Abstract
Enteric inhibitory neurotransmission is an important feature of the neural regulation of gastrointestinal motility. Purinergic neurotransmission, via P2Y1 receptors, mediates one phase of inhibitory neural control. For decades, ATP has been assumed to be the purinergic neurotransmitter and smooth muscle cells (SMCs) have been considered the primary targets for inhibitory neurotransmission. Recent experiments have cast doubt on both of these assumptions and suggested that another cell type, platelet-derived growth factor receptor-α-positive (PDGFRα+) cells, is the target for purinergic neurotransmission. We compared responses of PDGFRα+ cells and SMCs to several purine compounds to determine if these cells responded in a manner consistent with enteric inhibitory neurotransmission. ATP hyperpolarized PDGFRα+ cells but depolarized SMCs. Only part of the ATP response in PDGFRα+ cells was blocked by MRS 2500, a P2Y1 antagonist. ADP, MRS 2365, β-NAD, and adenosine 5-diphosphate-ribose, P2Y1 agonists, hyperpolarized PDGFRα+ cells, and these responses were blocked by MRS 2500. Adenosine 5-diphosphate-ribose was more potent in eliciting hyperpolarization responses than β-NAD. P2Y1 agonists failed to elicit responses in SMCs. Small hyperpolarization responses were elicited in SMCs by a small-conductance Ca2+-activated K+ channel agonist, cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine, consistent with the low expression and current density of small-conductance Ca2+-activated K+ channels in these cells. Large-amplitude hyperpolarization responses, elicited in PDGFRα+ cells, but not SMCs, by P2Y1 agonists are consistent with the generation of inhibitory junction potentials in intact muscles in response to purinergic neurotransmission. The responses of PDGFRα+ cells and SMCs to purines suggest that SMCs are unlikely targets for purinergic neurotransmission in colonic muscles.
Keywords: purinergic inhibitory neurotransmission, colonic motility, smooth muscle layer
enteric inhibitory motor neurotransmission consists of nitrergic, purinergic, and peptidergic components in the gastrointestinal (GI) tract (5, 7, 19). The purinergic component is characterized by a fast, transient inhibitory junction potential (fIJP) that is elicited by electrical field stimulation (EFS) of intrinsic nerves in the tunica muscularis (3, 8) and inhibited by the bee venom apamin (2). Activation of fIJPs causes membrane hyperpolarization in GI muscles, bringing transmembrane potential close to the equilibrium potential for K+ (EK). Hyperpolarization reduces the open probability of voltage-dependent Ca2+ channels and inhibits contractions. For many years, the purine neurotransmitter has been assumed to be ATP; however, more recent studies have questioned this assumption and shown that exogenous ATP does not mimic the endogenous neurotransmitter (33). These studies have suggested that β-nicotinamide adenine dinucleotide (β-NAD), or possibly its primary metabolite adenosine 5-diphosphate-ribose (ADPR), better satisfies the criteria for a neurotransmitter. These purines are released (or possibly formed after release, in the case of the metabolite) from motor nerve terminals (12, 26, 33). Postjunctional effects are mediated by P2Y1 purinoceptors, blocked completely by specific P2Y1 receptor antagonists (16, 20, 33), and absent in muscles of P2ry1−/− mice (15, 25). The postjunctional fIJP response depends partially on apamin-sensitive ion channels that have been identified as the product(s) of one or more of the paralogs of small-conductance Ca2+-activated K+ (SK) channels (Kcnn1–3) (16, 33, 39, 44).
The traditional view of enteric neurotransmission has been release of neurotransmitter from nerve varicosities and volume transmission to smooth muscle cell (SMC) receptors (38). However, the neurojunctional environment is complicated by the presence of at least two types of interstitial cells: interstitial cells of Cajal (ICC) and platelet-derived growth factor receptor-α-positive (PDGFRα+) cells (36). ICCs and PDGFRα+ cells express receptors for enteric inhibitory neurotransmitters and form gap junctions with SMCs (9, 23, 30, 31, 34). For example, PDGFRα+ cells express P2ry1, encoding P2Y1 receptors, and Kcnn3, encoding SK3. These cells also display Ca2+ transients and large-amplitude, apamin-sensitive outward currents in response to purines, and these responses are mediated by P2Y1 receptors (1, 31). Together, SMCs, ICCs, and PDGFRα+ cells form the postjunctional receptive field for enteric motor neurotransmitters, and we have referred to this integrative structure as the SMC-ICC-PDGFRα+ cell (SIP) syncytium (37). Because of the electrical coupling between the cells, conductance changes in any of the cells of the SIP syncytium affect the excitability of the greater syncytium. Thus neurotransmission to any of the cells of the SIP syncytium can regulate motor function. A comprehensive concept of enteric motor neurotransmission requires understanding of which cells, receptors, and effectors mediate responses in normal and abnormal muscles.
There has been controversy about the role of interstitial cells in enteric neurotransmission, and some investigators have discounted the functions of these cells as mediators of neurotransmission (18, 38). SMCs express purinergic receptors and SK channels (29, 41). However, previous studies have suggested that the current density attributable to SK channels is very small in SMCs (25, 30). Evidence for SK channel currents in SMCs has been collected at nonphysiological potentials, with nonphysiological ionic gradients used to emphasize resolution of these currents. Held at physiological membrane potentials, SMCs generate little or no outward currents in response to purines (26, 31). Thus questions remain regarding the relative contributions of SMCs and PDGFRα+ cells to hyperpolarization responses, which are the hallmark of purinergic neurotransmission in GI muscles (3, 8). In the present study we compared voltage responses to a variety of candidate purine neurotransmitters and selective P2Y1 receptor agonists under current-clamp conditions. Our results show that PDGFRα+ cells hyperpolarize rapidly and dramatically in response to purines but SMCs are depolarized or fail to respond to these compounds.
MATERIALS AND METHODS
Animals and Tissue Preparation
Pdgfratm11(EGFP)Sor/J heterozygote (PDGFRα-GFP) mice, which have enhanced green fluorescent protein (eGFP) in nuclei of PDGFRα+ cells throughout the body (22, 31), and their wild-type (C57BL/6) siblings were obtained from Jackson Laboratory (Bar Harbor, ME). Animals (3–6 wk postpartum) were anesthetized by isoflurane inhalation (AErrane, Baxter, Deerfield, IL) and killed by cervical dislocation. The abdomens were opened, and colons were removed and washed with Krebs-Ringer bicarbonate solution, as previously described (29). Mice were maintained and the experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Use and Care Committee at the University of Nevada approved experimental protocols.
Isolation of PDGFRα+ Cells and SMCs
Colonic muscles were equilibrated in Ca2+-free Hanks' solution, and cells were dispersed as described previously (29, 45). The resulting cell suspension was plated onto murine collagen-coated (2.5 μg/ml; BD Falcon, Franklin Lakes, NJ) glass coverslips in 35-mm culture dishes. The cells were allowed to settle for 10 min before addition of smooth muscle growth medium (Clonetics, San Diego, CA). The cells were placed in a 95% O2-5% CO2 incubator at 37°C and used for experiments within 1–5 h.
Electrophysiological Experiments
Patch clamping.
