphorylation (30, 44, 47, 49). Previous studies have shown the important role of the small GTPase Rho and its effector Rho-associated kinase (Rho-kinase/ROK/ROCK) on Ca2+-independent regulation of smooth muscle contraction (23). The RhoA/ROCK pathway modulates the phosphorylation of the myosin II light chain MLC20, mainly through the inhibition of myosin phosphatase, and contributes to agonist-induced Ca2+ sensitization in smooth muscle contraction (43, 44). The ROCK pathway is constitutively active, and stretch enhances ROCK pathway activation, contributing to myogenic tone generation in bladder wall smooth muscle, thereby affecting bladder compliance (41). Inhibiting ROCK with the inhibitor Y-27632 induced concentration-dependent relaxation of detrusor muscle strips at resting tension (41). Furthermore, spontaneously tonic smooth muscle, such as internal anal sphincter, has higher levels of RhoA/ROCK activity compared with phasic smooth muscle, indicating a role for ROCK in the maintenance of basal tone (35). The ROCK-mediated pathway is partly responsible for a high degree of force maintenance and slow relaxation in the DSM from decompensated obstructed bladder (7).
In this study, biaxial strain was applied by stretching the elastic membrane cyclically for 24 and 48 h to provide 2.5% strain. The strain level 2.5% used was sufficient to produce molecular changes without inducing apparent cell injury (12).
The strain may be different in different disease conditions of the bladder; thus future studies should consider the effect of various levels of strain on gene expressions. Bladders of long-term diabetics are abnormally stretched owing to the inability of the patient to sense bladder fullness. The same can be said for some patients with neurogenic bladders. These patients all show changes in the composition of the bladder wall, which in some cases can proceed to a fibrotic bladder and associated uropathies. However, there are no studies that report on the strain threshold, which initiates some of these changes. Obviously, these types of studies need to be carried out to be able to fully assess the relationship between strain and resultant uropathies.
In the present study, we applied cyclic, equibiaxial stretch rather than tonic stretch to cells grown on type I collagen-coated silicon membranes. Biaxial strain applied by stretching the elastic membrane of the wells cyclically for 24 h provides 2.5% strain and mimics the cyclic increase in tension with obstruction-induced high-pressure voiding in vitro (4, 52). During the filling and emptying of the bladder, DSM is subjected to cyclic physical deformation of the bladder environment. Thus the increased expression of the Ca2+ sensitization pathway proteins that helps to maintain DSM tone is not surprising.
The overexpression of RhoA and ROCK-β is seen at both the mRNA and protein levels, indicating that the synthesis of these proteins is upregulated following an increase in the intravesical pressure caused by an obstruction to bladder emptying. CPI-17 overexpression associated with the pBOO animal model is caused by increased GATA-6 binding to the CPI-17 promoter, suggesting that the pBOO-induced intravesical pressure alters the transcriptional regulation of CPI-17.
Our previous study showed that CPI-17 gene expression is regulated by GATA-6 and NF-κB transcription factors that bind multiple GATA and κB motifs, respectively, on the CPI-17 promoter and activate gene transcription (9). These component deficiencies decrease CPI-17 gene expression, resulting in impairment of smooth muscle contraction (9). Recently Kim et al. (24) reported that SP1 and SP3 regulate CPI-17 expression via promoter GC boxes through multiple kinase pathways. GATA-6 binding proximal to the GC box is also required for CPI-17 gene expression in aortic smooth muscle. This is in agreement with our findings that GATA motifs increase CPI-17 promoter activation in murine and human DSM cells. Furthermore, Kim et al. (24) demonstrated that silencing of NF-κB p65 (RelA) gene expression did not change the level of CPI-17 expression in aortic smooth muscle. However, our study demonstrated that NF-κB p50 and c-Rel positively regulate CPI-17 gene expression in murine and human DSM. This may be due to either tissue-specific regulation of this gene by NF-κB or the involvement of different NF-κB isoforms in the regulation of CPI-17 gene expression in aortic smooth muscle and BSM.
Although no data are presently available on the transcriptional regulation of RhoA and ROCK, our study provides evidence that stretch-activated GATA-6 and NF-κB directly control the expression of CPI-17, which is also involved in calcium sensitization. Stretch-induced aberrant activity of these transcription factors deregulates proteins involved in calcium sensitization, inhibiting contraction during smooth muscle remodeling and hypertrophy in pathological conditions. We reported previously (9) that the subunits of NF-κB, p50, and c-Rel are present in the nucleus at basal levels and nuclear localization increases in diseased DSM. It is possible that the bladder wall stretch in smooth muscle hypertrophy stimulates the increased accumulation of NF-κB p50 and c-Rel in the nucleus.