Cells from PDGFRα-eGFP mice were placed in a 300-μl chamber mounted on an inverted microscope. PDGFRα+ cells were identified by the fluorescence of eGFP in cell nuclei. SMCs were obtained from dispersion of wild-type siblings and identified on the basis of cell shapes. Whole cell configurations of the patch-clamp technique (dialyzing conditions for PDGFRα+ cells and SMCs and perforated patches using amphotericin B were also used for SMCs) were used to record membrane voltage under current clamp [current (I) = 0]. Pipette tip resistances were 5–7 MΩ. An Axopatch 200B amplifier with a CV-4 headstage (Axon Instruments, Foster City, CA) was used to measure membrane potentials. The recording (external) bath solutions and drugs dissolved in the external solutions at desired concentrations were applied to the cells by a gravity-fed pinch valve perfusion system (ValveLink8.2, AutoMate Scientific, Berkeley, CA). All experiments were performed at room temperature. The data were analyzed using Clampfit (pCLAMP, version 9.2, Axon Instruments) and GraphPad Prism (version 3.0, GraphPad Software, San Diego, CA) software.
Solutions and reagents for patch-clamp experiments.
The external solution for whole cell recording was Ca2+-containing physiological salt solution containing (mM) 5 KCl, 135 NaCl, 2 CaCl2, 10 glucose, 1.2 MgCl2, and 10 HEPES, with pH adjusted to 7.4 with Tris. Two types of pipette solutions were used: 1) with 20 nM Ca2+ (pipette solution A) and 2) with 100 nM Ca2+ (pipette solution B). Solution A contained, in addition to Ca2+, (in mM) 135 KCl, 0.0113 CaCl2, 3 MgATP, 0.1 NaGTP, 0.1 EGTA, and 10 HEPES, with pH adjusted to 7.2 with Tris. Solution B also contained (in mM) 135 KCl, 3.88 CaCl2, 3 MgATP, 0.1 NaGTP, 10 EGTA, and 10 HEPES, with pH adjusted to 7.2 with Tris. Free Ca2+ concentrations were calculated by MaxChelator software (http://maxchelator.stanford.edu). Adenosine 5′-triphosphate magnesium salt (ATP), adenosine 5′-diphosphate sodium salt (ADP), β-nicotinamide adenine dinucleotide hydrate (β-NAD), ADPR, and cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine (CyPPA), a selective activator of SK2 and SK3 channels, were obtained from Sigma-Aldrich (St. Louis, MO). MRS 2500 (a selective antagonist of P2Y1 receptor), MRS 2365 (a selective P2Y1 receptor agonist), and UCL 1684 (a nonpeptidic blocker of SK channels) were obtained from Tocris Bioscience (Ellisville, MO).
Statistical Analyses
Values are means ± SE of n cells. All statistical analyses were performed using GraphPad Prism. We used paired t-tests to estimate the effects of MRS 2500 or UCL 1684 on the responses to ATP, ADP, MRS 2365, β-NAD, and ADPR in PDGFRα+ cells and nonpaired t-test to investigate the difference of the responses between PDGFRα+ cells and SMCs to ATP and CyPPA. In all statistical analyses, P < 0.05 was considered statistically significant.
RESULTS
Giga seals were formed on SMCs and PDGFRα+ cells. SMCs were identified by standard morphological criteria and PDGFRα+ cells by the expression of eGFP in nuclei (22). The two types of cells were of significantly different size. Cell capacitances for SMCs averaged 34.1 ± 1.22 pF (n = 43 from 15 mice), whereas PDGFRα+ cells averaged 4.03 ± 0.27 pF (n = 61 from 51 mice). Experiments for this study were conducted in current-clamp mode, and under the conditions of our experiments (I = 0; see materials and methods), membrane potentials of SMCs averaged −26.7 ± 1.92 mV (n = 43 from 15 mice) and −19.8 ± 1.67 mV (n = 61 from 51 mice) for PDGFRα+ cells.
ATP Hyperpolarized PDGFRα+ Cells but Depolarized SMCs
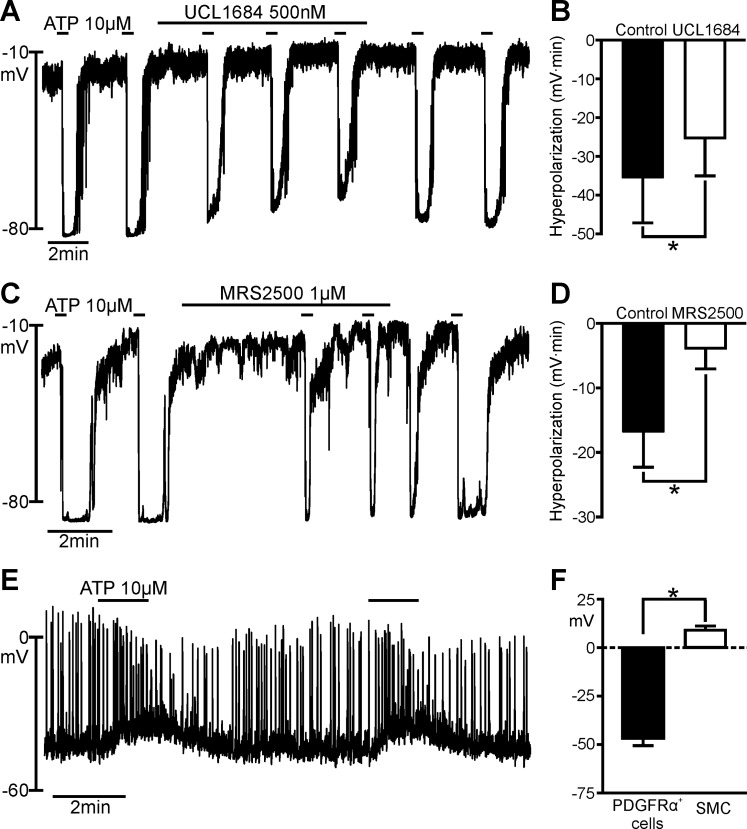
ATP is a potent ligand for purinergic receptors and can bind to most P2X and P2Y receptors (7). The effects of ATP on PDGFRα+ cells and SMCs were compared (Fig. 1) using pipette solution A. ATP caused rapid hyperpolarization of PDGFRα+ cells (averaging −46.70 ± 3.84 mV, n = 20) that reached a peak of about −80 mV (EK under the conditions of our experiments; Fig. 1, A and C). Eight of 11 cells tested displayed oscillatory responses to ATP. We used the SK channel blocker UCL 1684 (IC50 = 4.1 ± 0.2 nM) (32, 43) and the P2Y1 receptor-selective antagonist MRS 2500 (IC50 = 8.4 ± 0.8 nM) (28) to investigate the characteristics of purinergic hyperpolarization in PDGFRα+ cells. Hyperpolarization responses to ATP (10 μM) were reduced significantly by UCL 1684 (500 nM) and MRS 2500 (1 μM); however, neither drug blocked responses quantitatively (Fig. 1, A–D). Figure 1, B and D, shows summaries of the inhibitory effects of UCL 1684 and MRS 2500 on responses to ATP (10 μM) in PDGFRα+ cells. The average areas of hyperpolarization responses in Fig. 1B were −35.5 ± 11.61 and −25.3 ± 9.70 mV·min for control and UCL 1684-treated cells, respectively (n = 5). The inhibition of the response in Fig. 1B was 42.5 ± 12.07%. The average areas of the hyperpolarization responses in Fig. 1D were −16.8 ± 5.49 and −3.8 ± 3.19 mV·min for control and MRS 2500-treated cells, respectively (n = 6). Inhibition of the response in Fig. 1D was 89.3 ± 8.00%. The inhibitory effects of these drugs were reversible upon washout of the compounds (Fig. 1, A and C).