Bladder distention and cell stretching has been shown to induce AP-I subunit Fos B-mediated increased expression of extracellular matrix proteins in smooth muscle (37). The DNA-binding activity of AP-1 increased after stretch stimulation of smooth muscle cells in vitro in primary human bladder myocytes. In cultured pulmonary artery smooth muscle cells, mechanical stretch induces platelet-derived activating factor (PAF) receptor gene expression, mediated by NF-κB binding to the PAF receptor gene promoter. In these cells, protein kinase C activation is among the molecular features of NF-κB activation and translocation into the nucleus in mechanically stretched cells (13). Mechanical stretch also plays an important role in TGF-β1 gene upregulation and DNA binding of AP-1 through a stretch-induced signaling pathway in cultured human airway smooth muscle cells (31). Either treatment of human airway smooth muscle cells (HASMCs) with the inhibitors of RhoA, ROCK1/2, protein tyrosine kinase, phosphoinositide-3-kinase, MEK1/2, or AP-1 or transfection of HASMCs with AP-1 decoy oligonucleotide attenuated stretch-induced TGF-β1 expression through repressing the DNA-binding activity of AP-1. This suggests that RhoA and ROCK are involved in the stretch-induced transcriptional activation in smooth muscle and other tissues.
Gene transcription is rarely regulated by a single transcription factor in isolation, and combinatorial regulation offers a precise means to establish expression profiles. The combined action of two transcriptional regulators together generates a different transcriptional output from either factor acting alone. The timing, amplitude, or duration of the transcriptional response and the selection of target sites can be modulated in a combinatorial manner (21). The work presented herein exemplifies the fine tuning of gene expression within the transcriptional regulatory network. Our results show that stretch-activated GATA-6 and NF-κB induces CPI-17 transcription, and these findings offer new insight into a novel regulatory mechanism involving GATA-6 and NF-κB.
GRANTS
This study is supported by National Institutes of Health grant DK-052620 (O'Brien Urology Research Program) and partially by grants from AUA-Pfizer (WS1626383) and Bridge Funding from Perelman School of Medicine, University of Pennsylvania. Darshan Patel was a Summer Student Research Scholar supported through University of Pennsylvania O'Brien Urology Research Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.B. and S.C. conception and design of research; E.B., C.C.G., S.A.Z., B.M., R.C., and D.P.P. performed experiments; E.B. analyzed data; E.B. and A.J.W. interpreted results of experiments; E.B. prepared figures; E.B. drafted manuscript; E.B. and S.C. edited and revised manuscript; E.B. and S.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Edward J. Macarak, who designed the Tensorcell III stretch machine and helped with its use and the computer program.
REFERENCES
- 1.Adam RM, Eaton SH, Estrada C, Nimgaonkar A, Shih SC, Smith LE, Kohane IS, Bagli D, Freeman MR. Mechanical stretch is a highly selective regulator of gene expression in human bladder smooth muscle cells. Physiol Genomics 20: 36–44, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Adelstein RS, Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem 49: 921–956, 1980 [DOI] [PubMed] [Google Scholar]
- 3.Austin JC, Chacko SK, DiSanto M, Canning DA, Zderic SA. A male murine model of partial bladder outlet obstruction reveals changes in detrusor morphology, contractility and Myosin isoform expression. J Urol 172: 1524–1528, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bagli DJ, Joyner BD, Mahoney SR, McCulloch L. The hyaluronic acid receptor RHAMM is induced by stretch injury of rat bladder in vivo and influences smooth muscle cell contraction in vitro [corrected]. J Urol 162: 832–840, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Barbee KA, Macarak EJ, Thibault LE. Strain measurements in cultured vascular smooth muscle cells subjected to mechanical deformation. Ann Biomed Eng 22: 14–22, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Benotmane AM, Hoylaerts MF, Collen D, Belayew A. Nonisotopic quantitative analysis of protein-DNA interactions at equilibrium. Anal Biochem 250: 181–185, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Bing W, Chang S, Hypolite JA, DiSanto ME, Zderic SA, Rolf L, Wein AJ, Chacko S. Obstruction-induced changes in urinary bladder smooth muscle contractility: a role for Rho kinase. Am J Physiol Renal Physiol 285: F990–F997, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Boopathi E, Gomes CM, Goldfarb R, John M, Srinivasan VG, Alanzi J, Malkowicz SB, Kathuria H, Zderic SA, Wein