Fig. 1.
Effects of ATP on platelet-derived growth factor receptor-α-positive (PDGFRα+) cells and smooth muscle cells (SMCs). A and C: current-clamp recordings from PDGFRα+ cells in the whole cell configuration [current (I) = 0]. ATP (10 μM) elicited fast transient hyperpolarization with each application. Peak of hyperpolarization was about −80 mV [i.e., equilibrium potential for K+ (EK) under conditions of our experiments]. In A and C, hyperpolarization responses are reduced by UCL 1684 and MRS 2500; B and D show significantly reduced hyperpolarization responses. ATP responses recovered after washout of the inhibitors (A and C). B: summary of inhibitory effects of UCL 1684 on responses to ATP (n = 5). *P = 0.0260 (by paired t-test). D: summary of inhibitory effects of MRS 2500 on responses to ATP (n = 6). *P = 0.0073 (by paired t-test). Hyperpolarization responses in B and D are tabulated as area under response curves (mV·min). E: current-clamp recording from a SMC (I = 0) with perforated-patch, whole cell configuration. ATP (10 μM) elicited slowly developing depolarization in the SMC. F: summary of effects of ATP on PDGFRα+ cells and SMCs. ATP evoked opposite effects on resting membrane potentials (RMPs) of PDGFRα+ cells and SMCs. Average changes in membrane potentials were −46.7 ± 3.80 mV in PDGFRα+ cells (n = 20) and +13.5 ± 2.90 mV in SMCs (n = 7). *P < 0.0001 (by unpaired t-test).
In preliminary experiments using cell-dialyzing conditions, responses to purines were not resolved in SMCs, and it was thought that second messenger coupling might be compromised in these cells after dialysis. Therefore, we also studied SMCs using the perforated-patch technique (see materials and methods) in an attempt to enhance the sensitivity of SMCs to purinergic responses. We also tested a broader range of purine concentrations than that used for PDGFRα+ cells, and responses were consistent at higher concentrations. ATP (10 μM) failed to elicit hyperpolarization responses and, instead, evoked depolarization of SMCs, averaging +8.97 ± 2.16 mV (n = 7; Fig. 1E). Many SMCs generated spontaneous action potentials, which increased in frequency during the depolarization responses elicited by ATP. Figure 1F shows a summary of the hyperpolarization responses in PDGFRα+ cells and depolarization responses in SMCs elicited by ATP.
ADP Hyperpolarized PDGFRα+ Cells but Did Not Affect SMCs
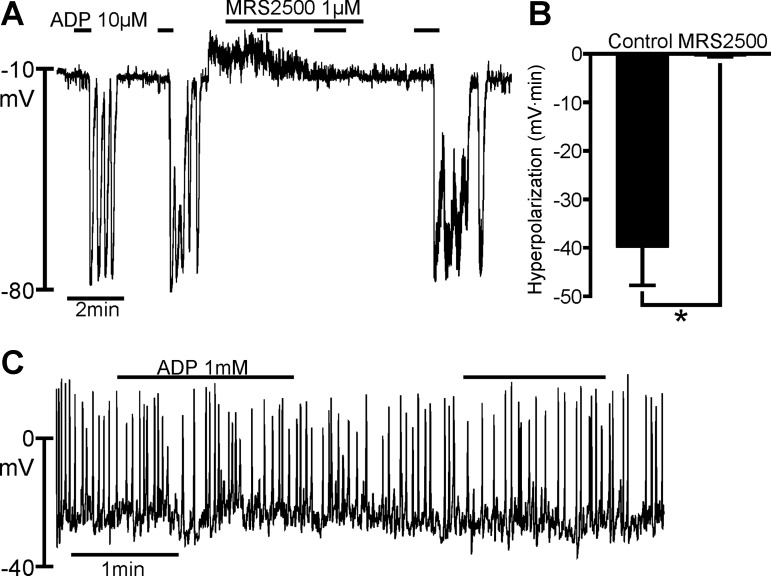
ATP breaks down to ADP rapidly when in contact with colonic muscles (12). Therefore, the effects of ATP in situ might be mediated partially by ADP, which is a more potent P2Y1 receptor agonist than ATP (7). The effects of ADP on PDGFRα+ cells and SMCs were compared using pipette solution A (Fig. 2). ADP provoked repeatable rapid hyperpolarization responses in PDGFRα+ cells, with peak membrane potential responses reaching EK (Fig. 2A). Five of six cells displayed oscillatory responses to ADP. The effects of ADP on PDGFRα+ cells were blocked reversibly by MRS 2500 (1 μM), and a summary of the effects of MRS 2500 is shown in Fig. 2B. The average areas of hyperpolarization responses were −39.8 ± 7.93 and −0.28 ± 0.28 mV·min for control and MRS 2500-treated cells, respectively (n = 6). Inhibition of the hyperpolarization responses by MRS 2500 averaged 99.4 ± 0.64%. In contrast to the responses of PDGFRα+ cells, ADP failed to elicit responses in SMCs (Fig. 2C).
Fig. 2.
Effects of ADP on PDGFRα+ cells and SMCs. A: current-clamp recording from a PDGFRα+ cell in the whole cell configuration (I = 0). ADP (10 μM) elicited transient hyperpolarizations with repetitive applications; peak hyperpolarization reached about −80 mV. Hyperpolarization responses in this cell were oscillatory in nature. Hyperpolarization response was blocked by MRS 2500 (1 μM). ADP effects recovered within a few minutes after removal of MRS 2500. B: summary of inhibitory effects of MRS 2500 on ADP responses (n = 6). *P = 0.0041 (by paired t-test). Hyperpolarization responses in B are tabulated as area under response curves (mV·min). C: current-clamp recording from a SMC with perforated-patch, whole cell recording (I = 0). ADP (1 mM) had no effect on RMP of SMCs.
P2Y1-Selective Agonist MRS 2365 Hyperpolarized PDGFRα+ Cells but Did Not Affect SMCs
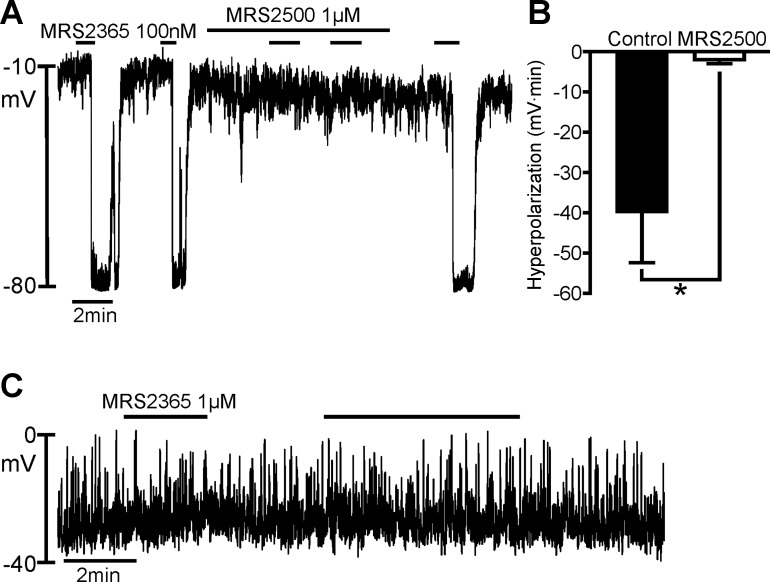
Blocking of ADP responses with MRS 2500 suggests that the responses are mediated primarily by P2Y1 receptors. Therefore, we compared the responses of a highly selective P2Y1 receptor agonist, MRS 2365 (EC50 = 0.40 ± 0.23 nM) (10, 35), on PDGFRα+ cells and SMCs using pipette solution A (Fig. 3). Repetitive application of MRS 2365 induced rapid hyperpolarizations, with the peak response in PDGFRα+ cells reaching EK (Fig. 3A). Four of five cells displayed oscillatory responses to MRS 2365. The response of PDGFRα+ cells to MRS 2365 was reversibly blocked by 95.8 ± 2.54% by MRS 2500 (1 μM), and a summary of the effects of MRS 2500 on MRS 2365 hyperpolarization is shown in Fig. 3B. The average areas of hyperpolarization were −39.96 ± 12.44 and −1.97 ± 1.01 mV·min for control and MRS 2500-treated cells, respectively (n = 5). As with ADP, MRS 2365 failed to produce responses in SMCs (Fig. 3C).
Fig. 3.
Effects of MRS 2365 on PDGFRα+ cells and SMCs. A: current-clamp recording in a PDGFRα+ cell with the dialyzed whole cell configuration (I = 0). MRS 2365 (100 nM) elicited repeatable fast transient hyperpolarization with a peak at about −80 mV (= EK) in the PDGFRα+ cell. This hyperpolarization was blocked by 1 μM MRS 2500 (A). As shown in B, this hyperpolarization was significantly blocked by MRS 2500 (1 μM). MRS 2365 effects recovered after washout of MRS 2500. B: summary of effects of MRS 2500 on MRS 2365 hyperpolarization responses (n = 5). *P = 0.0321 (by paired t-test). Hyperpolarization responses in B are tabulated as area under response curves (mV·min). C: current-clamp recording (I = 0) in a SMC with perforated-patch whole cell configuration. MRS 2365 (1 μM) did not affect RMP of SMCs (n = 6).
β-NAD and ADPR Hyperpolarize PDGFRα+ Cells but Do Not Affect SMCs
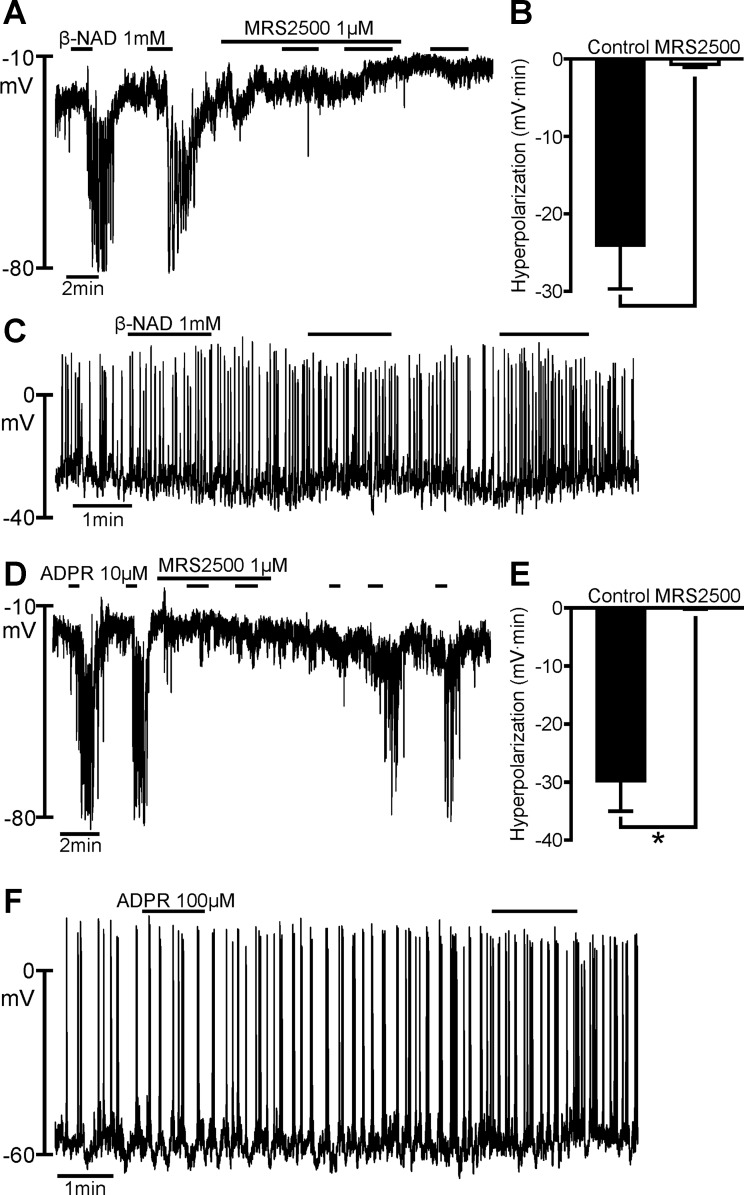
Using pipette solution A, we compared the effects of β-NAD and ADPR on PDGFRα+ cells and SMCs (Fig. 4). Recently, β-NAD was reported to be a P2Y1 agonist and was shown to mimic enteric inhibitory neurotransmitters better than exogenous ATP (26, 33). ADPR, a metabolite of β-NAD, is also a P2Y1 agonist (12, 21). β-NAD and ADPR evoked repeatable fast transient hyperpolarizations of PDGFRα+ cells that reached EK (Fig. 4, A and D). All cells displayed oscillatory responses to β-NAD and ADPR. MRS 2500 blocked hyperpolarizations evoked by β-NAD and ADPR in PDGFRα+ cells (Fig. 4, A and B), and effects of β-NAD and ADPR were restored after washout of MRS 2500 for 10 min. Responses to ADPR recovered more rapidly than responses to β-NAD after washout of MRS 2500 (Fig. 4, A and D). Figure 4, B and E, shows summaries of the inhibitory effects of MRS 2500 on responses to β-NAD (1 mM) and ADPR (10 μM), respectively. The average areas of hyperpolarization in Fig. 4B were −24.15 ± 5.54 and −0.65 ± 0.42 mV·min for control and MRS 2500-treated cells, respectively (n = 5). The inhibition of responses in Fig. 4B was 96.9 ± 2.19%. The average areas of hyperpolarization responses in Fig. 4E were −29.96 ± 5.02 and −0.12 ± 0.08 mV·min for control and MRS 2500-treated cells, respectively (n = 6). Inhibition of the response in Fig. 4E was 99.5 ± 0.31%. The effects of ADPR were elicited at far lower concentrations than the effects of β-NAD. In contrast to PDGFRα+ cells, SMCs were unaffected by β-NAD or ADPR (Fig. 4C).