AJ, Chacko S. Transcriptional repression of Caveolin-1 (CAV1) gene expression by GATA-6 in bladder smooth muscle hypertrophy in mice and human beings. Am J Pathol 178: 2236–2251, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boopathi E, Hypolite JA, Zderic SA, Gomes CM, Malkowicz B, Liou HC, Wein AJ, Chacko S. GATA-6 and NF-kappaB activate CPI-17 gene transcription and regulate Ca2+ sensitization of smooth muscle contraction. Mol Cell Biol 33: 1085–1102, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burch JB. Regulation of GATA gene expression during vertebrate development. Semin Cell Dev Biol 16: 71–81, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chang S, Hypolite JA, Mohanan S, Zderic SA, Wein AJ, Chacko S. Alteration of the PKC-mediated signaling pathway for smooth muscle contraction in obstruction-induced hypertrophy of the urinary bladder. Lab Invest 89: 823–832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaqour B, Han JS, Tamura I, Macarak E. Mechanical regulation of IGF-I and IGF-binding protein gene transcription in bladder smooth muscle cells. J Cell Biochem 84: 264–277, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Chaqour B, Howard PS, Richards CF, Macarak EJ. Mechanical stretch induces platelet-activating factor receptor gene expression through the NF-kappaB transcription factor. J Mol Cell Cardiol 31: 1345–1355, 1999 [DOI] [PubMed] [Google Scholar]
- 14.DiSanto ME, Stein R, Chang S, Hypolite JA, Zheng Y, Zderic S, Wein AJ, Chacko S. Alteration in expression of myosin isoforms in detrusor smooth muscle following bladder outlet obstruction. Am J Physiol Cell Physiol 285: C1397–C1410, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Dmochowski RR. Bladder outlet obstruction: etiology and evaluation. Rev Urol 7, Suppl 6: S3–S13, 2005 [PMC free article] [PubMed] [Google Scholar]
- 16.Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem 118: 1104–1107, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Gaspar-Pereira S, Fullard N, Townsend PA, Banks PS, Ellis EL, Fox C, Maxwell AG, Murphy LB, Kirk A, Bauer R, Caamano JH, Figg N, Foo RS, Mann J, Mann DA, Oakley F. The NF-kappaB subunit c-Rel stimulates cardiac hypertrophy and fibrosis. Am J Pathol 180: 929–939, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorfien SF, Winston FK, Thibault LE, Macarak EJ. Effects of biaxial deformation on pulmonary artery endothelial cells. J Cell Physiol 139: 492–500, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Guven A, Lin WY, Neuman P, Kogan BA, Levin R, Mannikarottu A. Effect of age on the role of Rho-kinase in short-term partial bladder outlet obstruction. Urology 71: 541–545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y, Macarak EJ, Korostoff JM, Howard PS. Compression and tension: differential effects on matrix accumulation by periodontal ligament fibroblasts in vitro. Connect Tissue Res 45: 28–39, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Istrail S, Davidson EH. Logic functions of the genomic cis-regulatory code. Proc Natl Acad Sci USA 102: 4954–4959, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanematsu A, Ramachandran A, Adam RM. GATA-6 mediates human bladder smooth muscle differentiation: involvement of a novel enhancer element in regulating alpha-smooth muscle actin gene expression. Am J Physiol Cell Physiol 293: C1093–C1102, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Kawano Y, Yoshimura T, Kaibuchi K. Smooth muscle contraction by small GTPase Rho. Nagoya J Med Sci 65: 1–8, 2002 [PubMed] [Google Scholar]
- 24.Kim JI, Urban M, Young GD, Eto M. Reciprocal regulation controlling the expression of CPI-17, a specific inhibitor protein for the myosin light chain phosphatase in vascular smooth muscle cells. Am J Physiol Cell Physiol 303: C58–C68, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko LJ, Engel JD. DNA-binding specificities of the GATA transcription factor family. Mol Cell Biol 13: 4011–4022, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lien CL, Wu C, Mercer B, Webb R, Richardson JA, Olson EN. Control of early cardiac-specific transcription of Nkx2–5 by a GATA-dependent enhancer. Development 126: 75–84, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Malmgren A, Sjogren C, Uvelius B, Mattiasson A, Andersson KE, Andersson PO. Cystometrical evaluation of bladder instability in rats with infravesical outflow obstruction. J Urol 137: 1291–1294, 1987 [DOI] [PubMed] [Google Scholar]