Fig. 4.
Effects of β-NAD and adenosine 5-diphosphate-ribose (ADPR) on PDGFRα+ cells and SMCs. A and D: current-clamp recordings from a PDGFRα+ cell in the dialyzed whole cell configuration (I = 0). β-NAD (1 mM) and ADPR (10 μM) elicited fast transient hyperpolarization, with peaks at about −80 mV. Hyperpolarization responses were blocked by MRS 2500 (1 μM). B and E: summary of inhibition of β-NAD and ADPR responses by MRS 2500 (n = 5 and 6, respectively). *P = 0.0141 and 0.0020, respectively (by paired t-test). Hyperpolarization responses in B and E are tabulated as area under response curves (mV·min). C and F: current-clamp recordings from a SMC in the perforated-patch whole cell configuration (I = 0). β-NAD (1 mM) and ADPR (10 μM) had no effect on RMPs of SMCs.
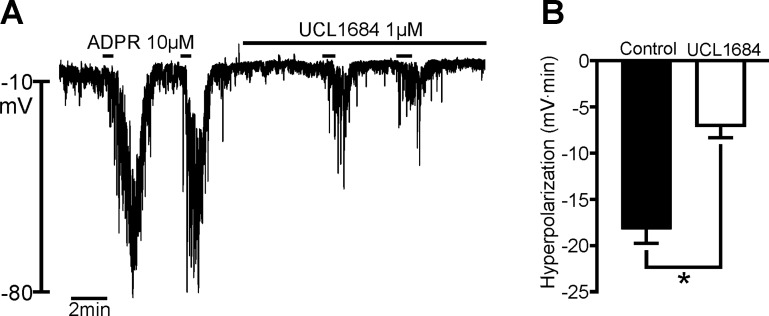
Responses of PDGFRα+ cells to ATP were not blocked totally by MRS 2500 or UCL 1684. This observation contrasts with responses in whole muscle, where MRS 2500 blocks fIJPs quantitatively (12, 14). There has been speculation that the partial block of fIJPs by apamin or UCL 1684 is due to the activation of conductance(s), in addition to SK channels, in response to purinergic neurotransmission. We tested the effects of ADPR (a potent P2Y1 agonist, the effects of which were blocked by MRS 2500; Fig. 4, D and E) before and after UCL 1648. Hyperpolarization responses to ADPR (10 μM) averaged −18.18 ± 1.55 mV·min in these experiments, and this response was reduced to −7.02 ± 1.32 mV·min after pretreatment with 1 μM UCL 1648 (59.5% block, n = 4; Fig. 5).
Fig. 5.
Effects of UCL 1684 on responses of PDGFRα+ cells to ADPR. A: effects of ADPR (10 μM) before and after pretreatment with UCL 1684 to block the small-amplitude Ca2+-activated K+ (SK) portion of the response. Addition of UCL 1684 (1 μM) blocked only part of the hyperpolarization response elicited by ADPR. B: summary of effects of UCL 1684 on ADPR responses. UCL 1684 significantly, but not quantitatively, reduced the hyperpolarization response to ADPR (n = 4). *P = 0.0214.
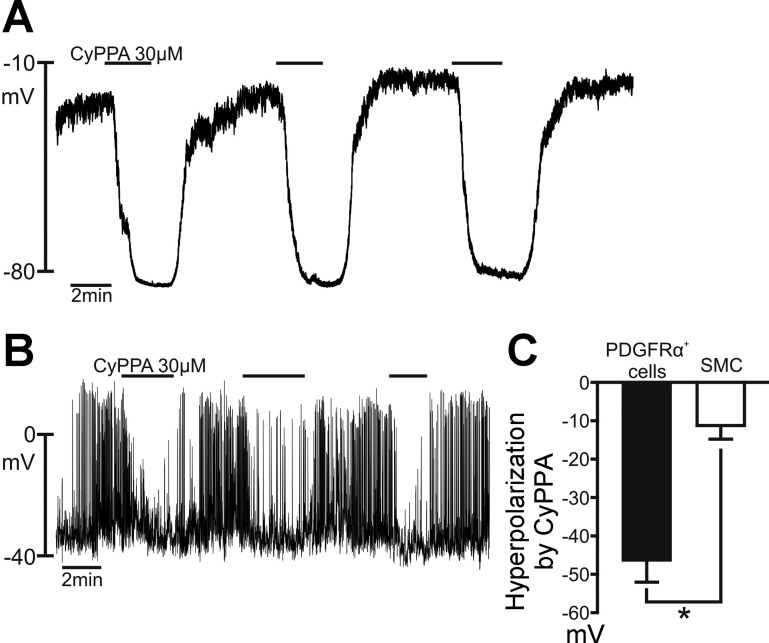
SK Channel Activator CyPPA Hyperpolarized PDGFRα+ Cells and SMCs
Previous studies showed that purines activate SK channels in PDGFRα+ cells (31). Other studies reported expression of SK channels and SK-like currents in SMCs (29, 41), so we also tested whether an SK channel activator, CyPPA, could mimic the effects of purines in these cells. CyPPA is a selective activator of SK2 and SK3 channels (EC50 = 14 ± 4 and 5.6 ± 1.6 μM for SK2 and SK3 channels, respectively) (24), and this drug (30 μM) induced repetitive hyperpolarization responses in PDGFRα+ cells, with a peak at EK, with use of pipette solution B (Fig. 6A). CyPPA (30 μM) caused smaller-amplitude hyperpolarization responses and tended to block spontaneous action potentials in SMCs with use of pipette solution B (Fig. 6B). Figure 6C shows a tabulation of the hyperpolarization responses induced by CyPPA in PDGFRα+ cells and SMCs. The average changes in membrane potential were −46.5 ± 5.50 and −11.2 ± 3.58 mV for PDGFRα+ cells and SMCs, respectively. These observations support the suggestion that SK channel availability is much greater in PDGFRα+ cells than in SMCs (31).
Fig. 6.
Effects of cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine (CyPPA) on PDGFRα+ cells and SMCs. A: current-clamp recording from a PDGFRα+ cell using the dialyzed whole cell configuration (I = 0). CyPPA (30 μM) evoked repeatable hyperpolarization responses in the PDGFRα+ cell, with a peak at about −80 mV. B: current-clamp recording (I = 0) in a SMC with the perforated-patch whole cell configuration. CyPPA (30 μM) elicited repeatable small hyperpolarization responses in the SMC. C: summary graphs of CyPPA effects in PDGFRα+ cells (n = 6) and SMCs (n = 6). Responses in SMCs were smaller than responses in PDGFRα+ cells. *P = 0.0003 (by unpaired t-test).
DISCUSSION
PDGFRα+ cells express P2ry1 and Kcnn3, and transcripts of these genes are far more abundant in PDGFRα+ cells than in the other cell types of the tunica muscularis (31, 34). In the present study we found that PDGFRα+ cells responded to purines and candidate purine neurotransmitters with hyperpolarization responses that drove membrane potential to EK, indicating that the responses were due to activation of a K+ conductance. All the purines tested, putative neurotransmitter compounds (ATP and β-NAD), primary metabolites of neurotransmitters (ADP and ADPR), and a selective P2Y1 agonist (MRS 2365), hyperpolarized PDGFRα+ cells. As with inhibitory junction potentials in intact muscles, purinergic hyperpolarizations were blocked by a selective P2Y1 receptor antagonist, mimicked by P2Y1 receptor agonists and an SK channel activator, and blocked by a selective SK channel blocker. The magnitude and rate of the hyperpolarization indicate a high current density of SK channels in these cells. SMCs express much lower levels of SK channel transcripts (34). SMCs showed no response to potent P2Y1 agonists, even though higher concentrations of these agonists were tested on SMCs than on PDGFRα+ cells. CyPPA caused slight hyperpolarization of SMCs, confirming low functional expression of SK channels in these cells, as previously reported (29). The purines tested elicited no response or depolarization (e.g., with ATP) in SMCs. When considered together with previous studies, our data suggest that SMCs are unlikely to mediate the purinergic component of enteric neurotransmission in the colon, because binding of the purinoceptors available on SMCs did not elicit the hyperpolarization responses that define purinergic inhibitory neurotransmission in GI muscles (3, 8, 16, 33). Our results strongly suggest that PDGFRα+ cells are the primary targets for the electrophysiological responses characteristic of purinergic neurotransmission. Our findings also further support the suggestion that β-NAD and/or ADPR are more likely than ATP to be the purinergic neurotransmitter(s) in the colon (12, 13, 26, 33). A schematic illustrating this concept is shown in Fig. 7.
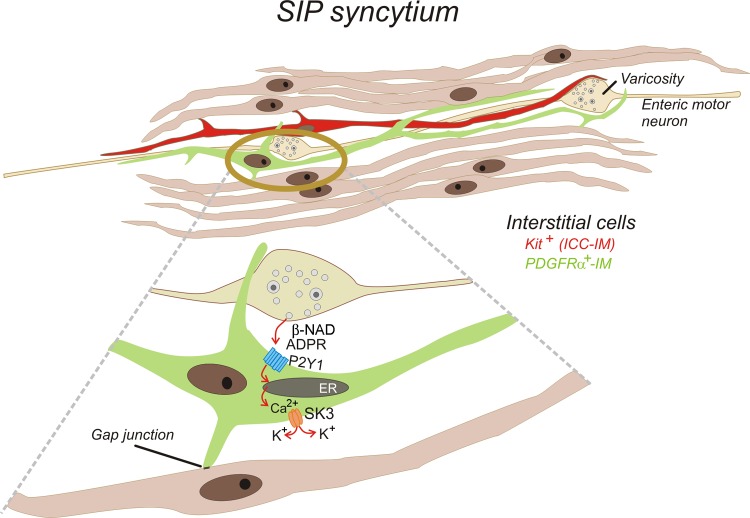
Fig. 7.
Schematic showing SIP syncytium and mechanism of purinergic neurotransmission from enteric inhibitory motor neurons to PDGFRα+ cells. SIP syncytium is composed of SMCs, intramuscular interstitial cells of Cajal (ICC-IM), and intramuscular PDGFRα+ cells (PDGFRα+-IM). These cells are electrically coupled through gap junctions. Thus conductance changes in any cell type lead to changes in syncytial input resistance and regulation of smooth muscle excitability. Current data favor the following concept for purinergic neurotransmission: β-NAD and/or ADPR are released from inhibitory motor neurons and bind to P2Y1 receptors expressed by PDGFRα+ cells. P2Y1 receptors couple through G (Gq/G11) proteins to PLCβ, leading to production of inositol-1,4,5-trisphosphate (not shown) and Ca2+ release from the endoplasmic reticulum (ER). Release of Ca2+ occurs as localized Ca2+ transients and waves. Increase in intracellular Ca2+ concentration activates small-conductance Ca2+-activated K+ (SK3) channels, which are expressed abundantly in PDGFRα+ cells. K+ efflux causes hyperpolarization that approaches the EK of PDGFRα+ cells. Hyperpolarization spreads via gap junctions to electrically coupled SMCs, resulting in the fast inhibitory junction potentials that are the hallmark of purinergic inhibitory neurotransmission in gastrointestinal muscles.
In conjunction with previous observations, the present study provides important insights into purinergic neurotransmission that can be summarized by the following reasoning and observations. 1) If ATP were the purinergic neurotransmitter, then fIJPs in intact muscles would be only partially blocked by MRS 2500 (Fig. 1). In fact, fIJPs in intact muscles are blocked completely by MRS 2500 (1 μM) (12, 14, 26). 2) If ATP was available to bind to purinoceptors on SMCs as a result of purinergic neurotransmission, then a depolarization response would be unmasked when P2Y1 receptors were blocked or genetically deactivated. No depolarization is observed in response to nerve stimulation when P2Y1 receptors are unavailable (12, 14, 15, 25, 26). 3) If SMCs transduced purinergic responses, then P2Y1 receptors could not be the purinoceptors involved in fIJPs, because agonists for these receptors had no effect on SMCs. In fact, loss of P2Y1 receptor availability completely blocks fIJPs in colonic muscles (15, 25). Thus postjunctional purinergic responses depend on a class of purinoceptors (i.e., P2Y1 receptors) that have low expression (34) and no obvious responsiveness in SMCs. 4) If ATP were a motor neurotransmitter, then its release would be inhibited by blocking nerve action potentials. Stimulating enteric neurons with agonists that bind to receptors expressed on somas or dendrites of motor neurons released ATP and β-NAD/ADPR (13). The release of β-NAD/ADPR was blocked by tetrodotoxin; however, the release of ATP was not blocked. These data suggest that β-NAD/ADPR is released from nerve terminals in muscle bundles but ATP comes largely from ganglionic sources.
PDGFRα+ cells were identified unequivocally in this study by expression of eGFP driven by the cell-specific endogenous promoters of Pdgfra (13). Previously, we showed colocalization of the reporter with antibodies against PDGFRα (31), and cells sorted on eGFP by fluorescein-activated cell sorting had significant enrichment in Pdgfra and greatly reduced expression of other cell-specific markers compared with the whole mixed-cell population obtained from enzymatic digestion of the tunica muscularis (34). These findings support the view that expression of the eGFP reporter reliably identifies PDGFRα+ cells in mixed-cell dispersions of the tunica muscularis.