- 28.McKay IA, Kirby L, Volyanik EV, Kumar V, Wong PW, Bustin SA. An enzyme-linked immunosorbent assay for the detection of agents which interfere with the DNA binding activities of transcription factors–exemplified by NF-IL6. Anal Biochem 265: 28–34, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Mirone V, Imbimbo C, Longo N, Fusco F. The detrusor muscle: an innocent victim of bladder outlet obstruction. Eur Urol 51: 57–66, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Mizuno Y, Isotani E, Huang J, Ding H, Stull JT, Kamm KE. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am J Physiol Cell Physiol 295: C358–C364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohamed JS, Boriek AM. Stretch augments TGF-beta1 expression through RhoA/ROCK1/2, PTK, and PI3K in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 299: L413–L424, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nitti VW. Pressure flow urodynamic studies: the gold standard for diagnosing bladder outlet obstruction. Rev Urol 7, Suppl 6: S14–S21, 2005 [PMC free article] [PubMed] [Google Scholar]
- 33.O'Neill LP, Turner BM. Histone H4 acetylation distinguishes coding regions of the human genome from heterochromatin in a differentiation-dependent but transcription-independent manner. EMBO J 14: 3946–3957, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orkin SH. GATA-binding transcription factors in hematopoietic cells. Blood 80: 575–581, 1992 [PubMed] [Google Scholar]
- 35.Patel CA, Rattan S. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol 291: G830–G837, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Polyak E, Boopathi E, Mohanan S, Deng M, Zderic SA, Wein AJ, Chacko S. Alterations in caveolin expression and ultrastructure after bladder smooth muscle hypertrophy. J Urol 182: 2497–2503, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Ramachandran A, Gong EM, Pelton K, Ranpura SA, Mulone M, Seth A, Gomez P, 3rd, Adam RM. FosB regulates stretch-induced expression of extracellular matrix proteins in smooth muscle. Am J Pathol 179: 2977–2989, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Development of a sensitive multi-well colorimetric assay for active NFkappaB. Nucleic Acids Res 29: E21, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakai Y, Nakagawa R, Sato R, Maeda M. Selection of DNA binding sites for human transcriptional regulator GATA-6. Biochem Biophys Res Commun 250: 682–688, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res 17: 6419, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiomi H, Takahashi N, Kawashima Y, Ogawa S, Haga N, Kushida N, Nomiya M, Yanagida T, Ishibashi K, Aikawa K, Yamaguchi O. Involvement of stretch-induced Rho-kinase activation in the generation of bladder tone. Neurourol Urodyn 32: 1019–1025, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Shirvani S, Xiang F, Koibuchi N, Chin MT. CHF1/Hey2 suppresses SM-MHC promoter activity through an interaction with GATA-6. Biochem Biophys Res Commun 339: 151–156, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522: 177–185, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stull JT, Tansey MG, Word RA, Kubota Y, Kamm KE. Myosin light chain kinase phosphorylation: regulation of the Ca2+ sensitivity of contractile elements. Adv Exp Med Biol 304: 129–138, 1991 [DOI] [PubMed] [Google Scholar]
- 46.van Berlo JH, Elrod JW, van den Hoogenhof MM, York AJ, Aronow BJ, Duncan SA, Molkentin JD. The transcription factor GATA-6 regulates pathological cardiac hypertrophy. Circ Res 107: 1032–1040, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang T, Kendig DM, Smolock EM, Moreland RS. Carbachol-induced rabbit bladder smooth muscle contraction: roles of protein kinase C and Rho kinase. Am J Physiol Renal Physiol 297: F1534–F1542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winston FK, Macarak EJ, Gorfien SF, Thibault LE. A system to reproduce and quantify the biomechanical environment of the cell. J Appl Physiol 67: 397–405, 1989 [DOI] [PubMed] [Google Scholar]
- 49.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol 535: 553–564, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xin M, Davis CA, Molkentin JD, Lien CL, Duncan SA, Richardson JA, Olson EN. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc Natl Acad Sci USA 103: 11189–11194, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi O. Response of bladder smooth muscle cells to obstruction: signal transduction and the role of mechanosensors. Urology 63: 11–16, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Yu G, Bo S, Xiyu J, Enqing X. Effect of bladder outlet obstruction on detrusor smooth muscle cell: an in vitro study. J Surg Res 114: 202–209, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Zhang EY, Stein R, Chang S, Zheng Y, Zderic SA, Wein AJ, Chacko S. Smooth muscle hypertrophy following partial bladder outlet obstruction is associated with overexpression of non-muscle caldesmon. Am J Pathol 164: 601–612, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]