The membrane potentials of isolated SMCs and PDGFRα+ cells were less negative than those recorded from cells in the colonic tunica muscularis in situ (39). However, additional factors affect the input resistances and, therefore, the resting membrane potentials of cells of the SIP syncytium in situ. For example, when spontaneous neural inputs were blocked with tetrodotoxin, cells in intact murine colonic muscles depolarized by 17 mV (39), demonstrating the tonic inhibition that regulates basal colonic excitability. Other factors available in intact muscles, such as prostaglandins and other autacoids, may further “tune” the membrane potential. After dispersion of cells, some forms of regulation are lost, and in the case of murine SMCs and PDGFRα+ cells, cells were more depolarized than they were likely to have been in situ. The fact, however, that PDGFRα+ cells hyperpolarized to values far more negative than the −40 to −50 mV resting potentials typically recorded in situ suggests that outward currents were activated in these cells that would tend to exert a potent hyperpolarizing effect on the SIP syncytium.
It is now clear from pharmacological studies and gene knockout studies that postjunctional responses to purines in colon muscles are mediated by P2Y1 receptors (14–17, 25, 26, 33, 44). As stated previously, appropriate receptors (P2Y1) and effectors (SK3 channels) are strongly expressed by PDGFRα+ cells, making these cells capable of generating responses with the same pharmacological profiles as fIJPs in intact muscles. SMCs and ICCs showed far lower expression of P2ry1, and ICCs were not included in this study, because very low relative expression of P2ry1 and Kcnn family transcripts make these cells unlikely targets for purinergic inhibitory responses (34). A generalized concept of postjunctional neural responses is beginning to emerge from studies of cells of the SIP syncytium (37). Different cellular components of the SIP syncytium express specialized receptors and ion channels that are fundamental to the responses of specific enteric neurotransmitters. Gene knockout studies in which ICCs fail to develop have suggested that postjunctional responses to cholinergic and nitrergic neural inputs are mediated primarily by ICCs, and the present study suggests that purinergic responses are contributed by PDGFRα+ cells (6, 42). Studies demonstrating direct responses of SMCs to neurotransmitters released from motor nerve terminals are lacking, except in the absence of ICCs (4).
A recent study showed that PDGFRα+ cells of GI muscles fire spontaneous localized Ca2+ transients that are mediated by release of Ca2+ from intracellular stores (1). Purinergic stimulation via P2Y1 receptors greatly enhanced the frequency of Ca2+ store release events and enhanced the development of Ca2+ waves in PDGFRα+ cells. P2Y1 receptors are typically coupled through G (Gq/G11) proteins to activation of phospholipase Cβ (PLC-β), generation of inositol-1,4,5-trisphosphate (IP3), and Ca2+ liberation from the endoplasmic reticulum via IP3 receptors (11). Ca2+ transients activated by purines were blocked by U-71322, an inhibitor of PLC-β, and 2-aminoethoxydiphenylborate, an inhibitor of IP3 receptors (1). Enhancement of Ca2+ release in PDGFRα+ cells may be the generalized mechanism by which binding of P2Y1 receptors couples to the activation of Ca2+-dependent outward current (via SK3 channels) and initiation of hyperpolarization responses in GI muscles.
It is important to highlight the observation that ADPR was far more potent than β-NAD in activating outward current responses. ADPR is a primary metabolite of β-NAD, and the kinetics for ADPR formation from β-NAD in colonic muscles are rapid (12). ADPR caused hyperpolarization and inhibition of contractions in intact colonic muscles, and these effects were blocked by MRS 2500 and apamin. These data suggest that ADPR could mediate a portion of the purinergic response in GI muscles. One might think that formation of a metabolite would be too slow to mediate the fIJP in muscles, but this question is not answerable, because there is no way to measure the actual rate of metabolite formation in situ. It has also not been determined whether ADPR is formed only in the postjunctional interstitium or whether ADPR is also released from enteric inhibitory neurons. Regardless of the source (i.e., released neurotransmitter or a metabolic product of β-NAD), ADPR is available to postjunctional receptors. As a P2Y1 agonist, ADPR must be considered, especially in light of its potency in activating outward currents in PDGFRα+ cells, as a potential mediator of purinergic neurotransmission (12). Moreover, ADPR could be a particularly important transmitter in human GI muscles, since it is prominent in tissue superfusates from these muscles during stimulation of intrinsic nerves (26).
Previous studies have described currents from SK channels in GI SMCs; however, the current density due to this conductance is low (29, 41). In our study the SK2 and SK3 channel agonist CyPPA (24) provided a positive control and confirmed that SK conductances are available in SMCs. CyPPA activated large-amplitude hyperpolarization in PDGFRα+ cells but much smaller responses in SMCs. These data are consistent with the differences in SK current density and channel expression by SMCs and PDGFRα+ cells (34). Under whole cell conditions, the net effects of the conductance(s) activated by purines in SMCs caused depolarization. Depolarization of SMCs could be achieved by ATP binding to P2X receptors or a nonselective cation conductance coupled to P2Y receptors. The nature of the conductance responsible for depolarization of SMCs was not explored in detail, because it was viewed as a purely pharmacological response that is not manifest in physiological responses to enteric inhibitory nerve stimulation.
Responses of PDGFRα+ cells to ATP were only partially blocked by a concentration of UCL 1684 sufficient to block SK channels quantitatively (32, 43). This observation suggests that a conductance in addition to SK channels is expressed by PDGFRα+ cells and linked to purinoceptors; however, the identity of this conductance is unknown. The fIJPs in colonic muscles of many laboratory animals and humans are also blocked only 40–60% by the SK channel blocker apamin (14, 16, 26, 27, 33, 40, 44), likewise suggesting the expression of an additional conductance. Further studies of PDGFRα+ cells may reveal the unknown conductance and its mechanism of activation in purinergic fIJPs.
We also noted that responses to ATP were not entirely blocked by MRS 2500, while hyperpolarization responses to other P2Y1 ligands were blocked more effectively by MRS 2500. These data suggest that ATP elicits hyperpolarization in PDGFRα+ cells by receptors in addition to P2Y1. Expression studies have shown low levels of P2ry2 and P2ry4 (34), and these receptors might transduce part of the response to ATP. As stated above, purinergic hyperpolarization responses are blocked quantitatively by MRS 2500 in intact muscles and purinergic fIJPs are absent in muscles of P2ry1−/− mice. Taken together, these findings suggest that ATP is unlikely to be the purine neurotransmitter released from enteric motor neurons, because if it were, fIJPs would be only partially blocked by P2Y1 antagonism or in the absence of these receptors. Availability of receptors in addition to P2Y1 receptors but strong dependence of fIJPs on P2Y1 receptors suggests that 1) the physiological neurotransmitter is a relatively selective P2Y1 agonist or 2) P2Y1 receptors are clustered selectively at postjunctional membranes, such that the neurotransmitter is free to bind only to this class of receptors.
In summary, PDGFRα+ cells display high expression levels of P2ry1 and Kcnn3, and these are the major receptor and effector required for postjunctional purinergic responses in GI muscles. PDGFRα+ cells respond to purines robustly with hyperpolarization responses that mimic the pharmacology and characteristics of the fIJPs evoked by stimulation of motor neurons in colonic muscles. SMCs depolarize in response to ATP and fail to respond to more selective P2Y1 agonists. These findings suggest that release of ATP from motor neurons and binding of ATP to SMC receptors is not likely to be an accurate concept of the purinergic component of enteric inhibitory neurotransmission. Responses of PDGFRα+ cells, in contrast, mimic the pharmacology and characteristics of purinergic responses in GI muscles, making these cells the likely targets for purinergic inhibitory neurotransduction.
GRANTS
This project was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-091336.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.K., V.M.-Y., S.D.K., and K.M.S. are responsible for conception and design of the research; M.K. performed the experiments; M.K., S.D.K., and K.M.S. analyzed the data; M.K., V.M.-Y., S.D.K., and K.M.S. interpreted the results of the experiments; M.K. and S.D.K. prepared the figures; M.K., S.D.K., and K.M.S. drafted the manuscript; M.K., V.M.-Y., S.D.K., and K.M.S. edited and revised the manuscript; M.K., V.M.-Y., S.D.K., and K.M.S. approved the final version of the manuscript.
REFERENCES
- 1.Baker SA, Hennig GW, Salter AK, Kurahashi M, Ward SM, Sanders KM. Distribution and Ca2+ signalling of fibroblast-like (PDGFRα+) cells in the murine gastric fundus. J Physiol 591: 6193–6208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks BE, Brown C, Burgess GM, Burnstock G, Claret M, Cocks TM, Jenkinson DH. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature 282: 415–417, 1979 [DOI] [PubMed] [Google Scholar]
- 3.Bennett MR, Burnstock G, Holman M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol 182: 541–558, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhetwal BP, Sanders KM, An C, Trappanese DM, Moreland RS, Perrino BA. Ca2+ sensitization pathways accessed by cholinergic neurotransmission in the murine gastric fundus. J Physiol 591: 2971–2986, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 345: 346–347, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA 93: 12008–12013, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci 64: 1471–1483, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G, Campbell G, Bennett M, Holman ME. Inhibition of the smooth muscle on the taenia coli. Nature 200: 581–582, 1963 [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Redelman D, Ro S, Ward SM, Ordog T, Sanders KM. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am J Physiol Cell Physiol 292: C497–C507, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther 311: 1038–1043, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol Cell Physiol 265: C577–C606, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Durnin L, Hwang SJ, Ward SM, Sanders KM, Mutafova-Yambolieva VN. Adenosine 5-diphosphate-ribose is a neural regulator in primate and murine large intestine along with β-NAD+. J Physiol 590: 1921–1941, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durnin L, Sanders KM, Mutafova-Yambolieva VN. Differential release of β-NAD+ and ATP upon activation of enteric motor neurons in primate and murine colons. Neurogastroenterol Motil 25: e194–204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallego D, Gil V, Aleu J, Martinez-Cutillas M, Clave P, Jimenez M. Pharmacological characterization of purinergic inhibitory neuromuscular transmission in the human colon. Neurogastroenterol Motil 23: 792-e338, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Gallego D, Gil V, Martinez-Cutillas M, Mane N, Martin MT, Jimenez M. Purinergic neuromuscular transmission is absent in the colon of P2Y1 knocked out mice. J Physiol 590: 1943–1956, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallego D, Hernandez P, Clave P, Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol 291: G584–G594, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gallego D, Vanden Berghe P, Farre R, Tack J, Jimenez M. P2Y1 receptors mediate inhibitory neuromuscular transmission and enteric neuronal activation in small intestine. Neurogastroenterol Motil 20: 159–168, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Goyal RK, Chaudhury A. Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut. Am J Physiol Gastrointest Liver Physiol 298: G10–G13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal RK, Rattan S, Said SI. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurones. Nature 288: 378–380, 1980 [DOI] [PubMed] [Google Scholar]
- 20.Grasa L, Gil V, Gallego D, Martin MT, Jimenez M. P2Y1 receptors mediate inhibitory neuromuscular transmission in the rat colon. Br J Pharmacol 158: 1641–1652, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustafsson AJ, Muraro L, Dahlberg C, Migaud M, Chevallier O, Khanh HN, Krishnan K, Li N, Islam MS. ADP ribose is an endogenous ligand for the purinergic P2Y1 receptor. Mol Cell Endocrinol 333: 8–19, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor-α receptor signaling mechanisms. Mol Cell Biol 23: 4013–4025, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/WV mouse small intestine. J Auton Nerv Syst 80: 142–147, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Hougaard C, Eriksen BL, Jorgensen S, Johansen TH, Dyhring T, Madsen LS, Strobaek D, Christophersen P. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br J Pharmacol 151: 655–665, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol 590: 1957–1972, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. β-Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology 140: 608–617 e606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keef KD, Du C, Ward SM, McGregor B, Sanders KM. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology 105: 1009–1016, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, Maddileti S, Marquez VE, Harden TK, Jacobson KA. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.10]hexane ring system locked in a Northern conformation: enhanced potency as P2Y1 receptor antagonists. J Med Chem 46: 4974–4987, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koh SD, Dick GM, Sanders KM. Small-conductance Ca2+-dependent K+ channels activated by ATP in murine colonic smooth muscle. Am J Physiol Cell Physiol 273: C2010–C2021, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Komuro T, Seki K, Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol 62: 295–316, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Kurahashi M, Zheng H, Dwyer L, Ward SM, Don Koh S, Sanders KM. A functional role for the “fibroblast-like cells” in gastrointestinal smooth muscles. J Physiol 589: 697–710, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malik-Hall M, Ganellin CR, Galanakis D, Jenkinson DH. Compounds that block both intermediate-conductance (IKCa) and small-conductance (SKCa) calcium-activated potassium channels. Br J Pharmacol 129: 1431–1438, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. β-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci USA 104: 16359–16364, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peri LE, Sanders KM, Mutafova-Yambolieva VN. Differential expression of genes related to purinergic signaling in smooth muscle cells, PDGFRα-positive cells and interstitial cells of Cajal in the murine colon. Neurogastroenterol Motil 25: e609–620, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson KA. Adenine nucleotide analogues locked in a Northern methanocarba conformation: enhanced stability and potency as P2Y1 receptor agonists. J Med Chem 45: 2090–2100, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol 588: 4621–4639, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility—insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol 9: 633–645, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarna SK. Are interstitial cells of Cajal plurifunction cells in the gut? Am J Physiol Gastrointest Liver Physiol 294: G372–G390, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Spencer NJ, Bywater RA, Holman ME, Taylor GS. Inhibitory neurotransmission in the circular muscle layer of mouse colon. J Auton Nerv Syst 70: 10–14, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Spencer NJ, Bywater RA, Holman ME, Taylor GS. Spontaneous and evoked inhibitory junction potentials in the circular muscle layer of mouse colon. J Auton Nerv Syst 69: 115–121, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Vogalis F, Goyal RK. Activation of small conductance Ca2+-dependent K+ channels by purinergic agonists in smooth muscle cells of the mouse ileum. J Physiol 502: 497–508, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci 20: 1393–1403, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wulff H, Zhorov BS. K+ channel modulators for the treatment of neurological disorders and autoimmune diseases. Chem Rev 108: 1744–1773, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Lomax AE, Paterson WG. P2Y1 receptors mediate apamin-sensitive and -insensitive inhibitory junction potentials in murine colonic circular smooth muscle. J Pharmacol Exp Ther 333: 602–611, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol 587: 4905–4918, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]